Chloride Resistance of Assembled Bridge Piers Reinforced with Epoxy-Coated Steel Bars
Abstract
:1. Introduction
2. Materials, Specimens, and Methods
2.1. Experimental Materials and Mix Proportions
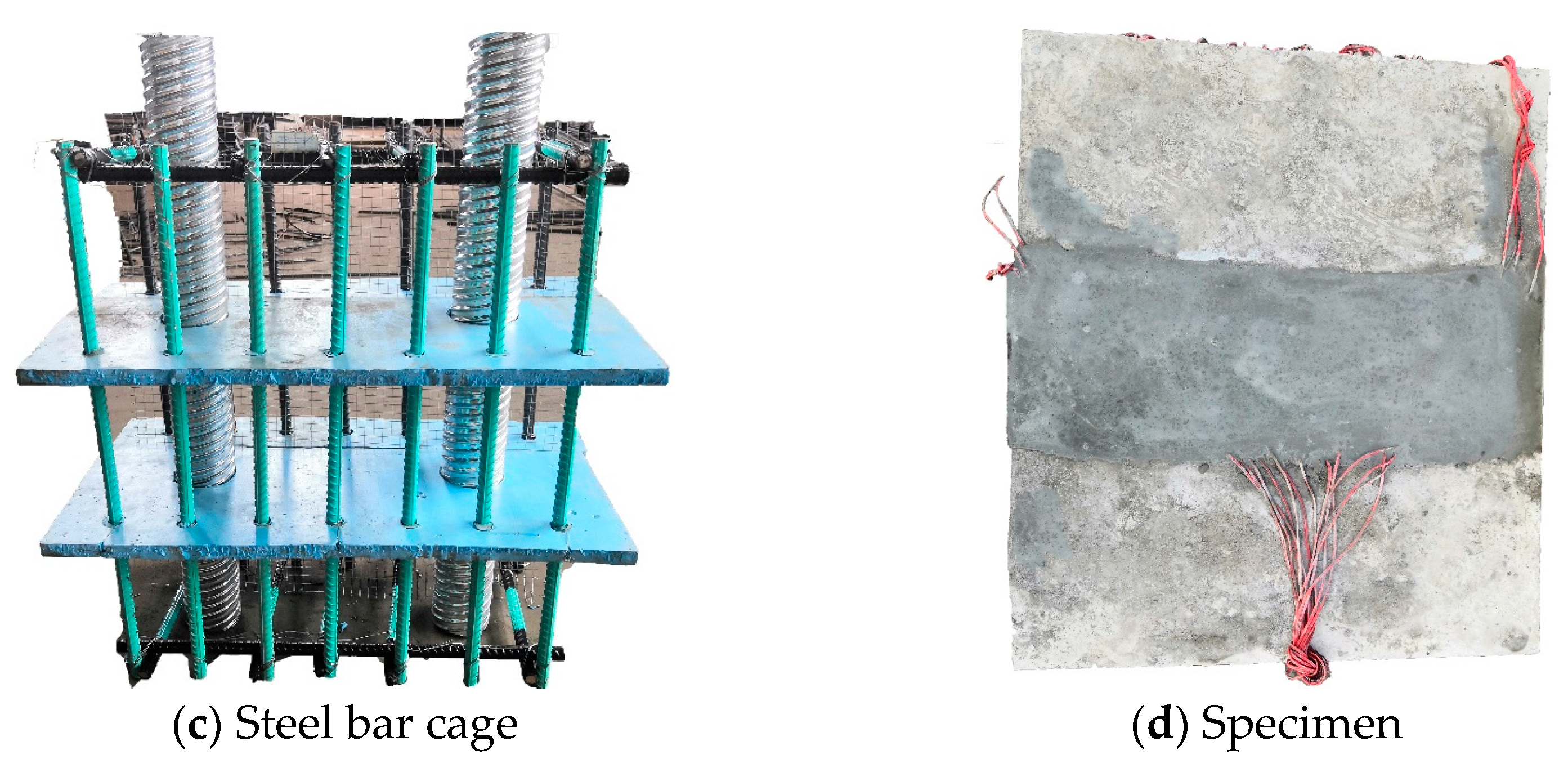
