The Effect of TiO2 on the Electrochemical Performance of Sb2O3 Anodes for Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Characterization
2.2. Preparation of TiO2@Sb2O3 Nanocomposite Material
2.3. Electrochemical Performance
2.3.1. Preparation of Electrode Solution
2.3.2. Li-ion Half-Cell Assembly
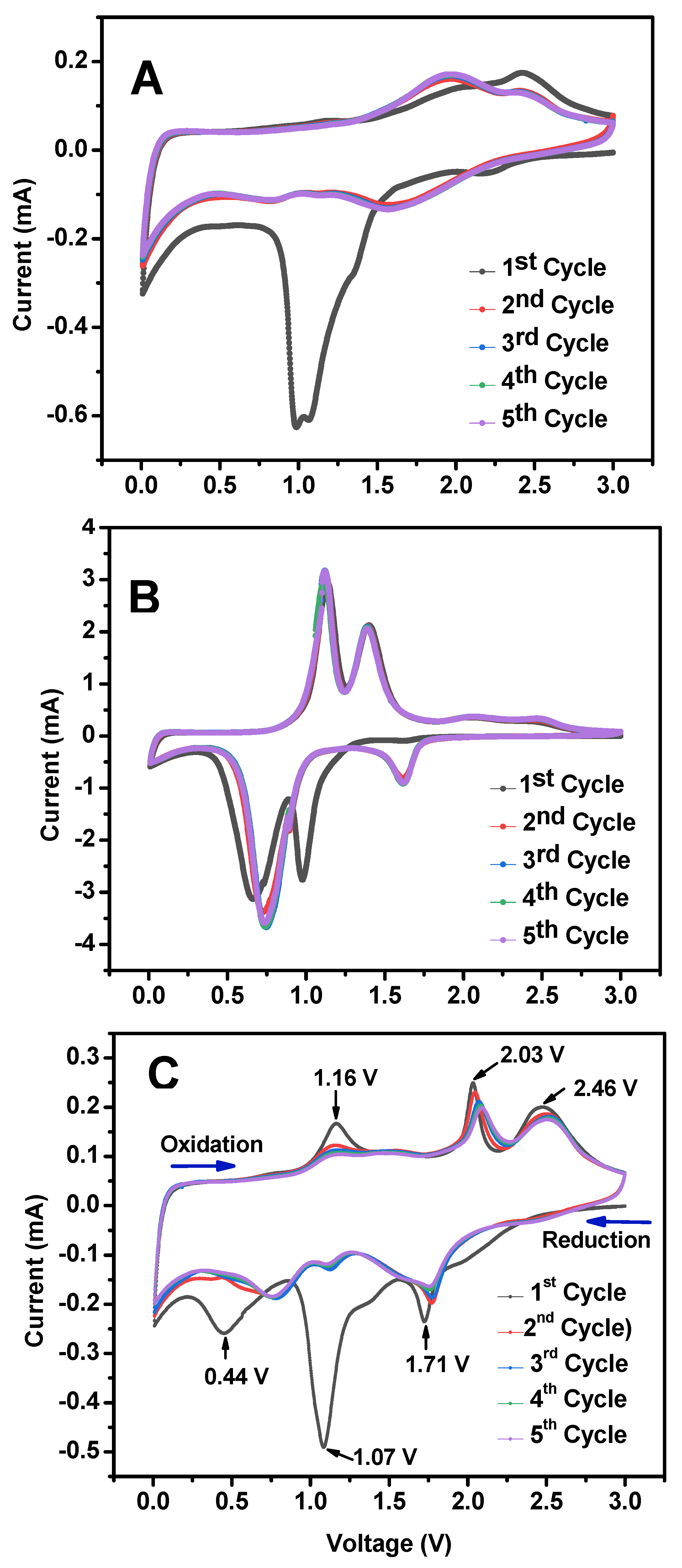
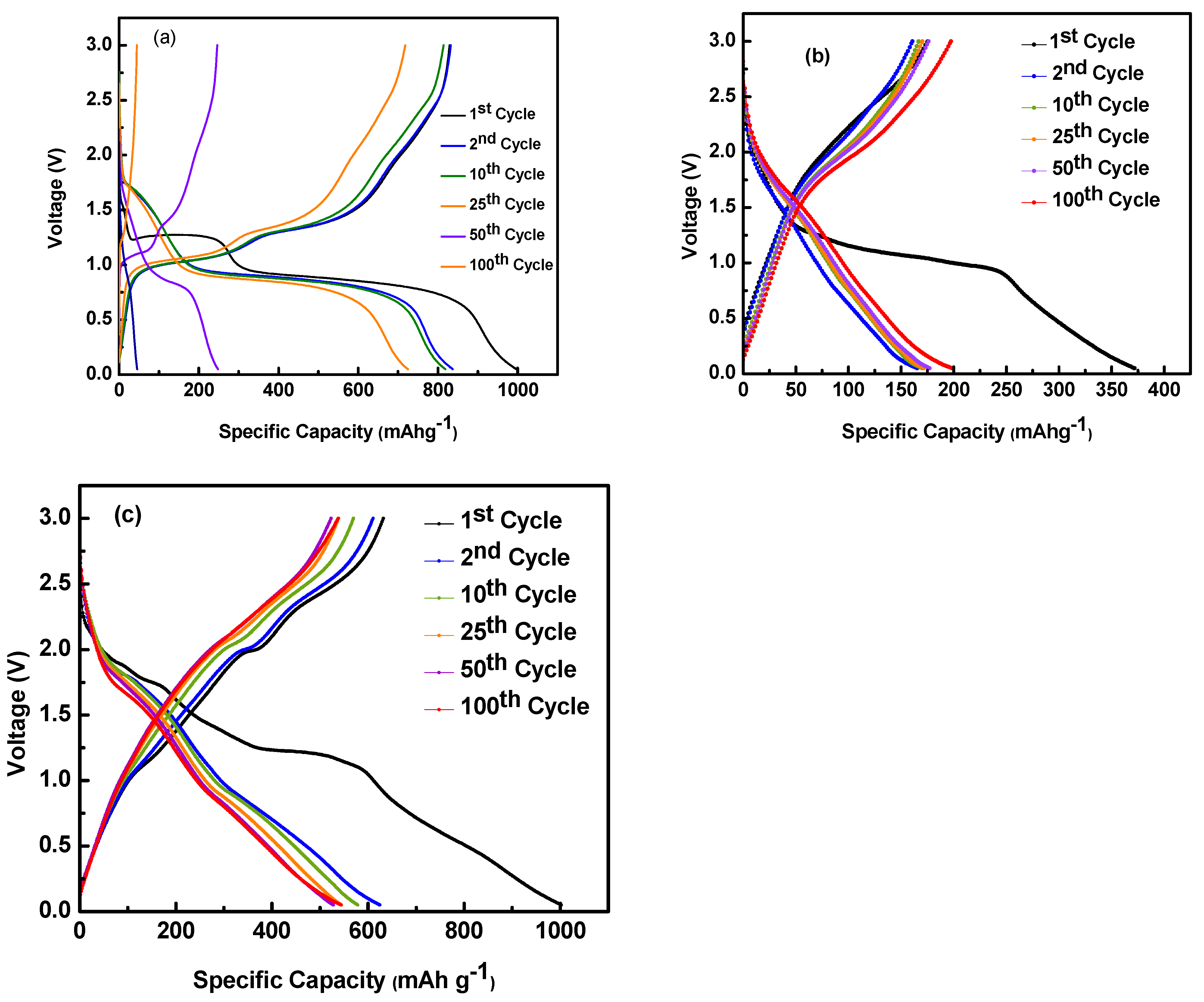
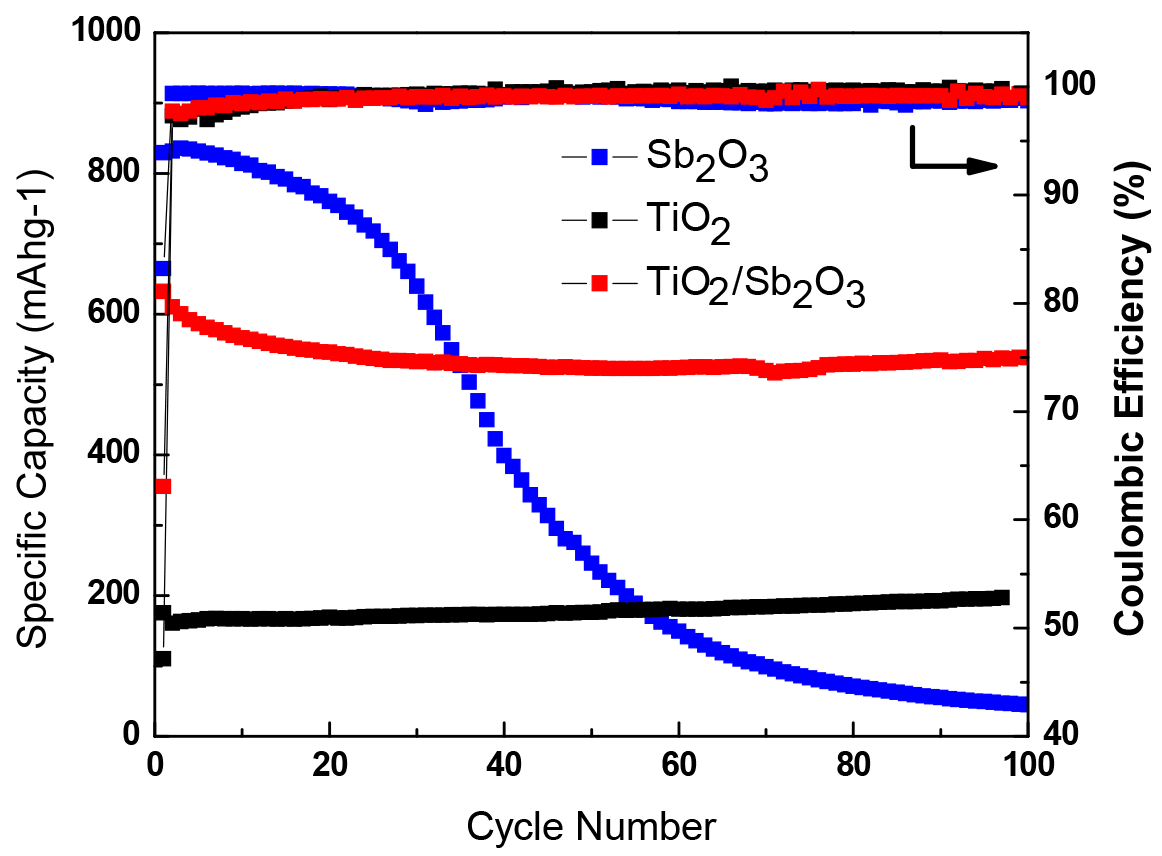
3. Results and Discussion
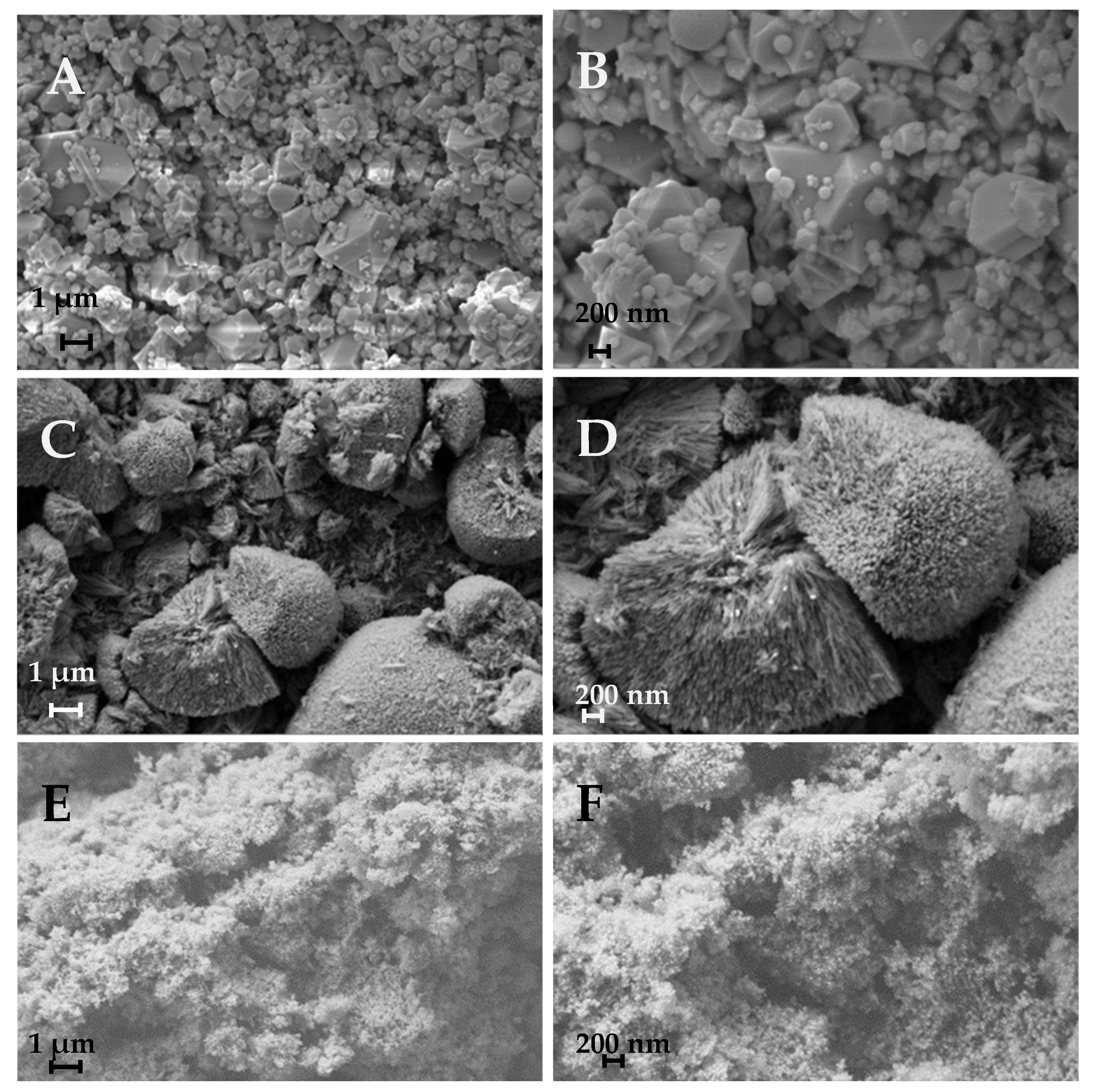
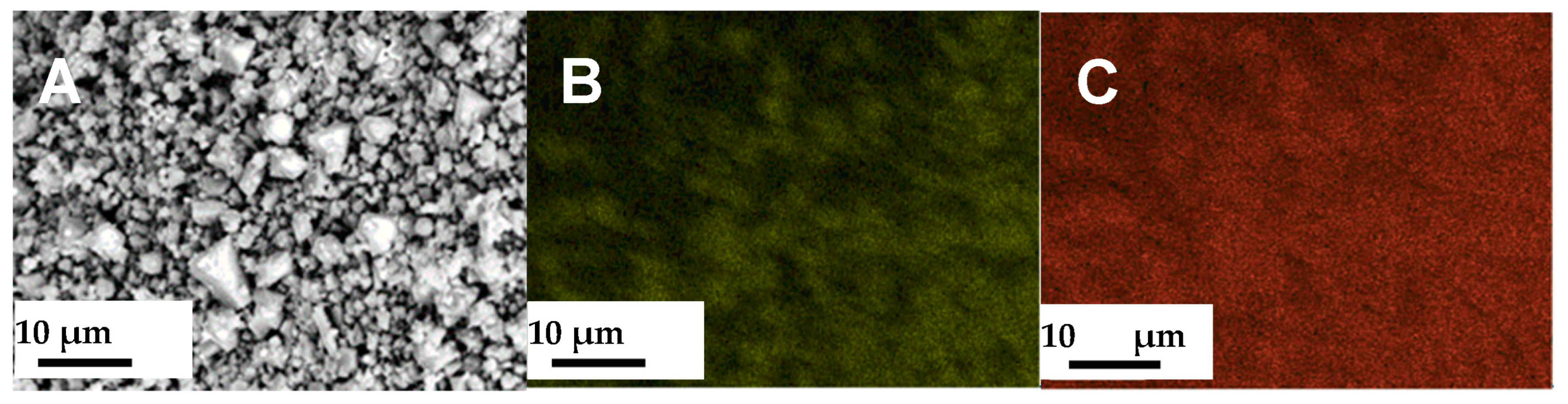
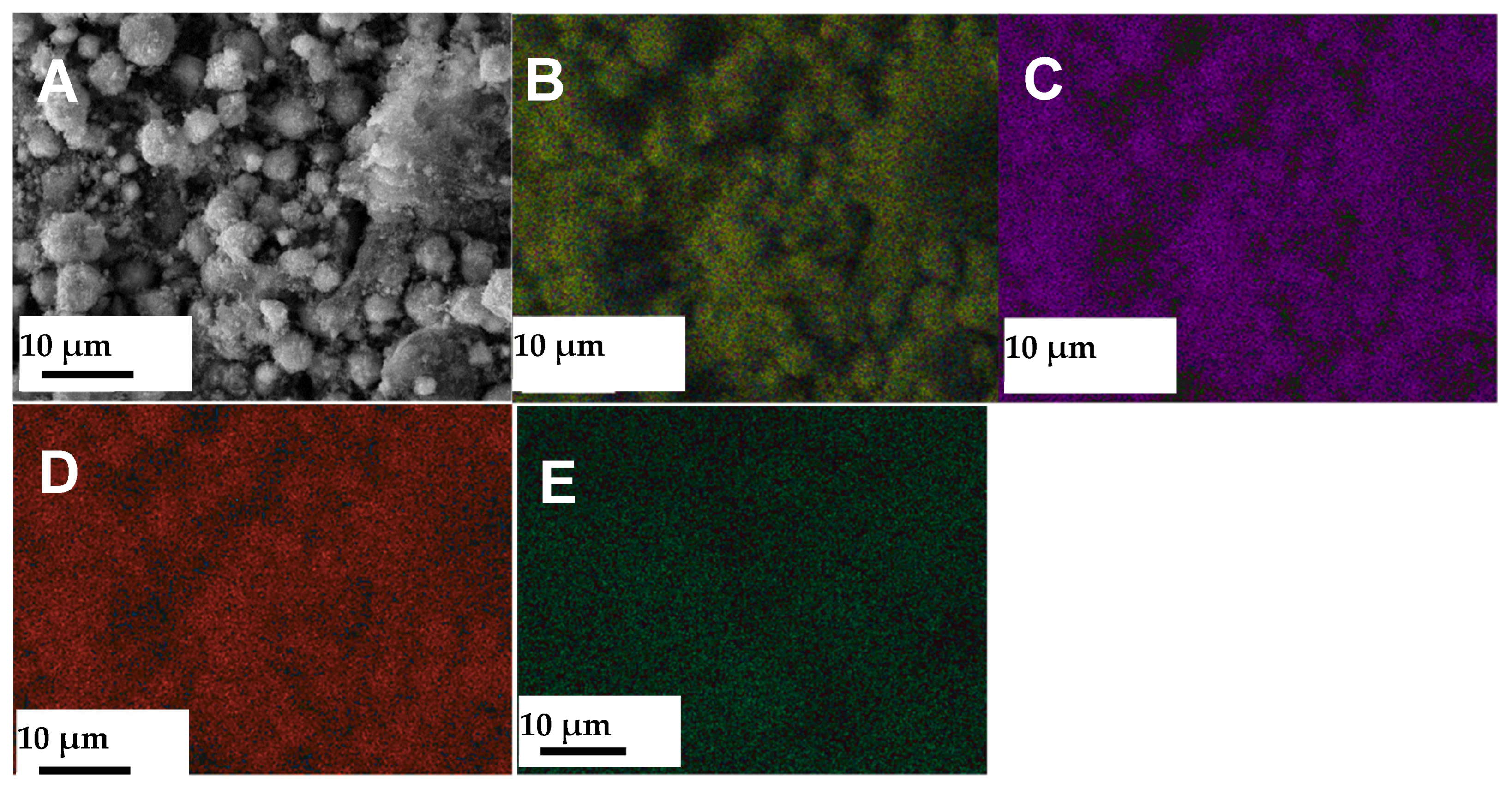
3.1. Characterization
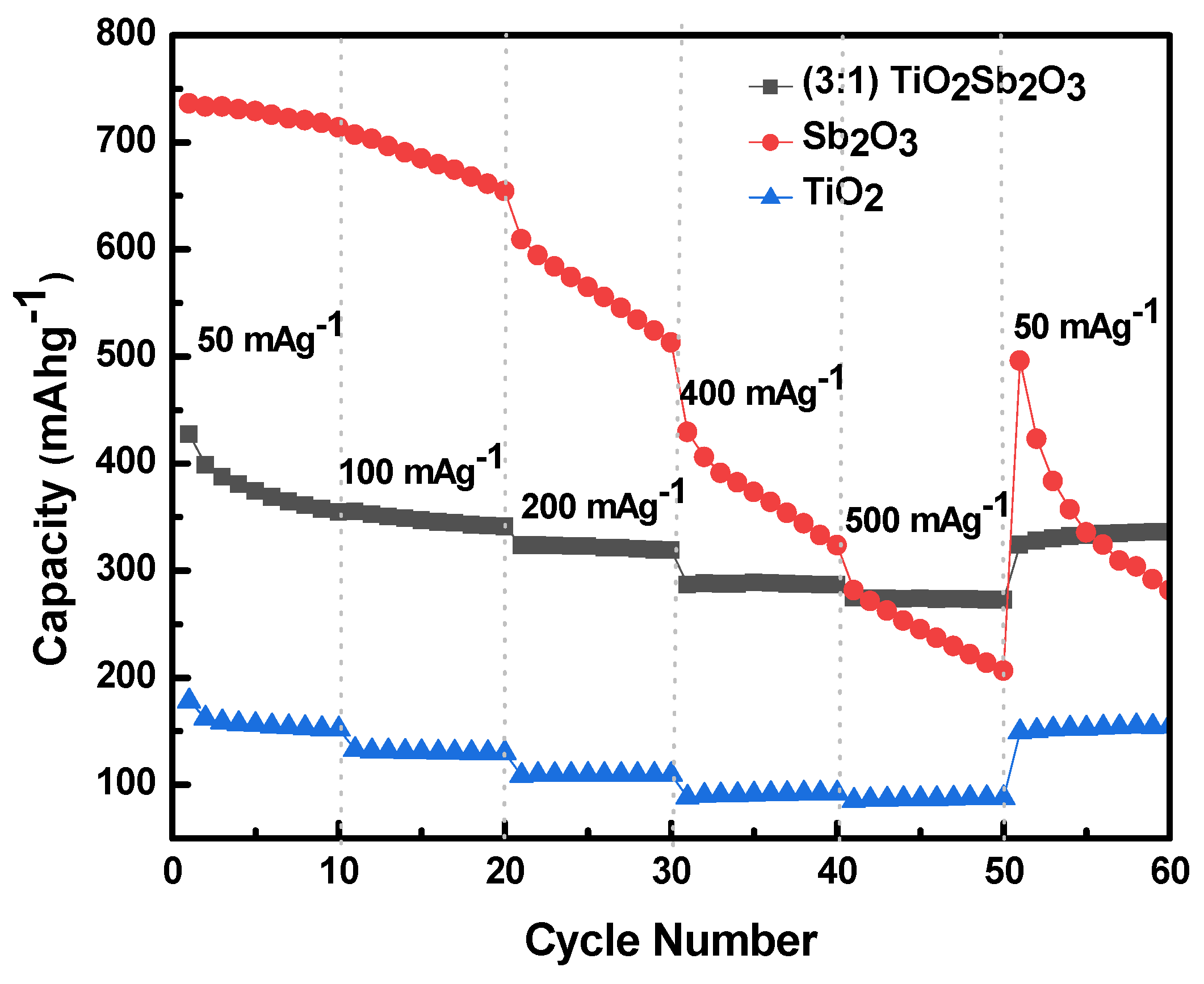
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.S.; Wang, L.; He, X.M. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Campos, H.; Villarreal, J.; Alcoutlabi, M. A comparative study on the performance of binary SnO2/NiO/C and Sn/C composite nanofibers as alternative anode materials for lithium ion batteries. Electrochim. Acta 2017, 224, 608–621. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Env. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, J.L.; Kintner-Meyer, M.C.W.; Lu, X.C.; Choi, D.W.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, H.; Yin, C.; Qiu, W. Si/SnSb alloy composite as high capacity anode materials for Li-ion batteries. J. Alloys Compd. 2006, 426, 277–280. [Google Scholar] [CrossRef]
- Nithyadharseni, P.; Reddy, M.; Nalini, B.; Ravindran, T.; Pillai, B.; Kalpana, M.; Chowdari, B. Electrochemical studies of CNT/Si–SnSb nanoparticles for lithium ion batteries. Mater. Res. Bull. 2015, 70, 478–485. [Google Scholar] [CrossRef]
- Park, M.-S.; Rajendran, S.; Kang, Y.-M.; Han, K.-S.; Han, Y.-S.; Lee, J.-Y. Si–Ni alloy–graphite composite synthesized by arc-melting and high-energy mechanical milling for use as an anode in lithium-ion batteries. J. Power Sources 2006, 158, 650–653. [Google Scholar] [CrossRef]
- Fang, Y.J.; Guan, B.Y.; Luan, D.Y.; Lou, X.W. Synthesis of CuS@CoS Double-Shelled Nanoboxes with Enhanced Sodium Storage Properties. Angew. Chem. Int. Ed. 2019, 58, 7739–7743. [Google Scholar] [CrossRef]
