Physiological, Biomechanical, and Thermographic Responses in Male Athletes during an Ultra-Endurance Race
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ultra-Endurance Event
2.3. Design and Procedure
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
5.1. Limitations of the Study
5.2. Future Research Lines
5.3. Practical Application
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaryski, C.; Smith, D.J. Training principles and issues for ultra-endurance athletes. Curr. Sports Med. Rep. 2005, 4, 165–170. [Google Scholar] [CrossRef]
- Belinchón-deMiguel, P.; Ruisoto, P.; Knechtle, B.; Nikolaidis, P.T.; Herrera-Tapias, B.; Clemente-Suárez, V.J. Predictors of Athlete’s Performance in Ultra-Endurance Mountain Races. Int. J. Environ. Res. Public Health 2021, 18, 956. [Google Scholar] [CrossRef] [PubMed]
- Scheer, V. Participation trends of ultra endurance events. Sports Med. Arthrosc. Rev. 2019, 27, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Belinchón-deMiguel, P.; Tornero-Aguilera, J.F.; Dalamitros, A.A.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B.; Clemente-Suárez, V.J. Multidisciplinary analysis of differences between finisher and non-finisher ultra-endurance mountain athletes. Front. Physiol. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M. Nutritional aspects in ultra-endurance exercise. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú, M.; Ruiz-Muñoz, A.; Roca, E.; Caminal, P.; Rodríguez, F.A.; Irurtia, A.; Perera, A. Assessment of Heart Rate Variability during an Endurance Mountain Trail Race by Multi-Scale Entropy Analysis. Entropy 2017, 19, 658. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. Cortical arousal and central nervous system fatigue after a mountain marathon. Cult. Cienc. Y Deporte 2017, 12, 143–148. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. Psychophysiological response and energy balance during a 14-h ultraendurance mountain running event. Appl. Physiol. Nutr. Metab. 2015, 40, 269–273. [Google Scholar] [CrossRef]
- Pokan, R.; Ocenasek, H.; Hochgatterer, R.; Miehl, M.; Vonbank, K.; Von Duvillard, S.P.; Franklin, B.; Würth, S.; Volf, I.; Wonisch, M.; et al. Myocardial dimensions and hemodynamics during 24-h ultraendurance ergometry. Med. Sci. Sports Exerc. 2014, 46, 268–275. [Google Scholar] [CrossRef]
- Belinchon-deMiguel, P.; Clemente-Suárez, V.J. Psychophysiological, body composition, biomechanical and autonomic modulation analysis procedures in an ultraendurance mountain race. J. Med. Syst. 2018, 42, 32. [Google Scholar] [CrossRef]
