Iron Removal from Low-Grade Pyrophyllite Ore by Microwave Irradiation and Dry Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, and Preparation
2.2. Sample Characterization
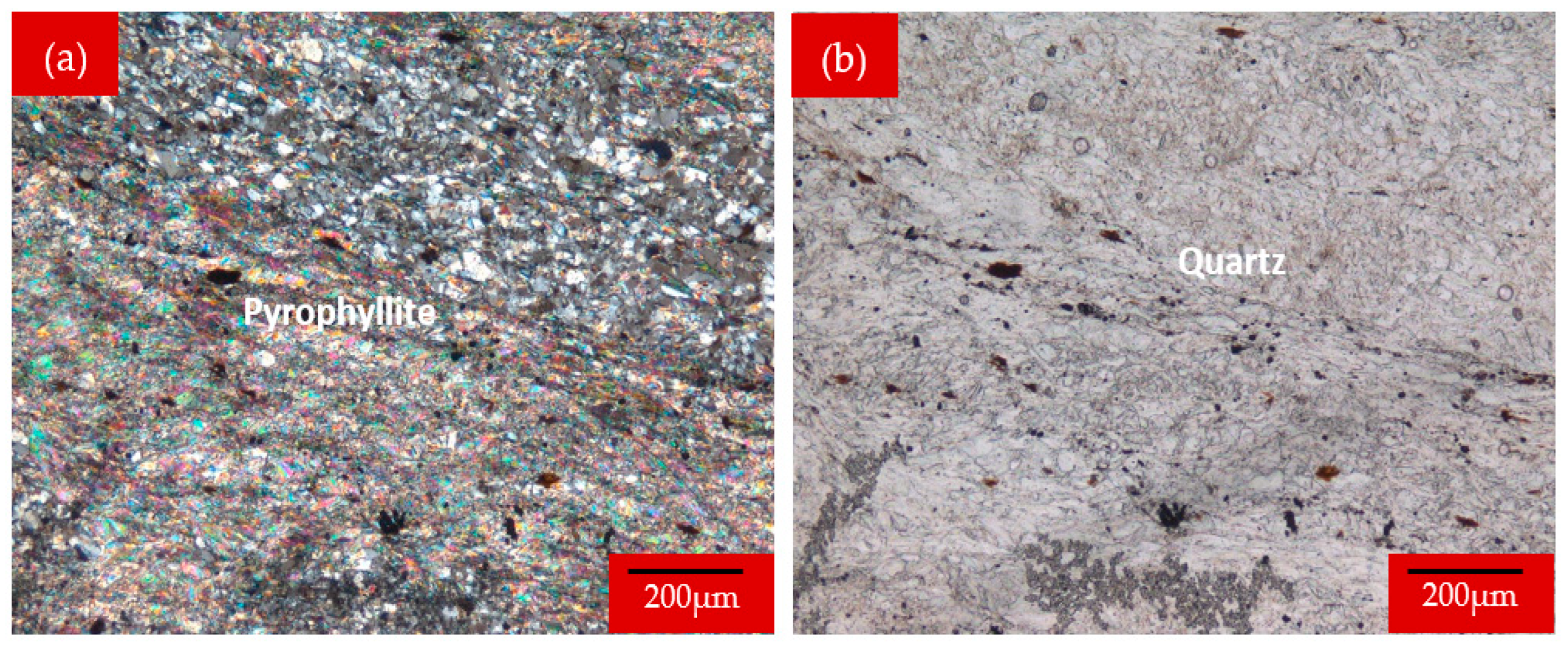
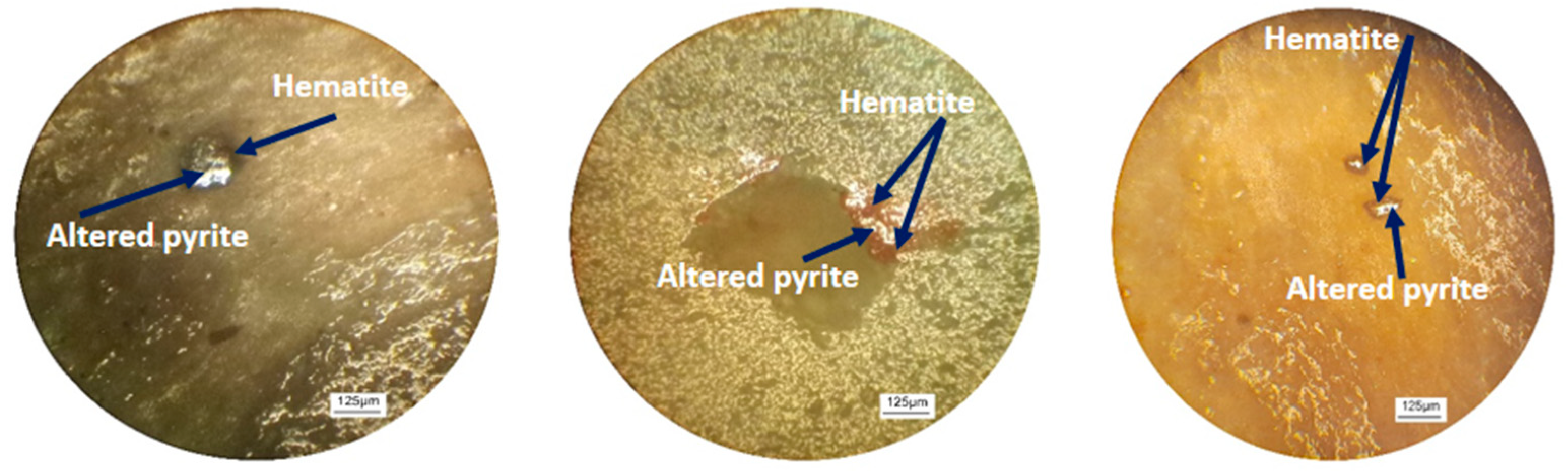
2.2.1. Microscopic Examination
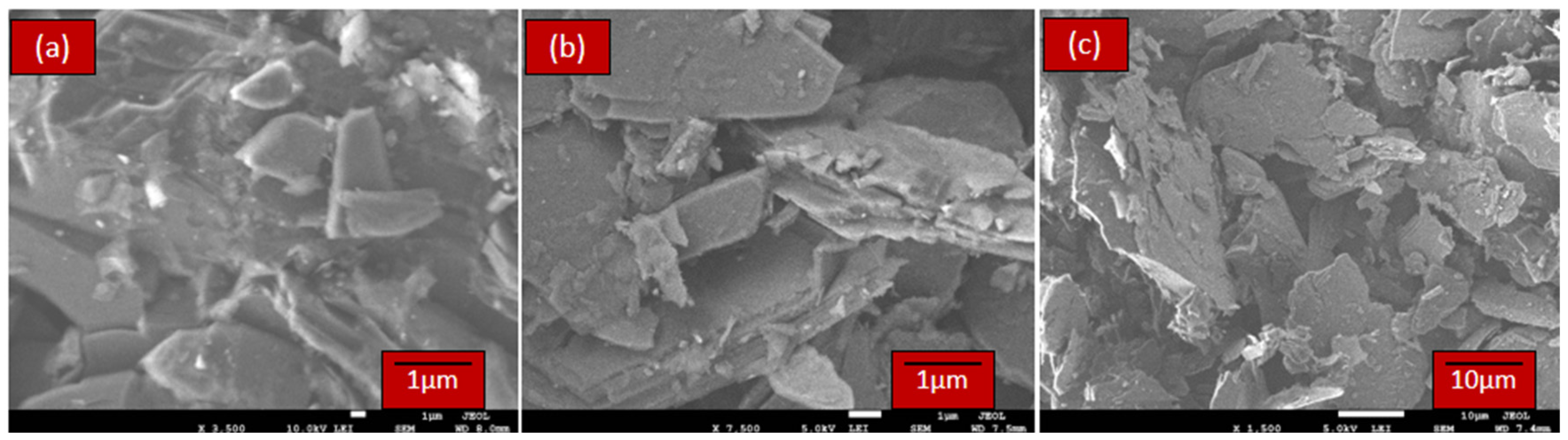
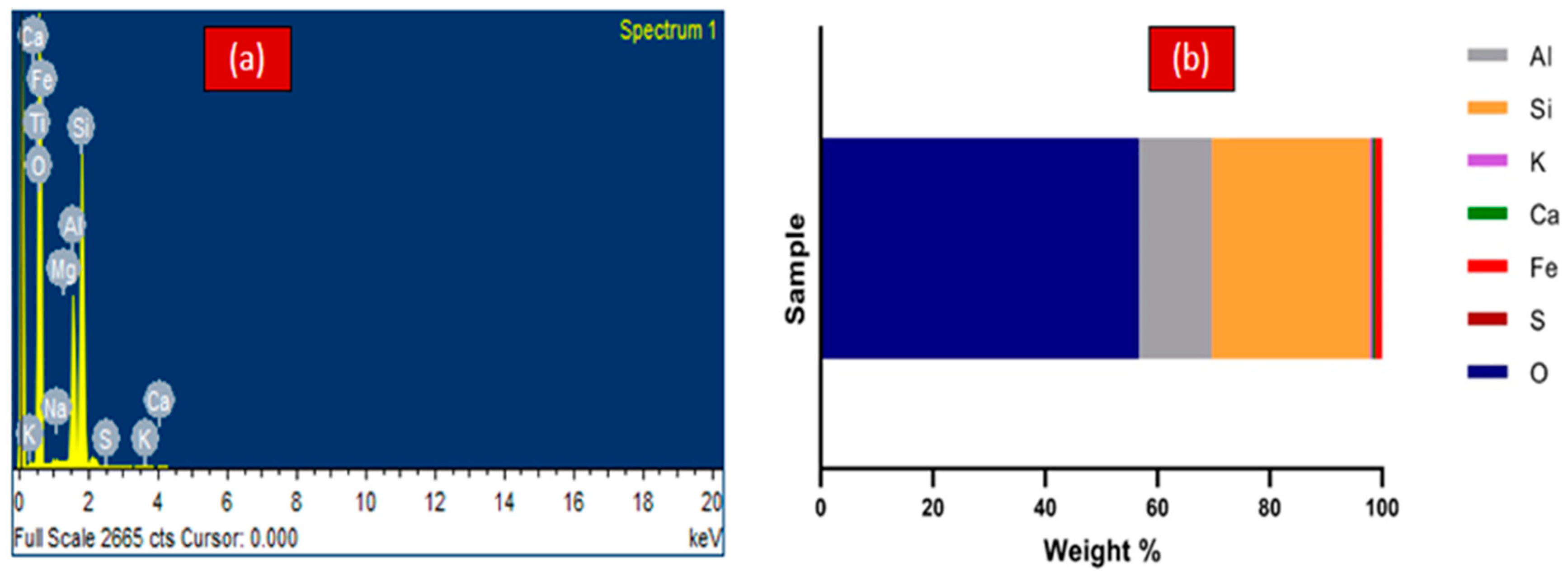
2.2.2. Scanning Electron Microscope (SEM)
2.2.3. X-ray Diffraction Analysis (XRD)
2.2.4. Chemical Analysis
2.3. Approach 1: Dry Magnetic Separation
2.4. Approach 2: Microwave Treatment Followed by Magnetic Separation
3. Results and Discussion
3.1. Characterization Results
3.1.1. Microscopic Analysis
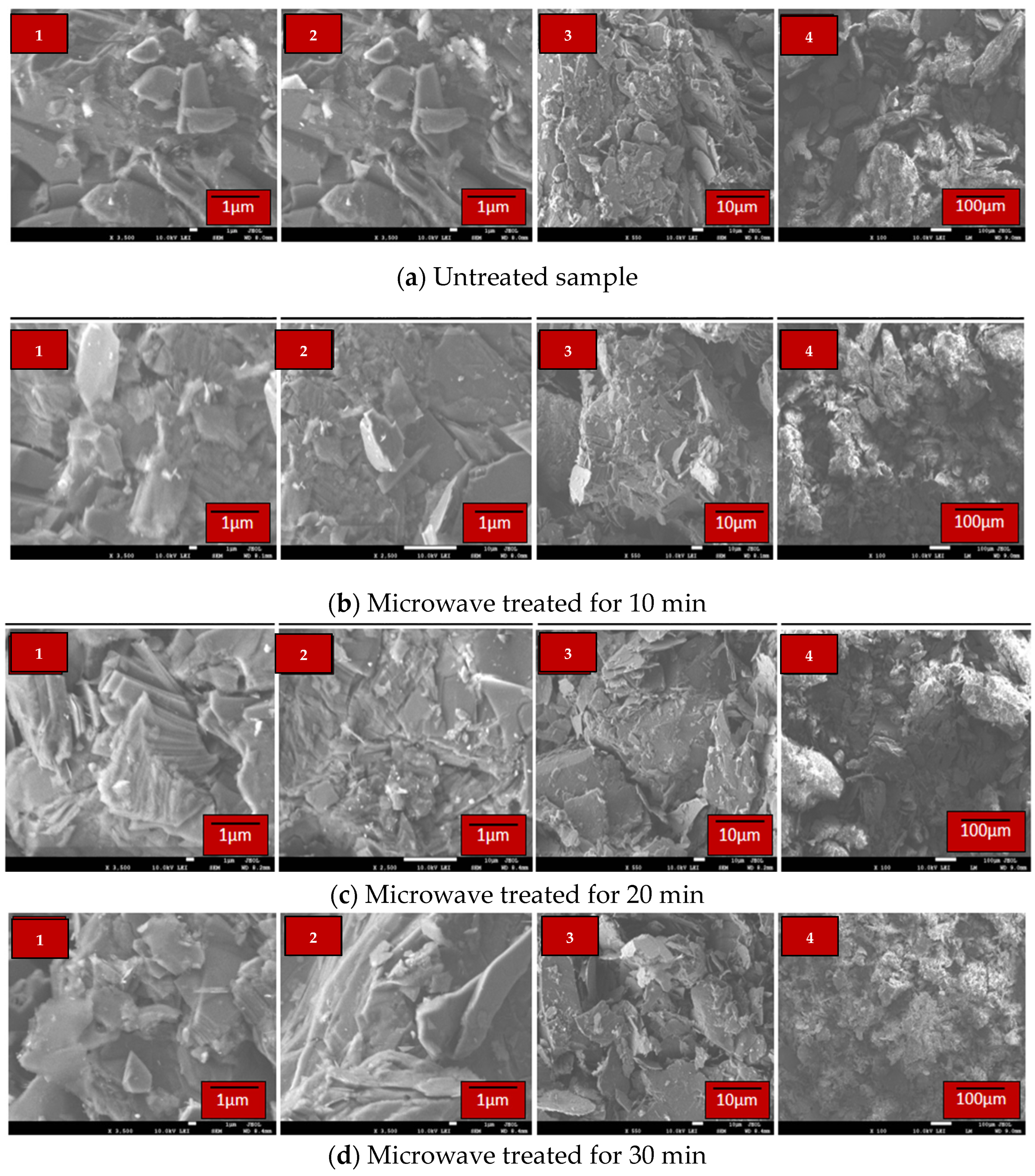
3.1.2. Scanning Electron Microscope (SEM)-EDX Characterization
3.1.3. Chemical Analysis
