Evaluation of Safety, Patient Perception and Efficacy of a New Cymenol-Based Mouth Rinse Formulation: A Randomized Clinical Trial
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Inclusion Criteria
- Age of 35–64 years.
- Periodontally treated patients undergoing SPC for at least 6 months, with the last SPC visit being within the previous 6 months.
- Patients should be generally healthy, according to the criteria of the American Society of Anesthesiologists (ASA), and categorized as ASA type I or II (refer to the exclusion criteria as well).
- There must be at least three assessable teeth in each quadrant.
- Inadequate plaque control (Turesky plaque index ≥ 1.5) [8].
- Patients should not have any orthodontic bands or removable prostheses.
- Subjects willing to participate and adhere to the study requirements.
- Subjects must report dentin hypersensitivity in at least one assessable tooth. Confirmation of dentin hypersensitivity was determined using an evaporative sensitivity test [9], with a minimum score of 1 [10]. To be eligible, the selected tooth must not currently undergo desensitizing therapy, should not have been restored within the last 3 months or have a crown or significant restoration. Only incisors, canines and premolars were considered [11].
2.1.2. Exclusion Criteria
- Presence of untreated or uncontrolled periodontitis.
- Regular use of mouth rinses containing antiseptics or anti-hypersensitivity agents.
- Antibiotic use within the past month.
- Excessive acid exposure due to conditions like eating disorders or chronic regurgitation.
- Chronic use of pain relievers or anti-inflammatory medications.
- Pregnancy.
- Any significant medical history (such as diabetes, osteoporosis or immunosuppression) or long-term medication use (including chemotherapy, immunosuppressive therapy and medications linked to gingival overgrowth, such as phenytoin, phenobarbital, lamotrigine, vigabatrin, ethosuximide, topiramate, primidone, nifedipine, amlodipine, verapamil and cyclosporine) that affects gingival health.
- Conditions which require antibiotic prophylaxis (infectious endocarditis, cardiac valve prosthesis…).
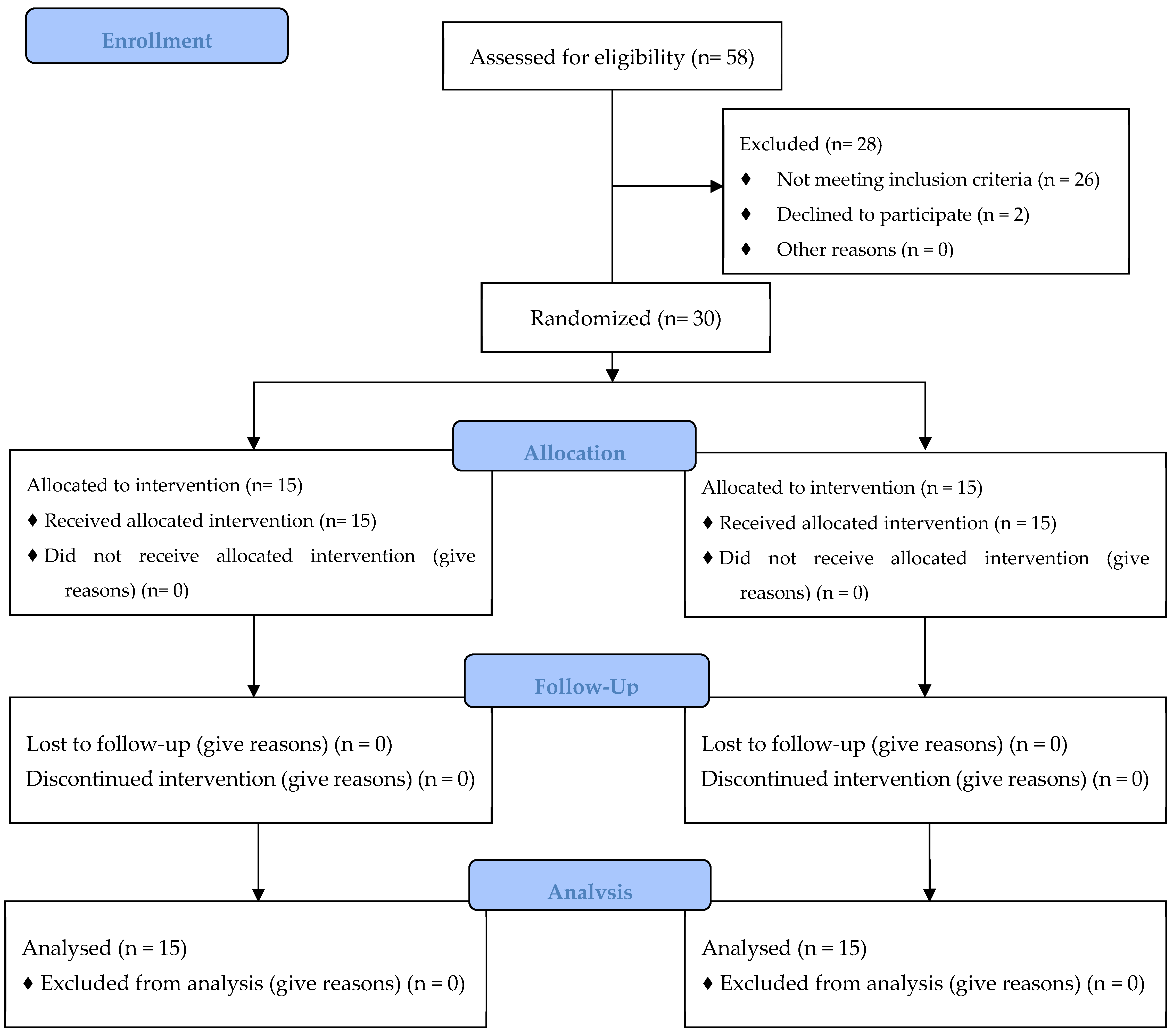
2.2. Study Design
2.2.1. Screening Visit
2.2.2. Baseline Visit
- (1)
- The experimental group used a supplied manual toothbrush with a sodium fluoride toothpaste three times a day, followed by the test mouth rinse (Lacer Oros Acción Integral—new formula, LACER SA, Barcelona, Spain), with O-Cymen-5-ol, potassium nitrate, zinc chloride, dipotassium glycyrrhizate, sodium fluoride, panthenol and xylitol.