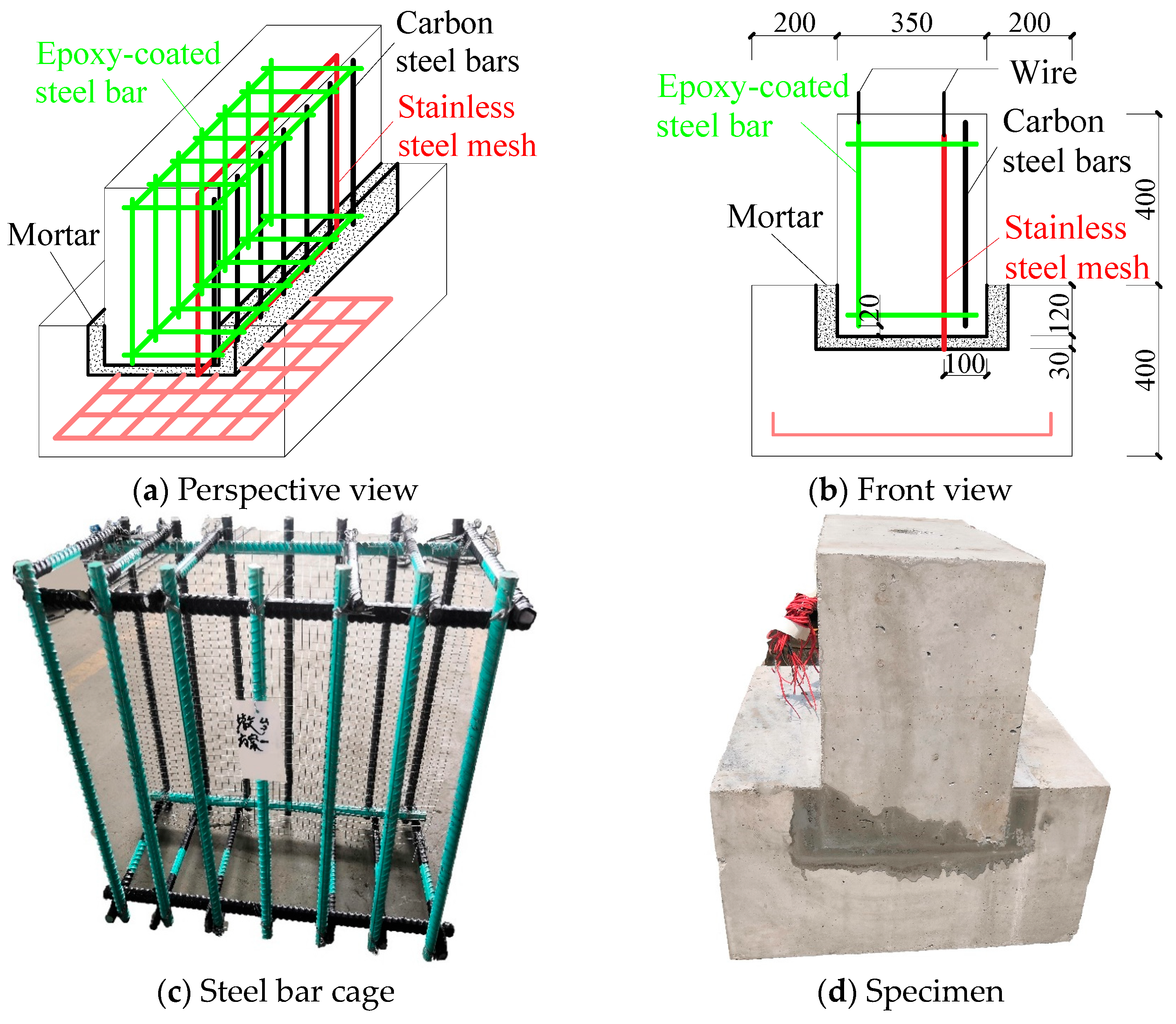
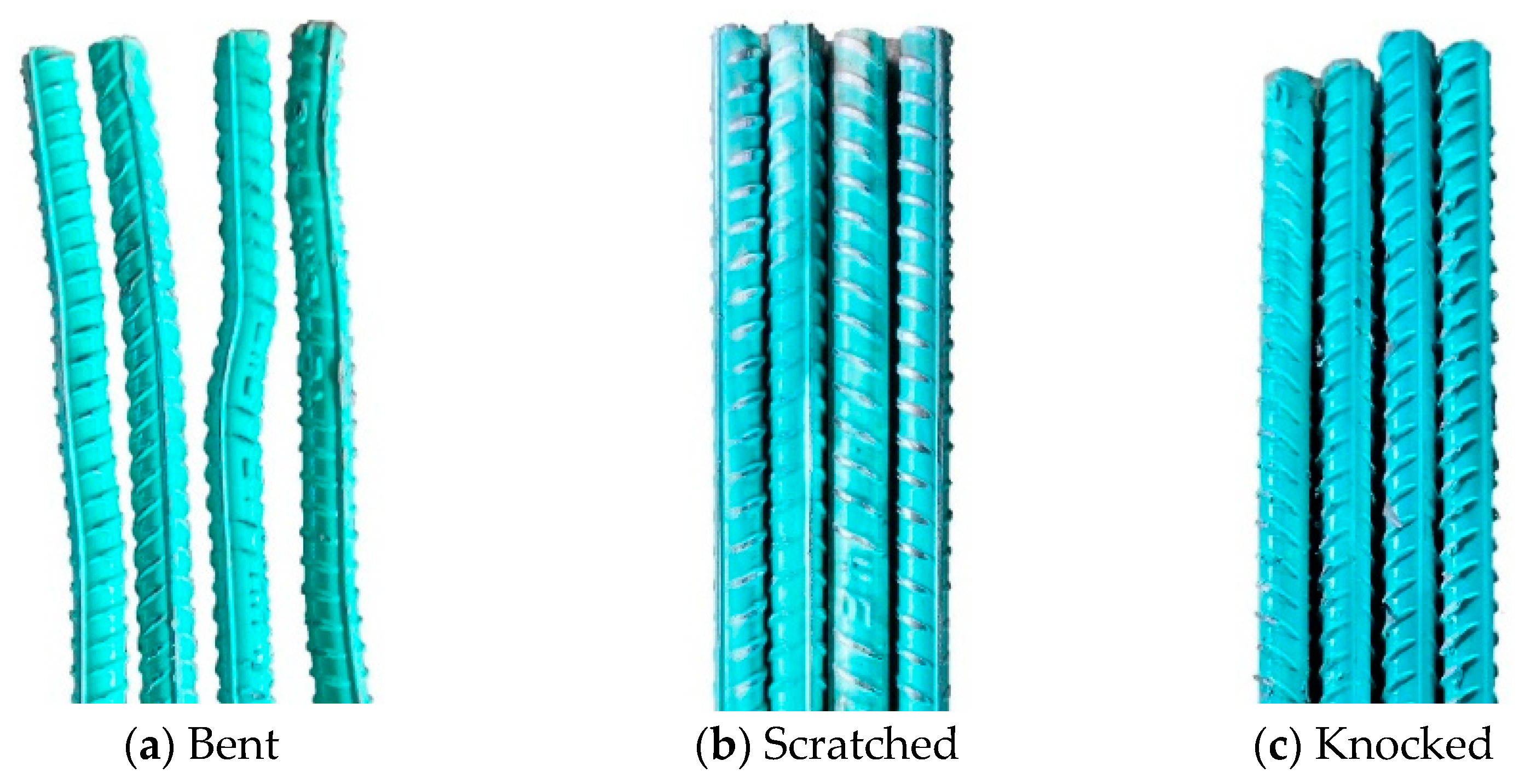
2.2. Specimen Design and Fabrication
2.3. Experimental Method
2.3.1. Electromigration Method
2.3.2. Electrochemical Testing Method
2.3.3. Chloride Ion Content Testing Method