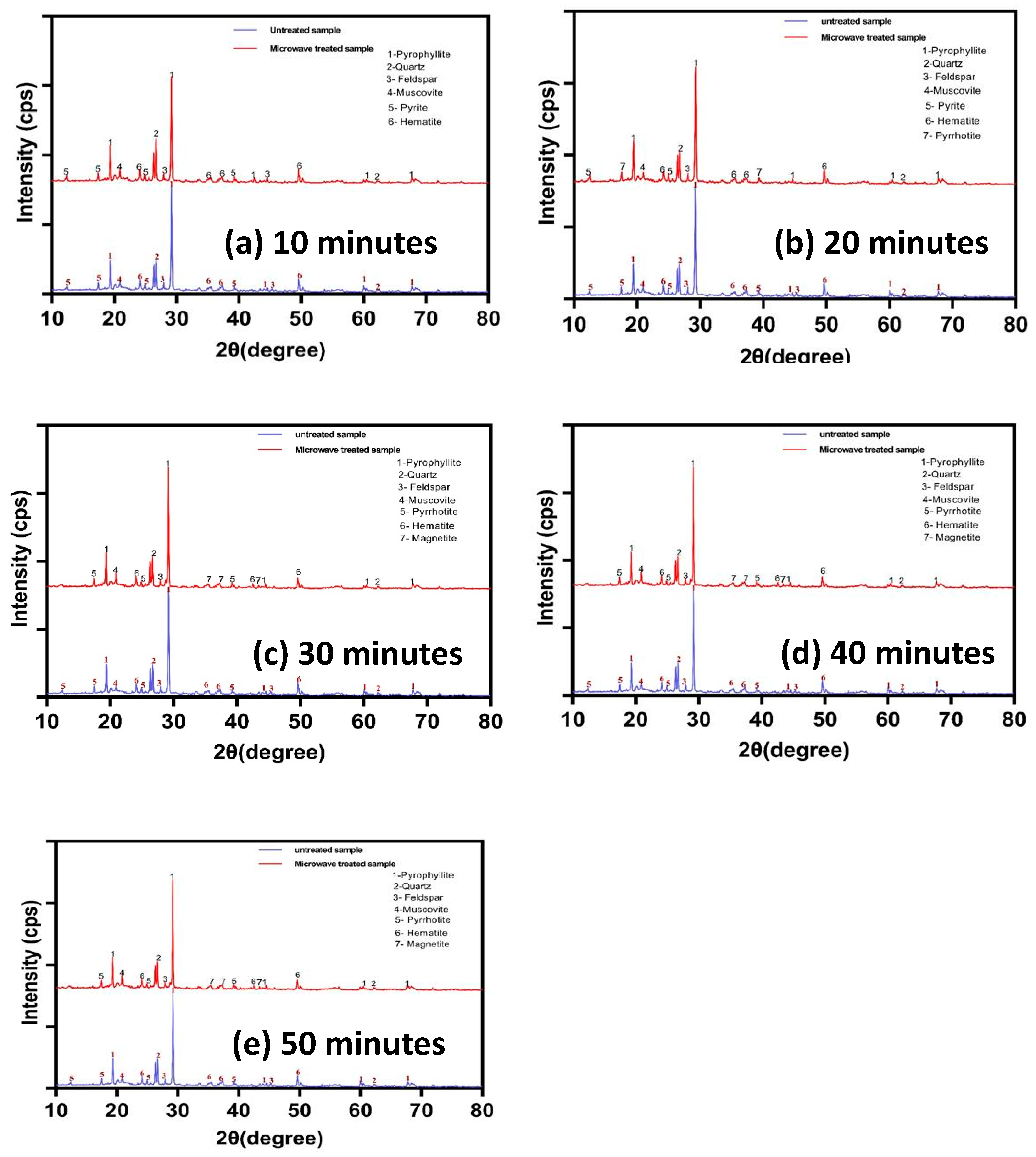
3.1.4. XRD
3.2. Dry High-Intensity Magnetic Separation (DHIMS)
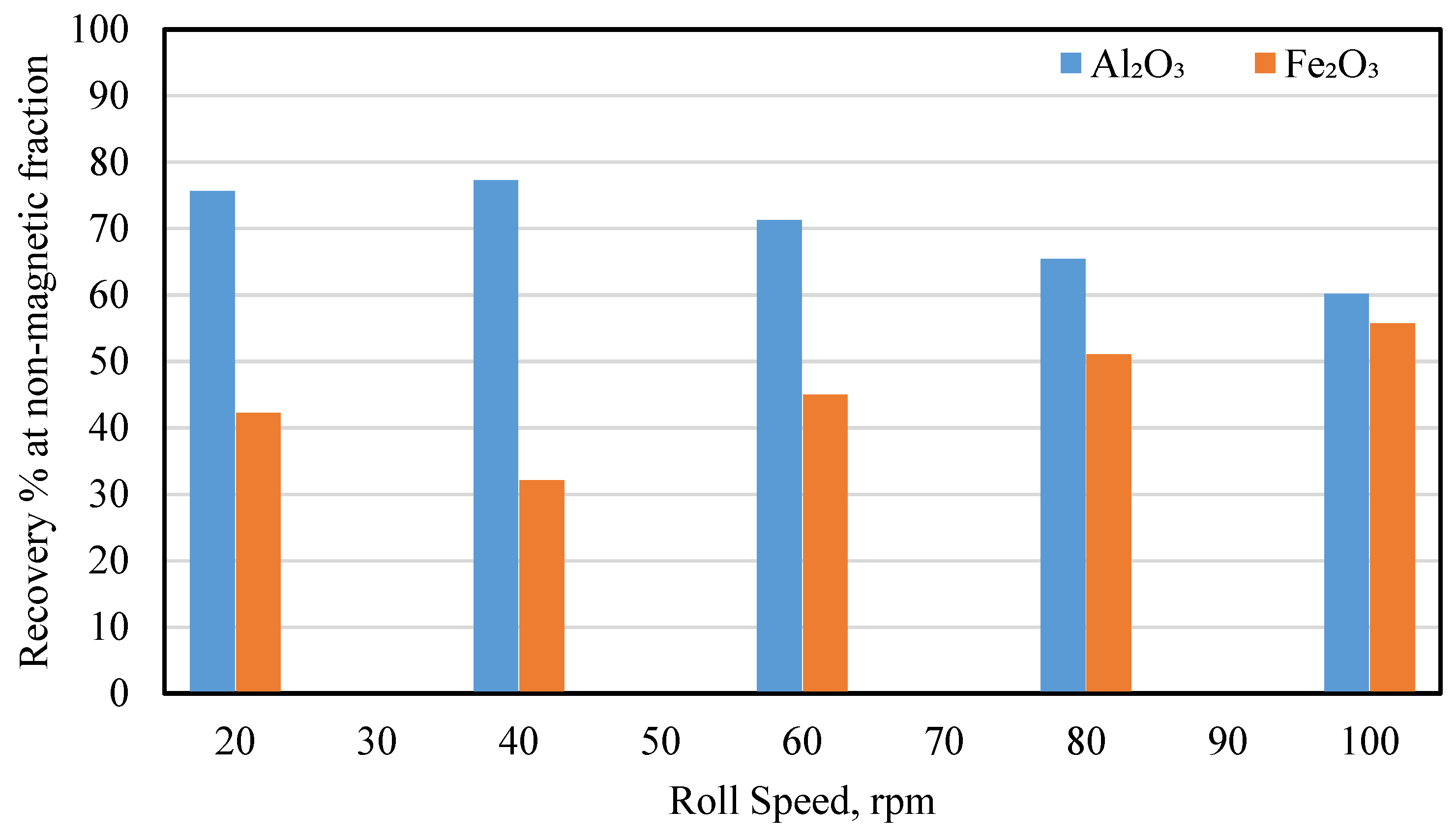
3.2.1. Effect of the Magnet Roll Speed
3.2.2. Effect of the Feed Flow Rate
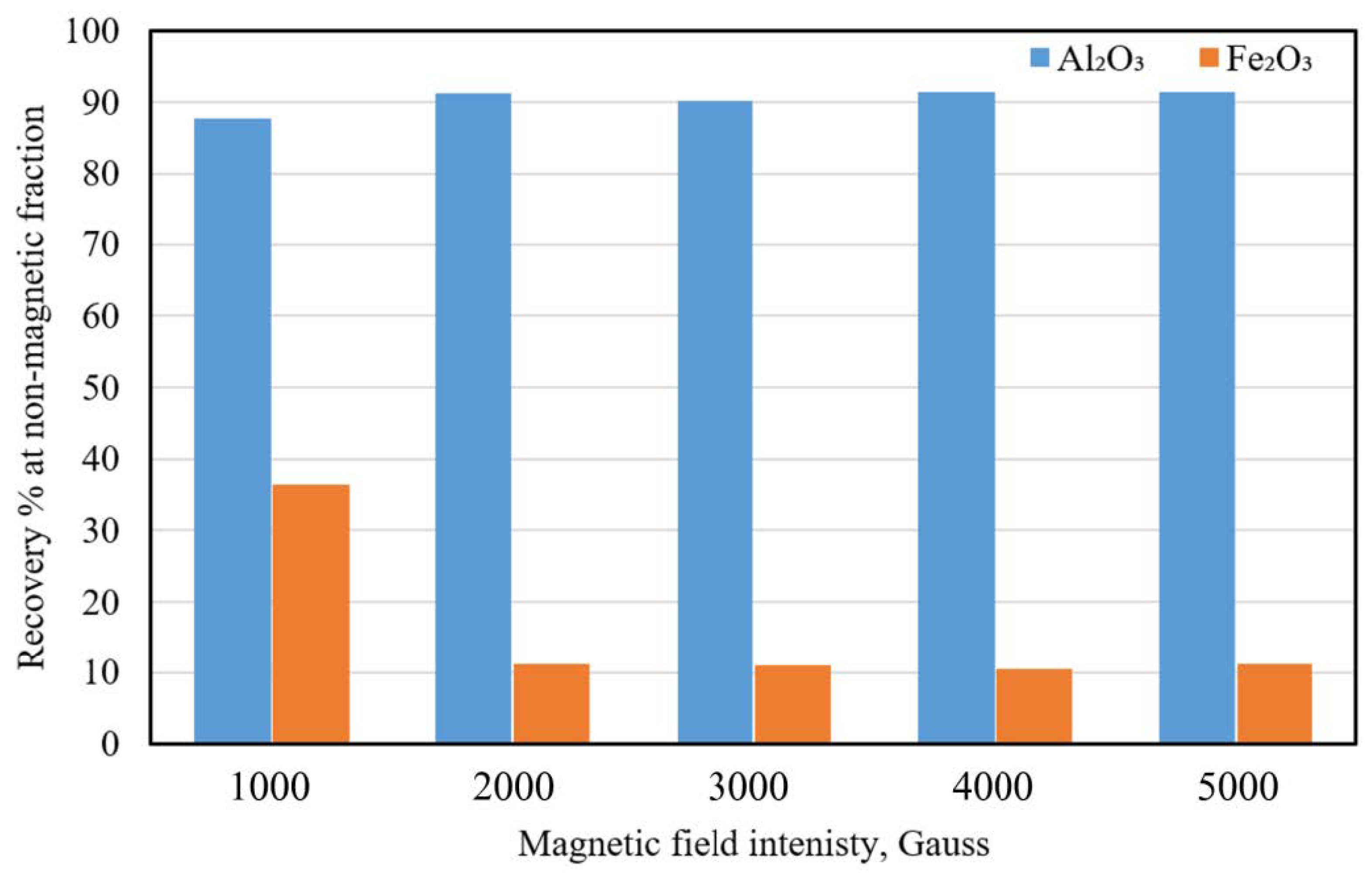
3.2.3. Effect of the Magnetic Field Intensity
3.2.4. Effect of the Particle Size
3.3. Microwave Treatment
3.3.1. Effect of Microwave Treatment on Impurities Phases
3.3.2. Effect of Microwave Irradiation on Iron Removal
Effect of Microwave Irradiation Time on Iron Removal
Effect of Magnetic Field Intensity on the Iron Removal from Microwave Irradiated Pyrophyllite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdrakhimova, E.S. Physicochemical studies of pyrophyllite of the Nikol’sk deposit. Refract. Ind. Ceram. 2010, 51, 6. [Google Scholar] [CrossRef]
