Trends and Opportunities in the Dairy Industry: A2 Milk and Processing Methods
Abstract
:1. Introduction
2. Global Market and General Trends
3. Important Milk Components
3.1. Proteins
3.2. Fats
4. A2 Milk
4.1. β-Casomorphin 7
4.2. β-Caseins A1 and A2—The Differences
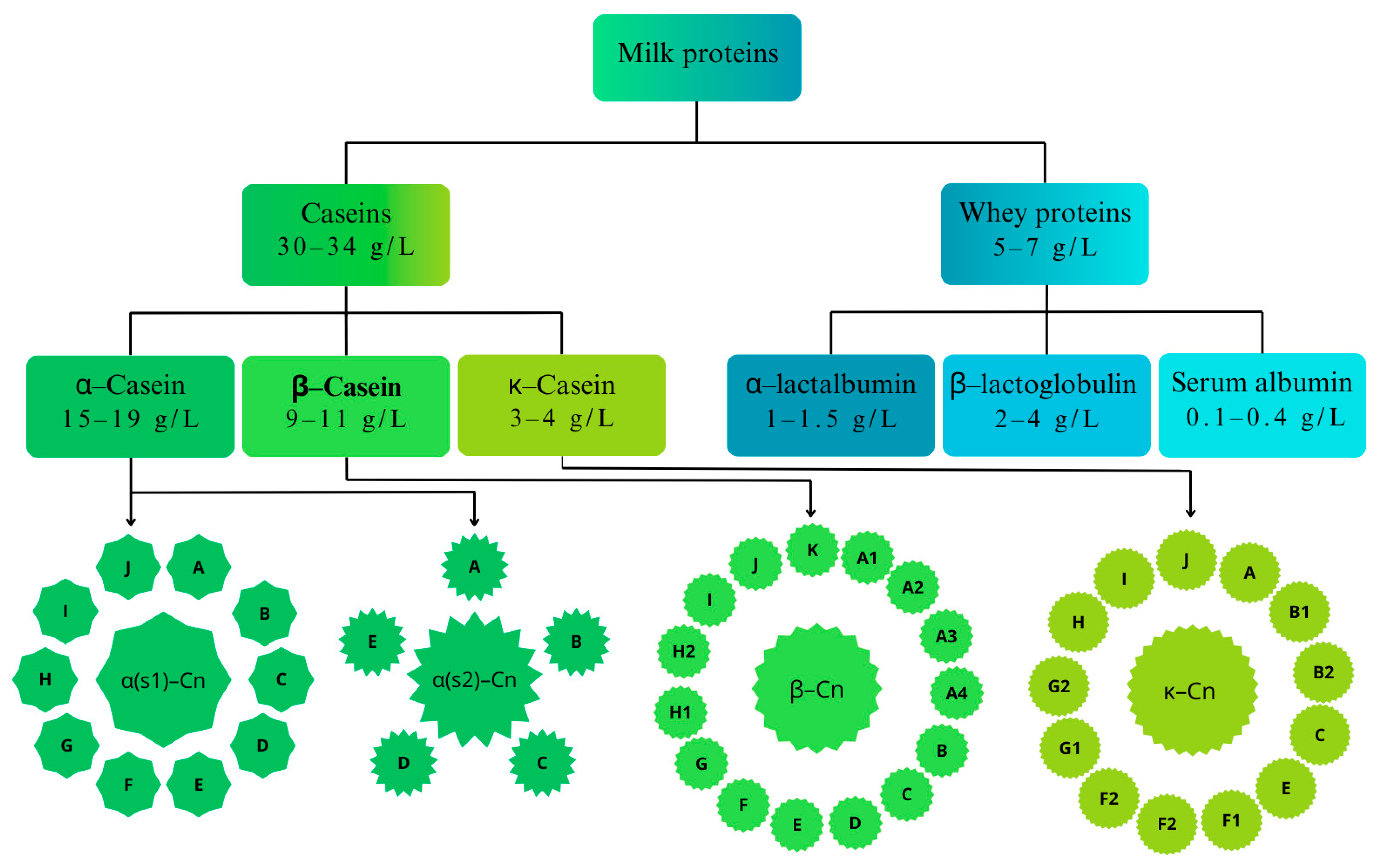
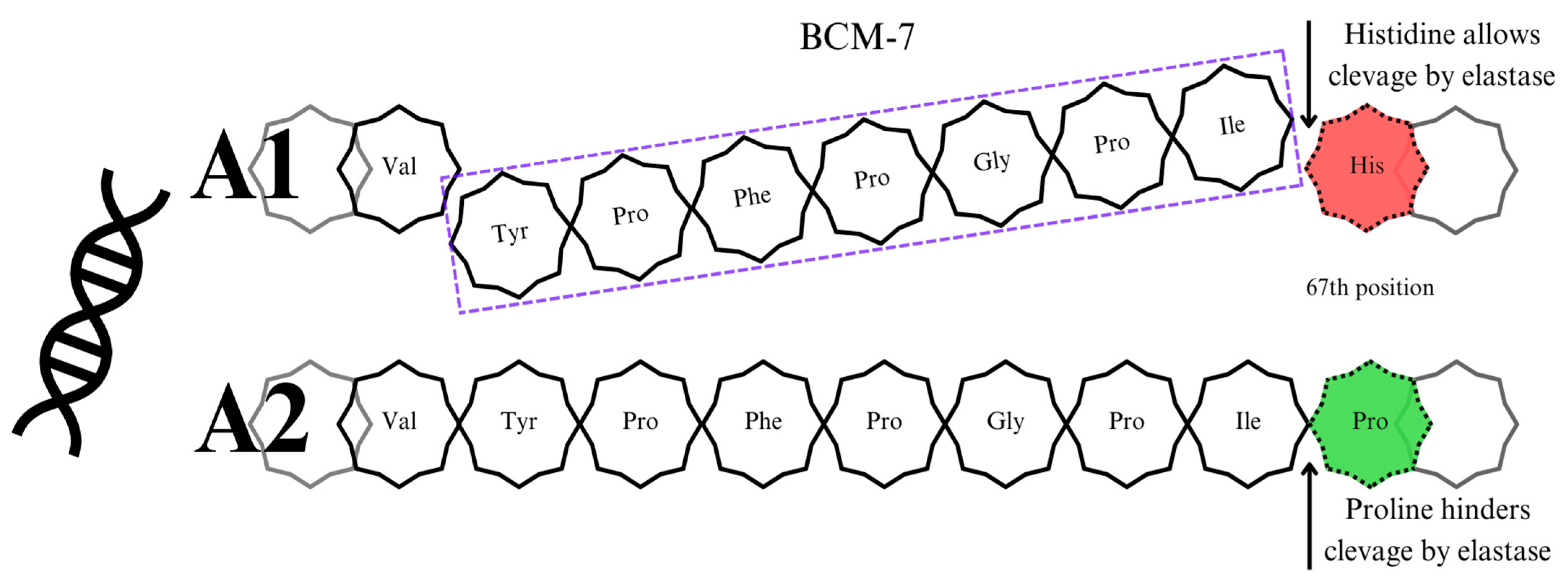
5. Milk Processing
5.1. Thermal Methods
5.1.1. Thermization
5.1.2. Pasteurization and Sterilization
5.1.3. Ohmic Heating (OH)
5.2. Non-Thermal Methods
| Method | Parameters | Study Subject | Impact | Ref. |
|---|---|---|---|---|
| Pulsed electric field (PEF) | 10 kV/cm Pulse width 30 µs | Microbial inactivation and the physical properties of low-fat milk |
| [76] |
| 55 kV/cm 90 Hz Pulse width 900 µs 100 s | Curd properties prepared with PEF-treated raw milk |
| [77] | |
| 20–26 kV Pulse width 34 µs | Physicochemical properties of whole milk-treated PEF (fat, xanthine oxidase, caseins, and whey proteins) |
| [78] | |
| Supercritical fluid technology | Liquid CO2 100–300 bar 50 min 60–70 °C | Mitigating β-LG antigenicity by supercritical fluid extraction in whole milk powder |
| [79] |
| Liquid CO2 15–25 MPa Co-solvent ethanol 10–50 mL 40–80 °C | Supercritical fluid extraction of cholesterol from whole milk powder |
| [80] | |
| Liquid CO2 80–180 bar 10–30 min 30–70 °C | Inactivation of alkaline phosphatase and Escherichia coli in raw whole milk |
| [81] | |
| UV radiation | UV-C lamp with total output power 18 W Flow rate 5–18 mL/min 4–25 °C | Raw milk bacterial load |
| [82] |
| UV-C lamp with total output power 19 W Flow rate 50–150 mL/min 20 °C | Effect of combined UV and heat to inactivate B. subtilis spores in skimmed and whole milk |
| [83] | |
| UV-C lamp with total output power 144 W Flow rate 3 L/min | Enhancing the quality of raw bovine milk |
| [84] | |
| Ionizing radiation | Gamma irradiation Dose 2–10 kGy Dose rate of 1.19 Gy/s | pH, acidity, and microbial contamination of raw milk |
| [85] |
| Electron beam (e-beam) irradiation 5–20 kGy Conveyor speed 80–400 cm/min | Antiproliferative, antidiabetic, and antioxidant activities of defatted cow milk |
| [86] | |
| Gamma irradiation 1–3 kGy Dose rate 45 Gy/min | Bacteriological and sensory quality of raw whole milk |
| [87] | |
| Nonthermal plasma (NTP) | Resonant frequency of 52 kHz 32 kV discharge and frequency 1 kHz | Reducing activity of Pseudomonas-secreted proteases in milk |
| [88] |
| 9 kV AC power supply <35 °C 0–20 min | Changes in protein, free fatty acids, and volatile profiles of whole raw milk |
| [89] | |
| N2-O2 plasma O2 plasma | Acid gelation properties of skim milk |
| [90] | |
| High-pressure processing (HPP) | 100–150 MPa 25 °C | Pseudomonas fluorescens protease inactivation in milk |
| [91] |
| 200–600 MPa 15 min 20 °C | Coagulation of protein and fat globules in whole and skim milk |
