Removal of Reactive Yellow 86 from Synthetic Wastewater in Lab-Scale Constructed Wetlands Planted with Cattail and Papyrus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Wastewater
2.2. Lab-Scale CWs
2.3. Operation of CWs
2.4. Screening RY86-Decolorzing Microorganisms
2.5. Analytical Procedures
3. Results
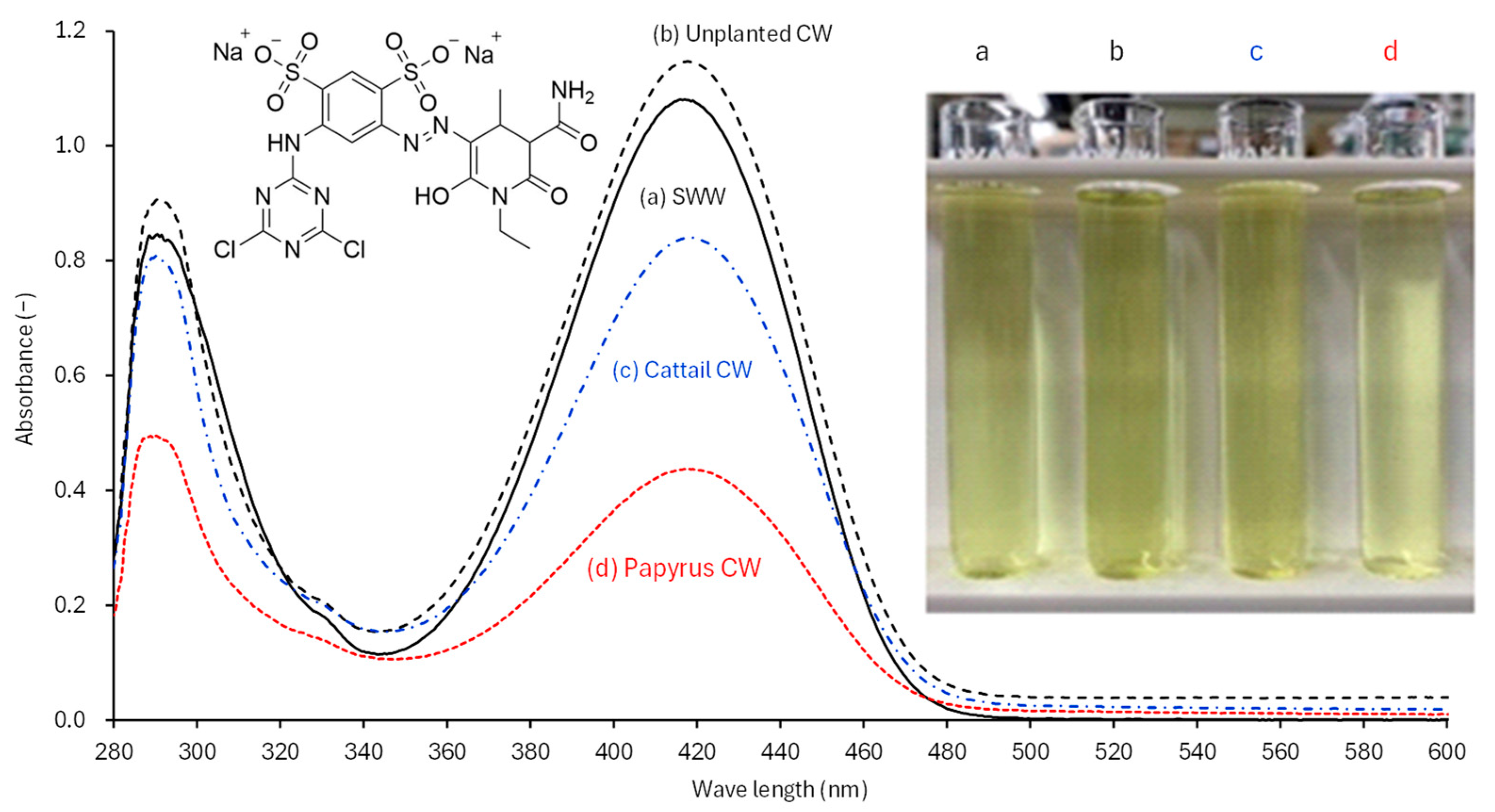
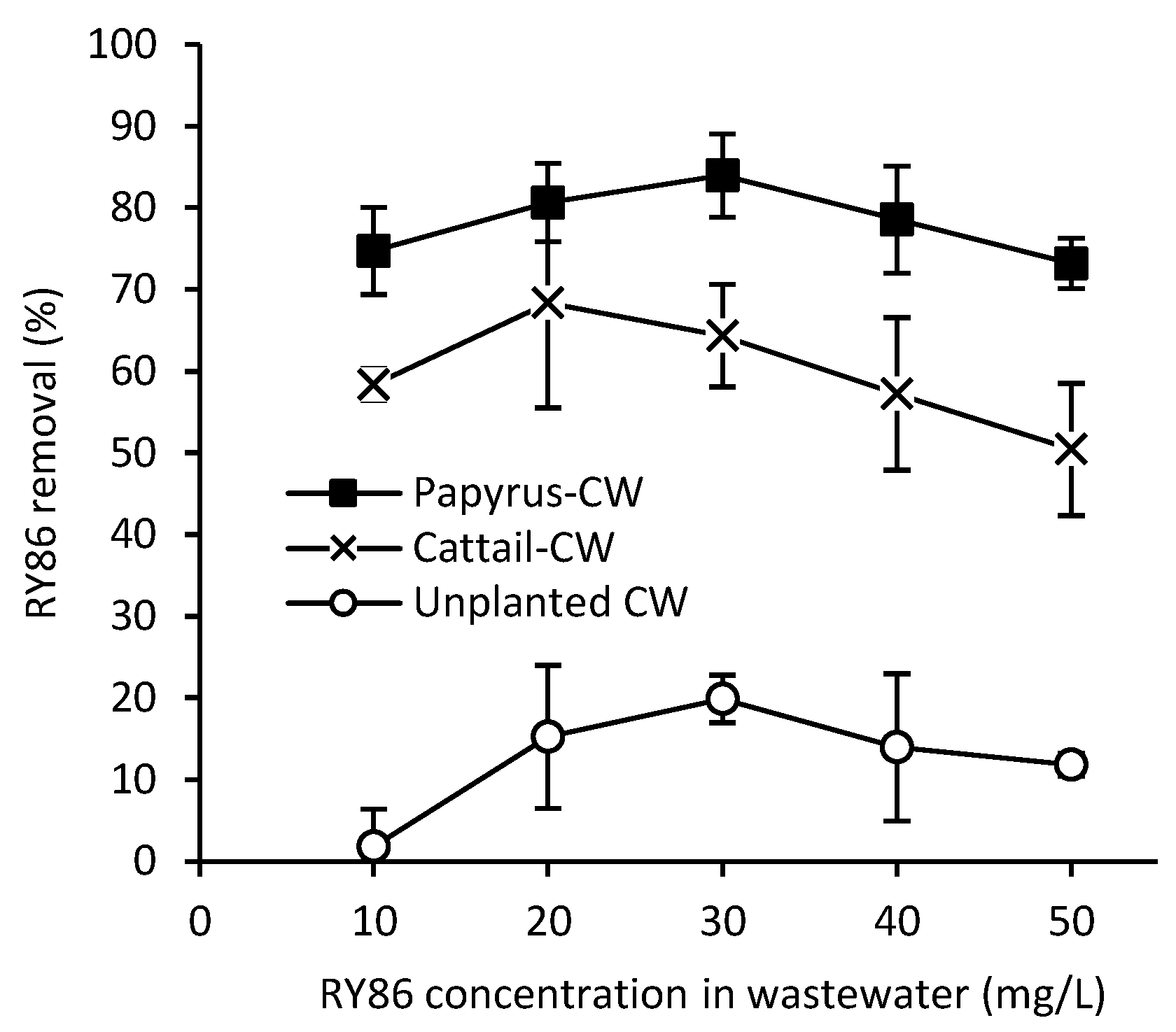
3.1. Effects of RY86 Concentration on CW Effluent Quality
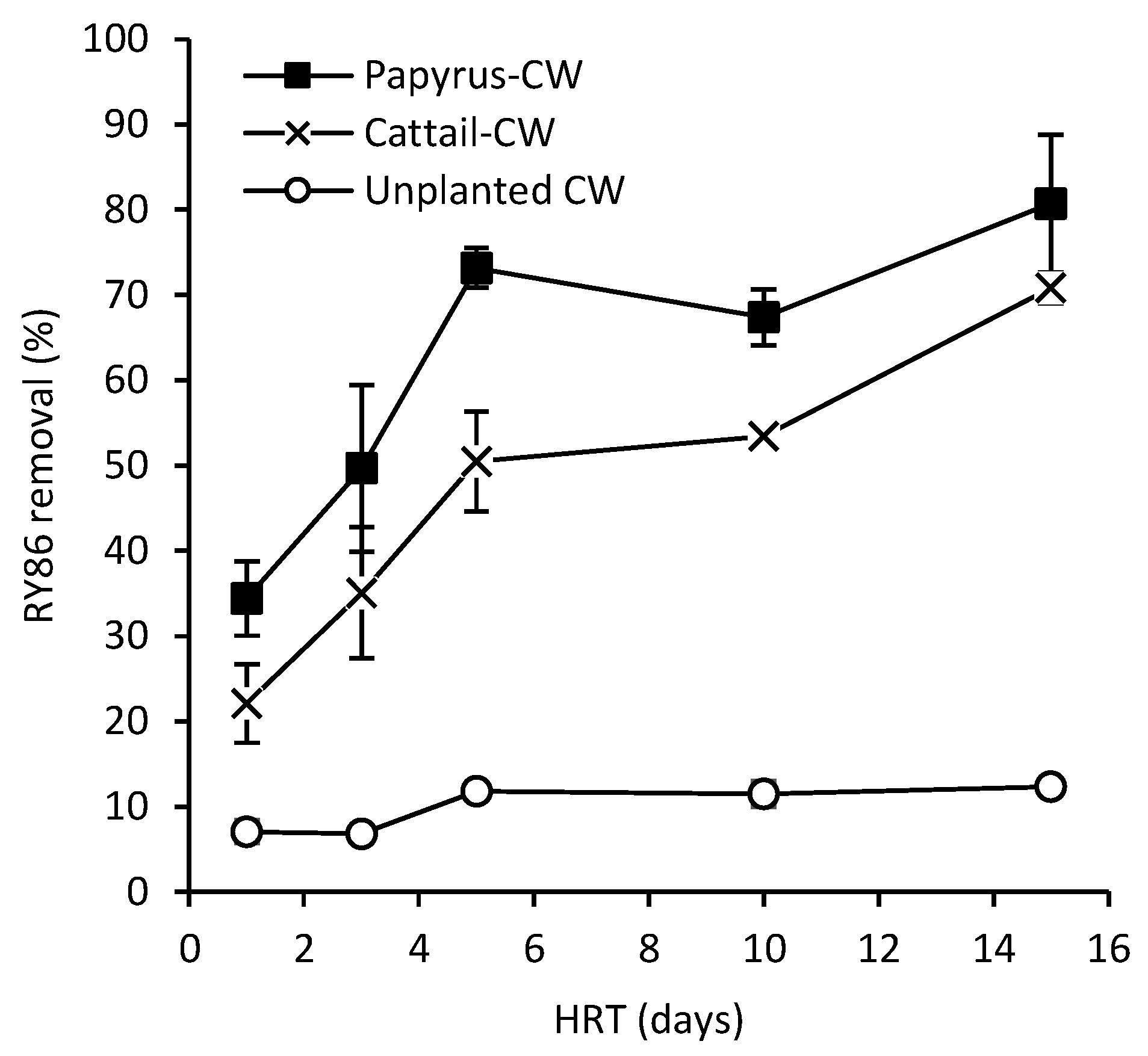
3.2. Effects of HRT on CW Effluent Quality
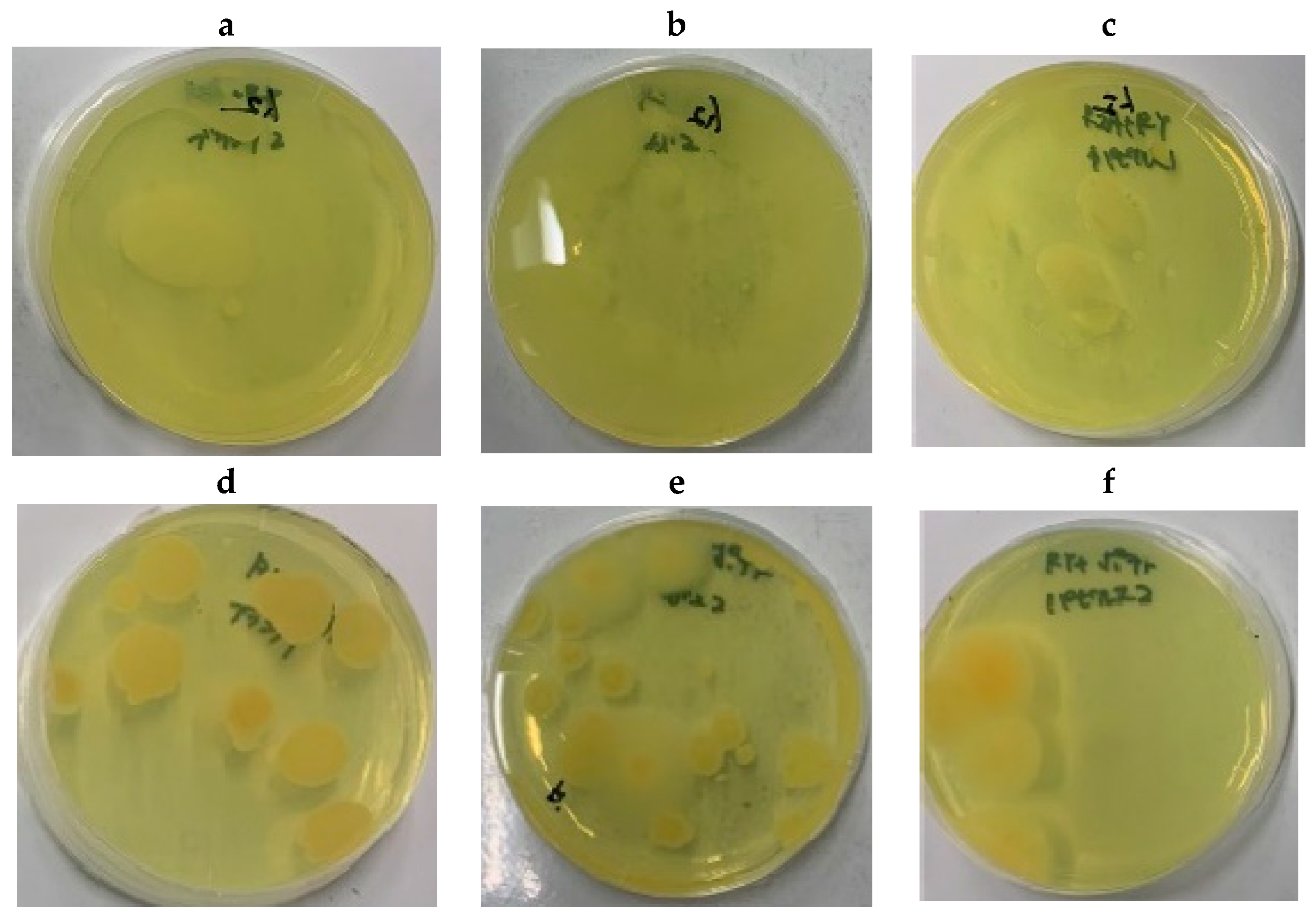
3.3. RY86-Degrading Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pramugani, A.; Soda, S.; Argo, T.A. Current situation of batik wastewater treatment in Pekalongan city, Indonesia. J. JSCE 2020, 8, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Daud, N.M.; Abdullah, S.R.S.; Hasan, H.A.; Ismail, N.; Dhokhikah, Y. Integrated physical-biological treatment system for batik industry wastewater: A review on process selection. Sci. Total Environ. 2022, 819, 152931. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Mahmud, K.; Faruk, O.; Billah, M.S. Textile dyeing industries in Bangladesh for sustainable development. Int. J. Environ. Sci. 2011, 2, 428–436. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Hua, Z.; Du, G. Research and application of biotechnology in textile industries in China. Enzyme Microb. Technol. 2007, 40, 1651–1655. [Google Scholar] [CrossRef]