- Jeong, Y.; Cho, K.; Kwon, E.E.; Tsang, Y.F.; Rinklebe, J.; Park, C. Evaluating the feasibility of pyrophyllite-based ceramic membranes for treating domestic wastewater in anaerobic ceramic membrane bioreactors. Chem. Eng. J. 2017, 328, 567–573. [Google Scholar] [CrossRef]
- Kogel, J.E.; Trivedi, N.C.; Barker, J.M.; Krukowski, S.T. Industrial Minerals & Rocks: Commodities, Markets, and Uses; SME: Littleton, CO, USA, 2006; Volume 44. [Google Scholar]
- Ali, M.A.; Ahmed, H.A.M. “A Review of Low-Grade Pyrophyllite ore Upgrading Techniques” Researchers: Abstract. Arab. J. Sci. Publ. 2021, 37, 19–31. [Google Scholar]
- Bentayeb, A.; Amouric, M.; Olives, J.; Dekayir, A.; Nadiri, A. XRD and HRTEM characterization of pyrophyllite from Morocco and its possible applications. Appl. Clay Sci. 2003, 22, 211–221. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, H.A.M.; Ahmed, H.M.; Hefni, M. Pyrophyllite: An Economic Mineral for Different Industrial Applications. Appl. Sci. 2021, 11, 11357. [Google Scholar] [CrossRef]
- González-Miranda, F.d.M.; Garzón, E.; Reca, J.; Pérez-Villarejo, L.; Martínez-Martínez, S.; Sánchez-Soto, P.J. Thermal behaviour of sericite clays as precursors of mullite materials. J. Therm. Anal. Calorim. 2018, 132, 967–977. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.; Hamza, A.I.; Dec, S.F.; Bronnimann, C.E. Solid-state 27Al and 29Si NMR and 1H CRAMPS studies of the thermal transformations of the 2:1 phyllosilicate pyrophyllite. J. Phys. Chem. 1996, 100, 17351–17360. [Google Scholar] [CrossRef]
- Zhao, X.; Ouyang, J.; Tan, Q.; Tan, X.; Yang, H. Interfacial characteristics between mineral fillers and phenolic resin in friction materials. Mater. Express 2020, 10, 70–80. [Google Scholar] [CrossRef]