| [92] | |
| 250–550 MPa 3–15 min 20 °C | Microbiological quality of skim milk |
| [93] | |
| Ultrasounds (US) | Frequency 24 kHz 200–400 W 2.5–10 min 20–55 °C | Chemical composition and sensory properties of the milk |
| [94] |
| 106–375 W Energy density 190.4, 570.7, 674.3, 2016.9 J/g 3–9 min 4 °C | Rheological and textural properties of rennet-coagulated skim milk |
| [95] | |
| 22.5 kHz 28 W 1–30 min | Protein changes in fresh skim milk |
| [96] | |
| Membrane technologies | Ultrafiltration Polyethersulfone membrane pore size 0.07 μm | Dairy wastewater filtration effectiveness |
| [97] |
| Microfiltration Polyvinylidene fluoride membrane Pore size 0.65 μm | Technology to isolate MFGM from raw and pausterized milk |
| [98] | |
| Microfiltration Silicon carbide ceramic membrane Pore size 1.4 μm | Fat separation from skim and raw milk |
| [99] |
5.2.1. Pulsed Electric Field (PEF)
5.2.2. Supercritical Fluid Technology
5.2.3. UV Radiation
5.2.4. Ionizing Radiation (e-Beam, Gamma)
5.2.5. Cold Plasma—Nonthermal Plasma (NTP)
5.2.6. High-Pressure Processing (HPP)
5.2.7. Ultrasounds (USs)
5.2.8. Membrane Technologies
6. Conclusions and Future Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, B.Q.; Smetana, S. Review on Milk Substitutes from an Environmental and Nutritional Point of View. Appl. Food Res. 2022, 2, 100105. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient Density and Nutritional Value of Milk and Plant-Based Milk Alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Curry, A. Archaeology: The Milk Revolution. Nature 2013, 500, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Thomas, M.G. The Evolution of Lactose Digestion. In Lactose; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–48. [Google Scholar]
- Gai, N.; Waldron, D.S.; Uniacke-Lowe, T.; Li, B.; O’Regan, J.; Goulding, D.A.; Kelly, A.L. Influence of β-Casein Genotype on Cheddar Cheese Making and Ripening. Int. Dairy J. 2024, 149, 105824. [Google Scholar] [CrossRef]
- Bisutti, V.; Pegolo, S.; Giannuzzi, D.; Mota, L.F.M.; Vanzin, A.; Toscano, A.; Trevisi, E.; Ajmone Marsan, P.; Brasca, M.; Cecchinato, A. The β-Casein (CSN2) A2 Allelic Variant Alters Milk Protein Profile and Slightly Worsens Coagulation Properties in Holstein Cows. J. Dairy Sci. 2022, 105, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Mendes, M.; Ferreira de Morais, M.; Ferreira Rodrigues, J. A2A2 Milk: Brazilian Consumers’ Opinions and Effect on Sensory Characteristics of Petit Suisse and Minas Cheeses. LWT 2019, 108, 207–213. [Google Scholar] [CrossRef]
- Dantas, A.; Kumar, H.; Prudencio, E.S.; de Avila, L.B.; Orellana-Palma, P.; Dosoky, N.S.; Nepovimova, E.; Kuča, K.; Cruz-Martins, N.; Verma, R.; et al. An Approach on Detection, Quantification, Technological Properties, and Trends Market of A2 Cow Milk. Food Res. Int. 2023, 167, 112690. [Google Scholar] [CrossRef]