- (2)
- The control group used the same provided manual toothbrush with a sodium fluoride dentifrice with the same frequency, but followed by a control mouth rinse (Lacer Oros Acción Integral—new formula, without active ingredients, LACER SA, Barcelona, Spain).
2.2.3. Six-Week Visit
2.2.4. Twelve-Week Visit
2.3. Outcome Variables
- Overall assessment of oral condition.
- Photographs of buccal area of lower and upper anterior teeth, from canine to canine.
- Dentin hypersensitivity.
- Plaque index (PlI).
- BOMP/GBI, evaluated during periodontal probing.
- Periodontal probing, including evaluation of BOMP/GBI, starting with the teeth evaluated in the first place for BOMP.
- Microbiological sampling.
2.3.1. Adverse Events’ Evaluation
2.3.2. Patient-Reported Outcomes (PROs)
2.3.3. Assessment of Compliance
2.3.4. Periodontal Clinical Parameters
- GBI [7], by dichotomously assessing bleeding after gentle probing.
2.3.5. Dentin Hypersensitivity
2.4. Microbiological Evaluation
2.4.1. Microbiological Sampling
2.4.2. Microbiological Processing
Culture
Quantitative Polymerase Chain Reaction (qPCR)
2.5. Data Analysis
2.5.1. Sample Size Calculation
2.5.2. Data Analysis
2.5.3. Calibration
3. Results
3.1. Safety and Tolerability
3.2. Patient-Reported Outcomes (PROs) and Compliance
3.3. Periodontal Clinical Outcomes
3.4. Dentin Hypersensitivity
3.5. Tooth Staining
3.6. Microbiological Outcomes—Culture
3.7. Microbiological Outcomes—qPCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants; Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- Serrano, J.; Escribano, M.; Roldan, S.; Martin, C.; Herrera, D. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S106–S138. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Roldan, S.; Santacruz, I.; Santos, S.; Masdevall, M.; Sanz, M. Differences in antimicrobial activity of four commercial 0.12% chlorhexidine mouthrinse formulations: An in vitro contact test and salivary bacterial counts study. J. Clin. Periodontol. 2003, 30, 307–314. [Google Scholar] [CrossRef]
- Food and Drug Administration; Department of Health and Human Services. Oral Health Care Drug Products for Over-the-Counter Human Use; Anti-Gingivitis/Anti-Plaque Drug Products; Establishment of a Monograph; Proposed Rules; Federal Register: Washington, DC, USA, 2003. [Google Scholar]
- ADA Council on Scientific Affairs. Acceptance Program Requirements. Chemotherapeutic Products for Control of Gingivitis; American Dental Association: Chicago, IL, USA, 2016. [Google Scholar]
- Van der Weijden, G.A.; Timmerman, M.F.; Nijboer, A.; Reijerse, E.; Van der Velden, U. Comparison of different approaches to assess bleeding on probing as indicators of gingivitis. J. Clin. Periodontol. 1994, 21, 589–594. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Turesky, S.; Gilmore, N.D.; Glickman, L. Reduced Plaque formation by the chloromethyl analogue of vitamin C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef]
- Schiff, T.; Dotson, M.; Cohen, S.; De Vizio, W.; McCool, J.; Volpe, A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: A twelve-week clinical study. J. Clin. Dent. 1994, 5, 87–92. [Google Scholar] [PubMed]
- West, N.; Newcombe, R.G.; Hughes, N.; Mason, S.; Maggio, B.; Sufi, F.; Claydon, N. A 3-day randomised clinical study investigating the efficacy of two toothpastes, designed to occlude dentine tubules, for the treatment of dentine hypersensitivity. J. Dent. 2013, 41, 187–194. [Google Scholar] [CrossRef]
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Bernimoulin, J.P.; Glynn, R.J. The effect of cigarette smoking on gingival bleeding. J. Periodontol. 2004, 75, 16–22. [Google Scholar] [CrossRef]
- Quigley, G.A.; Hein, J.W. Comparative cleansing efficiency of manual and power brushing. J. Am. Dent. Assoc. 1962, 65, 26–29. [Google Scholar] [CrossRef]