- Freeman, H.S.; Sokolowska, J. Developments in dyestuff chemistry. Rev. Prog. Color. Relat. Top. 1999, 29, 8–22. [Google Scholar] [CrossRef]
- Zakaria, N.; Rohani, R.; Mohtar, W.H.M.W.; Purwadi, R.; Sumampouw, G.A.; Indarto, A. Batik effluent treatment and decolorization—A review. Water 2023, 15, 1339. [Google Scholar] [CrossRef]
- Bergamini, R.B.M.; Azevedo, E.B.; de Araújo, L.R.R. Heterogeneous photocatalytic degradation of reactive dyes in aqueous TiO2 suspensions: Decolorization kinetics. Chem. Eng. J. 2009, 149, 215–220. [Google Scholar] [CrossRef]
- Suphitcha, W.; Aroonsrimorakot, S.; Thavipoke, P.; Kumsopa, A.; Sangjan, S. Removal of reactive dyes from textile dyeing industrial effluent by ozonation process. APCBEE Procedia 2013, 5, 279–282. [Google Scholar]
- Pramugani, A.; Shimizu, T.; Goto, S.; Argo, T.A.; Soda, S. Decolorization and biodegradability enhancement of synthetic Batik wastewater containing Reactive Black 5 and Reactive Orange 16 by ozonation. Water 2022, 14, 3330. [Google Scholar] [CrossRef]
- Nilratnisakorn, S.; Thiravetyan, P.; Nakbanpote, W.A. Constructed wetland model for synthetic reactive dye wastewater treatment by narrow-leaved cattails (Typha angustifolia Linn.). Water Sci. Technol. 2009, 60, 1565–1574. [Google Scholar] [CrossRef]