- Juan, B.; Trujillo, A.-J. Acid and Rennet Coagulation Properties of A2 Milk. Foods 2022, 11, 3648. [Google Scholar] [CrossRef]
- Hemar, Y.; Banjar, W.; Otter, D.; Yang, Z. Viscosity, Size, Structural and Interfacial Properties of Sodium Caseinate Obtained from A2 Milk. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126163. [Google Scholar] [CrossRef]
- Daniloski, D.; McCarthy, N.A.; Vasiljevic, T. Impact of Heating on the Properties of A1/A1, A1/A2, and A2/A2 β-Casein Milk Phenotypes. Food Hydrocoll. 2022, 128, 107604. [Google Scholar] [CrossRef]
- Daniloski, D.; McCarthy, N.A.; Gazi, I.; Vasiljevic, T. Rheological and Structural Properties of Acid-Induced Milk Gels as a Function of β-Casein Phenotype. Food Hydrocoll. 2022, 131, 107846. [Google Scholar] [CrossRef]
- Kaskous, S. A1- and A2-Milk and Their Effect on Human Health. J. Food Eng. Technol. 2020, 9, 15–21. [Google Scholar] [CrossRef]
- D’Incecco, P.; Limbo, S.; Hogenboom, J.A.; Pellegrino, L. Novel Technologies for Extending the Shelf Life of Drinking Milk: Concepts, Research Trends and Current Applications. LWT 2021, 148, 111746. [Google Scholar] [CrossRef]
- Foroutan, A.; Guo, A.C.; Vazquez-Fresno, R.; Lipfert, M.; Zhang, L.; Zheng, J.; Badran, H.; Budinski, Z.; Mandal, R.; Ametaj, B.N.; et al. Chemical Composition of Commercial Cow’s Milk. J. Agric. Food Chem. 2019, 67, 4897–4914. [Google Scholar] [CrossRef] [PubMed]
- US Department of Agriculture. Major Producers of Cow Milk Worldwide in 2023, by Country (in Million Metric Tons); US Department of Agriculture: Washington, DC, USA, 2023. [Google Scholar]
- US Department of Agriculture. Total Milk Production in the United States from 1999 to 2024 (in Million Pounds)*; US Department of Agriculture: Washington, DC, USA, 2024. [Google Scholar]
- USDA Foreign Agricultural Service. Cow Milk Production Worldwide from 2015 to 2023 (in Million Metric Tons); USDA Foreign Agricultural Service: Washington, DC, USA, 2023. [Google Scholar]
- Dg Agri Dashboard: Dairy Products. 2024. Available online: https://agriculture.ec.europa.eu/document/download/c9474932-d061-4169-80f1-38951aa8615e_en?filename=dashboard-dairy_en.pdf (accessed on 25 March 2024).
- Bojovic, M.; McGregor, A. A Review of Megatrends in the Global Dairy Sector: What Are the Socioecological Implications? Agric. Hum. Values 2023, 40, 373–394. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Annual Consumption of Fluid Cow Milk Worldwide in 2023, by Country (in 1,000 Metric Tons); USDA Foreign Agricultural Service: Washington, DC, USA, 2022. [Google Scholar]
- Shahbandeh, M. Global Consumption of Milk per Year by Country 2023; Statista: Hamburg, Germany, 2023; Available online: https://www.statista.com/statistics/272003/global-annual-consumption-of-milk-by-region/ (accessed on 2 April 2024).
