Looking for Microbial Biosignatures in All the Right Places: Clues for Identifying Extraterrestrial Life in Lava Tubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.1.1. Site Description El Malpais National Monument, New Mexico, USA
2.1.2. Site Description Hawai`i, USA
2.1.3. Sampling
2.2. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
2.3. Geochemistry
2.4. DNA Extraction and Amplification
2.5. Sequencing and Phylogenetic Analyses
3. Results
3.1. General Results
3.2. Alpha Diversity
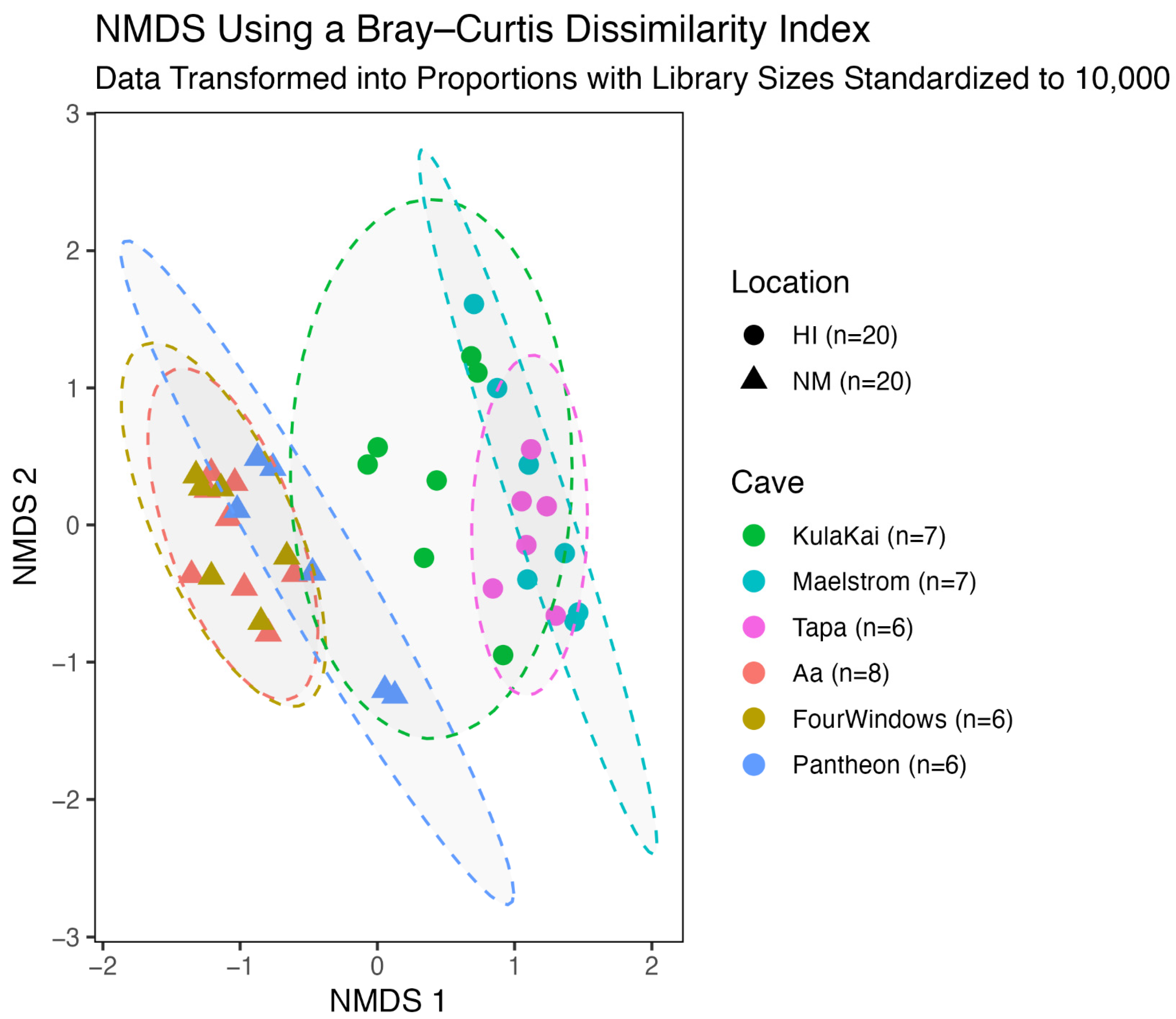
3.3. Beta Diversity
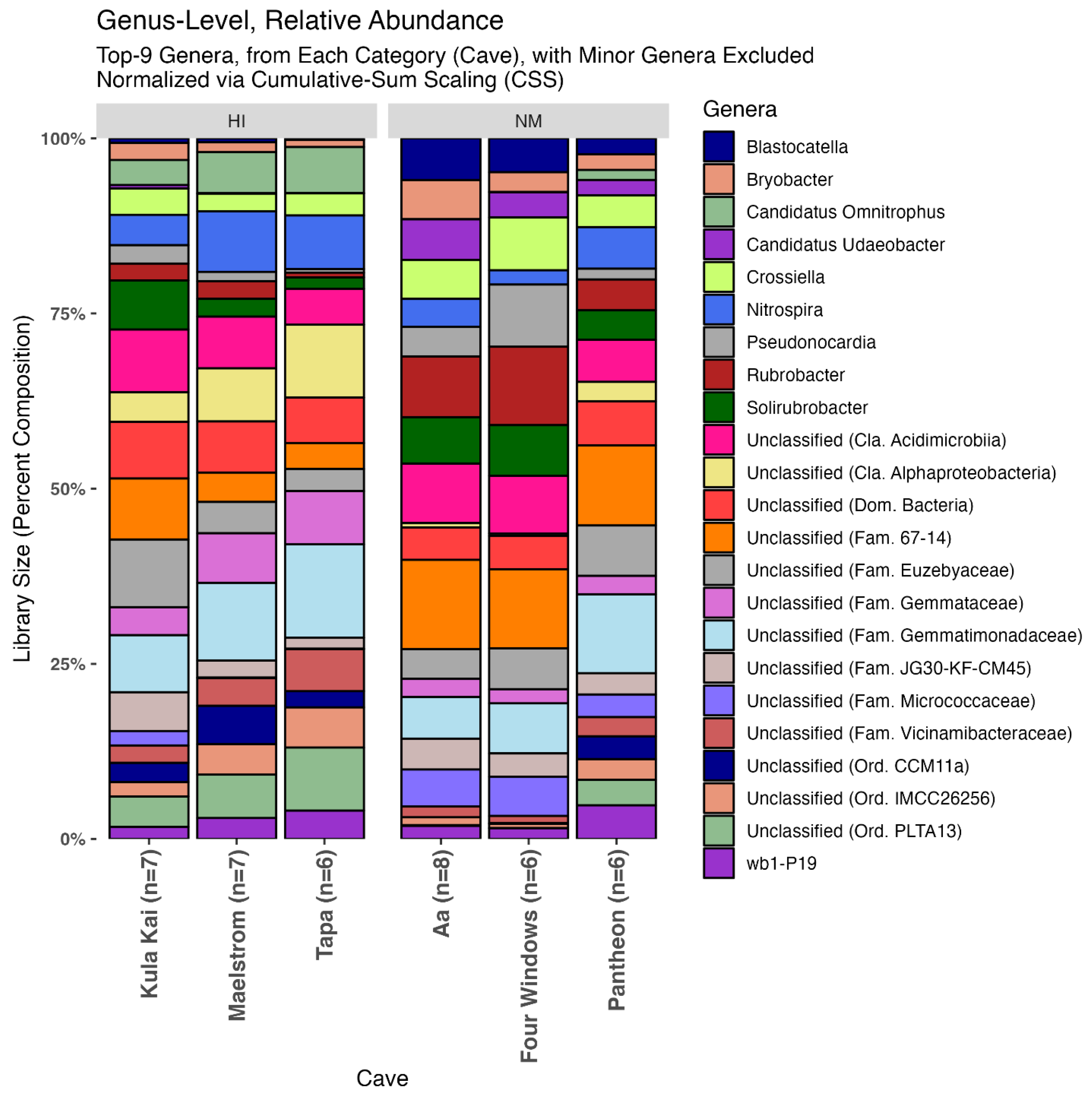
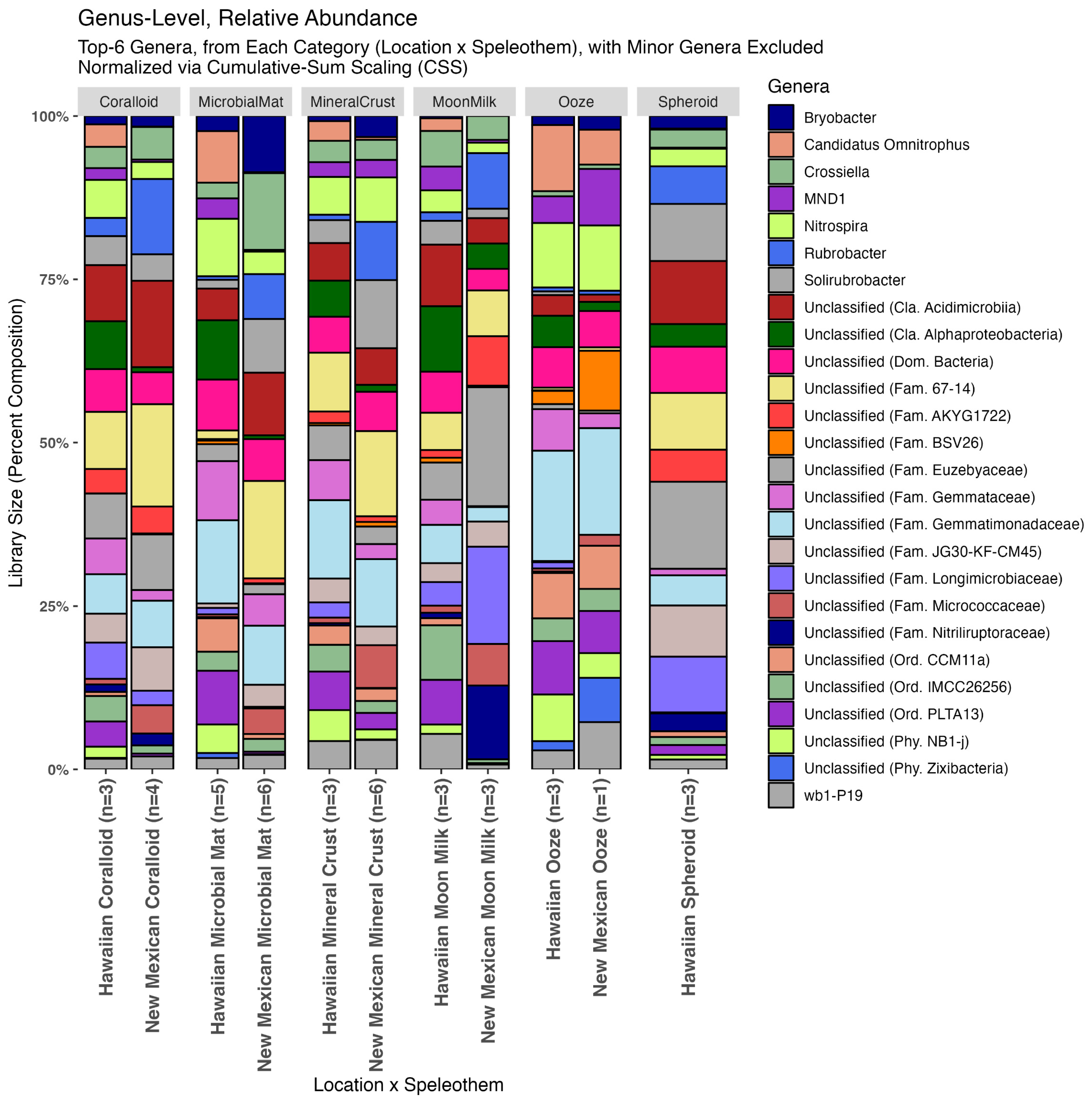
3.4. Relative Abundance
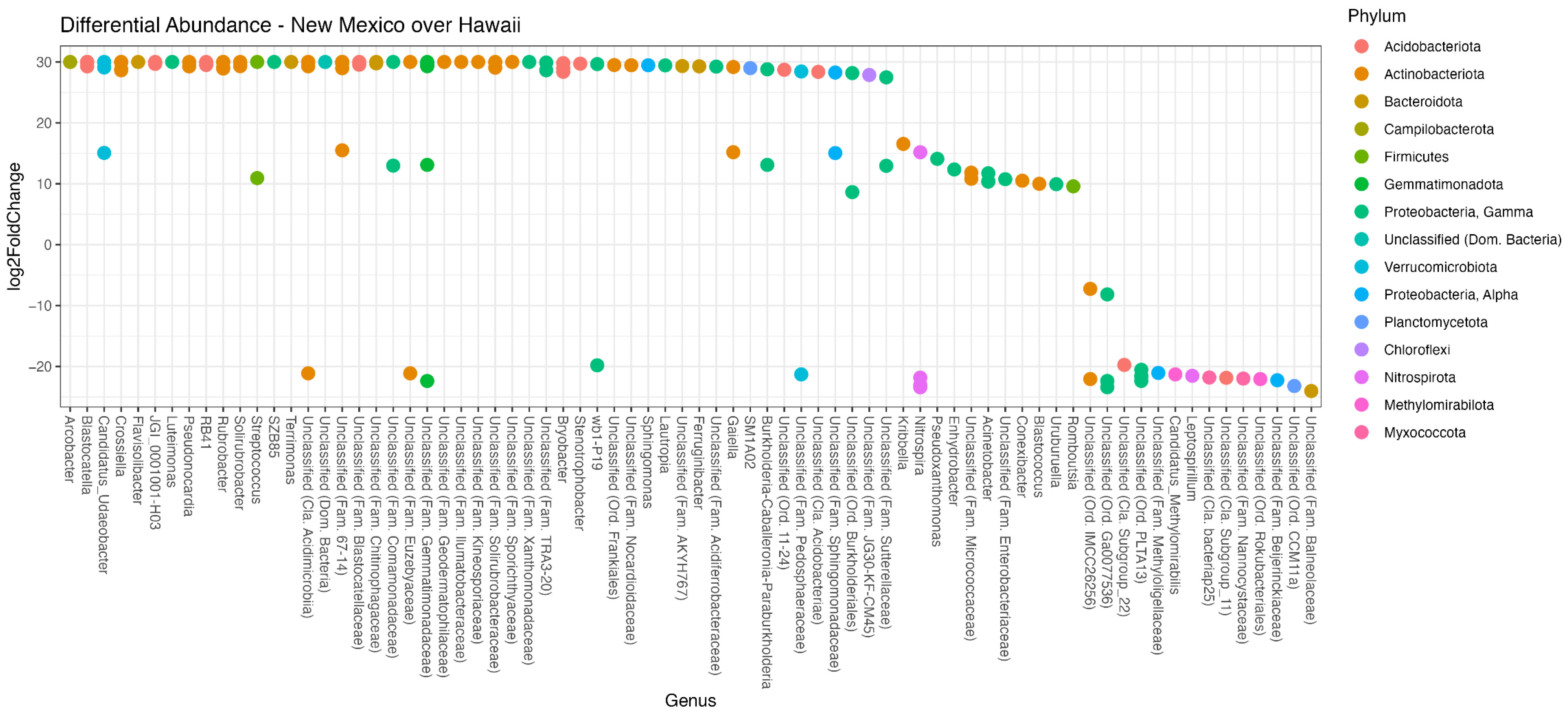
3.5. Differential Abundance Analysis
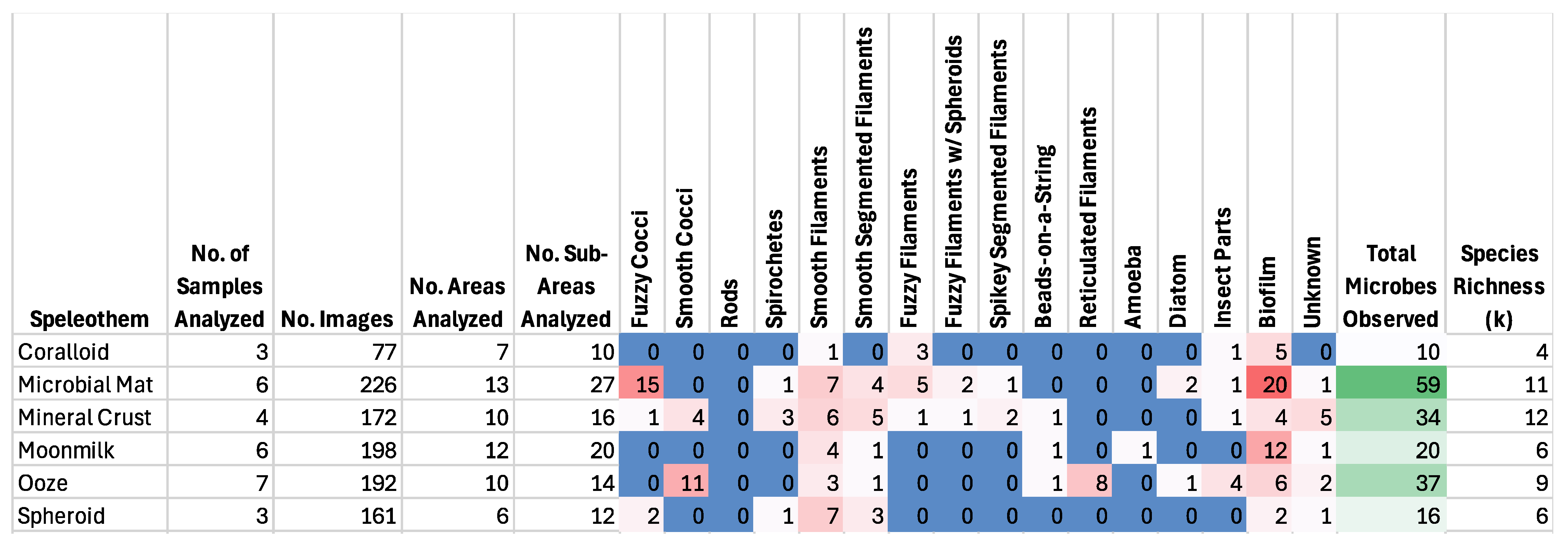
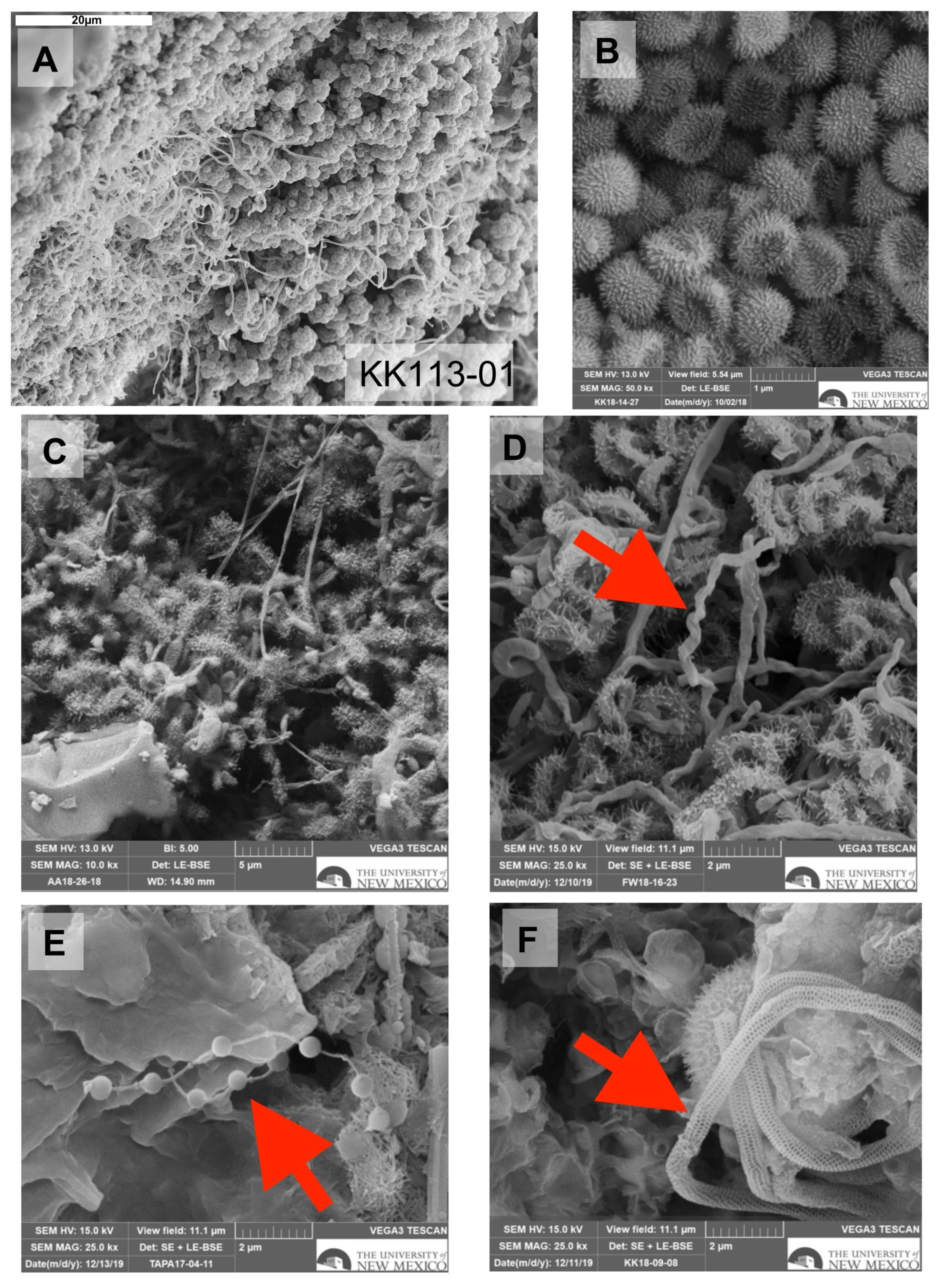
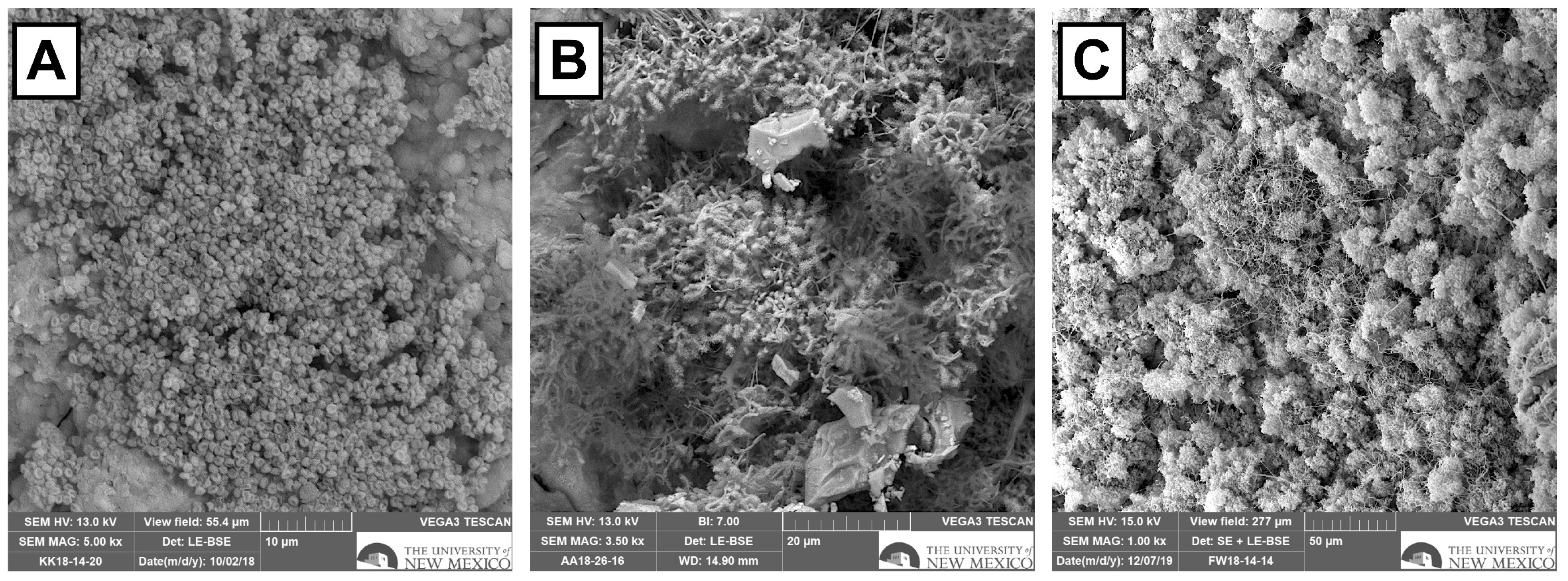
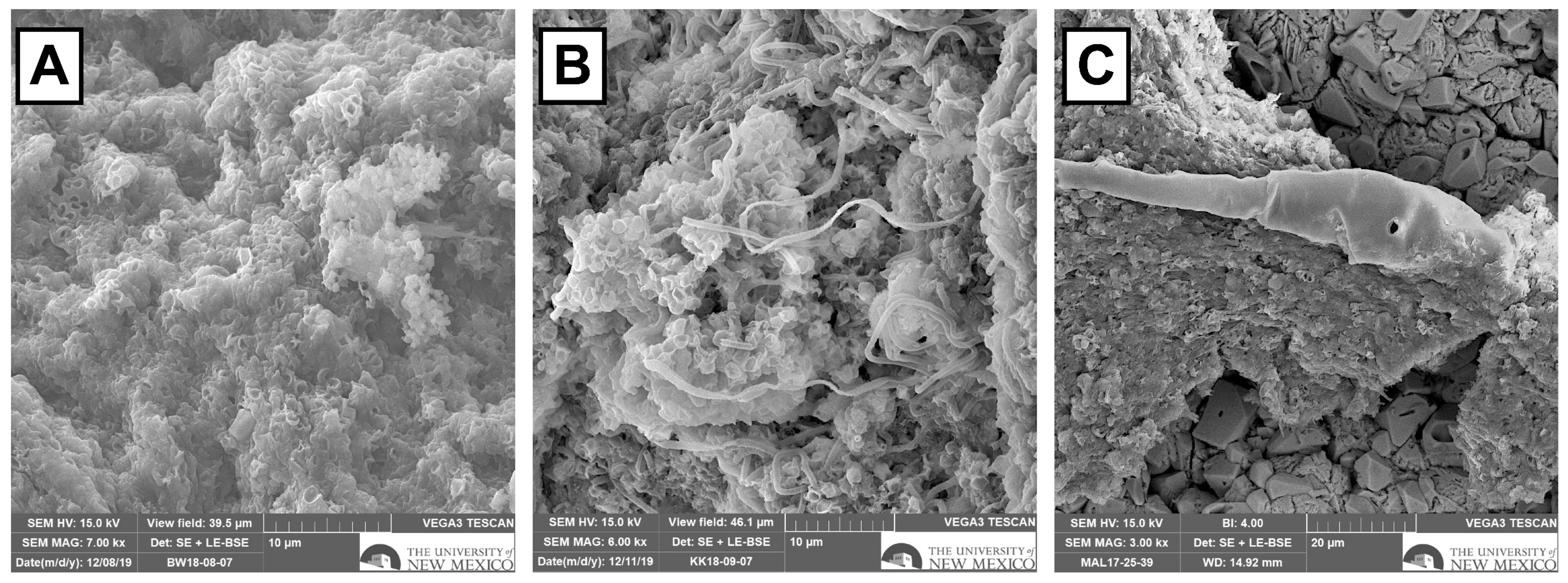
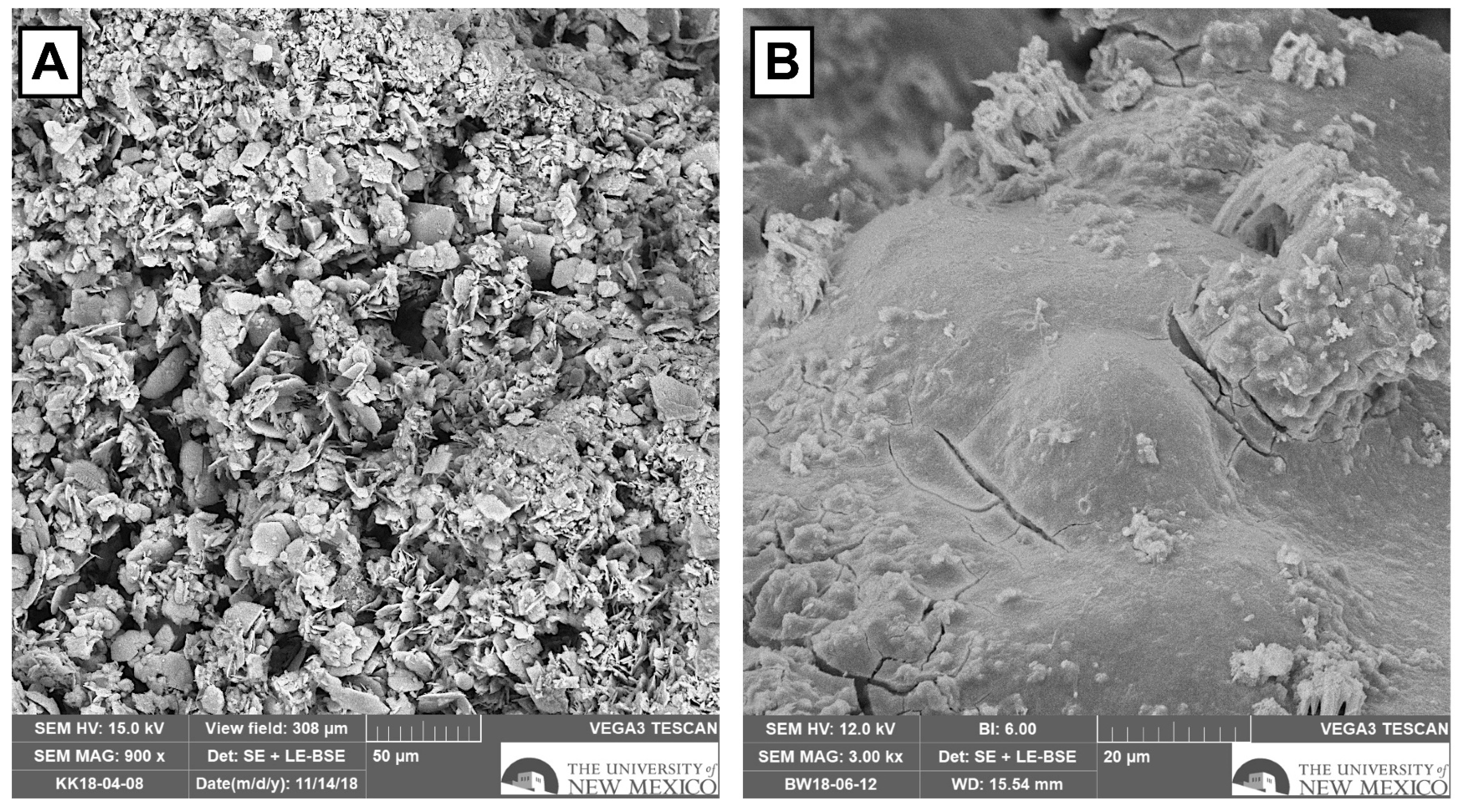
3.6. SEM Results
3.7. Geochemistry
4. Discussion
5. Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hathaway, J.J.M.; Garcia, M.G.; Balasch, M.M.; Spilde, M.N.; Stone, F.D.; Dapkevicius, M.D.L.N.E.; Amorim, I.R.; Gabriel, R.; Borges, P.A.V.; Northup, D.E. Comparison of Bacterial Diversity in Azorean and Hawai’ian Lava Cave Microbial Mats. Geomicrobiol. J. 2014, 31, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Northup, D.E.; Connolly, C.A.; Trent, A.; Peck, V.M.; Spilde, M.N.; Welbourn, W.C.; Natvig, D.O. The Nature of Bacterial Communities in Four Windows Cave, El Malpais National Monument, New Mexico, USA. In Proceedings of the X, XI, and XII International Symposia on Vulcanospeleology; Espinasa-Pereña, R., Pint, J., Eds.; Association for Mexican Cave Studies: Austin, TX, USA, 2004; pp. 119–125. [Google Scholar]