- Lie, M.A.; Timmerman, M.F.; Van der Velden, U.; Van der Weijden, G.A. Evaluation of 2 methods to assess gingival bleeding in smokers and non-smokers in natural and experimental gingivitis. J. Clin. Periodontol. 1998, 25, 695–700. [Google Scholar] [CrossRef]
- Gründemann, L.J.; Timmerman, M.F.; IJzerman, Y.; Van der Weijden, G.A.; Van der Weijden, G. Stain, plaque and gingivitis reduction by combining chlorhexidine and peroxyborate. J. Clin. Periodontol. 2000, 27, 9–15. [Google Scholar] [CrossRef]
- Koertge, T.E.; Gunsolley, J.C.; Domke, T.W.; Nelson, B.J. Comparison of two dentifrices in the control of chlorhexidine-induced stain. J. Clin. Dent. 1993, 4, 1–5. [Google Scholar]
- Lobene, R.R. Effect of dentifrices on tooth stains with controlled brushing. J. Am. Dent. Assoc. 1968, 77, 849–855. [Google Scholar] [CrossRef]
- Syed, S.A.; Loesche, W.J. Survival of human dental plaque flora in various transport media. Appl. Microbiol. 1972, 24, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Olle, E.; Frias, J. Improved, low-cost selective culture medium for Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 2001, 39, 509–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marin, M.J.; Ambrosio, N.; Herrera, D.; Sanz, M.; Figuero, E. Validation of a multiplex qPCR assay for the identification and quantification of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: In vitro and subgingival plaque samples. Arch. Oral Biol. 2018, 88, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A. Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol. Int. J. Toxicol. 2006, 25 (Suppl. S1), 29–127. [Google Scholar] [CrossRef]
- Kakar, A.; Newby, E.E.; Kakar, K.; Ghosh, S.; Targett, D.; Bosma, M.L. A randomised clinical trial to assess maintenance of gingival health by a novel dentifrice containing 0.1%w/w o-cymen-5-ol and 0.6%w/w zinc chloride. Int. Dent. J. 2011, 61 (Suppl. S3), 13–20. [Google Scholar] [CrossRef]
- Payne, D.; Gordon, J.J.; Nisbet, S.; Karwal, R.; Bosma, M.L. A randomised clinical trial to assess control of oral malodour by a novel dentifrice containing 0.1%w/w o-cymen-5-ol, 0.6%w/w zinc chloride. Int. Dent. J. 2011, 61 (Suppl. S3), 60–66. [Google Scholar] [CrossRef]
- Suarez-Rodriguez, B.; Regueira-Iglesias, A.; Blanco-Pintos, T.; Balsa-Castro, C.; Vila-Blanco, N.; Carreira, M.J.; Tomas, I. Short-term anti-plaque effect of a cymenol mouthwash analysed using the DenTiUS Deep Plaque software: A randomised clinical trial. BMC Oral Health 2023, 23, 560. [Google Scholar] [CrossRef]
- West, N.X.; Seong, J.; Davies, M. Management of dentine hypersensitivity: Efficacy of professionally and self-administered agents. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S256–S302. [Google Scholar] [CrossRef]
- Pereira, R.; Chava, V.K. Efficacy of a 3% potassium nitrate desensitizing mouthwash in the treatment of dentinal hypersensitivity. J. Periodontol. 2001, 72, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Pizzey, R.L.; Marquis, R.E.; Bradshaw, D.J. Antimicrobial effects of o-cymen-5-ol and zinc, alone & in combination in simple solutions and toothpaste formulations. Int. Dent. J. 2011, 61 (Suppl. S3), 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Bruhn, G.; Heumann, C.; Netuschil, L.; Brecx, M.; Hoffmann, T. Effect of two new chlorhexidine mouthrinses on the development of dental plaque, gingivitis, and discolouration. A randomized, investigator-blind, placebo-controlled, 3-week experimental gingivitis study. J. Clin. Periodontol. 2006, 33, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lobene, R.R.; Soparker, P.M.; Newman, M.B. Use of Dental Floss. Effect of plaque and gingivitis. Clin. Prev. Dent. 1982, 4, 5–8. [Google Scholar]
- Van der Weijden, G.A.; Timmerman, M.F.; Saxton, C.A.; Russell, J.I.; Huntington, E.; Van der Velden, U. Intra-/inter-examiner reproducibility study of gingival bleeding. J. Periodontal. Res. 1994, 29, 236–241. [Google Scholar] [CrossRef]