- Agarwal, S.; Beausire, R.L.W.; Patel, S.; Patel, H. Innovative Uses of Milk Protein Concentrates in Product Development. J. Food Sci. 2015, 80, A23–A29. [Google Scholar] [CrossRef] [PubMed]
- Goulding, D.A.; Fox, P.F.; O’Mahony, J.A. Milk Proteins: An Overview. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 21–98. [Google Scholar]
- Bentivoglio, D.; Finco, A.; Bucci, G.; Staffolani, G. Is There a Promising Market for the A2 Milk? Analysis of Italian Consumer Preferences. Sustainability 2020, 12, 6763. [Google Scholar] [CrossRef]
- Magan, J.B.; O′Callaghan, T.F.; Kelly, A.L.; McCarthy, N.A. Compositional and Functional Properties of Milk and Dairy Products Derived from Cows Fed Pasture or Concentrate-based Diets. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2769–2800. [Google Scholar] [CrossRef]
- Dhillon, B.; Singh Sodhi, K.; Chaudhary, R. Is A2 Milk a Healthier Choice than A1 Milk? A Review. Adv. Bio. Res. 2021, 12, 253–259. [Google Scholar]
- O’callaghan, T.F. An Overview of the A1/A2 Milk Hypothesis. In Dairy Nutrition Forum; The National Dairy Council: Dublin, Ireland, 2020; Volume 12, pp. 1–4. [Google Scholar]
- European Food Safety Authority (EFSA). Review of the Potential Health Impact of β-Casomorphins and Related Peptides. EFSA J. 2009, 7, 231r. [Google Scholar] [CrossRef]
- Küllenberg de Gaudry, D.; Lohner, S.; Schmucker, C.; Kapp, P.; Motschall, E.; Hörrlein, S.; Röger, C.; Meerpohl, J.J. Milk A1 β-Casein and Health-Related Outcomes in Humans: A Systematic Review. Nutr. Rev. 2019, 77, 278–306. [Google Scholar] [CrossRef]
- de la Fuente, M.A.; Juárez, M. Milk and Dairy Products. In Handbook of Mineral Elements in Food; Wiley: Hoboken, NJ, USA, 2015; pp. 645–668. [Google Scholar]
- Górska-Warsewicz, H.; Rejman, K.; Laskowski, W.; Czeczotko, M. Milk and Dairy Products and Their Nutritional Contribution to the Average Polish Diet. Nutrients 2019, 11, 1771. [Google Scholar] [CrossRef] [PubMed]
- Dor, C.; Stark, A.H.; Dichtiar, R.; Keinan-Boker, L.; Shimony, T.; Sinai, T. Milk and Dairy Consumption Is Positively Associated with Height in Adolescents: Results from the Israeli National Youth Health and Nutrition Survey. Eur. J. Nutr. 2022, 61, 429–438. [Google Scholar] [CrossRef]
- Givens, D.I. MILK Symposium Review: The Importance of Milk and Dairy Foods in the Diets of Infants, Adolescents, Pregnant Women, Adults, and the Elderly. J. Dairy Sci. 2020, 103, 9681–9699. [Google Scholar] [CrossRef]
- Franzoi, M.; Niero, G.; Penasa, M.; Cassandro, M.; De Marchi, M. Technical Note: Development and Validation of a New Method for the Quantification of Soluble and Micellar Calcium, Magnesium, and Potassium in Milk. J. Dairy Sci. 2018, 101, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.W.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2020, 8, 878–889.e3. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.M. Chemical Composition of Milk and Milk Products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–34. [Google Scholar]
- Boro, P.; Debnath, J.; Das, T.; Naha, B.; Debbarma, P.; Debbarma, C.; Suinti, L.; Devi, B.; Gynashwari, T. Milk Composition and Factors Affecting It in Dairy Buffaloes: A Review. J. Entomol. Zool. Stud. 2018, 6, 340–343. [Google Scholar]
- Manuyakorn, W.; Tanpowpong, P. Cow Milk Protein Allergy and Other Common Food Allergies and Intolerances. Paediatr. Int. Child Health 2019, 39, 32–40. [Google Scholar] [CrossRef]
- Jiménez-Montenegro, L.; Alfonso, L.; Mendizabal, J.A.; Urrutia, O. Worldwide Research Trends on Milk Containing Only A2 β-Casein: A Bibliometric Study. Animals 2022, 12, 1909. [Google Scholar] [CrossRef]
- Nie, C.; Zhao, Y.; Wang, X.; Li, Y.; Fang, B.; Wang, R.; Wang, X.; Liao, H.; Li, G.; Wang, P.; et al. Structure, Biological Functions, Separation, Properties, and Potential Applications of Milk Fat Globule Membrane (MFGM): A Review. Nutrients 2024, 16, 587. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J. Casein Micelles as an Emerging Delivery System for Bioactive Food Components. Foods 2021, 10, 1965. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.Y.; Dar, T.A.; Singh, L.R. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; InTech: London, UK, 2016. [Google Scholar]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. The Caseins: Structure, Stability, and Functionality. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–92. [Google Scholar]
- Davoodi, S.H.; Shahbazi, R.; Esmaeili, S.; Sohrabvandi, S.; Mortazavian, A.; Jazayeri, S.; Taslimi, A. Health-Related Aspects of Milk Proteins. Iran. J. Pharm. Res. 2016, 15, 573–591. [Google Scholar] [PubMed]
- Runthala, A.; Mbye, M.; Ayyash, M.; Xu, Y.; Kamal-Eldin, A. Caseins: Versatility of Their Micellar Organization in Relation to the Functional and Nutritional Properties of Milk. Molecules 2023, 28, 2023. [Google Scholar] [CrossRef] [PubMed]
- Giribaldi, M.; Lamberti, C.; Cirrincione, S.; Giuffrida, M.G.; Cavallarin, L. A2 Milk and BCM-7 Peptide as Emerging Parameters of Milk Quality. Front. Nutr. 2022, 9, 842375. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Malca, J.A.; Tirado-Kulieva, V.A.; Abanto-López, M.S.; Aldana-Juárez, W.L.; Palacios-Zapata, C.M. Worldwide Research on the Health Effects of Bovine Milk Containing A1 and A2 β-Casein: Unraveling the Current Scenario and Future Trends through Bibliometrics and Text Mining. Curr. Res. Food Sci. 2023, 7, 100602. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk Intolerance, Beta-Casein and Lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef] [PubMed]