- Song, S.; Wu, Z.; Nie, J. Paper fillers innovations: From design of particles to preparing filler composites. BioResources 2020, 15, 2117. [Google Scholar] [CrossRef]
- DeArmitt, C. Functional Fillers for Plastics. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- McGonigle, F.; Ciullo, P.A. Industrial Minerals and Their Uses: A Handbook and Formulary, 1st ed.; Ciullo, P.A., Ed.; Noyes Publications: Westwood, NJ, USA, 1996. [Google Scholar]
- Hasanbegović, E.; Huremović, J.; Žero, S. Adsorption capacity of nitrate from artificial fertilizers and soil on pyrophyllite. Int. J. Environ. Sci. Technol. 2021, 18, 3731–3738. [Google Scholar] [CrossRef]
- Qin, X.; Zhao, J.; Wang, J.; He, M. Atomic structure, electronic, and mechanical properties of pyrophyllite under pressure: A first-principles study. Minerals 2020, 10, 778. [Google Scholar] [CrossRef]
- Shayakhmetov, U.S.; Shayakhmetov, A.U.; Zakharov, A.V.; Khamidullin, A.R.; Gazizova, A.T. Refractory Composites Based on Pyrophyllite Raw Materials. Refract. Ind. Ceram. 2018, 59, 241–246. [Google Scholar] [CrossRef]
- Harvey, C.C.; Murray, H.H. Industrial clays in the 21st century: A perspective of exploration, technology and utilization. Appl. Clay Sci. 1997, 11, 285–310. [Google Scholar] [CrossRef]
- Decleer, J.G.M. Introduction to Industrial Minerals, 1st ed.; Chapman & Hall: London, UK, 1996; Volume 10. [Google Scholar]
- Sarikaya, M.; Depci, T.; Aydogmus, R.; Yucel, A.; Kizilkaya, N. Production of Nano Amorphous SiO2 from Malatya Pyrophyllite. IOP Conf. Ser. Earth Environ. Sci. 2016, 44, 052004. [Google Scholar] [CrossRef]