- Noonpui, S.; Thiravetyan, P. Treatment of reactive azo dye from textile wastewater by burhead (Echinodorus cordifolius L.) in constructed wetland: Effect of molecular size. J. Environ. Sci. Health A 2011, 46, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Saba, B.; Jabeen, M.; Khalid, A.; Aziz, I.; Christy, A. Effectiveness of rice agriculture wastes, microbes and wetland plants in the removal of Reactive Blak-5 azo dye in microcosm constructed wetland. Int. J. Phytoremediat. 2015, 17, 1060–1067. [Google Scholar] [CrossRef]
- Dogdu, G.; Yalcuk, A. Evaluation of the treatment performance of lab-scaled vertical flow constructed wetlands in removal of organic compounds, color and nutrients in azo dye-containing wastewater. Int. J. Phytoremediat. 2016, 18, 171–183. [Google Scholar] [CrossRef]
- Hussein, A.; Scholz, M. Dye wastewater treatment by vertical-flow constructed wetlands. Ecol. Eng. 2017, 101, 28–38. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, J.; Kumar, S.; Singh, P.; Al-Rashed, S.; Kaur, H.; Rawat, M. An efficient and viable photodegradation of a textile Reactive Yellow-86 dye under direct sunlight by multi-structured Fe2O3 encapsulated with phytochemicals of R. Indica. J. Mater. Sci. Mater. Electron. 2020, 31, 21233–21247. [Google Scholar] [CrossRef]
- Eren, T.; Törün, H.; Yola, M.L.; Ertan, B.; Atar, N. Removal of reactive yellow 86 usingTiO2 nanoparticle- colemanite waste. J. Chem. Eng. Res. Stud. 2014, 1, 102. [Google Scholar]