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of Bacterial Communities from Lava Cave Microbial Mats to Overlying Surface Soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339. [Google Scholar] [CrossRef]
- Staley, J.T.; Crawford, R. The Biologist’s Chamber: Lava Tube Slime. Cascade Caver 1975, 14, 20–21. [Google Scholar]
- Howarth, F.G. Community Structure and Niche Differentiation in Hawaiian Lava Tubes. In Island Ecosystems: Biological Organization in Selected Hawaiian Communities; Hutchinson Ross Pub. Co.: Stroudsburg, PA, USA, 1981; pp. 318–336. ISBN 0-87933-381-2. [Google Scholar]
- Léveillé, R.J.; Fyfe, W.S.; Longstaffe, F.J. Geomicrobiology of Carbonate–Silicate Microbialites from Hawaiian Basaltic Sea Caves. Chem. Geol. 2000, 169, 339–355. [Google Scholar] [CrossRef]
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow Coloured Mats from Lava Tubes of La Palma (Canary Islands, Spain) Are Dominated by Metabolically Active Actinobacteria. Sci. Rep. 2018, 8, 1944. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, D.-S.; Lee, K.C.; Kim, Y.-H.; Ahn, U.-S.; Lee, M.-K.; Lee, J.-S. Yellow-Colored Mats in Jeju Island Lava Tubes. J. Korean Appl. Sci. Technol. 2019, 36, 1338–1348. [Google Scholar] [CrossRef]