- Mattsson, C.M.; Flockhart, M.; Söderlund, K.; Hendo, G.; Jakobsson, M.; Pontén, M.; Ekblom, B. Effects of prolonged low intensity exercise with energy deficit (military training operation) on markers of muscle protein turnover. In Proceedings of the ECSS, 20th Annual Congress of the European College of Sport Science, Malmö, Sweden, 24–27 June 2015. [Google Scholar]
- Hermand, E.; Chabert, C.; Hue, O. Ultra-endurance events in tropical environments and countermeasures to optimize performances and health. Int. J. Hyperth. 2019, 36, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Neves, E.B.; Matos, F.; da Cunha, R.M.; Reis, V.M. Thermography to monitoring of Sports Training: An Overview. Pan Am. J. Med. Thermol. 2015, 2, 18–22. [Google Scholar] [CrossRef]
- Sillero-Quintana, M.; Gomez-Carmona, P.M.; Fernández-Cuevas, I. Infrared thermography as a means of monitoring and preventing sports injuries. In Research Anthology on Business Strategies, Health Factors, and Ethical Implications in Sports and eSports; IGI Global: Hershey, PA, USA, 2021. [Google Scholar] [CrossRef]
- Côrte, A.C.; Pedrinelli, A.; Marttos, A.; Souza, I.F.G.; Grava, J.; Hernandez, A.J. Infrared thermography study as a complementary method of screening and prevention of muscle injuries: Pilot study. BMJ Open Sport. Exerc. Med. 2019, 5, e000431. [Google Scholar] [CrossRef] [PubMed]
- Hillen, B.; Pfirrmann, D.; Nägele, M.; Simon, P. Infrared thermography in exercise physiology: The dawning of exercise radiomics. Sports Med. 2020, 50, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Valverde, D.; Tomás-Carús, P.; Timón, R.; Batalha, N.; Sánchez-Ureña, B.; Gutiérrez-Vargas, R.; Olcina, G. Short-term skin temperature responses to endurance exercise: A systematic review of methods and future challenges in the use of infrared thermography. Life 2021, 11, 1286. [Google Scholar] [CrossRef]
- Comassi, M.; Vitolo, E.; Pratali, L.; Del Turco, S.; Dellanoce, C.; Rossi, C.; Santini, E.; Solini, A. Acute effects of different degrees of ultra-endurance exercise on systemic inflammatory responses. Intern. Med. J. 2015, 45, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Viegas, F.; Mello, M.T.D.; Rodrigues, S.A.; Costa, C.M.A.; Freitas, L.D.S.N.; Rodrigues, E.L.; Silva, A. The use of thermography and its control variables: A systematic review. Rev. Bras. Med. Esporte 2020, 26, 82–86. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J. The application of cortical arousal assessment to control neuromuscular fatigue during strength training. J. Mot. Behav. 2017, 49, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.F.T.; Elias, V.F.; Clemente-Suárez, V.J. Autonomic and cortical response of soldiers in different combat scenarios. BMJ Mil. Health 2021, 167, 172–176. [Google Scholar] [CrossRef]
- Hummelink, S.; Kruit, A.S.; van Vlaenderen, A.R.W.; Schreinemachers, M.J.M.; Steenbergen, W.; Ulrich, D.J.O. Post-operative monitoring of free flaps using a low-cost thermal camera: A pilot study. Eur. J. Plast. Surg. 2020, 43, 589–596. [Google Scholar] [CrossRef]
- Aguilar-Ferrándiz, M.E.; Casas-Barragán, A.; Tapia-Haro, R.M.; Rus, A.; Molina, F.; Correa-Rodríguez, M. Evaluation of sympathetic adrenergic branch of cutaneous neural control throughout thermography and its relationship to nitric oxide levels in patients with fibromyalgia. J. Therm. Biol. 2021, 95, 102813. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Sundén, P.; Zetterquist, S. Leg temperature profiles with a simplified thermographic technique in the diagnosis of acute venous thromboses. Scand. J. Clin. Lab. Investig. 1979, 39, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; Alacid, F.; Soto-Méndez, F.; Alcaraz, P.E.; López-Román, F.J.; Rubio-Arias, J.Á. Muscle damage, physiological changes, and energy balance in ultra-endurance mountain-event athletes. Appl. Physiol. Nutr. Metab. 2016, 41, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Swart, A.; Constantinou, D. The effects of a 3-day mountain bike cycling race on the autonomic nervous system (ANS) and heart rate variability in amateur cyclists: A prospective quantitative research design. BMC Sports Sci. Med. Rehabil. 2023, 15, 2. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Fazackerley, L.A.; Fell, J.W.; Kitic, C.M. The effect of an ultra-endurance running race on heart rate variability. Eur. J. Appl. Physiol. 2019, 119, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Kajaia, T.; Maskhulia, L.; Chelidze, K.; Akhalkatsi, V.; Kakhabrishvili, Z. The effects of non-functional overreaching and overtraining on autonomic nervous system function in highly trained Georgian athletes. Georgian Med. Newa 2017, 3, 97–101. [Google Scholar]