- Kaur, H.; Singh, J.; Rani, P.; Kaur, N.; Kumar, S.; Rawat, M. A novel and one-pot synthesis of Punica granatum mediated copper oxide having flower-like morphology as an efficient visible-light driven photocatalyst for degradation of textile dyes in waste water. J. Mol. Liq. 2022, 355, 118966. [Google Scholar] [CrossRef]
- Katsumata, H.; Koike, S.; Kaneco, S.; Suzuki, T.; Ohta, K. Degradation of Reactive Yellow 86 with photo-Fenton process driven by solar light. J. Environ. Sci. 2010, 22, 1455–1461. [Google Scholar] [CrossRef]
- Khandegar, K.; Saroha, A.K. Electrochemical treatment of effluent from small-scale dyeing unit. Ind. Chem. Eng. 2013, 55, 112–120. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, K.K.; Jacob, S.; Gakhar, S.K. Molecular docking of laccase protein from Bacillus safensis DSKK5 isolated from earthworm gut: A novel method to study dye decolorization potential. Water Air Soil Pollut. 2014, 225, 2175. [Google Scholar] [CrossRef]
- García-Ávila, F.; Patiño-Chávez, J.; Zhinín-Chimbo, F.; Donoso-Moscoso, S.; del Pino, L.F.; Avilés-Añazco, A. Performance of Phragmites australis and Cyperus papyrus in the treatment of municipal wastewater by vertical flow subsurface constructed wetlands. Int. Soil Water Conserv. Res. 2019, 7, 286–296. [Google Scholar] [CrossRef]
- Hamad, M.T.M.H. Comparative study on the performance of Typha latifolia and Cyperus papyrus on the removal of heavy metals and enteric bacteria from wastewater by surface constructed wetlands. Chemosphere 2020, 260, 127551. [Google Scholar] [CrossRef]