3. Results and Discussion
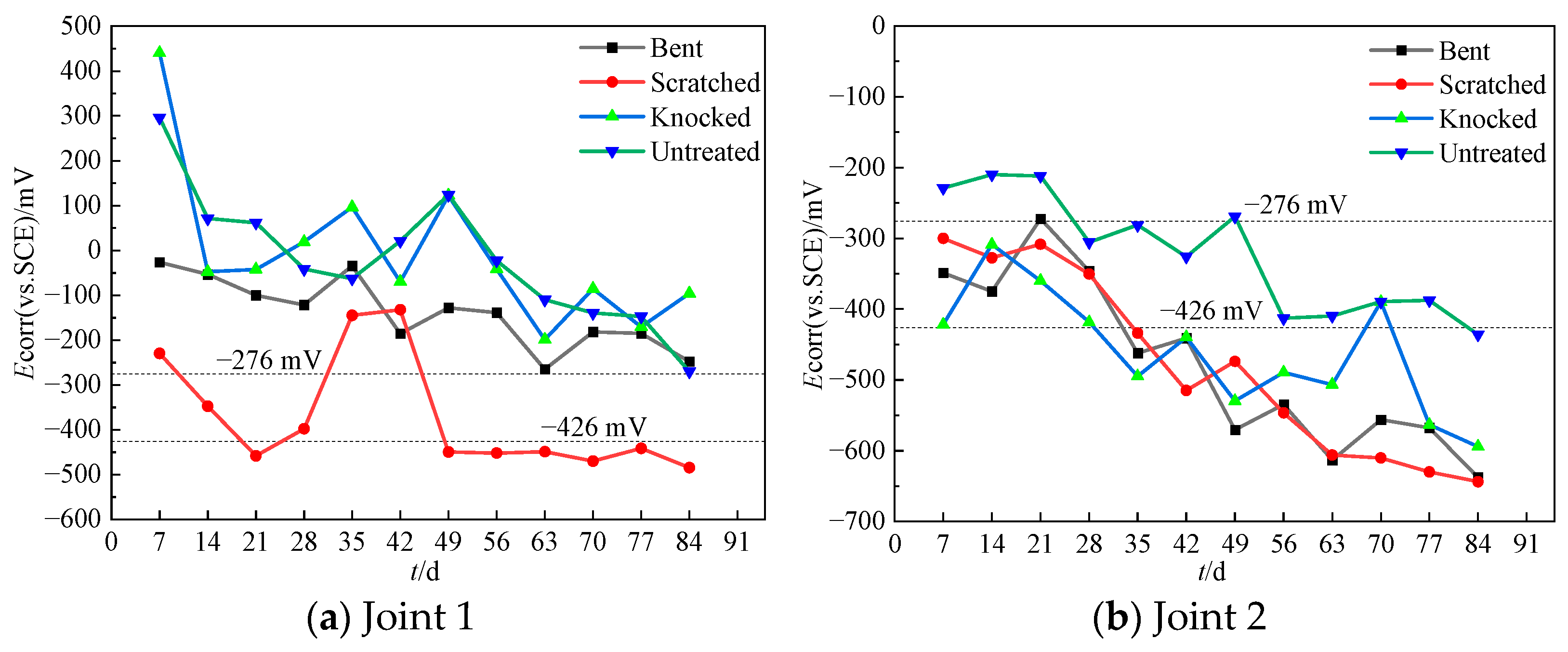
3.1. Self-Corrosion Potential ()
3.2. Corrosion Current Density ()
3.3. Polarization Resistance ()
3.4. Chloride Ion Content ()
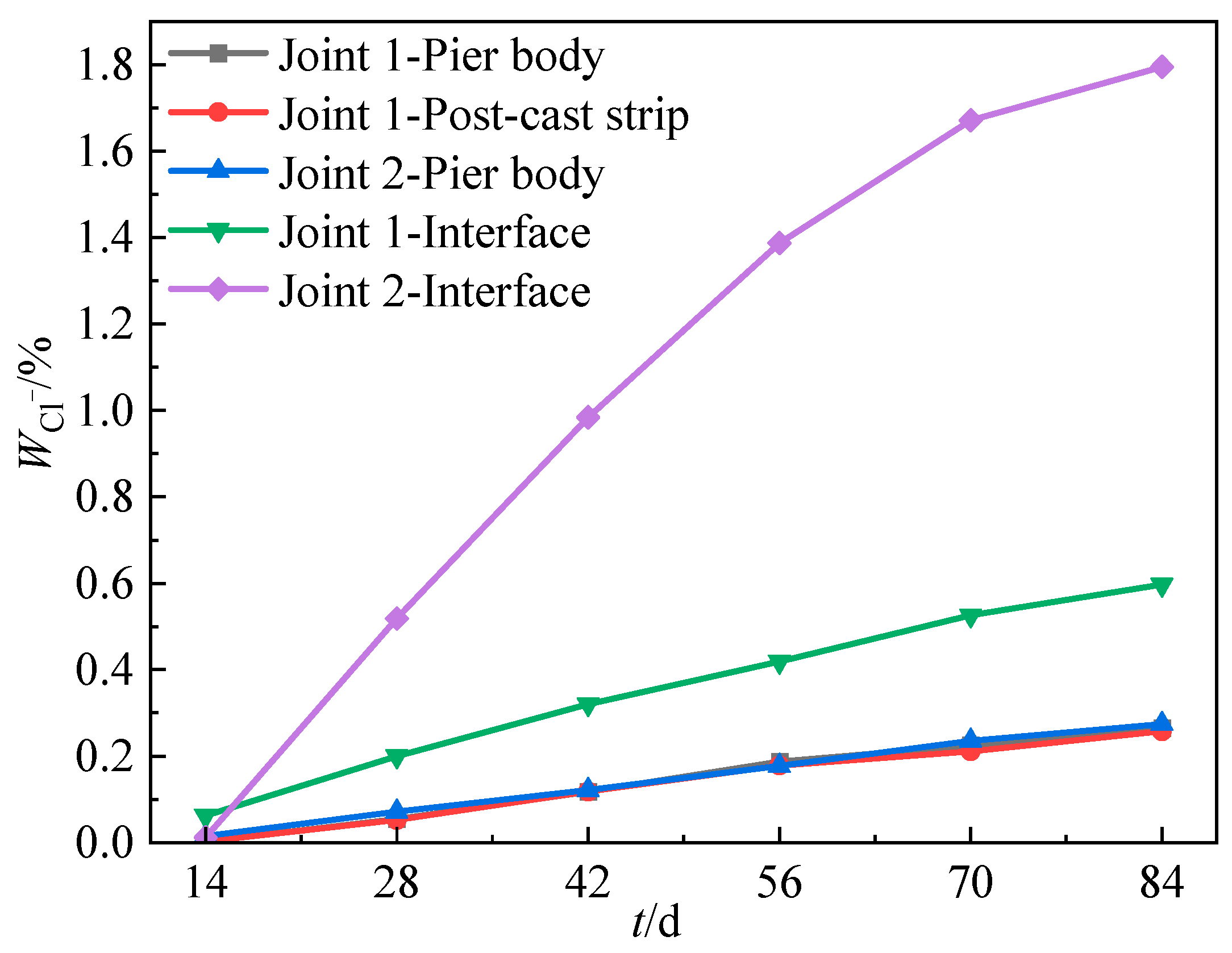