| Test | Control | p-Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | |||||
| Male | 6 | 40 | 4 | 26.7 | 0.439 |
| Female | 9 | 60 | 11 | 73.3 | |
| Tobacco | |||||
| Non-smoker | 14 | 93.3 | 14 | 93.3 | 1.000 |
| Smoker | 1 | 6.7 | 1 | 6.7 | |
| Systemic Diseases | |||||
| No | 10 | 66.7 | 12 | 80 | 0.409 |
| Yes | 5 | 33.3 | 3 | 20 | |
| Medication | |||||
| No Medication | 7 | 46.7 | 7 | 46.7 | 1.000 |
| Medication | 8 | 53.3 | 8 | 53.3 | |
| Stress | |||||
| No Stress | 13 | 86.7 | 12 | 80 | 0.624 |
| Stress | 2 | 13.3 | 3 | 20 | |
| Allergies | |||||
| No Allergies | 13 | 86.7 | 13 | 86.7 | 1.000 |
| Allergies | 2 | 13.3 | 2 | 13.3 | |
| 6 Weeks | 12 Weeks | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | N | Mean | SD | Mean Diff. | 95% CI | p Value | N | Mean | SD | Mean Diff. | 95% CI | p Value | ||
| Lower | Upper | Lower | Upper | ||||||||||||
| Liquid left | Control | 15 | 877.2 | 2540.0 | −20.2 | −1916.8 | 1876.5 | 0.603 | 15 | 778.8 | 2557.4 | 567.5 | −789.6 | 1924.5 | 0.256 |
| Test | 15 | 897.4 | 2531.4 | 15 | 211.3 | 207.1 | |||||||||
| n miss | Control | 15 | 10.7 | 13.8 | −0.9 | −9.7 | 7.8 | 0.454 | 15 | 11.9 | 13.2 | −7.0 | −20.1 | 6.1 | 0.327 |
| Test | 15 | 11.6 | 9.0 | 15 | 18.9 | 21.1 | |||||||||
| n right | Control | 15 | 106.9 | 32.5 | −7.5 | −25.3 | 10.4 | 0.884 | 15 | 89.8 | 46.0 | −17.3 | −44.6 | 10.0 | 0.442 |
| Test | 15 | 114.4 | 9.0 | 15 | 107.1 | 21.1 | |||||||||
| n not registered | Control | 15 | 8.4 | 32.5 | 8.4 | −9.6 | 26.4 | 0.317 | 15 | 24.2 | 50.2 | 24.2 | −3.6 | 52.0 | 0.073 |
| Test | 15 | 0.0 | 0.0 | 15 | 0.0 | 0.0 | |||||||||
| Q1 | Control | 15 | 8.1 | 1.5 | 0.8 | −0.4 | 2.0 | 0.194 | 15 | 8.0 | 0.8 | 0.7 | −0.3 | 1.7 | 0.490 |
| Test | 15 | 7.3 | 1.8 | 15 | 7.3 | 1.7 | |||||||||
| Q2 | Control | 15 | 5.5 | 2.3 | 0.3 | −1.1 | 1.7 | 0.720 | 15 | 5.5 | 2.0 | 0.3 | −1.2 | 1.9 | 0.556 |
| Test | 15 | 5.3 | 1.3 | 15 | 5.1 | 2.1 | |||||||||
| Q3 | Control | 15 | 5.4 | 1.3 | 0.5 | −0.4 | 1.4 | 0.190 | 15 | 5.3 | 0.8 | 0.2 | −0.6 | 1.0 | 0.758 |
| Test | 15 | 4.9 | 1.1 | 15 | 5.1 | 1.3 | |||||||||
| Q4 | Control | 15 | 1.5 | 2.1 | −0.2 | −1.6 | 1.2 | 0.343 | 15 | 2.0 | 1.5 | 0.1 | −1.2 | 1.5 | 0.357 |
| Test | 15 | 1.7 | 1.8 | 15 | 1.9 | 2.0 | |||||||||
| Q5 | Control | 15 | 1.3 | 1.0 | −1.8 | −3.3 | −0.3 | 0.016 | 15 | 1.9 | 1.8 | −1.3 | −3.1 | 0.5 | 0.122 |
| Test | 15 | 3.1 | 2.6 | 15 | 3.3 | 2.9 | |||||||||
| Q6 | Control | 15 | 1.0 | 0.0 | −0.7 | −1.7 | 0.3 | 0.073 | 15 | 1.2 | 0.6 | −0.5 | −1.6 | 0.7 | 0.916 |
| Test | 15 | 1.7 | 1.8 | 15 | 1.7 | 2.1 | |||||||||
| Q7 | Control | 15 | 1.6 | 2.3 | −0.2 | −1.6 | 1.2 | 0.053 | 15 | 1.5 | 1.5 | −1.8 | −3.7 | 0.1 | 0.046 |
| Test | 15 | 1.8 | 1.1 | 15 | 3.3 | 3.1 | |||||||||
| Q8 | Control | 15 | 8.3 | 1.5 | 1.8 | 0.4 | 3.2 | 0.022 | 15 | 8.2 | 1.2 | 1.0 | −0.3 | 2.3 | 0.165 |
| Test | 15 | 6.5 | 2.3 | 15 | 7.2 | 2.1 | |||||||||
| Q9 | Control | 15 | 6.1 | 2.5 | 0.5 | −1.2 | 2.3 | 0.322 | 15 | 6.3 | 1.8 | 0.4 | −1.3 | 2.1 | 0.831 |
| Test | 15 | 5.6 | 2.1 | 15 | 5.9 | 2.8 | |||||||||
| Baseline | 6 Weeks | 12 Weeks | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Sites | Group | N | Mean | SD | Mean Diff. | 95% CI | p Value | N | Mean | SD | Mean Diff. | 95% CI | p Value | N | Mean | SD | Mean Diff. | 95% CI | p Value | |||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||||||||||
| n teeth | Control | 15 | 23.6 | 4.5 | 1.0 | −1.7 | 3.6 | 0.181 | 15 | 23.6 | 4.5 | 1.1 | −1.6 | 3.8 | 0.162 | 15 | 23.6 | 4.5 | 1.1 | −1.6 | 3.8 | 0.156 | |
| Test | 15 | 22.6 | 2.4 | 15 | 22.5 | 2.5 | 15 | 22.5 | 2.4 | ||||||||||||||
| PlI (0–5) | All | Control | 15 | 1.7 | 0.5 | 0.1 | −0.2 | 0.5 | 0.384 | 15 | 1.7 | 0.6 | 0.0 | −0.4 | 0.4 | 0.983 | 15 | 1.7 | 0.5 | −0.2 | −0.6 | 0.2 | 0.290 |
| Test | 15 | 1.6 | 0.4 | 15 | 1.7 | 0.3 | 15 | 1.8 | 0.6 | ||||||||||||||