- Fang, Y.J.; Luan, D.Y.; Chen, Y.; Gao, S.Y.; Lou, X.W. Synthesis of Copper-Substituted CoS@CuS Double-Shelled Nanoboxes by Sequential Ion Exchange for Efficient Sodium Storage. Angew. Chem. Int. Ed. 2020, 59, 2644–2648. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.J.; Luan, D.Y.; Lou, X.W. Recent Advances on Mixed Metal Sulfides for Advanced Sodium-Ion Batteries. Adv. Mater. 2020, 32, 2002976. [Google Scholar] [CrossRef]
- Mishra, A.K.; Monika; Patial, B.S. A review on recent advances in anode materials in lithium ion batteries. Mater. Today Electron. 2024, 7, 100089. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; Flores, D.; Villareal, J.; Alcoutlabi, M. Composite Nanofibers as Advanced Materials for Li-ion, Li-O and Li-S Batteries. Electrochim. Acta 2016, 192, 529–550. [Google Scholar] [CrossRef]
- Agubra, V.A.; Zuniga, L.; De la Garza, D.; Gallegos, L.; Pokhrel, M.; Alcoutlabi, M. Forcespinning: A new method for the mass production of Sn/C composite nanofiber anodes for lithium ion batteries. Solid State Ion. 2016, 286, 72–82. [Google Scholar] [CrossRef]
- Zuniga, L.; Agubra, V.; Flores, D.; Campos, H.; Villareal, J.; Alcoutlabi, M. Multichannel hollow structure for improved electrochemical performance of TiO/Carbon composite nanofibers as anodes for lithium ion batteries. J. Alloys Compd. 2016, 686, 733–743. [Google Scholar] [CrossRef]
- Murugesan, S.; Harris, J.T.; Korgel, B.A.; Stevenson, K.J. Copper-coated amorphous silicon particles as an anode material for lithium-ion batteries. Chem. Mater. 2012, 24, 1306–1315. [Google Scholar] [CrossRef]
- Xue, L.; Xu, G.; Li, Y.; Li, S.; Fu, K.; Shi, Q.; Zhang, X. Carbon-coated Si nanoparticles dispersed in carbon nanotube networks as anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 21–25. [Google Scholar] [CrossRef]
- Li, X.F.; Dhanabalan, A.; Gu, L.; Wang, C.L. Three-Dimensional Porous Core-Shell Sn@Carbon Composite Anodes for High-Performance Lithium-Ion Battery Applications. Adv. Energy Mater. 2012, 2, 238–244. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.Q.; Zhai, T.Y.; Li, H.Q. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455. [Google Scholar] [CrossRef]