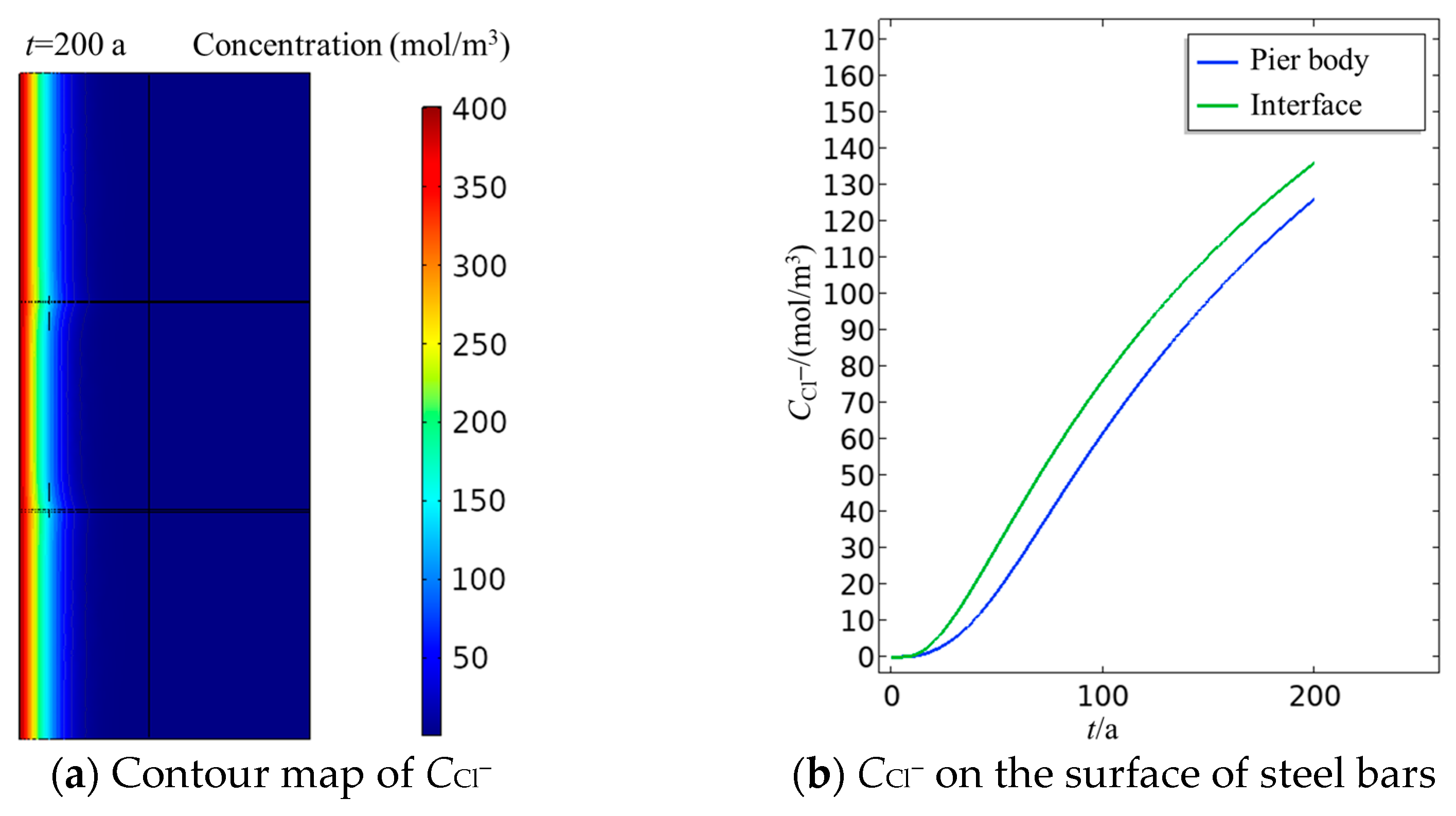
3.4.1. Spatial and Temporal Distribution of Chloride Ion Content
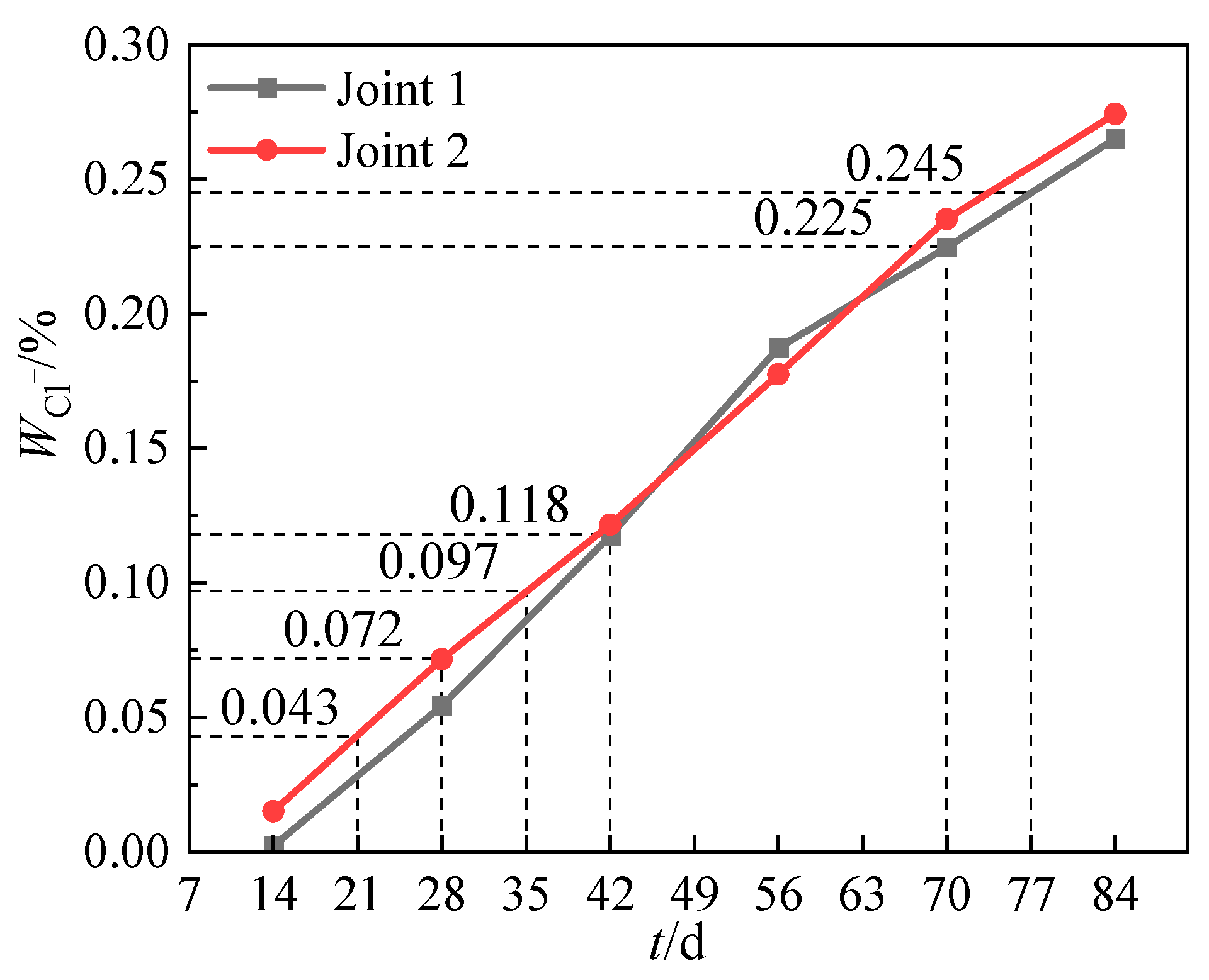
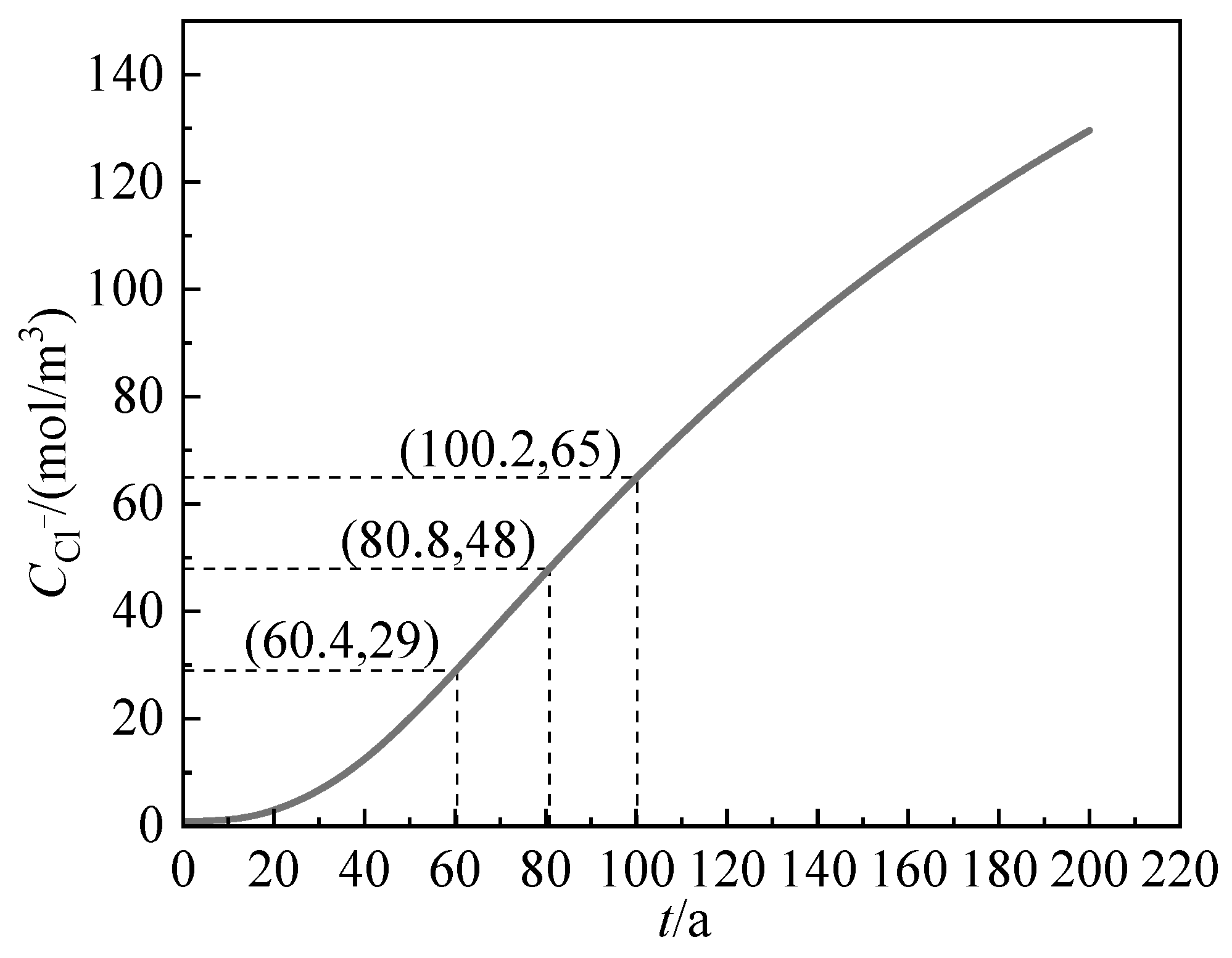
3.4.2. Chloride Ion Content on the Surface of Steel Bars