- Chia, J.S.J.; McRae, J.L.; Kukuljan, S.; Woodford, K.; Elliott, R.B.; Swinburn, B.; Dwyer, K.M. A1 Beta-Casein Milk Protein and Other Environmental Pre-Disposing Factors for Type 1 Diabetes. Nutr. Diabetes 2017, 7, e274. [Google Scholar] [CrossRef] [PubMed]
- Kuellenberg de Gaudry, D.; Lohner, S.; Bischoff, K.; Schmucker, C.; Hoerrlein, S.; Roeger, C.; Schwingshackl, L.; Meerpohl, J.J. A1- and A2 Beta-Casein on Health-Related Outcomes: A Scoping Review of Animal Studies. Eur. J. Nutr. 2022, 61, 1–21. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between Milk Proteins and Polyphenols: Binding Mechanisms, Related Changes, and the Future Trends in the Dairy Industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Edwards, T.S.; Dawson, K.L.; Keenan, J.I.; Day, A.S. A Simple Method to Generate β-Casomorphin-7 by in Vitro Digestion of Casein from Bovine Milk. J. Funct. Foods 2021, 85, 104631. [Google Scholar] [CrossRef]
- MG, P.; Gourkhede, D.P.; HB, V.; Shinde, B.; Mishra, B.P.; Wankhade, P.R.; Belore, B.; Lalthanmawii, J.; Koneti, P.B. Delving into the A1/A2 Milk Hypothesis: A Comprehensive Analysis of Milk Proteins and Their Impact on Human Health. Int. J. Vet. Sci. Anim. Husb. 2024, 9, 594–605. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, B.; De, A.K. Milk Proteins, Health Issues and Its Implications on National Livestock Breeding Policy of India. Curr. Sci. 2018, 115, 1393. [Google Scholar] [CrossRef]
- Jeong, H.; Park, Y.-S.; Yoon, S.-S. A2 Milk Consumption and Its Health Benefits: An Update. Food Sci. Biotechnol. 2024, 33, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Vigolo, V.; Visentin, E.; Ballancin, E.; Lopez-Villalobos, N.; Penasa, M.; De Marchi, M. β-Casein A1 and A2: Effects of Polymorphism on the Cheese-Making Process. J. Dairy Sci. 2023, 106, 5276–5287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.H.; Schwendel, H.; Harland, D.; Day, L. Differences in the Yoghurt Gel Microstructure and Physicochemical Properties of Bovine Milk Containing A1A1 and A2A2 β-Casein Phenotypes. Food Res. Int. 2018, 112, 217–224. [Google Scholar] [CrossRef] [PubMed]
- de Vitte, K.; Kerziene, S.; Klementavičiūtė, J.; de Vitte, M.; Dilbiene, V.; Stankevičius, R. Relationship between β-Casein Genotypes (A1A1, A1A2, and A2A2) and Coagulation Properties of Milk and the Fatty Acid Composition and Sensory Characteristics of Dairy Products (Soft Cheese, Sour Cream, and Butter). Acta Agric. Scand. A Anim. Sci. 2022, 71, 21–32. [Google Scholar] [CrossRef]
- de Vitte, K.; Kerziene, S.; Klementavičiūtė, J.; de Vitte, M.; Mišeikienė, R.; Kudlinskienė, I.; Čepaitė, J.; Dilbiene, V.; Stankevičius, R. Relationship of β-Casein Genotypes (A1A1, A1A2 and A2A2) to the Physicochemical Composition and Sensory Characteristics of Cows’ Milk. J. Appl. Anim. Res. 2022, 50, 161–166. [Google Scholar] [CrossRef]
- Deeth, H.C.; Lewis, M.J. Heat Treatments of Milk—Thermisation and Pasteurisation. In High Temperature Processing of Milk and Milk Products; Wiley: Hoboken, NJ, USA, 2017; pp. 15–39. [Google Scholar]
- Albarella, S.; Selvaggi, M.; D’Anza, E.; Cosenza, G.; Caira, S.; Scaloni, A.; Fontana, A.; Peretti, V.; Ciotola, F. Influence of the Casein Composite Genotype on Milk Quality and Coagulation Properties in the Endangered Agerolese Cattle Breed. Animals 2020, 10, 892. [Google Scholar] [CrossRef]
- Sempiira, E.J.; Mugisa, D.J.; Galiwango, J.; Kisaalita, W.S. Combining Thermization and Evaporative Cooling toward Milk Freshness Preservation at the Smallholder Farm Level. J. Food Process Eng. 2020, 43, e13529. [Google Scholar] [CrossRef]
- Rukke, E.O.; Sørhaug, T.; Stepaniak, L. HEAT TREATMENT OF MILK|Thermization of Milk. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2011; pp. 693–698. [Google Scholar]
- Panthi, R.R.; Jordan, K.N.; Kelly, A.L.; (Diarmuid) Sheehan, J.J. Selection and Treatment of Milk for Cheesemaking. In Cheese; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–50. [Google Scholar]
- Dhotre, A.V. Milk Pasteurization and Equipment. In Animal Products Technology; Mandal, P.K., Biswas, A.K., Eds.; Studium Press (India) Pvt.Ltd.: New Delhi, India, 2014; pp. 51–78. [Google Scholar]
- Myer, P.R.; Parker, K.R.; Kanach, A.T.; Zhu, T.; Morgan, M.T.; Applegate, B.M. The Effect of a Novel Low Temperature-Short Time (LTST) Process to Extend the Shelf-Life of Fluid Milk. Springerplus 2016, 5, 660. [Google Scholar] [CrossRef] [PubMed]
- Indumathy, M.; Sobana, S.; Panda, B.; Panda, R.C. Modelling and Control of Plate Heat Exchanger with Continuous High-Temperature Short Time Milk Pasteurization Process—A Review. Chem. Eng. J. Adv. 2022, 11, 100305. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of Different Heat Treatments on the Maillard Reaction Products, Volatile Compounds and Glycation Level of Milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Cabral, L.; Guimarães, J.T.; Noronha, M.F.; Cappato, L.P.; Cruz, A.G.; Sant’Ana, A.S. Conventional and Ohmic Heating Pasteurization of Fresh and Thawed Sheep Milk: Energy Consumption and Assessment of Bacterial Microbiota during Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2022, 76, 102947. [Google Scholar] [CrossRef]