- Tian, J.J.; Yang, H.; Fu, C.M.; Sun, M.L.; Wang, L.N.; Liu, T.X. In-situ synthesis of microspherical Sb@C composite anode with high tap density for lithium/sodium-ion batteries. Compos. Commun. 2020, 17, 177–181. [Google Scholar] [CrossRef]
- Moolayadukkam, S.; Bopaiah, K.A.; Parakkandy, P.K.; Thomas, S. Antimony (Sb)-Based Anodes for Lithium-Ion Batteries: Recent Advances. Condens. Matter 2022, 7, 27. [Google Scholar] [CrossRef]
- Hou, H.S.; Cao, X.Y.; Yang, Y.C.; Fang, L.B.; Pan, C.C.; Yang, X.M.; Song, W.X.; Ji, X.B. NiSb alloy hollow nanospheres as anode materials for rechargeable lithium ion batteries. Chem. Commun. 2014, 50, 8201–8203. [Google Scholar] [CrossRef] [PubMed]
- Ke, F.S.; Huang, L.; Solomon, B.C.; Wei, G.Z.; Xue, L.J.; Zhang, B.; Li, J.T.; Zhou, X.D.; Sun, S.G. Three-dimensional nanoarchitecture of Sn-Sb-Co alloy as an anode of lithium-ion batteries with excellent lithium storage performance. J. Mater. Chem. 2012, 22, 17511–17517. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.T.; Wu, C.; Wen, Y.R.; Yin, K.B.; Chiang, F.K.; Hu, R.Z.; Liu, J.W.; Sun, L.T.; Gu, L.; et al. New Nanoconfined Galvanic Replacement Synthesis of Hollow Sb@C Yolk-Shell Spheres Constituting a Stable Anode for High-Rate Li/Na-Ion Batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Bryngelsson, H.; Eskhult, J.; Nyholm, L.; Herranen, M.; Alm, O.; Edström, K. Electrodeposited Sb and Sb/SbO nanoparticle coatings as anode materials for Li-ion batteries. Chem. Mater. 2007, 19, 1170–1180. [Google Scholar] [CrossRef]
- Ramireddy, T.; Rahman, M.M.; Xing, T.; Chen, Y.; Glushenkov, A.M. Stable anode performance of an Sb-carbon nanocomposite in lithium-ion batteries and the effect of ball milling mode in the course of its preparation. J. Mater. Chem. A 2014, 2, 4282–4291. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.J.; Zhu, J.; Xia, Y.; Müller-Buschbaum, P. A chronicle review of nonsilicon (Sn, Sb, Ge)-based lithium/sodium-ion battery alloying anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.; Chowdari, B. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Deng, M.; Li, S.; Hong, W.; Jiang, Y.; Xu, W.; Shuai, H.; Zou, G.; Hu, Y.; Hou, H.; Wang, W. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage. Mater. Chem. Phys. 2019, 223, 46–52. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yao, T.H.; Dong, H.; Ge, Q.J.; Chen, S.Q.; Ma, Z.H. Ultrafine SbO Nanoparticle-Decorated Reduced Graphene Oxide as an Anode Material for Lithium-Ion Batteries. Energy Fuel 2023, 37, 5586–5594. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, L.; Chen, H.; Hou, Q.; Chen, X. Synthesis of a symmetric bundle-shaped Sb2O3 and its application for anode materials in lithium ion batteries. Mater. Lett. 2018, 212, 103–106. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yang, K.; Yang, Y.; Ma, J.; Pan, K.; Wang, G.; Ren, F.; Pang, H. Enhanced electrochemical performance of Sb2O3 as an anode for lithium-ion batteries by a stable cross-linked binder. Appl. Sci. 2019, 9, 2677. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Armstrong, G.; Canales, J.; García, R.; Bruce, P.G. Lithium-ion intercalation into TiO2-B nanowires. Adv. Mater. 2005, 17, 862–865. [Google Scholar] [CrossRef]
- Batteries, L. New Materials, Developments and Perspectives. Ind. Chem. Libr. 1994, 5, 137–165. [Google Scholar]
- Han, Q.G.; Sun, Y.B.; Zhang, W.Q.; Li, X.; Li, Y.; Zhang, X.; Deng, Y.S. Synthesis of one-dimensional yolk-shell SbO/TiO composite as an anode material for enhanced lithium-storage properties. Ionics 2020, 26, 1221–1228. [Google Scholar] [CrossRef]
- Huang, M.; Chu, Y.T.; Xi, B.J.; Shi, N.X.; Duan, B.; Zhang, C.H.; Chen, W.H.; Feng, J.K.; Xiong, S.L. TiO-Based Heterostructures with Different Mechanism: A General Synergistic Effect toward High-Performance Sodium Storage. Small 2020, 16, 2004054. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, N.N.; Liu, Y.Z.; Liu, W.; Liu, J.R. Improved electrochemical performances derived from synergistic titanium dioxide and iron titanate porous nanohybrids serving as lithium-ion battery anodes. J. Alloys Compd. 2017, 714, 583–592. [Google Scholar] [CrossRef]