- Nicolosi, G.; Gonzalez-Pimentel, J.L.; Piano, E.; Isaia, M.; Miller, A.Z. First Insights into the Bacterial Diversity of Mount Etna Volcanic Caves. Microb. Ecol. 2023, 86, 1632–1645. [Google Scholar] [CrossRef]
- Lavoie, K.H.; Northup, D.E.; Barton, H.A. Microbe–Mineral Interactions: Cave Geomicrobiology. In Geomicrobiology; Jain, S.K., Khan, A.A., Rai, M.K., Eds.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4398-4510-3. [Google Scholar]
- Willems, L.; Compère, P.; Hatert, F.; Pouclet, A.; Vicat, J.P.; Ek, C.; Boulvain, F. Karst in Granitic Rocks, South Cameroon: Cave Genesis and Silica and Taranakite Speleothems. Terra Nova 2002, 14, 355–362. [Google Scholar] [CrossRef]
- Romaní, J.R.V.; Rodriguez, M.V. Types of Granite Cavities and Associated Speleothems: Genesis and Evolution. Nat. Conserv. 2007, 63, 41–46. [Google Scholar]
- Miller, A.Z.; Pereira, M.F.C.; Calaforra, J.M.; Forti, P.; Dionísio, A.; Saiz-Jimenez, C. Siliceous Speleothems and Associated Microbe-Mineral Interactions from Ana Heva Lava Tube in Easter Island (Chile). Geomicrobiol. J. 2014, 31, 236–245. [Google Scholar] [CrossRef]
- Tebo, B.M.; Davis, R.E.; Anitori, R.P.; Connell, L.B.; Schiffman, P.; Staudigel, H. Microbial Communities in Dark Oligotrophic Volcanic Ice Cave Ecosystems of Mt. Erebus, Antarctica. Front. Microbiol. 2015, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.Z.; García-Sánchez, A.M.; Coutinho, M.L.; Costa Pereira, M.F.; Gázquez, F.; Calaforra, J.M.; Forti, P.; Martínez-Frías, J.; Toulkeridis, T.; Caldeira, A.T.; et al. Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach. Coatings 2020, 10, 1134. [Google Scholar] [CrossRef]