| Buccal | Control | 15 | 1.5 | 0.5 | 0.1 | −0.2 | 0.4 | 0.548 | 15 | 1.5 | 0.6 | 0.0 | −0.4 | 0.4 | 0.983 | 15 | 1.5 | 0.5 | −0.3 | −0.8 | 0.2 | 0.272 | |
| Test | 15 | 1.4 | 0.4 | 15 | 1.5 | 0.4 | 15 | 1.8 | 0.7 | ||||||||||||||
| Lingual | Control | 15 | 2.0 | 0.7 | 0.1 | −0.3 | 0.6 | 0.548 | 15 | 1.9 | 0.7 | 0.0 | −0.5 | 0.4 | 0.663 | 15 | 1.8 | 0.6 | −0.1 | −0.4 | 0.3 | 0.934 | |
| Test | 15 | 1.8 | 0.6 | 15 | 1.9 | 0.5 | 15 | 1.9 | 0.5 | ||||||||||||||
| Prox | Control | 15 | 2.0 | 0.5 | 0.2 | −0.2 | 0.5 | 0.419 | 15 | 1.9 | 0.6 | 0.0 | −0.4 | 0.4 | 0.917 | 15 | 1.9 | 0.5 | −0.1 | −0.5 | 0.2 | 0.373 | |
| Test | 15 | 1.8 | 0.4 | 15 | 1.9 | 0.4 | 15 | 2.0 | 0.5 | ||||||||||||||
| GBI (%) | All | Control | 15 | 41.9% | 12.8% | 2.0% | −6.1% | 10.2% | 0.756 | 15 | 31.5% | 10.3% | 1.7% | −6.1% | 9.6% | 0.619 | 15 | 26.4% | 10.4% | −0.3% | −7.2% | 6.5% | 0.694 |
| Test | 15 | 39.9% | 8.6% | 15 | 29.7% | 10.6% | 15 | 26.7% | 7.7% | ||||||||||||||
| Buccal | Control | 15 | 33.7% | 14.4% | 1.9% | −7.3% | 11.1% | 0.868 | 15 | 23.4% | 11.6% | 0.9% | −7.0% | 8.8% | 0.575 | 15 | 20.4% | 9.2% | 1.0% | −5.8% | 7.8% | 0.803 | |
| Test | 15 | 31.8% | 10.0% | 15 | 22.5% | 9.5% | 15 | 19.4% | 9.0% | ||||||||||||||
| Lingual | Control | 15 | 50.2% | 14.3% | 2.1% | −8.3% | 12.6% | 0.740 | 15 | 39.5% | 14.4% | 2.6% | −8.5% | 13.7% | 0.724 | 15 | 32.4% | 14.6% | −1.7% | −10.9% | 7.4% | 0.351 | |
| Test | 15 | 48.1% | 13.7% | 15 | 36.9% | 15.2% | 15 | 34.1% | 9.2% | ||||||||||||||
| Prox | Control | 15 | 46.1% | 12.2% | 5.6% | −2.6% | 13.7% | 0.237 | 15 | 31.6% | 9.9% | 0% | −8% | 8% | 0.967 | 15 | 28.2% | 10.1% | 1% | −7% | 8% | 0.803 | |
| Test | 15 | 40.5% | 9.4% | 15 | 31.5% | 11.0% | 15 | 27.4% | 9.8% | ||||||||||||||
| BOMP (0–2) | All | Control | 15 | 0.4 | 0.2 | 0.0 | −0.1 | 0.2 | 0.507 | 15 | 0.3 | 0.2 | 0.1 | 0.0 | 0.2 | 0.078 | 15 | 0.2 | 0.1 | 0.0 | −0.1 | 0.1 | 0.967 |
| Test | 15 | 0.4 | 0.2 | 15 | 0.2 | 0.1 | 15 | 0.2 | 0.1 | ||||||||||||||
| Buccal | Control | 15 | 0.3 | 0.2 | 0.0 | −0.1 | 0.1 | 0.481 | 15 | 0.2 | 0.1 | 0.0 | −0.1 | 0.1 | 0.481 | 15 | 0.2 | 0.1 | 0.0 | −0.1 | 0.1 | 0.934 | |
| Test | 15 | 0.3 | 0.1 | 15 | 0.2 | 0.1 | 15 | 0.2 | 0.1 | ||||||||||||||
| Lingual | Control | 15 | 0.6 | 0.2 | 0.1 | −0.1 | 0.3 | 0.330 | 15 | 0.4 | 0.3 | 0.2 | 0.0 | 0.3 | 0.093 | 15 | 0.3 | 0.2 | 0.0 | −0.1 | 0.2 | 0.663 | |
| Test | 15 | 0.5 | 0.2 | 15 | 0.3 | 0.2 | 15 | 0.3 | 0.1 | ||||||||||||||
| Prox | Control | 15 | 0.5 | 0.2 | 0.1 | 0.0 | 0.2 | 0.237 | 15 | 0.3 | 0.2 | 0.1 | 0.0 | 0.2 | 0.147 | 15 | 0.2 | 0.1 | 0.0 | −0.1 | 0.1 | 0.724 | |
| Test | 15 | 0.4 | 0.2 | 15 | 0.2 | 0.1 | 15 | 0.2 | 0.1 | ||||||||||||||
| PD (mm) | All | Control | 15 | 2.4 | 0.4 | 0.1 | −0.1 | 0.4 | 0.254 | 15 | 2.5 | 0.4 | 0.1 | −0.1 | 0.4 | 0.221 | 15 | 2.4 | 0.4 | 0.1 | −0.2 | 0.3 | 0.395 |
| Test | 15 | 2.3 | 0.2 | 15 | 2.3 | 0.3 | 15 | 2.3 | 0.2 | ||||||||||||||
| Buccal | Control | 15 | 2.4 | 0.3 | 0.2 | −0.1 | 0.4 | 0.141 | 15 | 2.4 | 0.3 | 0.1 | −0.1 | 0.4 | 0.237 | 15 | 2.3 | 0.3 | 0.1 | −0.1 | 0.3 | 0.494 | |
| Test | 15 | 2.2 | 0.2 | 15 | 2.3 | 0.2 | 15 | 2.2 | 0.2 | ||||||||||||||
| Lingual | Control | 15 | 2.5 | 0.5 | 0.1 | −0.2 | 0.4 | 0.330 | 15 | 2.5 | 0.4 | 0.1 | −0.1 | 0.4 | 0.245 | 15 | 2.5 | 0.4 | 0.0 | −0.2 | 0.3 | 0.534 | |
| Test | 15 | 2.4 | 0.3 | 15 | 2.4 | 0.4 | 15 | 2.4 | 0.3 | ||||||||||||||
| Prox | Control | 15 | 2.7 | 0.4 | 0.1 | −0.1 | 0.4 | 0.191 | 15 | 2.7 | 0.4 | 0.1 | −0.2 | 0.3 | 0.494 | 15 | 2.6 | 0.4 | 0.0 | −0.2 | 0.2 | 0.820 | |
| Test | 15 | 2.6 | 0.3 | 15 | 2.6 | 0.3 | 15 | 2.6 | 0.2 | ||||||||||||||
| %PD < 4 mm | Control | 15 | 87% | 8% | −5% | −11% | 0% | 0.065 | 15 | 89% | 8% | −3% | −8% | 2% | 0.309 | 15 | 89% | 8% | −2% | −7% | 3% | 0.443 | |
| Test | 15 | 93% | 5% | 15 | 92% | 6% | 15 | 91% | 5% | ||||||||||||||