- Page, V.; Schwitzguebel, J. The role of cytochromes P450 and peroxidases in the detoxification of sulfonated anthroquinones by rhubarb and common sorrel plants cultivated under hydroponic conditions. Environ. Sci. Pollut. Res. Int. 2009, 16, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Q.; Zandstra, M.B.; Gupta, S.; Joner, E.J. Utilizing the synergy between plants and rhizosphere organisms to enhance the breakdown of the organic pollutants in the environment. Environ. Sci. Pollut. Res. 2005, 12, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Inter. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Fujita, M.; Era, A.; Ike, M.; Soda, S.; Miyata, N.; Hirao, T. Decolorization of heat-treatment liquor of waste sludge by a bioreactor using polyurethane foam-immobilized white rot fungus equipped with an ultramembrane filtration unit. J. Biosci. Bioeng. 2000, 90, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.S.; Udayanga, D.; Jayasanka, D.J.; Hewawasam, C. Textile dye decolorization by white rot fungi—A review. Bioresour. Technol. Rep. 2024, 25, 101687. [Google Scholar] [CrossRef]
- Saha, P.; Rao, K.V.B. Biotransformation of Reactive Orange 16 by alkaliphilic bacterium Bacillus fexus VITSP6 and toxicity assessment of biotransformed metabolites. Int. J. Environ. Sci. Technol. 2020, 17, 99–114. [Google Scholar] [CrossRef]