- Luis-Vargas, M.N.; López-Martínez, R.A.; Vilchis-Nestor, A.R.; Daza, R.; Alcántara-Hernández, R.J. Bacterial Insights into the Formation of Opaline Stromatolites from the Chimalacatepec Lava Tube System, Mexico. Geomicrobiol. J. 2019, 36, 694–704. [Google Scholar] [CrossRef]
- Gonzalez-Pimentel, J.L.; Martin-Pozas, T.; Jurado, V.; Miller, A.Z.; Caldeira, A.T.; Fernandez-Lorenzo, O.; Sanchez-Moral, S.; Saiz-Jimenez, C. Prokaryotic Communities from a Lava Tube Cave in La Palma Island (Spain) Are Involved in the Biogeochemical Cycle of Major Elements. PeerJ 2021, 9, e11386. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.M.; Zaikova, E.; Millan, M.; Williams, A.J.; McAdam, A.C.; Knudson, C.A.; Fuqua, S.R.; Wagner, N.Y.; Craft, K.; Kobs Nawotniak, S.; et al. Life Underground: Investigating Microbial Communities and Their Biomarkers in Mars-Analog Lava Tubes at Craters of the Moon National Monument and Preserve. J. Geophys. Res. Planets 2022, 127, e2022JE007268. [Google Scholar] [CrossRef]
- Barton, H.A.; Northup, D.E. Geomicrobiology in Cave Environments: Past, Current and Future Perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Boston, P.J.; Ivanov, M.V.; McKay, C.P. On the Possibility of Chemosynthetic Ecosystems in Subsurface Habitats on Mars. Icarus 1992, 95, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Cushing, G.E.; Titus, T.N.; Wynne, J.J.; Christensen, P.R. THEMIS Observes Possible Cave Skylights on Mars. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Cushing, G.E. Mars Global Cave Candidate Catalog Archive Bundle; US Geological Survey: Troy, NY, USA, 2015.