- Rocha, R.S.; Silva, R.; Ramos, G.L.P.; Cabral, L.A.; Pimentel, T.C.; Campelo, P.H.; Blumer Zacarchenco, P.; Freitas, M.Q.; Esmerino Erick, A.; Silva, M.C.; et al. Ohmic Heating Treatment in High-Protein Vanilla Flavored Milk: Quality, Processing Factors, and Biological Activity. Food Res. Int. 2022, 161, 111827. [Google Scholar] [CrossRef]
- Ahmad, T.; Butt, M.Z.; Aadil, R.M.; Inam-ur-Raheem, M.; Abdullah; Bekhit, A.E.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Esmerino, E.A.; et al. Impact of Nonthermal Processing on Different Milk Enzymes. Int. J. Dairy Technol. 2019, 72, 481–495. [Google Scholar] [CrossRef]
- Abrahamsen, R.K.; Narvhus, J.A. Can Ultrasound Treatment Replace Conventional High Temperature Short Time Pasteurization of Milk? A Critical Review. Int. Dairy J. 2022, 131, 105375. [Google Scholar] [CrossRef]
- Masotti, F.; Cattaneo, S.; Stuknytė, M.; De Noni, I. Current Insights into Non-Thermal Preservation Technologies Alternative to Conventional High-Temperature Short-Time Pasteurization of Drinking Milk. Crit. Rev. Food Sci. Nutr. 2023, 63, 5643–5660. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.J.; Han, B.K.; Choi, H.J.; Kang, S.H.; Baick, S.C.; Lee, D.-U. Inactivation of Escherichia Coli, Saccharomyces Cerevisiae, and Lactobacillus Brevis in Low-Fat Milk by Pulsed Electric Field Treatment: A Pilot-Scale Study. Korean J. Food Sci. Anim. Resour. 2015, 35, 800–806. [Google Scholar] [CrossRef]
- Preeti, B.; Ravindra, M.R.; Shivaram, M.; Gajanan, D.P.; Singh, A.M. Effect of Pulsed Electric Field Treated on Quality of Curd. Food Sci. Technol. Int. 2023, 29, 598–609. [Google Scholar] [CrossRef]
- Sharma, P.; Oey, I.; Everett, D.W. Thermal Properties of Milk Fat, Xanthine Oxidase, Caseins and Whey Proteins in Pulsed Electric Field-Treated Bovine Whole Milk. Food Chem. 2016, 207, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Venkatram, R.; García-Cano, I.; Jiménez-Flores, R. Reduction in the Antigenicity of Beta-Lactoglobulin in Whole Milk Powder via Supercritical CO2 Treatment. J. Dairy Sci. 2024, 107, 4216–4234. [Google Scholar] [CrossRef] [PubMed]
- Dey Paul, I.; Jayakumar, C.; Niwas Mishra, H. Optimization of Process Parameters for Supercritical Fluid Extraction of Cholesterol from Whole Milk Powder Using Ethanol as Co-solvent. J. Sci. Food Agric. 2016, 96, 4885–4895. [Google Scholar] [CrossRef] [PubMed]
- Ceni, G.; Fernandes Silva, M.; Valério, C., Jr.; Cansian, R.L.; Oliveira, J.V.; Dalla Rosa, C.; Mazutti, M.A. Continuous Inactivation of Alkaline Phosphatase and Escherichia Coli in Milk Using Compressed Carbon Dioxide as Inactivating Agent. J. CO2 Util. 2016, 13, 24–28. [Google Scholar] [CrossRef]
- Atik, A.; Gumus, T. The Effect of Different Doses of UV-C Treatment on Microbiological Quality of Bovine Milk. LWT 2021, 136, 110322. [Google Scholar] [CrossRef]
- Ansari, J.A.; Ismail, M.; Farid, M. Investigate the Efficacy of UV Pretreatment on Thermal Inactivation of Bacillus Subtilis Spores in Different Types of Milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 387–393. [Google Scholar] [CrossRef]
- Makararpong, D.; Tantayanon, S.; Gowanit, C.; Jareonsawat, J.; Samgnamnim, S.; Wataradee, S.; Hogeveen, H.; Inchaisri, C. Enhancing Raw Bovine Milk Quality Using Ultraviolet-C (UV-C) Irradiation: A Microbial and Lipid Peroxidation Study. Food Sci. Anim. Resour. 2024, 44, 372–389. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, M.; Ghorbani-HasanSaraei, A.; Sadeghzadeh, N. Evaluation of the Effect of Gamma Irradiation in Combination with Ascorbic Acid on the Chemical Parameters and the Decontamination of Raw Milk. Radiat. Phys. Chem. 2022, 201, 110462. [Google Scholar] [CrossRef]
- Harizi, N.; Madureira, J.; Haffani, Y.Z.; Zouari, A.; Ayadi, M.A.; Verde, S.C.; Boudhrioua, N. E-Beam Irradiation of Defatted Liquid Camel and Cow Milk Fractions: Antiproliferative, Antidiabetic and Antioxidant Activities. Innov. Food Sci. Emerg. Technol. 2023, 89, 103457. [Google Scholar] [CrossRef]
- de Oliveira Silva, A.C.; de Oliveira, L.A.T.; de Jesus, E.F.O.; Cortez, M.A.S.; Alves, C.C.C.; Monteiro, M.L.G.; Conte Junior, C.A. Effect of Gamma Irradiation on the Bacteriological and Sensory Analysis of Raw Whole Milk under Refrigeration. J. Food Process Preserv. 2015, 39, 2404–2411. [Google Scholar] [CrossRef]
- Mohammadpour, H.; Zarei, M.; Cullen, P.J.; Valtchev, P.; Schindeler, A.; Dehghani, F. Potential Application of Non-Thermal Atmospheric Plasma in Reducing the Activity of Pseudomonas-Secreted Proteases in Milk. Int. Dairy J. 2021, 120, 105078. [Google Scholar] [CrossRef]
- Korachi, M.; Ozen, F.; Aslan, N.; Vannini, L.; Guerzoni, M.E.; Gottardi, D.; Ekinci, F.Y. Biochemical Changes to Milk Following Treatment by a Novel, Cold Atmospheric Plasma System. Int. Dairy J. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.K. Effect of Atmospheric Cold Plasma Treatment on Acid Gelation Properties of Skim Milk: Rheology and Textural Studies. Food Res. Int. 2023, 172, 113212. [Google Scholar] [CrossRef]