- Ruscasso, F.; Cavello, I.; Butler, M.; Loveira, E.; Curutchet, G.; Cavalitto, S. Biodegradation and detoxification of reactive orange 16 by Candida sake 41E. Bioresour. Technol. Rep. 2021, 15, 100726. [Google Scholar] [CrossRef]



| Runs | Period (2022) | RY86 (mg/L) | Influent (mL/Batch) | HRT (days) |
|---|---|---|---|---|
| 1–3 | 12 September–26 September | 10 | 300 | 5 |
| 4–6 | 26 September–11 October | 20 | 200 | |
| 7–9 | 17 October–1 November | 30 | ||
| 10–12 | 1 November–16 November | 40 | ||
| 13–15 | 16 November–26 November | 50 | ||
| 16 | 26 November–28 November | 3 | ||
| 17 | 29 November–30 November | 1 | ||
| 18 | 30 November–10 December | 10 | ||
| 19 | 10 December–25 December | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, A.; Eguchi, H.; Soda, S. Removal of Reactive Yellow 86 from Synthetic Wastewater in Lab-Scale Constructed Wetlands Planted with Cattail and Papyrus. Appl. Sci. 2024, 14, 6584. https://doi.org/10.3390/app14156584
Yamamoto A, Eguchi H, Soda S. Removal of Reactive Yellow 86 from Synthetic Wastewater in Lab-Scale Constructed Wetlands Planted with Cattail and Papyrus. Applied Sciences. 2024; 14(15):6584. https://doi.org/10.3390/app14156584
Chicago/Turabian StyleYamamoto, Akihiro, Hiroki Eguchi, and Satoshi Soda. 2024. "Removal of Reactive Yellow 86 from Synthetic Wastewater in Lab-Scale Constructed Wetlands Planted with Cattail and Papyrus" Applied Sciences 14, no. 15: 6584. https://doi.org/10.3390/app14156584