- Sims, K.W.W.; Ackert, R.P.; Ramos, F.C.; Sohn, R.A.; Murrell, M.T.; DePaolo, D.J. Determining Eruption Ages and Erosion Rates of Quaternary Basaltic Volcanism from Combined U-Series Disequilibria and Cosmogenic Exposure Ages. Geology 2007, 35, 471. [Google Scholar] [CrossRef]
- Laughlin, A.W.; Poths, J.; Healey, H.A.; Reneau, S.; WoldeGabriel, G. Dating of Quaternary Basalts Using the Cosmogenic 3He and 14C Methods with Implications for Excess 40Ar. Geology 1994, 22, 135–138. [Google Scholar] [CrossRef]
- Laughlin, A.W.; WoldeGabriel, G. Dating the Zuni-Bandera Volcanic Field. In Natural History of El Malpais National Monument; Mabery, K., Ed.; New Mexico Bureau of Geology and Mineral Resources: Socorro, NM, USA, 1997; pp. 25–29. ISBN 1-883905-01-X. [Google Scholar]
- Cañaveras, J.C.; Cuezva, S.; Sanchez-Moral, S.; Lario, J.; Laiz, L.; Gonzalez, J.M.; Saiz-Jimenez, C. On the Origin of Fiber Calcite Crystals in Moonmilk Deposits. Naturwissenschaften 2006, 93, 27–32. [Google Scholar] [CrossRef]
- Larson, C.V. Nomenclature of Lava Tube Features. In Proceedings of the Sixth International Symposium on Vulcanospeleology, Hilo, HI, USA, 5–9 August 1991; Rea, G.T., Ed.; National Speleological Society: Huntsville, AL, USA, 1992; pp. 231–248, ISBN 1-879961-02-4. [Google Scholar]
- Spilde, M.N.; Northup, D.E.; Caimi, N.A.; Boston, P.J.; Stone, F.D.; Smith, S. Microbial Mat Communities in Hawaiian Lava Caves; GSA: Denver, CO, USA, 2016; Volume 48. [Google Scholar]