- de Oliveira, M.M.; de Castro Leite Júnior, B.R.; Tribst, A.A.L.; Cristianini, M. Use of High Pressure Homogenization to Reduce Milk Proteolysis Caused by Pseudomonas Fluorescens Protease. LWT 2018, 92, 272–275. [Google Scholar] [CrossRef]
- He, X.; Yang, M.; Yuan, F.; Singh, H.; Ye, A. High-Pressure Processing of Bovine Milk: Effects on the Coagulation of Protein and Fat Globules during Dynamic in Vitro Gastric Digestion. Curr. Res. Food Sci. 2022, 5, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Liepa, M.; Zagorska, J.; Galoburda, R.; Kostascuka, S. Effect of High-Pressure Processing on Microbial Quality of Skimmed Milk. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2018, 72, 118–122. [Google Scholar] [CrossRef]
- Juraga, E.; Vukušić Pavičić, T.; Gajdoš Kljusurić, J.; Brnčić, M.; Juraga, T.; Herceg, Z. Properties of Milk Treated with High-Power Ultrasound and Bactofugation. Food Technol. Biotechnol. 2021, 59, 92–102. [Google Scholar] [CrossRef]
- Hammam, A.R.A.; Martinez-Monteagudo, S.I.; Metzger, L.E.; Alsaleem, K.A. Effect of Ultrasound Intensity on the Functional Characteristics of Rennet-coagulated Skim Milk. J. Food Process Eng. 2021, 44, e13800. [Google Scholar] [CrossRef]
- Yang, J.; Yang, M.; Qin, J.; Zeng, Q.; Wang, Y.; Han, N. Effect of Ultrasound on the Structural Characteristics of Fresh Skim Milk. Food Sci. Technol. Int. 2020, 26, 222–230. [Google Scholar] [CrossRef]
- Al-Tayawi, A.N.; Gulyás, N.S.; Gergely, G.; Fazekas, Á.F.; Szegedi, B.; Hodúr, C.; Lennert, J.R.; Kertész, S. Enhancing Ultrafiltration Performance for Dairy Wastewater Treatment Using a 3D Printed Turbulence Promoter. Environ. Sci. Pollut. Res. 2023, 30, 108907–108916. [Google Scholar] [CrossRef]
- Hansen, S.F.; Petrat-Melin, B.; Rasmussen, J.T.; Larsen, L.B.; Ostenfeld, M.S.; Wiking, L. Placing Pasteurisation before or after Microfiltration Impacts the Protein Composition of Milk Fat Globule Membrane Material. Int. Dairy J. 2018, 81, 35–41. [Google Scholar] [CrossRef]
- Dons, T.; Candelario, V.; Andersen, U.; Ahrné, L.M. Gentle Milk Fat Separation Using Silicon Carbide Ceramic Membranes. Innov. Food Sci. Emerg. Technol. 2023, 84, 103299. [Google Scholar] [CrossRef]
- Shabbir, M.A.; Ahmed, H.; Maan, A.A.; Rehman, A.; Afraz, M.T.; Iqbal, M.W.; Khan, I.M.; Amir, R.M.; Ashraf, W.; Khan, M.R.; et al. Effect of Non-Thermal Processing Techniques on Pathogenic and Spoilage Microorganisms of Milk and Milk Products. Food Sci. Technol. 2021, 41, 279–294. [Google Scholar] [CrossRef]
- Alirezalu, K.; Munekata, P.E.S.; Parniakov, O.; Barba, F.J.; Witt, J.; Toepfl, S.; Wiktor, A.; Lorenzo, J.M. Pulsed Electric Field and Mild Heating for Milk Processing: A Review on Recent Advances. J. Sci. Food Agric. 2020, 100, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Pavan, M.S.; SaiPrasanna, N.; Kant, R. Applications of Super Critical Fluid Extraction in Milk and Dairy Industry: A Review. J. Food Process. Technol. 2018, 9, 12. [Google Scholar] [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Cappato, L.P.; Guimaraes, J.T.; Alvarenga, V.O.; Esmerino, E.A.; Portela, J.B.; Sant’ Ana, A.S.; Freitas, M.Q.; et al. Dairy Processing Using Supercritical Carbon Dioxide Technology: Theoretical Fundamentals, Quality and Safety Aspects. Trends Food Sci. Technol. 2017, 64, 94–101. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Safety of UV-treated Milk as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2016, 14, 4370. [Google Scholar] [CrossRef]
- Delorme, M.M.; Guimarães, J.T.; Coutinho, N.M.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Margalho, L.P.; Pimentel, T.C.; Silva, M.C.; Freitas, M.Q.; et al. Ultraviolet Radiation: An Interesting Technology to Preserve Quality and Safety of Milk and Dairy Foods. Trends Food Sci. Technol. 2020, 102, 146–154. [Google Scholar] [CrossRef]
- Jermann, C.; Koutchma, T.; Margas, E.; Leadley, C.; Ros-Polski, V. Mapping Trends in Novel and Emerging Food Processing Technologies around the World. Innov. Food Sci. Emerg. Technol. 2015, 31, 14–27. [Google Scholar] [CrossRef]
- Bhullar, M.S.; Patras, A.; Kilanzo-Nthenge, A.; Pokharel, B.; Yannam, S.K.; Rakariyatham, K.; Pan, C.; Xiao, H.; Sasges, M. Microbial Inactivation and Cytotoxicity Evaluation of UV Irradiated Coconut Water in a Novel Continuous Flow Spiral Reactor. Food Res. Int. 2018, 103, 59–67. [Google Scholar] [CrossRef]
- Cilliers, F.P.; Gouws, P.A.; Koutchma, T.; Engelbrecht, Y.; Adriaanse, C.; Swart, P. A Microbiological, Biochemical and Sensory Characterisation of Bovine Milk Treated by Heat and Ultraviolet (UV) Light for Manufacturing Cheddar Cheese. Innov. Food Sci. Emerg. Technol. 2014, 23, 94–106. [Google Scholar] [CrossRef]
- Wen, C.; Peng, Y.; Zhang, L.; Chen, Y.; Yu, J.; Bai, J.; Yang, K.; Ding, W. Effect of Electron Beam Irradiation on Raw Goat Milk: Microbiological, Physicochemical and Protein Structural Analysis. J. Sci. Food Agric. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lung, H.-M.; Cheng, Y.-C.; Chang, Y.-H.; Huang, H.-W.; Yang, B.B.; Wang, C.-Y. Microbial Decontamination of Food by Electron Beam Irradiation. Trends Food Sci. Technol. 2015, 44, 66–78. [Google Scholar] [CrossRef]