- Saito, S. Does fatigue exist in a quantitative measurement of eye movements? Ergonomics 1992, 35, 607–615. [Google Scholar] [CrossRef]
- Doppelmayr, M.M.; Finkernagel, H.; Doppelmayr, H.I. Changes in Cognitive Performance during a 216 Kilometer, Extreme Endurance Footrace: A Descriptive and Prospective Study. Percept. Mot. Skills abril de 2005, 100, 473–487. [Google Scholar] [CrossRef]
- Wollseiffen, P.; Schneider, S.; Martin, L.A.; Kerhervé, H.A.; Klein, T.; Solomon, C. The effect of 6 h of running on brain activity, mood, and cognitive performance. Exp. Brain Res. 2016, 234, 1829–1836. [Google Scholar] [CrossRef]
- Bachasson, D.; Temesi, J.; Gruet, M.; Yokoyama, K.; Rupp, T.; Millet, G.Y.; Verges, S. Transcranial magnetic stimulation intensity affects exercise-induced changes in corticomotoneuronal excitability and inhibition and voluntary activation. Neuroscience 2016, 314, 125–133. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; De Paz, J.A.; Garatachea, N.; Cabeza De Vaca, S.; Chavarren, J. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J. Appl. Physiol. 2003, 94, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef]
- Golriz, S.; Peiffer, J.J.; Walker, B.F.; Foreman, K.B.; Hebert, J.J. The effect of backpack load placement on physiological and self-reported measures of exertion. Work 2018, 61, 273–279. [Google Scholar] [CrossRef]
- Crowder, T.A.; Beekley, M.D.; Sturdivant, R.X.; Johnson, C.A.; Lumpkin, A. Metabolic effects of soldier performance on a simulated graded road march while wearing two functionally equivalent military ensembles. Mil. Med. 2007, 172, 596–602. [Google Scholar] [CrossRef]
- Hough, P.; Earle, J. Energy Balance during a Self-Sufficient, Multistage Ultramarathon. J. Hum. Perform. Extrem. Environ. 2017, 13. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramarathon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef]
- Martinez-Navarro, I.; Sanchez-Gómez, J.M.; Aparicio, I.; Priego-Quesada, J.I.; Pérez-Soriano, P.; Collado, E.; Hernando, B.; Hernando, C. Effect of mountain ultramarathon distance competition on biochemical variables, respiratory and lower-limb fatigue. PLoS ONE 2020, 15, e0238846. [Google Scholar] [CrossRef]
- Méndez-Alonso, D.; Prieto-Saborit, J.A.; Bahamonde, J.R.; Jiménez-Arberás, E. Influence of psychological factors on the success of the ultra-trail runner. Int. J. Environ. Res. Public Health 2021, 18, 2704. [Google Scholar] [CrossRef]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and peripheral fatigue in physical exercise explained: A narrative review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef]