| %PD 4–5 mm | Control | 15 | 12% | 8% | 5% | 0% | 10% | 0.120 | 15 | 10% | 8% | 3% | −2% | 8% | 0.351 | 15 | 11% | 7% | 3% | −2% | 7% | 0.372 | |
| Test | 15 | 7% | 4% | 15 | 7% | 5% | 15 | 8% | 5% | ||||||||||||||
| %PD > 5 mm | Control | 15 | 1% | 2% | 0% | −1% | 1% | 0.714 | 15 | 1% | 1% | 0% | −1% | 1% | 0.963 | 15 | 0% | 1% | 0% | −1% | 0% | 0.449 | |
| Test | 15 | 1% | 1% | 15 | 1% | 1% | 15 | 1% | 1% | ||||||||||||||
| REC (mm) | All | Control | 15 | 0.7 | 0.8 | −0.3 | −0.9 | 0.4 | 0.361 | 15 | 0.7 | 0.8 | −0.2 | −0.9 | 0.5 | 0.419 | 15 | 0.7 | 0.8 | −0.2 | −0.9 | 0.4 | 0.330 |
| Test | 15 | 1.0 | 1.0 | 15 | 0.9 | 1.1 | 15 | 0.9 | 1.1 | ||||||||||||||
| Buccal | Control | 15 | 0.8 | 0.9 | −0.2 | −0.9 | 0.5 | 0.290 | 15 | 0.8 | 0.9 | −0.2 | −0.9 | 0.5 | 0.330 | 15 | 0.8 | 0.9 | −0.2 | −0.9 | 0.5 | 0.372 | |
| Test | 15 | 1.0 | 1.0 | 15 | 1.0 | 1.0 | 15 | 1.0 | 1.0 | ||||||||||||||
| Lingual | Control | 15 | 0.6 | 0.7 | −0.3 | −1.0 | 0.4 | 0.419 | 15 | 0.6 | 0.7 | −0.2 | −0.9 | 0.5 | 0.678 | 15 | 0.6 | 0.7 | −0.3 | −0.9 | 0.4 | 0.604 | |
| Test | 15 | 0.9 | 1.1 | 15 | 0.8 | 1.1 | 15 | 0.8 | 1.1 | ||||||||||||||
| Prox | Control | 15 | 0.6 | 0.7 | −0.3 | −1.0 | 0.4 | 0.395 | 15 | 0.6 | 0.7 | −0.2 | −0.9 | 0.5 | 0.648 | 15 | 0.6 | 0.7 | −0.3 | −0.9 | 0.4 | 0.520 | |
| Test | 15 | 0.8 | 1.1 | 15 | 0.8 | 1.1 | 15 | 0.8 | 1.1 | ||||||||||||||
| CAL (mm) | All | Control | 15 | 1.7 | 0.9 | 0.4 | −0.3 | 1.1 | 0.110 | 15 | 1.8 | 0.8 | 0.4 | −0.3 | 1.1 | 0.178 | 15 | 1.7 | 0.9 | 0.3 | −0.4 | 1.0 | 0.178 |
| Test | 15 | 1.3 | 1.0 | 15 | 1.4 | 1.1 | 15 | 1.4 | 1.1 | ||||||||||||||
| Buccal | Control | 15 | 1.6 | 1.0 | 0.4 | −0.3 | 1.1 | 0.059 | 15 | 1.6 | 1.0 | 0.4 | −0.4 | 1.1 | 0.106 | 15 | 1.5 | 1.0 | 0.3 | −0.4 | 1.1 | 0.237 | |
| Test | 15 | 1.2 | 0.9 | 15 | 1.3 | 1.0 | 15 | 1.2 | 1.0 | ||||||||||||||
| Lingual | Control | 15 | 1.9 | 0.8 | 0.4 | −0.3 | 1.1 | 0.178 | 15 | 1.9 | 0.7 | 0.4 | −0.4 | 1.1 | 0.254 | 15 | 1.9 | 0.8 | 0.3 | −0.4 | 1.0 | 0.340 | |
| Test | 15 | 1.5 | 1.0 | 15 | 1.6 | 1.2 | 15 | 1.6 | 1.2 | ||||||||||||||
| Prox | Control | 15 | 2.2 | 0.8 | 0.4 | −0.2 | 1.1 | 0.085 | 15 | 2.1 | 0.8 | 0.3 | −0.4 | 1.0 | 0.237 | 15 | 2.1 | 0.8 | 0.3 | −0.5 | 1.0 | 0.419 | |
| Test | 15 | 1.8 | 1.0 | 15 | 1.8 | 1.1 | 15 | 1.8 | 1.1 | ||||||||||||||
| n open pock. | All | Control | 15 | 3.5 | 3.4 | 1.0 | −1.7 | 3.7 | 0.134 | 15 | 2.7 | 2.8 | 0.7 | −1.4 | 2.8 | 0.328 | 15 | 1.5 | 1.1 | 0.1 | −0.9 | 1.1 | 0.588 |
| Test | 15 | 2.5 | 3.7 | 15 | 1.9 | 2.8 | 15 | 1.3 | 1.6 | ||||||||||||||
| Prevalence | Bacteria | Group | Baseline | 6 Weeks | 12 Weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | p Value | N | n | % | p Value | N | n | % | p Value | |||
| Culture | Aa | Control | 15 | 0 | 0.0 | 15 | 0 | 0.0 | 14 | 0 | 0.0 | |||
| Test | 15 | 0 | 0.0 | 14 | 0 | 0.0 | 14 | 0 | 0.0 | |||||
| Pg | Control | 15 | 7 | 46.7 | 0.713 | 15 | 6 | 40.0 | 0.876 | 14 | 5 | 35.7 | 0.256 | |
| Test | 15 | 6 | 40.0 | 14 | 6 | 42.9 | 14 | 8 | 57.1 | |||||
| Pi | Control | 15 | 4 | 26.7 | 0.361 | 15 | 1 | 6.7 | 0.054 | 14 | 7 | 50.0 | 0.445 | |
| Test | 15 | 2 | 13.3 | 14 | 5 | 35.7 | 14 | 9 | 64.3 | |||||
| Tf | Control | 15 | 1 | 6.7 | 0.309 | 15 | 0 | 0.0 | 0.292 | 14 | 4 | 28.6 | 0.663 | |
| Test | 15 | 0 | 0.0 | 14 | 1 | 7.1 | 14 | 3 | 21.4 | |||||
| Pm | Control | 15 | 0 | 0.0 | 0.032 | 15 | 3 | 20.0 | 0.316 | 14 | 0 | 0.0 | 0.309 | |
| Test | 15 | 4 | 26.7 | 14 | 1 | 7.1 | 14 | 1 | 7.1 | |||||
| Fn | Control | 15 | 13 | 86.7 | 1.000 | 15 | 8 | 53.3 | 0.550 | 14 | 13 | 92.9 | 0.541 | |
| Test | 15 | 13 | 86.7 | 14 | 9 | 64.3 | 14 | 12 | 85.7 | |||||
| Cr | Control | 15 | 3 | 20.0 | 0.624 | 15 | 0 | 0.0 | 14 | 3 | 21.4 | 0.067 | ||
| Test | 15 | 2 | 13.3 | 14 | 0 | 0.0 | 14 | 0 | 0.0 | |||||
| Ec | Control | 15 | 3 | 20.0 | 0.232 | 15 | 2 | 13.3 | 0.033 | 14 | 7 | 50.0 | 0.705 | |
| Test | 15 | 6 | 40.0 | 14 | 7 | 50.0 | 14 | 6 | 42.9 | |||||