- Odueke, O.B.; Chadd, S.A.; Baines, R.N.; Farag, K.W.; Jansson, J. Effects of Gamma Irradiation on the Shelf-Life of a Dairy-like Product. Radiat. Phys. Chem. 2018, 143, 63–71. [Google Scholar] [CrossRef]
- Carvalho Santos, I.; Pinto, J.; Pimenta, A.I.; Madureira, J.; Matos, P.; Viegas, C.; Raposo, A.; Margaça, F.M.A.; Cabo Verde, S. Use of Gamma Radiation in Sheep Butter Manufacturing Process for Shelf-Life Extension. Int. Dairy J. 2017, 71, 43–49. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kahar, S.P.; Ranveer, R.C.; Annapure, U.S. Cold Plasma an Emerging Nonthermal Technology for Milk and Milk Products: A Review. Int. J. Dairy Technol. 2021, 74, 615–626. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Stelmasiak, A.; Wierzbicka, A.; Poltorak, A. Green Technology for Pork Loin Wet Curing—Unconventional Use of Cow and Soy Milk Treated with Non-Thermal Atmospheric Plasma. Foods 2022, 11, 2523. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Keener, K.M. The Effects of Cold Plasma Technology on Physical, Nutritional, and Sensory Properties of Milk and Milk Products. LWT 2022, 154, 112729. [Google Scholar] [CrossRef]
- Lee, T.-A.; Lin, Y.-H.; Li, P.-H.; Ho, J.-H. The Effects of Corona Discharge from a Cold Plasma Source on the Physicochemical Properties and Shelf-Life of Milk. Food Biosci. 2024, 103980. [Google Scholar] [CrossRef]
- Lim, S.H.; Chin, N.L.; Sulaiman, A.; Tay, C.H.; Wong, T.H. Sensory Analysis for Cow Milk Product Development Using High Pressure Processing (HPP) in the Dairy Industry. Foods 2022, 11, 1233. [Google Scholar] [CrossRef]
- Rodríguez-Alcalá, L.M.; Castro-Gómez, P.; Felipe, X.; Noriega, L.; Fontecha, J. Effect of Processing of Cow Milk by High Pressures under Conditions up to 900 MPa on the Composition of Neutral, Polar Lipids and Fatty Acids. LWT Food Sci. Technol. 2015, 62, 265–270. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of High Pressure Processing on the Safety, Shelf Life and Quality of Raw Milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Chávez-Martínez, A.; Reyes-Villagrana, R.A.; Rentería-Monterrubio, A.L.; Sánchez-Vega, R.; Tirado-Gallegos, J.M.; Bolivar-Jacobo, N.A. Low and High-Intensity Ultrasound in Dairy Products: Applications and Effects on Physicochemical and Microbiological Quality. Foods 2020, 9, 1688. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Huppertz, T. Effect of Nonthermal Processing on Milk Protein Interactions and Functionality. In Milk Proteins; Elsevier: Amsterdam, The Netherlands, 2020; pp. 293–324. [Google Scholar]
- Reig, M.; Vecino, X.; Cortina, J.L. Use of Membrane Technologies in Dairy Industry: An Overview. Foods 2021, 10, 2768. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ukuku, D.O. The Role of Emerging Technologies to Ensure the Microbial Safety of Fresh Produce, Milk and Eggs. Curr. Opin. Food Sci. 2018, 19, 145–154. [Google Scholar] [CrossRef]
- Steinhauer, T.; Marx, M.; Bogendörfer, K.; Kulozik, U. Membrane Fouling during Ultra- and Microfiltration of Whey and Whey Proteins at Different Environmental Conditions: The Role of Aggregated Whey Proteins as Fouling Initiators. J. Membr. Sci. 2015, 489, 20–27. [Google Scholar] [CrossRef]
- Hansen, S.F.; Hogan, S.A.; Tobin, J.; Rasmussen, J.T.; Larsen, L.B.; Wiking, L. Microfiltration of Raw Milk for Production of High-Purity Milk Fat Globule Membrane Material. J. Food Eng. 2020, 276, 109887. [Google Scholar] [CrossRef]
- Yao, D.; Ranadheera, C.S.; Shen, C.; Wei, W.; Cheong, L.-Z. Milk Fat Globule Membrane: Composition, Production and Its Potential as Encapsulant for Bioactives and Probiotics. Crit. Rev. Food Sci. Nutr. 2023, 1–16. [Google Scholar] [CrossRef]
| Study Subject | Results A1 | Results A2 | Ref. |
|---|---|---|---|
| Acid and rennet coagulation |
|
| [9] |
| |||
| Rennet coagulation and cheese making |
|
| [5] |
| |||
| Impact of heating |
|
| [11] |
| Cheese yield, curd nutrient recovery, whey composition, and cheese composition |
|
| [58] |
| |||
| Calcium distribution, acid gelation, foaming properties, and microstructure of acid gels |
|
| [59] |
| Coagulation, fatty acids composition, and sensory characteristics |
|
| [60] |
| |||
| Main components, fatty acids composition, amino acids composition, and sensory characteristics |
|
| [61] |
| Milk composition, rennet coagulation properties, and cheese-making properties |
|
| [6] |
| |||
| Sensory characteristics and consumers opinions |
|
| [7] |
| |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żbik, K.; Onopiuk, A.; Górska-Horczyczak, E.; Wierzbicka, A. Trends and Opportunities in the Dairy Industry: A2 Milk and Processing Methods. Appl. Sci. 2024, 14, 6513. https://doi.org/10.3390/app14156513
Żbik K, Onopiuk A, Górska-Horczyczak E, Wierzbicka A. Trends and Opportunities in the Dairy Industry: A2 Milk and Processing Methods. Applied Sciences. 2024; 14(15):6513. https://doi.org/10.3390/app14156513
Chicago/Turabian StyleŻbik, Klara, Anna Onopiuk, Elżbieta Górska-Horczyczak, and Agnieszka Wierzbicka. 2024. "Trends and Opportunities in the Dairy Industry: A2 Milk and Processing Methods" Applied Sciences 14, no. 15: 6513. https://doi.org/10.3390/app14156513