- Belli, T.; Macedo, D.V.; De Araujo, G.G.; Dos Reis, I.G.M.; Scariot, P.P.M.; Lazarim, F.L.; Nunes, L.A.S.; Brenzikofer, R.; Gobatto, C.A. Mountain ultramarathon induces early increases of muscle damage, inflammation, and risk for acute renal injury. Front. Physiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Svensson, M.; Rosvall, P.; Boza-Serrano, A.; Andersson, E.; Lexell, J.; Deierborg, T. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress 2016, 5, 8–18. [Google Scholar] [CrossRef]
- Tyler, C.J.; Reeve, T.; Hodges, G.J.; Cheung, S.S. The effects of heat adaptation on physiology, perception and exercise performance in the heat: A meta-analysis. Sports Med. 2016, 46, 1699–1724. [Google Scholar] [CrossRef]
- Kenny, G.P.; Jay, O. Thermometry, Calorimetry, and Mean Body Temperature during Heat Stress. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 1689–1719. [Google Scholar] [CrossRef]
- Marino, F.E. Methods, advantages, and limitations of body cooling for exercise performance. Br. J. Sports Med. 2002, 36, 89–94. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Casa, D.J.; Millard-Stafford, M.; Moran, D.S.; Pyne, S.W.; Roberts, W.O. Exertional heat illness during training and competition. Med. Sci. Sports Exerc. 2007, 39, 556–572. [Google Scholar] [CrossRef]
- Lim, C.L.; Byrne, C.; Lee, J.K. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann. Acad. Med. Singap. 2008, 37, 347–353. [Google Scholar] [CrossRef]
- Cramer, M.N.; Jay, O. Biophysical aspects of human thermoregulation during heat stress. Auton. Neurosci. 2016, 196, 3–13. [Google Scholar] [CrossRef]
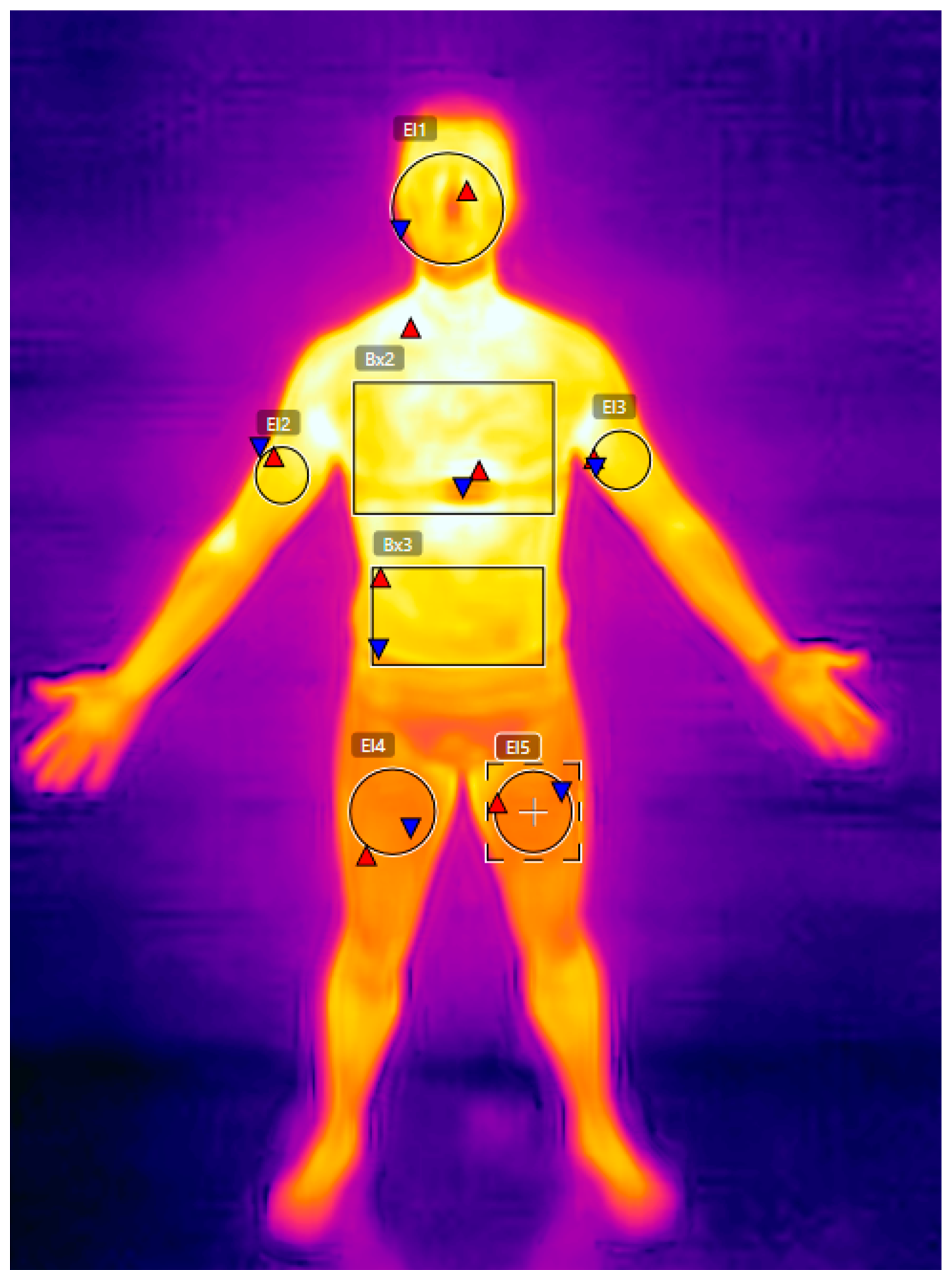
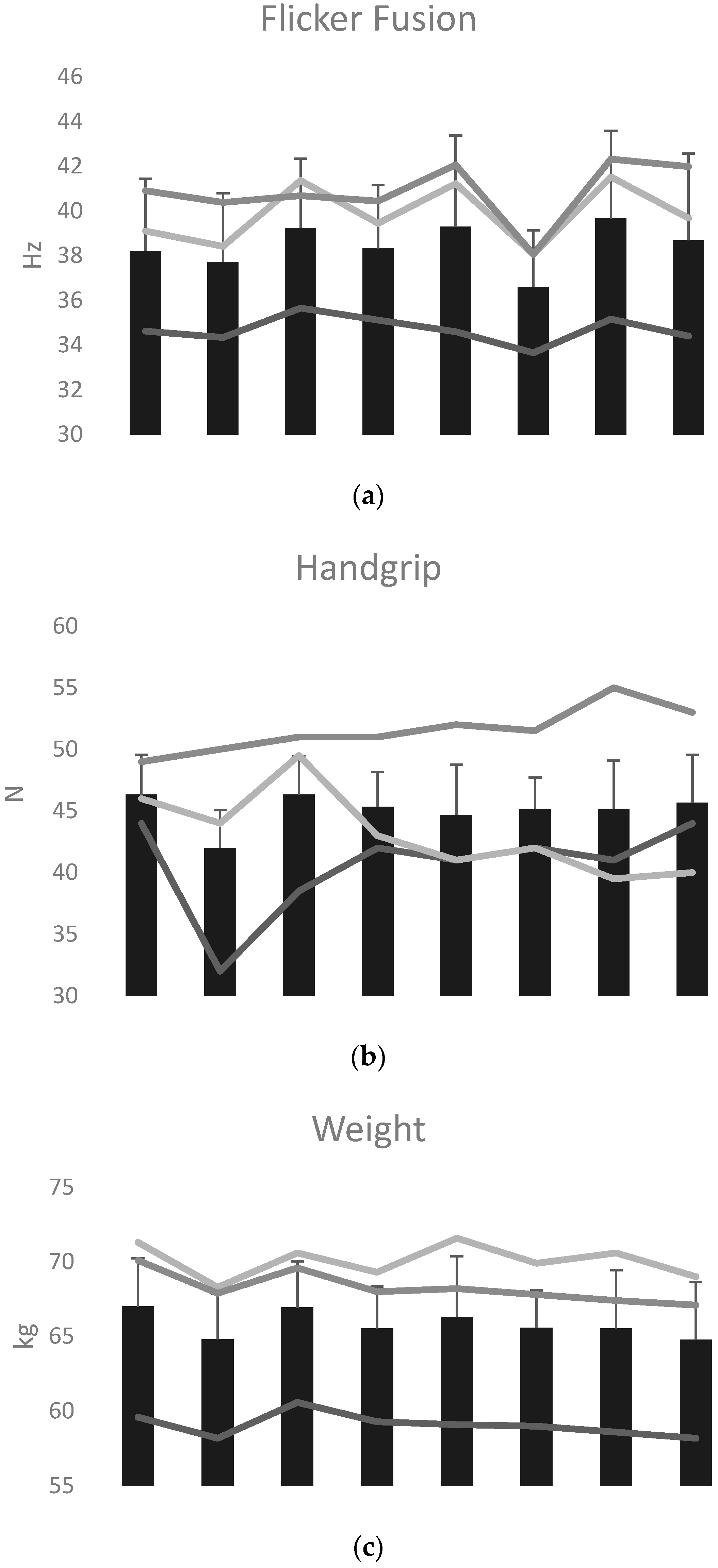



| Pre-Event | Day 1 | Day 2 | Day 3 | Day 4 | Cohen’s d (Pre vs. Day 4) | |
|---|---|---|---|---|---|---|
| Time-domain | ||||||
| Mean HR (bpm) | 70.0 ± 15.2 | 91.9 ± 15.3 | 95.8 ± 18.3 | 88.3 ± 10.7 | 95.1 ± 11.4 | 1.65 |
| Min HR (bpm) | 53.0 ± 14.3 | 62.9 ± 8.9 | 64.1 ± 8.5 | 73.6 ± 10.2 | 65.2 ± 12.6 | 0.85 |
| Max HR (bpm) | 80.4 ± 9.3 | 126.6 ± 20.3 | 145.8 ± 12.2 | 137.1 ± 18.3 | 147.6 ± 14.6 | 7.23 |
| RMSSD (ms) | 64.4 ± 20.3 | 16.5 ± 18.6 | 18.4 ± 14.3 | 15.3 ± 12.4 | 18.5 ± 15.2 | −2.26 |
| pNN50 (%) | 28.3 ± 9.9 | 1.3 ± 3.3 | 1.7 ± 4.3 | 1.2 ± 5.2 | 1.3 ± 4.8 | −2.73 |
| Frequency domain | ||||||