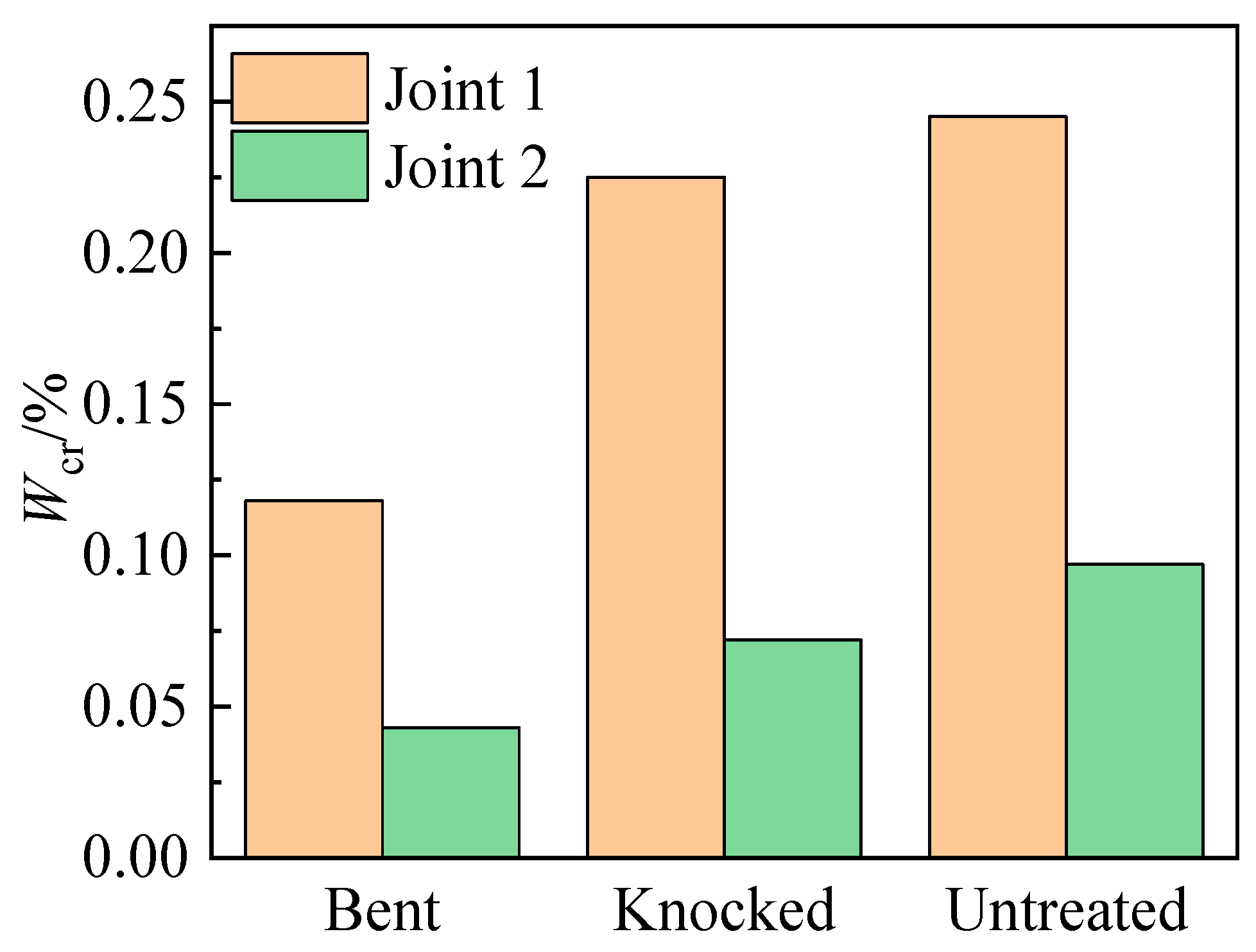
3.4.3. Critical Chloride Ion Content
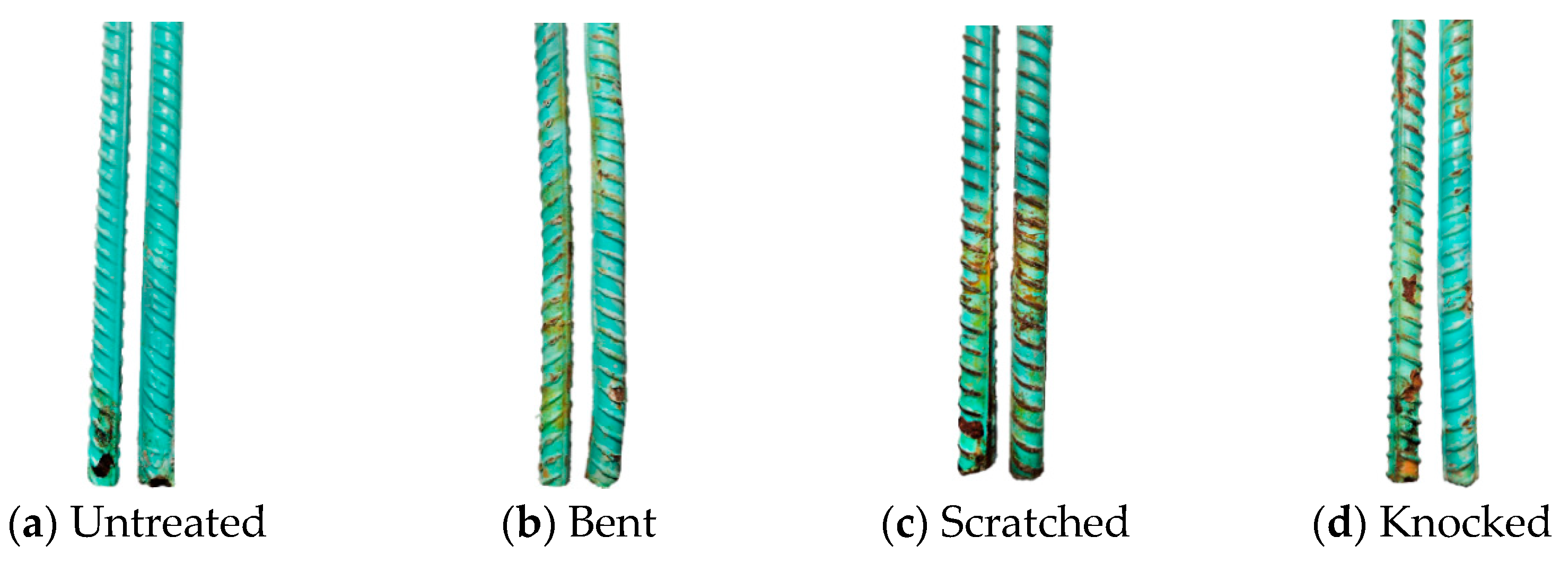
3.5. Corrosion Morphology
4. Service Life Prediction
4.1. Determination of DNSSD
4.1.1. and
4.1.2. The Correction of Diffusion Coefficient
4.1.3. Experimental Determination of

- : applied voltage, V;
- : average value of the initial and final temperatures in the anolyte solution, °C;
- : thickness of the specimen;
- : average value of the penetration depths, mm;
- : test duration, hour.