- Northup, D.E.; Melim, L.A.; Spilde, M.N.; Hathaway, J.J.M.; Garcia, M.G.; Moya, M.; Stone, F.D.; Boston, P.J.; Dapkevicius, M.L.N.E.; Riquelme, C. Lava Cave Microbial Communities Within Mats and Secondary Mineral Deposits: Implications for Life Detection on Other Planets. Astrobiology 2011, 11, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.A.; Forti, P. Speleothems. In Cave Minerals of the World; National Speleological Society: Huntsville, AL, USA, 1997; pp. 45–116. ISBN 978-1-879961-07-4. [Google Scholar]
- Giovannoni, S.J.; DeLong, E.F.; Schmidt, T.M.; Pace, N.R. Tangential Flow Filtration and Preliminary Phylogenetic Analysis of Marine Picoplankton. Appl. Environ. Microbiol. 1990, 56, 2572–2575. [Google Scholar] [CrossRef] [PubMed]
- Takada-Hoshino, Y.; Matsumoto, N. An Improved DNA Extraction Method Using Skim Milk from Soils That Strongly Adsorb DNA. Microbes Environ. 2004, 19, 13–19. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact Sequence Variants Should Replace Operational Taxonomic Units in Marker-Gene Data Analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package 2024.
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Posit Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; The R Project for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Vavrek, M.J. Fossil: Palaeoecological and Palaeogeographical Analysis Tools. Palaeontol. Electron. 2011, 14, 16. [Google Scholar]
- McMurdie, P.J.; Holmes, S. Phyloseq 2017.
- Joseph Nathaniel Paulson, H.T. metagenomeSeq 2017.
- Love, M.; Anders, S.; Ahlmann-Eltze, C.; Forbes, K.; Huber, W. DESeq2 2017.