| LF Power (n.u) | 70.8 ± 6.2 | 83.2 ± 7.8 | 92.2 ± 4.1 | 85.3 ± 6.3 | 89.1 ± 5.9 | 2.95 |
| HF Power (n.u) | 29.2 ± 6.7 | 16.7 ± 5.0 | 7.8 ± 3.2 | 14.7 ± 6.8 | 10.9 ± 4.6 | −2.73 |
| Ratio LF/HF | 3.7 ± 0.9 | 5.0 ± 1.1 | 11.8 ± 2.0 | 5.8 ± 1.8 | 8.2 ± 2.3 | 5.00 |
| Nonlinear | ||||||
| SD1 (ms) | 45.6 ± 9.6 | 11.7 ± 8.9 | 13.0 ± 11.3 | 10.8 ± 12.4 | 13.1 ± 10.8 | −3.39 |
| SD2 (ms) | 95.2 ± 25.6 | 29.0 ± 10.5 | 42.6 ± 21.3 | 34.2 ± 18.3 | 34.4 ± 14.5 | −2.38 |
| ApEn | 1.2 ± 0.2 | 1.4 ± 0.6 | 1.2 ± 0.5 | 1.2 ± 0.4 | 1.3 ± 0.2 | 0.50 |
| SampEn | 1.3 ± 0.4 | 1.5 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.2 | 0.00 |
| Units | Post-1st Stage | Post-2nd Stage | Post-3rd Stage | Post-4th Stage | p | |
|---|---|---|---|---|---|---|
| pH urine | 5.33 ± 0.58 | 5.33 ± 0.58 | 6.00 ± 0.00 | 6.00 ± 0.00 | 0.112 | |
| Colorimetry urine | 7.33 ± 0.58 | 4.67 ± 3.21 | 4.33 ± 3.1 | 5.00 ± 2.65 | 0.142 | |
| Glucose urine | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1.000 | |
| Creatinine Kinase | µmol/L | n/a | 418.33 ± 112.77 | 300.67 ± 80.41 | 219.33 ± 63.01 | 0.097 |
| Protein urine | 0 ± 0 | 0 ± 0 | 343.34 ± 271.34 | 53.35 ± 40.40 | 0.033 |
| Body Part | Pre | Post | % Difference | Cohen’s d (Pre vs. Post) |
|---|---|---|---|---|
| Face | 29.4 ± 0.8 | 29.1 ± 1.1 | 1.0 | −0.38 |
| Chest | 30.9 ± 0.6 | 30.2 ± 1.0 | 2.4 | −1.17 |
| Abdomen | 30.5 ± 0.9 | 30.3 ± 1.2 | 0.7 | −0.22 |
| Right Arm | 30.3 ± 0.9 | 29.9 ± 0.9 | 1.4 | −0.44 |
| Left Arm | 30.4 ± 0.9 | 29.5 ± 1.4 | 3.2 | −1.00 |
| Right Leg | 26.3 ± 0.7 | 26.4 ± 2.4 | −0.6 | 0.14 |
| Left Leg | 26.3 ± 0.8 | 28.3 ± 2.3 | −7.9 | 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belinchón-deMiguel, P.; Ramos-Campo, D.J.; Clemente-Suárez, V.J. Physiological, Biomechanical, and Thermographic Responses in Male Athletes during an Ultra-Endurance Race. Appl. Sci. 2024, 14, 6511. https://doi.org/10.3390/app14156511
Belinchón-deMiguel P, Ramos-Campo DJ, Clemente-Suárez VJ. Physiological, Biomechanical, and Thermographic Responses in Male Athletes during an Ultra-Endurance Race. Applied Sciences. 2024; 14(15):6511. https://doi.org/10.3390/app14156511
Chicago/Turabian StyleBelinchón-deMiguel, Pedro, Domingo Jesús Ramos-Campo, and Vicente Javier Clemente-Suárez. 2024. "Physiological, Biomechanical, and Thermographic Responses in Male Athletes during an Ultra-Endurance Race" Applied Sciences 14, no. 15: 6511. https://doi.org/10.3390/app14156511