| Specimen No. | U/V | T/°C | L/mm | t/h | xd/mm | Dnssm | |
|---|---|---|---|---|---|---|---|
| Value | Average | ||||||
| C50-A | 60 | 16 | 50 | 24 | 10.6 | 2.326 | 2.765 |
| C50-B | 60 | 24 | 14.5 | 3.243 | |||
| C50-C | 60 | 24 | 12.3 | 2.725 | |||
| C60-A | 60 | 24 | 8.7 | 1.884 | 2.319 | ||
| C60-B | 60 | 24 | 12.2 | 2.701 | |||
| C60-C | 60 | 24 | 10.8 | 2.373 | |||
| M-A | 60 | 48 | 13.5 | 1.503 | 1.602 | ||
| M-B | 60 | 48 | 14.3 | 1.598 | |||
| M-C | 60 | 48 | 15.2 | 1.704 | |||
| CC-A | 50 | 24 | 37.6 | 10.513 | 11.673 | ||
| CC-B | 50 | 24 | 41.5 | 11.644 | |||
| CC-C | 50 | 24 | 45.7 | 12.863 | |||
| CM-A | 60 | 24 | 24.4 | 5.593 | 6.151 | ||
| CM-B | 60 | 24 | 27.1 | 6.238 | |||
| CM-C | 60 | 24 | 28.7 | 6.621 | |||
4.2. Modeling Methods
4.3. Simulation Results
5. Conclusions
- (1)
- The corrosion resistance of the steel bars is directly related to the integrity of the epoxy coating. The ranking from highest to lowest corrosion resistance is untreated > knocked > bent > scratched. The corrosion risk and corrosion rate of steel bars increase significantly when the damaged area ratio of the epoxy coating is higher than 5%.
- (2)
- For assembled bridge piers, the quality of the connection interfaces between components significantly impacts the chloride resistance. The grouting interface of Joint 2 exhibits more defects than the prestressed post-cast strip interface of Joint 1. The chloride ion transport rate at the interface of Joint 2 is about 5 times that of Joint 1.
- (3)
- The types of assembled interface affect the corrosion behavior of the steel bars. The critical chloride ion content of the same type of steel bars in Joint 2 is less than 40% of that in Joint 1, and the initial rust time is less than 1/2 of that in Joint 1.
- (4)
- The numerical simulation suggests that the integrity of the epoxy coating and the type of joint significantly influence the service life. For Joint 1, the service life can reach up to 100 years regardless of the treatment of the steel bars. In contrast, Joint 2 achieves a service life of approximately 100 years only when using untreated epoxy-coated steel bars. This underscores the importance of proper joint design and the prevention of epoxy coating damage as critical strategies to enhance the durability of assembled piers in cross-sea bridges.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, P.K. Durability of Concrete—Fifty Years of Progress? ACI Symp. Publ. 1991, 126, 1–32. [Google Scholar] [CrossRef]
- Wei, J.; Wei, Y.; Li, J.; Zhao, H.; Lv, C.; Dong, J.; Ke, W.; He, X. Corrosion Behavior of Damaged Epoxy Coated Steel Bars Under the Coupling Effect of Chloride Ion and Carbonization. Acta Metall. Sin. 2020, 56, 885–897. [Google Scholar] [CrossRef]
- Elmoaty, A.E.M.A. Four-years carbonation and chloride induced steel corrosion of sulfate-contaminated aggregates concrete. Constr. Build. Mater. 2018, 163, 539–556. [Google Scholar] [CrossRef]
- Yan, L.; Deng, W.; Wang, N.; Xue, X.; Hua, J.; Chen, Z. Anti-Corrosion Reinforcements Using Coating Technologies—A Review. Polymers 2022, 14, 4782. [Google Scholar] [CrossRef]
- Yadav, M.; Dey, I.; Ghosh, S.K. A comparative study on the microstructure, hardness and corrosion resistance of epoxy coated and plain rebars. Mater. Res. Express 2022, 9, 056504. [Google Scholar] [CrossRef]
- Pérez-Claros, E.; Andrawes, B. Concrete Bridge Deck Reinforced with Textured and Nontextured Epoxy-Coated Bars. J. Bridge Eng. 2023, 28, 04023023. [Google Scholar] [CrossRef]
- Ahmad, S.; Al-Tholaia, M.M.H. Evaluation of corrosion resistance of coated steel strips embedded in mortar under chloride exposure. Anti-Corros. Methods Mater. 2015, 62, 29–36. [Google Scholar] [CrossRef]
- Huang, G.; Wu, B.; Shen, Y.; Wang, L.; Li, G. Comparative experiment of steel bar corrosion at concrete construction joints. Front. Mater. 2022, 9, 1094696. [Google Scholar] [CrossRef]
- Wang, X.-H.; Gao, Y. Corrosion behavior of epoxy-coated reinforced bars in RC test specimens subjected to pre-exposure loading and wetting-drying cycles. Constr. Build. Mater. 2016, 119, 185–205. [Google Scholar] [CrossRef]
- Assaad, J.J.; Issa, C.A. Bond strength of epoxy-coated bars in underwater concrete. Constr. Build. Mater. 2012, 30, 667–674. [Google Scholar] [CrossRef]
- Kayyali, O.A.; Yeomans, S.R. Bond and slip of coated reinforcement in concrete. Constr. Build. Mater. 1995, 9, 219–226. [Google Scholar] [CrossRef]
- Manning, D.G. Corrosion performance of epoxy-coated reinforcing steel: North American experience. Constr. Build. Mater. 1996, 10, 349–365. [Google Scholar] [CrossRef]
- Keßler, S.; Angst, U.; Zintel, M.; Gehlen, C. Defects in epoxy coated reinforcement and their impact on service life of concrete structures. Struct. Concr. 2015, 16, 398–405. [Google Scholar] [CrossRef]
- Cao, H.; Lyu, Z.; Dong, W.; Zhao, Z.; Gan, W.; Wang, Y. Corrosion Experimental Research on Local Damage of Epoxy-Coated Steel Bars in Concrete under Marine Environment. Front. Mater. 2022, 8, 821716. [Google Scholar] [CrossRef]
- Guaygua, B.; Sánchez-Garrido, A.J.; Yepes, V. A systematic review of seismic-resistant precast concrete buildings. Structures 2023, 58, 105598. [Google Scholar] [CrossRef]
- Carrara, P.; Lorenzis, L.D. Consistent identification of the interfacial transition zone in simulated cement microstructures. Cem. Concr. Compos. 2017, 80, 224–234. [Google Scholar] [CrossRef]