- Anderson, M.J. A New Method for Non-parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Kenett, R.S., Longford, N.T., Piegorsch, W.W., Ruggeri, F., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 1–15. ISBN 978-1-118-44511-2. [Google Scholar]
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities, 2nd ed.; MjM Software Design: Gleneden Beach, OR, USA, 2002; ISBN 978-0-9721290-0-8. [Google Scholar]
- Gonzalez-Pimentel, J.L.; Martin-Pozas, T.; Jurado, V.; Laiz, L.; Fernandez-Cortes, A.; Sanchez-Moral, S.; Saiz-Jimenez, C. The Marine Bacterial Genus Euzebya Is Distributed Worldwide in Terrestrial Environments: A Review. Appl. Sci. 2023, 13, 9644. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Melim, L.A.; Northup, D.E.; Spilde, M.N.; Boston, P.J. Update: Living Reticulated Filaments from Herbstlabyrinth-Adventhöhle Cave System, Germany. J. Cave Karst Stud. 2015, 77, 87–90. [Google Scholar] [CrossRef]
- Cailleau, G.; Verrecchia, E.P.; Braissant, O.; Emmanuel, L. The Biogenic Origin of Needle Fibre Calcite. Sedimentology 2009, 56, 1858–1875. [Google Scholar] [CrossRef]
- Bindschedler, S.; Cailleau, G.; Braissant, O.; Millière, L.; Job, D.; Verrecchia, E.P. Unravelling the Enigmatic Origin of Calcitic Nanofibres in Soils and Caves: Purely Physicochemical or Biogenic Processes? Biogeosciences 2014, 11, 2809–2825. [Google Scholar] [CrossRef]
- Rowe, M.C.; Lassiter, J.C. Chlorine Enrichment in Central Rio Grande Rift Basaltic Melt Inclusions: Evidence for Subduction Modification of the Lithospheric Mantle. Geology 2009, 37, 439–442. [Google Scholar] [CrossRef]
- Peters, T.J.; Menzies, M.; Thirlwall, M.; Kyle, P.R. Zuni–Bandera Volcanism, Rio Grande, USA—Melt Formation in Garnet- and Spinel-Facies Mantle Straddling the Asthenosphere–Lithosphere Boundary. Lithos 2008, 102, 295–315. [Google Scholar] [CrossRef]
- Oberbeck, V.R.; Quaide, W.L.; Greeley, R. On the Origin of Lunar Sinuous Rilles. Mod. Geol. 1969, 1, 75–80. [Google Scholar]
- Greeley, R. Lava Tubes and Channels in the Lunar Marius Hills. Moon 1971, 3, 289–314. [Google Scholar] [CrossRef]
- Carr, M.H. Volcanism on Mars. J. Geophys. Res. 1973, 78, 4049–4062. [Google Scholar] [CrossRef]
- Baker, V.R.; Strom, R.G.; Gulick, V.C.; Kargel, J.S.; Komatsu, G.; Kale, V.S. Ancient Oceans, Ice Sheets and the Hydrological Cycle on Mars. Nature 1991, 352, 589–594. [Google Scholar] [CrossRef]
- Westall, F.; Brack, A.; Hofmann, B.; Horneck, G.; Kurat, G.; Maxwell, J.; Ori, G.G.; Pillinger, C.; Raulin, F.; Thomas, N.; et al. An ESA Study for the Search for Life on Mars. Planet. Space Sci. 2000, 48, 181–202. [Google Scholar] [CrossRef]
- Michalski, J.R.; Cuadros, J.; Niles, P.B.; Parnell, J.; Deanne Rogers, A.; Wright, S.P. Groundwater Activity on Mars and Implications for a Deep Biosphere. Nat. Geosci. 2013, 6, 133–138. [Google Scholar] [CrossRef]
- Kasting, J.F.; Whitmire, D.P.; Reynolds, R.T. Habitable Zones around Main Sequence Stars. Icarus 1993, 101, 108–128. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; O’Malley-James, J.; Parnell, J. Circumstellar Habitable Zones for Deep Terrestrial Biospheres. Planet. Space Sci. 2013, 85, 312–318. [Google Scholar] [CrossRef]
- Boston, P.J.; Spilde, M.N.; Northup, D.E.; Melim, L.A.; Soroka, D.S.; Kleina, L.G.; Lavoie, K.H.; Hose, L.D.; Mallory, L.M.; Dahm, C.N.; et al. Cave Biosignature Suites: Microbes, Minerals, and Mars. Astrobiology 2001, 1, 25–55. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, W.S. Petrology and Petrogenesis of Plio-Pleistocene Basaltic Rocks from the Central Rio Grande Rift, New Mexico, and Their Relation to Rift Structure. In Rio Grande Rift: Tectonics and Magmatism; American Geophysical Union (AGU): Washington, DC, USA, 1979; pp. 323–354. ISBN 978-1-118-66498-8. [Google Scholar]
- Palma, V.; González-Pimentel, J.L.; Jimenez-Morillo, N.T.; Sauro, F.; Gutiérrez-Patricio, S.; De La Rosa, J.M.; Tomasi, I.; Massironi, M.; Onac, B.P.; Tiago, I.; et al. Connecting Molecular Biomarkers, Mineralogical Composition, and Microbial Diversity from Mars Analog Lava Tubes. Sci. Total Environ. 2024, 913, 169583. [Google Scholar] [CrossRef]
- Ouyang, E.; Liu, Y.; Ouyang, J.; Wang, X. Effects of Different Wastewater Characteristics and Treatment Techniques on the Bacterial Community Structure in Three Pharmaceutical Wastewater Treatment Systems. Environ. Technol. 2019, 40, 329–341. [Google Scholar] [CrossRef]