- Wang, J.; Ran, R.; Tade, M.O.; Shao, Z.P. Self-assembled mesoporous TiO/carbon nanotube composite with a three-dimensional conducting nanonetwork as a high-rate anode material for lithium-ion battery. J. Power Sources 2014, 254, 18–28. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Y.F.; Zhang, T.; Chen, Q.; Guo, S.Y. Co O hollow nanospheres/carbon-assembled mesoporous polyhedron with internal bubbles encapsulating TiO nanosphere for high-performance lithium ion batteries. Nanotechnology 2019, 30, 355401. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Rahman, M.A.; Sharif, S.B.; Kim, J.-H.; Siddiqui, S.-E.-T.; Hossain, M.A.M. TiO2 as an Anode of high-performance lithium-ion batteries: A Comprehensive Review towards Practical Application. Nanomaterials 2022, 12, 2034. [Google Scholar] [CrossRef] [PubMed]
- Pfanzelt, M.; Kubiak, P.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. TiO2 rutile—An alternative anode material for safe lithium-ion batteries. J. Power Sources 2011, 196, 6815–6821. [Google Scholar] [CrossRef]
- Laskova, B.; Zukalova, M.; Zukal, A.; Bousa, M.; Kavan, L. Capacitive contribution to Li-storage in TiO2 (B) and TiO2 (anatase). J. Power Sources 2014, 246, 103–109. [Google Scholar] [CrossRef]
- Yang, L.R.; Niu, Z.R.; Wang, C.M.; Liu, Z.G.; Feng, X.X. Preparation and electrochemical performance of biomass-derived porous carbon/MoO2@TiO2 composite as anode materials for lithium-ion batteries. Solid State Ion. 2023, 389, 116110. [Google Scholar] [CrossRef]
- Gonzalez, G.; Hasan, M.T.; Ramirez, D.; Parsons, J.; Alcoutlabi, M. Synthesis of SnO/TiO micro belt fibers from polymer composite precursors and their applications in Li-ion batteries. Polym. Eng. Sci. 2022, 62, 360–372. [Google Scholar] [CrossRef]
- Rhee, D.Y.; Kim, J.; Moon, J.; Park, M.S. Off-stoichiometric TiO-decorated graphite anode for high-power lithium-ion batteries. J. Alloys Compd. 2020, 843, 156042. [Google Scholar] [CrossRef]
- Leal, J.H.; Cantu, Y.; Gonzalez, D.F.; Parsons, J.G. Brookite and anatase nanomaterial polymorphs of TiO synthesized from TiCl. Inorg. Chem. Commun. 2017, 84, 28–32. [Google Scholar] [CrossRef]
- Wang, Z.M.; Cheng, Y.; Li, Q.; Chang, L.M.; Wang, L.M. Facile synthesis of one-dimensional hollow SbO@TiO composites as anode materials for lithium ion batteries. J. Power Sources 2018, 389, 214–221. [Google Scholar] [CrossRef]