| qPCR | Aa | Control | 15 | 1 | 6.7 | 1.000 | 15 | 1 | 6.7 | 1.000 | 15 | 1 | 6.7 | 1.000 |
| Test | 15 | 0 | 0.0 | 15 | 0 | 0.0 | 15 | 0 | 0.0 | |||||
| Pg | Control | 15 | 6 | 40.0 | 0.705 | 15 | 12 | 80.0 | 0.427 | 15 | 10 | 66.7 | 0.705 | |
| Test | 15 | 5 | 33.3 | 15 | 9 | 60.0 | 15 | 9 | 60.0 | |||||
| Tf | Control | 15 | 13 | 86.7 | 0.390 | 15 | 13 | 86.7 | 1.000 | 15 | 12 | 80.0 | 0.427 | |
| Test | 15 | 10 | 66.7 | 15 | 12 | 80.0 | 15 | 9 | 60.0 | |||||
| Method | Variable | Group | Baseline | 6 Weeks | 12 Weeks | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Mean Diff. | 95% CI | p Value | N | Mean | SD | Mean Diff. | 95% CI | p Value | N | Mean | SD | Mean Diff. | 95% CI | p Value | |||||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||||||||||||||
| Culture | Counts (CFU/mL) | All | Control | 15 | 1.3 × 106 | 1.8 × 106 | 3.9 × 105 | −7.1 × 105 | 1.5 × 106 | 0.787 | 15 | 6.5 × 105 | 1.2 × 106 | −1.7 × 106 | −4.4 × 106 | 1.1 × 106 | 0.116 | 14 | 2.7 × 106 | 5.3 × 106 | 1.8 × 106 | −1.3 × 106 | 4.9 × 106 | 0.383 |
| Test | 15 | 9.3 × 105 | 1.1 × 106 | 14 | 2.3 × 106 | 5.1 × 106 | 14 | 8.8 × 105 | 1.3 × 106 | |||||||||||||||
| Aa | Control | 15 | 0.0 | 0.0 | 15 | 0.0 | 14 | 0.0 | ||||||||||||||||
| Test | 15 | 0.0 | 0.0 | 14 | 0.0 | 14 | 0.0 | |||||||||||||||||
| Pg | Control | 15 | 2.5 × 105 | 5.9 × 105 | 1.4 × 105 | −1.9 × 105 | 4.6 × 105 | 0.854 | 15 | 6.6 × 103 | 1.0 × 104 | −4.2 × 105 | −1.1 × 106 | 2.5 × 105 | 0.608 | 14 | 3.4 × 105 | 1.1 × 106 | 2.2 × 105 | −3.9 × 105 | 8.4 × 105 | 0.424 | ||
| Test | 15 | 1.1 × 105 | 1.9 × 105 | 14 | 4.3 × 105 | 1.2 × 106 | 14 | 1.1 × 105 | 2.5 × 105 | |||||||||||||||
| Pi | Control | 15 | 1.2 × 105 | 4.0 × 105 | 1.2 × 105 | −1.0 × 105 | 3.4 × 105 | 0.299 | 15 | 6.7 × 101 | 2.6 × 102 | −4.2 × 103 | −1.0 × 104 | 2.0 × 103 | 0.049 | 14 | 1.1 × 105 | 3.7 × 105 | 9.5 × 104 | −1.1 × 105 | 3.0 × 105 | 0.720 | ||
| Test | 15 | 6.7 × 102 | 2.6 × 103 | 14 | 4.2 × 103 | 1.1 × 104 | 14 | 1.2 × 104 | 2.1 × 104 | |||||||||||||||
| Tf | Control | 15 | 3.3 × 103 | 1.3 × 104 | 3.3 × 103 | −3.8 × 103 | 1.0 × 104 | 0.317 | 15 | 0.0 | 0.0 | −1.8 × 103 | −5.6 × 103 | 2.1 × 103 | 0.301 | 14 | 5.1 × 103 | 1.3 × 104 | −2.9 × 103 | −1.5 × 104 | 9.0 × 103 | 0.856 | ||
| Test | 15 | 0.0 | 0.0 | 14 | 1.8 × 103 | 6.7 × 103 | 14 | 8.0 × 103 | 1.7 × 104 | |||||||||||||||
| Pm | Control | 15 | 0.0 | 0.0 | −3.7 × 103 | −9.4 × 103 | 2.1 × 103 | 0.035 | 15 | 6.1 × 104 | 2.3 × 105 | 5.6 × 104 | −7.2 × 104 | 1.8 × 105 | 0.344 | 14 | 0.0 | 0.0 | −2.9 × 103 | −9.0 × 103 | 3.3 × 103 | 0.317 | ||
| Test | 15 | 3.7 × 103 | 1.0 × 104 | 14 | 5.0 × 103 | 1.9 × 104 | 14 | 2.9 × 103 | 1.1 × 104 | |||||||||||||||
| Fn | Control | 15 | 5.3 × 104 | 1.2 × 105 | 3.7 × 104 | −2.8 × 104 | 1.0 × 105 | 0.394 | 15 | 5.6 × 103 | 8.7 × 103 | −2.2 × 104 | −4.2 × 104 | −1.3 × 103 | 0.108 | 14 | 2.5 × 104 | 3.7 × 104 | −7.1 × 101 | −2.8 × 104 | 2.8 × 104 | 0.854 | ||
| Test | 15 | 1.6 × 104 | 2.1 × 104 | 14 | 2.7 × 104 | 3.5 × 104 | 14 | 2.5 × 104 | 3.6 × 104 | |||||||||||||||
| Cr | Control | 15 | 2.1 × 103 | 5.6 × 103 | 6.7 × 102 | −3.4 × 103 | 4.7 × 103 | 0.632 | 15 | 0.0 | 1.000 | 14 | 3.6 × 104 | 1.3 × 105 | 3.6 × 104 | −4.1 × 104 | 1.1 × 105 | 0.072 | ||||||
| Test | 15 | 1.4 × 103 | 5.2 × 103 | 14 | 0.0 | 14 | 0.0 | 0.0 | ||||||||||||||||
| Ec | Control | 15 | 2.2 × 103 | 5.6 × 103 | −1.3 × 103 | −5.6 × 103 | 3.1 × 103 | 0.293 | 15 | 1.3 × 103 | 4.4 × 103 | −6.5 × 103 | −1.7 × 104 | 3.5 × 103 | 0.043 | 14 | 6.6 × 102 | 1.1 × 103 | −1.1 × 103 | −3.2 × 103 | 1.0 × 103 | 1.000 | ||
| Test | 15 | 3.5 × 103 | 6.1 × 103 | 14 | 7.8 × 103 | 1.7 × 104 | 14 | 1.7 × 103 | 3.5 × 103 | |||||||||||||||
| Proportions (%) | Aa | Control | 15 | 0.0 | 15 | 0.0 | 14 | 0.0 | ||||||||||||||||
| Test | 15 | 0.0 | 14 | 0.0 | 14 | 0.0 | ||||||||||||||||||