- Semendary, A.A.; Svecova, D. Factors affecting bond between precast concrete and cast in place ultra high performance concrete (UHPC). Eng. Struct. 2020, 216, 110746. [Google Scholar] [CrossRef]
- Emmons, P.H.; Vaysburd, A.M.; Mcdonald, J.E. Concrete repair in the future turn of the century-Any problem? Concr. Int. 1994, 16, 42–49. [Google Scholar] [CrossRef]
- Qin, R.; Hao, H.; Rousakis, T.C.; Lau, D. Effect of shrinkage reducing admixture on new-to-old concrete interface. Compos. Part B Eng. 2019, 167, 346–355. [Google Scholar] [CrossRef]
- Saito, M.; Ishimori, H. Chloride permeability of concrete under static and repeated compressive loading. Cem. Concr. Res. 1995, 25, 803–808. [Google Scholar] [CrossRef]
- ASTM C-876-22b; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshehoken, PA, USA, 2022. [CrossRef]
- Mundra, S.; Samson, G.; Masi, G.; Achenbach, R.; Bastidas, D.M.; Bernal, S.A.; Bignozzi, M.C.; Criado, M.; Cyr, M.; Gartner, N.; et al. Application of electrochemical methods for studying steel corrosion in alkali-activated materials. Mater. Corros. 2023, 74, 988–1008. [Google Scholar] [CrossRef]
- Chai, W.; Li, W.; Ba, H. Experimental Study on Predicting Service Life of Concrete in the Marine Environment. Open Civ. Eng. J. 2011, 5, 93–99. [Google Scholar] [CrossRef]
- Videm, K.; Myrdal, R. Electrochemical Behavior of Steel in Concrete and Evaluation of the Corrosion Rate. Corrosion 1997, 53, 734–742. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, S.; Liu, K.; Li, T.; Yang, H. Research on the critical chloride content of reinforcement corrosion in marine concrete—A review. J. Build. Eng. 2023, 79, 107838. [Google Scholar] [CrossRef]
- Cao, Y.; Gehlen, C.; Angst, U.; Wang, L.; Wang, Z.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cem. Concr. Res. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- NT BUILD 492; Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady-State Migration Experiments. Nordtest: Espoo, Finland, 1999.
- Zhang, Q.M.; Li, Q.; Li, K.; Fan, Z.H. Quality control for concrete durability when subjected to chloride diffusion in Hong Kong-Zhuhai-Macau sea link project. Gongcheng Lixue/Eng. Mech. 2015, 32, 175–182. [Google Scholar] [CrossRef]
- Collepardi, M.; Marcialis, A.; Turriziani, R. Penetration of Chloride Ions into Cement Pastes and Concretes. J. Am. Ceram. Soc. 1972, 55, 534–535. [Google Scholar] [CrossRef]
- Sergi, G.; Yu, S.W.; Page, C.L. Diffusion of chloride and hydroxyl ions in cementitious materials exposed to a saline environment. Mag. Concr. Res. 1992, 44, 63–69. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.; De Schutter, G.; Audenaert, K.; Deng, D. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Martín-Pérez, B.; Zibara, H.; Hooton, R.D.; Thomas, M.D.A. A study of the effect of chloride binding on service life predictions. Cem. Concr. Res. 2000, 30, 1215–1223. [Google Scholar] [CrossRef]
- Safehian, M.; Ramezanianpour, A.A. Prediction of RC structure service life from field long term chloride diffusion. Comput. Concr. 2015, 15, 589–606. [Google Scholar] [CrossRef]
- Li, Q.; Li, K.; Zhou, X.G.; Zhang, Q.; Fan, Z.H. Model-based durability design of concrete structures in Hong Kong-Zhuhai-Macau sea link project. Struct. Saf. 2015, 53, 1–12. [Google Scholar] [CrossRef]
- Valipour, M.; Pargar, F.; Shekarchi, M.; Khani, S.; Moradian, M. In situ study of chloride ingress in concretes containing natural zeolite, metakaolin and silica fume exposed to various exposure conditions in a harsh marine environment. Constr. Build. Mater. 2013, 46, 63–70. [Google Scholar] [CrossRef]
- Gjørv, O.E. Durability of Concrete Structures. Arab. J. Sci. Eng. 2011, 36, 151–172. [Google Scholar] [CrossRef]













| Strength Grade | Mix Proportion/(kg·m−3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Water | Cement | Fine Aggregate | Coarse Aggregate | Fly Ash | GGBS | PCA®-IV | HME®-II | |
| C50 | 183 | 340 | 736 | 942 | 60 | 100 | 3.5 | 0 |
| C60 | 142.5 | 325 | 639 | 1135 | 50 | 50 | 3.5 | 75 |
| Joint Type | Joint 1 | Joint 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Scratched | Bent | Knocked | Untreated | Scratched | Bent | Knocked | Untreated |
| Initial corrosion time/d | 7 | 42 | 70 | 77 | 7 | 21 | 28 | 35 |
| Cs (mol/m3) | c (mm) | W (mm) | DNSSD (×10−12m2/s) | |||||
|---|---|---|---|---|---|---|---|---|
| DC50 | DC60 | DM | DCC | DCM | D0 | |||
| 400.587 | 70 | 5 | 0.395 | 0.331 | 0.229 | 1.668 | 0.879 | 2032 |
| Joint Type | Joint 1 | Joint 2 | ||||
|---|---|---|---|---|---|---|
| Treatment Method | Bent | Knocked | Untreated | Bent | Knocked | Untreated |
| (mol/m3) | 79 | 150 | 163 | 29 | 48 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Wang, H.; Xu, H.; He, T. Chloride Resistance of Assembled Bridge Piers Reinforced with Epoxy-Coated Steel Bars. Appl. Sci. 2024, 14, 6609. https://doi.org/10.3390/app14156609
Fan D, Wang H, Xu H, He T. Chloride Resistance of Assembled Bridge Piers Reinforced with Epoxy-Coated Steel Bars. Applied Sciences. 2024; 14(15):6609. https://doi.org/10.3390/app14156609
Chicago/Turabian StyleFan, Dazhang, Hailong Wang, Hongquan Xu, and Tingquan He. 2024. "Chloride Resistance of Assembled Bridge Piers Reinforced with Epoxy-Coated Steel Bars" Applied Sciences 14, no. 15: 6609. https://doi.org/10.3390/app14156609