- Prescott, R.D.; Zamkovaya, T.; Donachie, S.P.; Northup, D.E.; Medley, J.J.; Monsalve, N.; Saw, J.H.; Decho, A.W.; Chain, P.S.G.; Boston, P.J. Islands Within Islands: Bacterial Phylogenetic Structure and Consortia in Hawaiian Lava Caves and Fumaroles. Front. Microbiol. 2022, 13, 934708. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Feng, C.; Zhang, Z.; Lei, Z.; Shimizu, K.; Cao, X.; Liu, H.; Liu, H. Microbial Vanadium (V) Reduction in Groundwater with Different Soils from Vanadium Ore Mining Areas. Chemosphere 2018, 202, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Song, A.; Yang, H.; Müller, W.E.G. Impact of Rocky Desertification Control on Soil Bacterial Community in Karst Graben Basin, Southwestern China. Front. Microbiol. 2021, 12, 636405. [Google Scholar] [CrossRef] [PubMed]
- Stomeo, F.; Portillo, M.C.; Gonzalez, J.M.; Laiz, L.; Saiz-Jimenez, C. Pseudonocardia in White Colonizations in Two Caves with Paleolithic Paintings. Int. Biodeterior. Biodegrad. 2008, 62, 483–486. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, F.; Wang, W.; He, D.; Gu, J.-D.; Feng, H.; Chen, T.; Liu, G.; An, L. The Microbial Community Characteristics of Ancient Painted Sculptures in Maijishan Grottoes, China. PLoS ONE 2017, 12, e0179718. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Tai, X.; Sun, L.; Wu, M.; Zhang, W.; Chen, X.; Zhang, G.; Chen, T.; Liu, G.; et al. Variation in Actinobacterial Community Composition and Potential Function in Different Soil Ecosystems Belonging to the Arid Heihe River Basin of Northwest China. Front. Microbiol. 2019, 10, 2209. [Google Scholar] [CrossRef]
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Cañaveras, J.C.; Benavente, D.; Duarte, E.; Saiz-Jimenez, C.; Sanchez-Moral, S. New Insights into the Structure, Microbial Diversity and Ecology of Yellow Biofilms in a Paleolithic Rock Art Cave (Pindal Cave, Asturias, Spain). Sci. Total Environ. 2023, 897, 165218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xiang, X.; Yun, Y.; Wang, W.; Wang, H.; Bodelier, P.L.E. Archaea and Their Interactions with Bacteria in a Karst Ecosystem. Front. Microbiol. 2023, 14, 1068595. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, J.J.M.; Moser, D.P.; Blank, J.G.; Northup, D.E. A Comparison of Primers in 16S rRNA Gene Surveys of Bacteria and Archaea from Volcanic Caves. Geomicrobiol. J. 2021, 38, 741–754. [Google Scholar] [CrossRef]
- Baker, B.J.; De Anda, V.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, Ecology and Evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
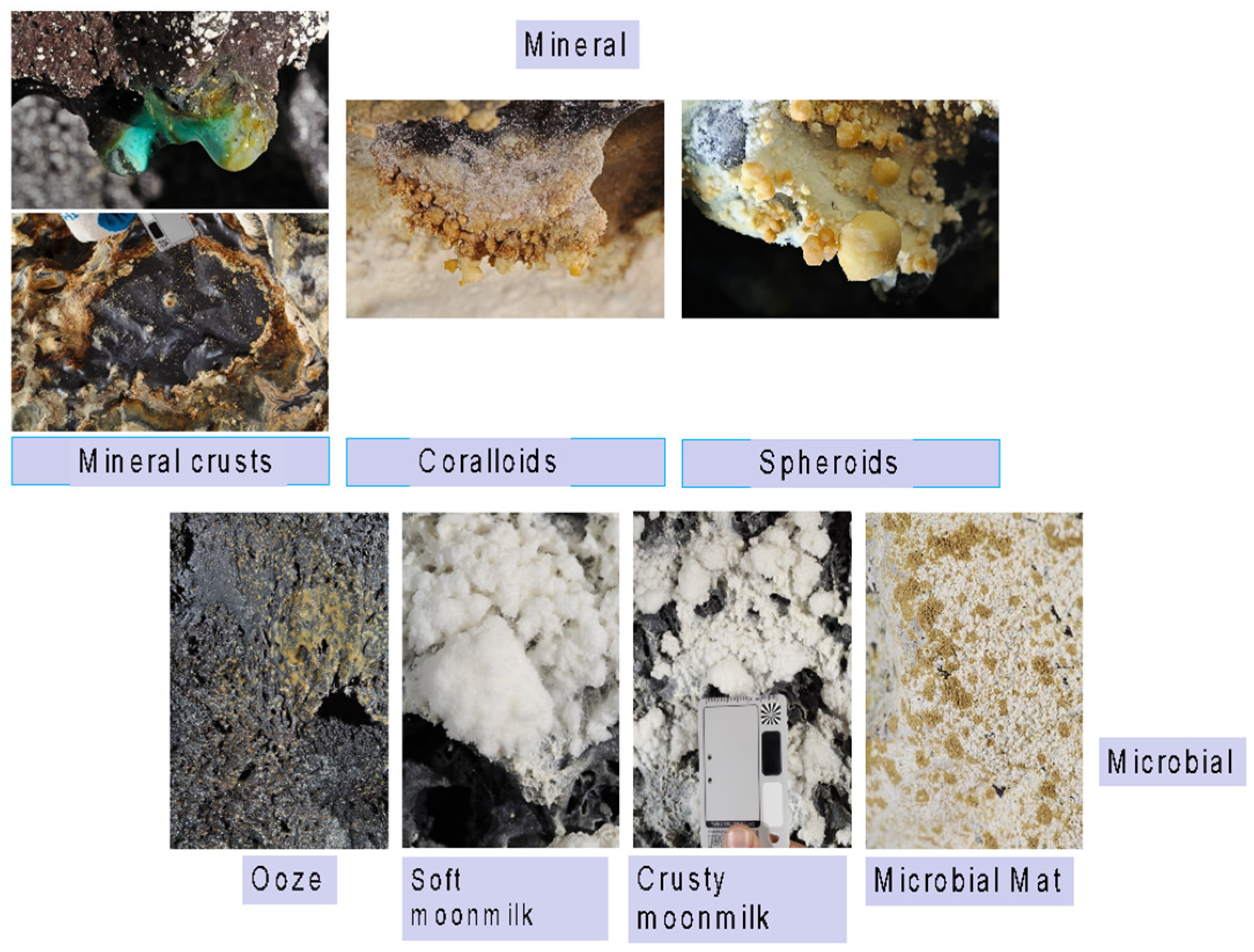





| New Mexico | Hawaii | |||||
|---|---|---|---|---|---|---|
| Cave | Four-Windows | Pantheon | A`a Cave | Maelstrom | Kula Kai | Tapa |
| Sample No.s | FW190831-01 | BW180325-14 | AA18113-05 | MAL171220-11, -20 | KK171218-6 | TAP171219-3 |
| Wt% | ||||||
| P2O5 | 0.30 | 0.31 | 0.17 | 0.24–0.26 | 0.25 | 0.23–0.25 |
| MnO | 0.18 | 0.18 | 0.17 | 0.17–0.18 | 0.174–0.18 | 0.17–0.18 |
| SO3 | 0.02 | 0.03 | 0.01 | 0.01–0.02 | 0.007–0.010 | 0.01–0.02 |
| FeO | 10.9820 | 11.34 | 10.350 | 10.6270 | 10.44–10.89 | 10.35–10.80 |
| ppm | ||||||
| Cu | 70 | 159 | 72 | 121–145 | 117–133 | 122–135 |
| V | 282 | na | na | 329 | 299–322 | 276–302 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medley, J.J.; Hathaway, J.J.M.; Spilde, M.N.; Northup, D.E. Looking for Microbial Biosignatures in All the Right Places: Clues for Identifying Extraterrestrial Life in Lava Tubes. Appl. Sci. 2024, 14, 6500. https://doi.org/10.3390/app14156500
Medley JJ, Hathaway JJM, Spilde MN, Northup DE. Looking for Microbial Biosignatures in All the Right Places: Clues for Identifying Extraterrestrial Life in Lava Tubes. Applied Sciences. 2024; 14(15):6500. https://doi.org/10.3390/app14156500
Chicago/Turabian StyleMedley, Joseph J., Jennifer J. M. Hathaway, Michael N. Spilde, and Diana E. Northup. 2024. "Looking for Microbial Biosignatures in All the Right Places: Clues for Identifying Extraterrestrial Life in Lava Tubes" Applied Sciences 14, no. 15: 6500. https://doi.org/10.3390/app14156500
APA StyleMedley, J. J., Hathaway, J. J. M., Spilde, M. N., & Northup, D. E. (2024). Looking for Microbial Biosignatures in All the Right Places: Clues for Identifying Extraterrestrial Life in Lava Tubes. Applied Sciences, 14(15), 6500. https://doi.org/10.3390/app14156500