- Han, Q.G.; Sheng, Y.L.; Han, Z.W.; Li, X.; Zhang, W.Q.; Li, Y.; Zhang, X. Metallic Sb nanoparticles embedded into a yolk-shell SbO@TiO composite as anode materials for lithium ion batteries. New J. Chem. 2020, 44, 13430–13438. [Google Scholar] [CrossRef]
- Rodriguezcarvajal, J. Recent Advances in Magnetic-Structure Determination by Neutron Powder Diffraction. Physics B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Meagher, E.; Lager, G.A. Polyhedral thermal expansion in the TiO2 polymorphs; refinement of the crystal structures of rutile and brookite at high temperature. Can. Mineral. 1979, 17, 77–85. [Google Scholar]
- Sarnstrand, C. Crystal-Structure of Antimony(Iii) Chloride Oxide Sb4O5Cl2. Acta Crystallogr. B 1978, 34, 2402–2407. [Google Scholar] [CrossRef]
- Svensson, C. Refinement of Crystal-Structure of Cubic Antimony Trioxide, Sb2O3. Acta Crystallogr. B 1975, 31, 2016–2018. [Google Scholar] [CrossRef]
- Svensson, C. Crystal-Structure of Orthorhombic Antimony Trioxide, Sb2o3. Acta Crystallogr. B 1974, 30, 458–461. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Song, D.; Hrbek, J.; Osgood, R. Formation of TiO nanoparticles by reactive-layer-assisted deposition and characterization by XPS and STM. Nano Lett. 2005, 5, 1327–1332. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, Y.C. Contact Angle Relaxation and Long-Lasting Hydrophilicity of Sputtered Anatase TiO Thin Films by Novel Quantitative XPS Analysis. Langmuir 2019, 35, 2066–2077. [Google Scholar] [CrossRef]
- Matouk, Z.; Islam, M.; Gutiérrez, M.; Pireaux, J.J.; Achour, A. X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO Films. Nanomaterials. 2022, 12, 3692. [Google Scholar] [CrossRef]
- Zeng, D.W.; Xie, C.S.; Zhu, B.L.; Song, W.L. Characteristics of SbO nanoparticles synthesized from antimony by vapor condensation method. Mater. Lett. 2004, 58, 312–315. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Salunkhe, T.T.; Nguyen, T.A.; Nguyen, T.L.; Kim, I. Optimal tailored preparation of Sb/Sb4O5Cl2 nanosheet composite anodes for efficient sodium-ion storage. Electrochim. Acta 2022, 436, 141429. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhao, S.N.; Meng, W.J.; Guo, M.Q.; Wang, G.W.; Guo, C.L.; Bai, Z.C.; Li, Z.Q.; Ye, J.Y.; Song, H.; et al. Electrochemical Performance and Stress Distribution of Sb/SbO Nanoparticles as Anode Materials for Sodium-Ion Batteries. Batteries 2023, 9, 98. [Google Scholar] [CrossRef]
- Liu, G.R.; Zhang, S.C.; Wu, X.M.; Lin, R.X. Fabrication of rutile TiO nanorod arrays on a copper substrate for high-performance lithiumion batteries. Rsc. Adv. 2016, 6, 55671–55675. [Google Scholar] [CrossRef]
- Gan, Y.P.; Zhu, L.Y.; Qin, H.P.; Xia, Y.; Xiao, H.; Xu, L.S.; Ruan, L.Y.; Liang, C.; Tao, X.Y.; Huang, H.; et al. Hybrid nanoarchitecture of rutile TiO nanoneedle/graphene for advanced lithium-ion batteries. Solid State Ion. 2015, 269, 44–50. [Google Scholar] [CrossRef]
- McNulty, D.; Carroll, E.; O’Dwyer, C. Rutile TiO Inverse Opal Anodes for Li-Ion Batteries with Long Cycle Life, High-Rate Capability, and High Structural Stability. Adv. Energy Mater. 2017, 7, 1602291. [Google Scholar] [CrossRef]
- Han, Z.; Zheng, J.; Kong, F.; Tao, S.; Qian, B. One dimensional SbO2/Sb2O3@ NC microrod as anode for lithium-ion and sodium-ion batteries. Nano Sel. 2021, 2, 425–432. [Google Scholar] [CrossRef]
- Cheng, Y.; Yi, Z.; Wang, C.; Wang, L.; Wu, Y.; Wang, L. Nanostructured carbon/antimony composites as anode materials for lithium-ion batteries with long life. Chem. Asian J. 2016, 11, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Z.; Zhang, Z.F.; Lu, X.F.; Lv, X.Y.; Ma, G.F.; Wang, Q.T.; Lei, Z.Q. SbO Nanoparticles Anchored on Graphene Sheets via Alcohol Dissolution-Reprecipitation Method for Excellent Lithium-Storage Properties. Acs Appl. Mater. Inter. 2017, 9, 34927–34936. [Google Scholar] [CrossRef]
- Lv, H.; Qiu, S.; Lu, G.; Fu, Y.; Li, X.; Hu, C.; Liu, J. Nanostructured antimony/carbon composite fibers as anode material for lithium-ion battery. Electrochim. Acta 2015, 151, 214–221. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Jung, D.-W.; Shin, E.W.; Oh, E.-S. Boron-doped TiO2 anode materials for high-rate lithium ion batteries. J. Alloys Compd. 2014, 604, 226–232. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Yang, F.; Zhang, D.L.; Zhang, X.; Xue, C.L.; Zuo, Y.H.; Li, C.B.; Cheng, B.W.; Wang, Q.M. A binder-free Si-based anode for Li-ion batteries. Rsc. Adv. 2015, 5, 15940–15943. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Wang, L.; Zhou, D.; Wu, T.L.; Xiao, Z.B. A Flower-Like SbOCl Cluster-based material as anode for potassium ion batteries. Appl. Surf. Sci. 2022, 583, 152509. [Google Scholar] [CrossRef]

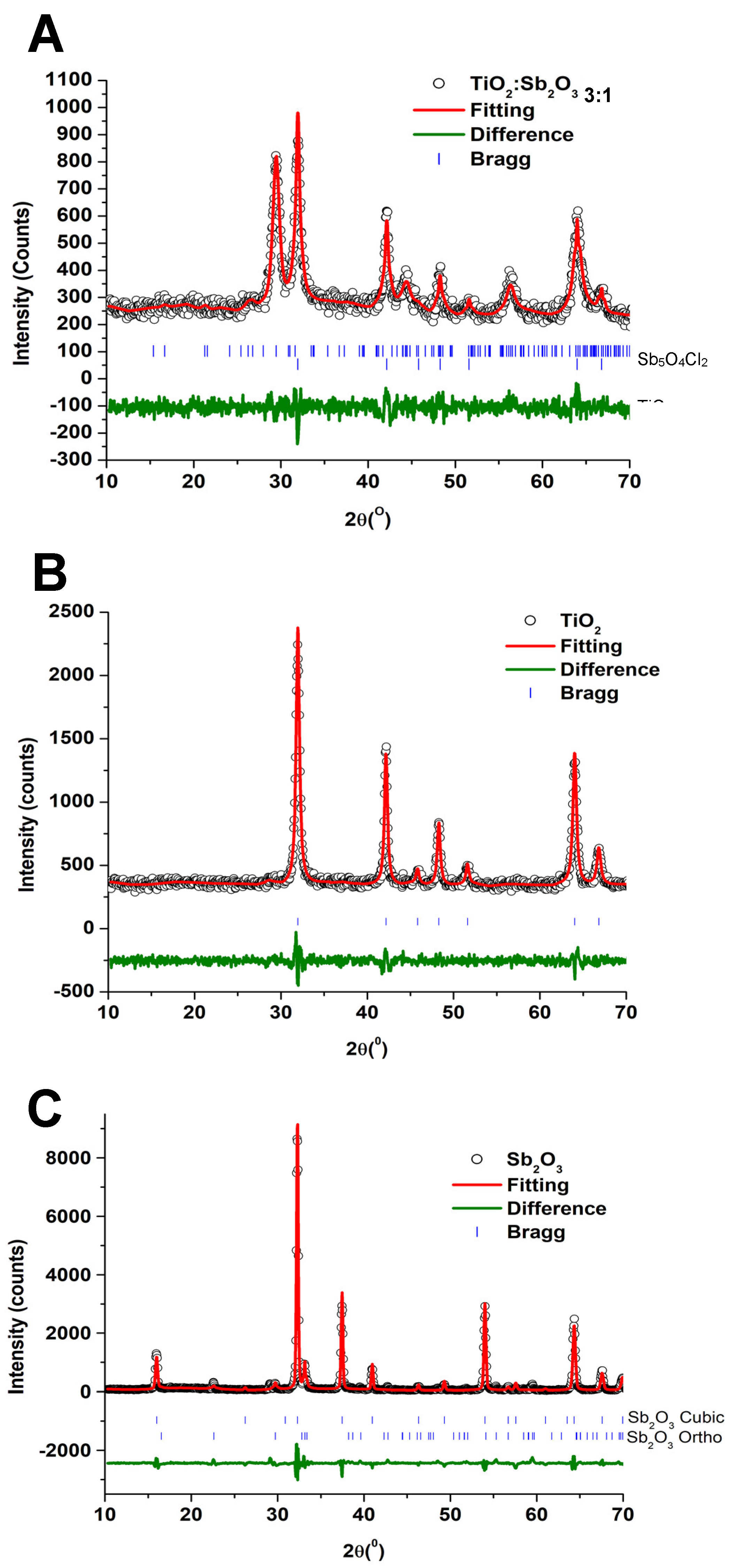
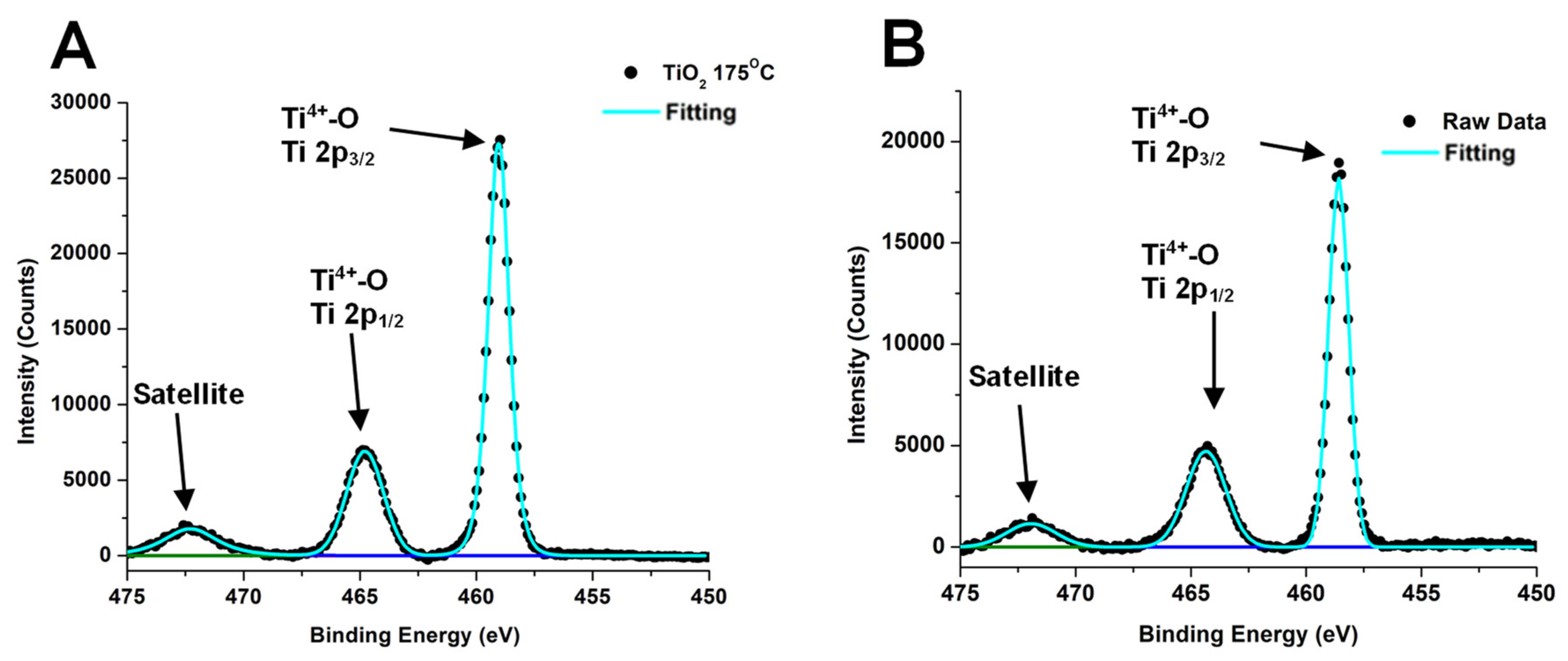

| Sample | Phase | Space Group | a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Rutile | P42/mnm | 4.59 (8) | 4.59 (8) | 2.95 (9) | 90.0 | 90.0 | 90.0 | 2.41 |
| Sb2O3 | Cubic | Fm-3m | 11.15 (3) | 11.15 (3) | 11.15 (3) | 90.0 | 90.0 | 90.0 | 3.15 |
| Orthorhombic | Pccn | 4.91 (0) | 12.46 (5) | 5.40 (5) | 90.0 | 90.0 | 90.0 | ||
| TiO2@Sb2O3 3:1 | Sb5O4Cl2 | P21/C | 6.21 (9) | 5.11 (6) | 13.49 (9) | 90.0 | 97.3 | 90.0 | 1.28 |
| TiO2 | P42/mnm | 4.59 (6) | 4.59 (6) | 2.95 (8) | 90.0 | 90.0 | 90.0 |
| Sample | Energy (eV) | Ti 2p3/2/2p½ | Energy (eV) | Ti | Energy (eV) | O 1 s | Energy (eV) | Sb 3d5/2/3d3/2 |
|---|---|---|---|---|---|---|---|---|
| (A) TiO2 | 458.6/464.3 | Ti4+-O | 471.9 | Satellite | 530.4 529.8 | O-Ti O-H | N/A | |
| (B) TiO2@Sb2O3 3:1 | 458.6/464.4 | Ti4+-O | 471.9 | Satellite | 531.4 | O-M | 528.4/537.7 530.7/540.4 | Sb(0) Sb3+-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez, K.; Fletes, E.; Parsons, J.G.; Alcoutlabi, M. The Effect of TiO2 on the Electrochemical Performance of Sb2O3 Anodes for Li-Ion Batteries. Appl. Sci. 2024, 14, 6598. https://doi.org/10.3390/app14156598
Gomez K, Fletes E, Parsons JG, Alcoutlabi M. The Effect of TiO2 on the Electrochemical Performance of Sb2O3 Anodes for Li-Ion Batteries. Applied Sciences. 2024; 14(15):6598. https://doi.org/10.3390/app14156598
Chicago/Turabian StyleGomez, Kithzia, Elizabeth Fletes, Jason G. Parsons, and Mataz Alcoutlabi. 2024. "The Effect of TiO2 on the Electrochemical Performance of Sb2O3 Anodes for Li-Ion Batteries" Applied Sciences 14, no. 15: 6598. https://doi.org/10.3390/app14156598