| Pg | Control | 15 | 9.4 | 15.1 | 0.3 | −11.7 | 12.3 | 0.731 | 15 | 2.6 | 7.1 | −6.1 | −18.0 | 5.8 | 0.696 | 14 | 5.0 | 11.3 | −2.2 | −10.3 | 5.9 | 0.272 | ||
| Test | 15 | 9.1 | 16.8 | 14 | 8.7 | 19.8 | 14 | 7.1 | 9.4 | |||||||||||||||
| Pi | Control | 15 | 2.4 | 7.7 | 2.3 | −1.9 | 6.6 | 0.285 | 15 | 0.0 | 0.0 | −0.6 | −1.5 | 0.3 | 0.049 | 14 | 2.3 | 4.1 | 1.3 | −1.1 | 3.8 | 0.720 | ||
| Test | 15 | 0.1 | 0.3 | 14 | 0.6 | 1.6 | 14 | 1.0 | 1.3 | |||||||||||||||
| Tf | Control | 15 | 0.1 | 0.2 | 0.1 | −0.1 | 0.2 | 0.317 | 15 | 0.0 | 0.0 | −1.4 | −4.6 | 1.7 | 0.301 | 14 | 0.6 | 2.0 | 0.1 | −1.1 | 1.3 | 0.809 | ||
| Test | 15 | 0.0 | 0.0 | 14 | 1.4 | 5.4 | 14 | 0.5 | 1.0 | |||||||||||||||
| Pm | Control | 15 | 0.0 | 0.0 | −0.9 | −1.9 | 0.1 | 0.035 | 15 | 3.9 | 13.9 | 2.9 | −5.0 | 10.7 | 0.344 | 14 | 0.0 | 0.0 | −0.2 | −0.5 | 0.2 | 0.317 | ||
| Test | 15 | 0.9 | 1.8 | 14 | 1.0 | 3.8 | 14 | 0.2 | 0.6 | |||||||||||||||
| Fn | Control | 15 | 3.8 | 3.7 | 1.0 | −1.5 | 3.4 | 0.383 | 15 | 1.9 | 3.1 | −6.5 | −17.8 | 4.8 | 0.353 | 14 | 2.4 | 3.0 | −5.5 | −14.3 | 3.2 | 0.250 | ||
| Test | 15 | 2.8 | 2.9 | 14 | 8.3 | 19.4 | 14 | 7.9 | 15.0 | |||||||||||||||
| Cr | Control | 15 | 0.1 | 0.3 | −0.1 | −0.4 | 0.2 | 0.725 | 15 | 0.0 | 14 | 0.8 | 2.1 | 0.8 | −0.4 | 2.1 | 0.072 | |||||||
| Test | 15 | 0.2 | 0.5 | 14 | 0.0 | 14 | 0.0 | 0.0 | ||||||||||||||||
| Ec | Control | 15 | 0.3 | 0.8 | −0.2 | −0.8 | 0.4 | 0.250 | 15 | 0.8 | 2.8 | 0.1 | −1.6 | 1.7 | 0.040 | 14 | 0.2 | 0.3 | −0.3 | −0.9 | 0.2 | 0.653 | ||
| Test | 15 | 0.5 | 0.8 | 14 | 0.7 | 1.0 | 14 | 0.6 | 1.0 | |||||||||||||||
| qPCR | Counts (CFU/mL) | Aa | Control | 15 | 6.5 × 103 | 2.5 × 104 | 6.5 × 103 | −7.5 × 103 | 2.0 × 104 | 0.317 | 15 | 3.7 × 102 | 1.4 × 103 | 3.7 × 102 | −4.2 × 102 | 1.2 × 103 | 0.317 | 15 | 6.1 × 104 | 2.4 × 105 | 6.1 × 104 | −7.0 × 104 | 1.9 × 105 | 0.317 |
| Test | 15 | 0.0 | 0.0 | 15 | 0.0 | 0.0 | 15 | 0.0 | 0.0 | |||||||||||||||
| Pg | Control | 15 | 1.3 × 105 | 2.6 × 105 | −5.3 × 103 | −2.1 × 105 | 2.0 × 105 | 0.792 | 15 | 8.2 × 104 | 2.5 × 105 | −1.1 × 105 | −4.1 × 105 | 1.9 × 105 | 0.690 | 15 | 5.5 × 104 | 1.5 × 105 | −3.1 × 104 | −1.8 × 105 | 1.2 × 105 | 0.750 | ||
| Test | 15 | 1.4 × 105 | 2.9 × 105 | 15 | 1.9 × 105 | 5.1 × 105 | 15 | 8.5 × 104 | 2.5 × 105 | |||||||||||||||
| Tf | Control | 15 | 1.7 × 106 | 3.4 × 106 | 8.7 × 105 | −1.1 × 106 | 2.9 × 106 | 0.327 | 15 | 5.9 × 105 | 1.2 × 106 | −6.3 × 105 | −2.2 × 106 | 9.4 × 105 | 0.633 | 15 | 7.5 × 105 | 1.6 × 106 | −3.3 × 105 | −1.6 × 106 | 9.7 × 105 | 0.344 | ||
| Test | 15 | 7.9 × 105 | 1.6 × 106 | 15 | 1.2 × 106 | 2.6 × 106 | 15 | 1.1 × 106 | 1.8 × 106 | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araoz, A.; Figuero, E.; Serrano, J.; Roldán, S.; Alonso, B.; Sanz, M.; Herrera, D. Evaluation of Safety, Patient Perception and Efficacy of a New Cymenol-Based Mouth Rinse Formulation: A Randomized Clinical Trial. Appl. Sci. 2024, 14, 6595. https://doi.org/10.3390/app14156595
Araoz A, Figuero E, Serrano J, Roldán S, Alonso B, Sanz M, Herrera D. Evaluation of Safety, Patient Perception and Efficacy of a New Cymenol-Based Mouth Rinse Formulation: A Randomized Clinical Trial. Applied Sciences. 2024; 14(15):6595. https://doi.org/10.3390/app14156595
Chicago/Turabian StyleAraoz, Ana, Elena Figuero, Jorge Serrano, Silvia Roldán, Bettina Alonso, Mariano Sanz, and David Herrera. 2024. "Evaluation of Safety, Patient Perception and Efficacy of a New Cymenol-Based Mouth Rinse Formulation: A Randomized Clinical Trial" Applied Sciences 14, no. 15: 6595. https://doi.org/10.3390/app14156595
APA StyleAraoz, A., Figuero, E., Serrano, J., Roldán, S., Alonso, B., Sanz, M., & Herrera, D. (2024). Evaluation of Safety, Patient Perception and Efficacy of a New Cymenol-Based Mouth Rinse Formulation: A Randomized Clinical Trial. Applied Sciences, 14(15), 6595. https://doi.org/10.3390/app14156595