- Mansour, S.M. Use of natural pyrophyllite as cement substitution in ultra performance polypropylene fiber concrete. Int. J. Eng. Res. Africa 2021, 56, 123–135. [Google Scholar] [CrossRef]
- Ambikadevi, V.R.; Lalithambika, M. Effect of organic acids on ferric iron removal from iron-stained kaolinite. Appl. Clay Sci. 2000, 16, 133–145. [Google Scholar] [CrossRef]
- Kim, B.J.; Cho, K.H.; Chang, B.; Kim, H.S.; Lee, S.G.; Park, C.Y.; Lee, S.; Choi, N.C. Sequential microwave roasting and magnetic separation for removal of Fe and Ti impurities in low-grade pyrophyllite ore from Wando mine, South Korea. Miner. Eng. 2019, 140, 105881. [Google Scholar] [CrossRef]
- Mowla, D.; Karimi, G.; Ostadnezhad, K. Removal of hematite from silica sand ore by reverse flotation technique. Sep. Purif. Technol. 2008, 58, 419–423. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.K.; Ghatak, S.; Maiti, H.S. Pyrophyllite as raw material for ceramic applications in the perspective of its pyro-chemical properties. Ceram. Int. 2010, 36, 909–916. [Google Scholar] [CrossRef]
- Chatterjee, K.K. Uses of Industrial Minerals, Rocks and Freshwater; Nova Science Publishers: New York, NY, USA, 2009. [Google Scholar]
- Perepelitsyn, V.A.; Proshkin, V.A.; Rytvin, V.M.; Ignatenko, V.G.; Yarosh, I.A.; Abyzov, A.N. Non-traditional domestic refractory materials for aluminum metallurgy. Refract. Ind. Ceram. 2008, 49, 257–260. [Google Scholar] [CrossRef]
- Jena, S.K.; Singh, S.; Rao, D.S.; Dhawan, N.; Misra, P.K.; Das, B. Characterization and removal of iron from pyrophyllite ore for industrial applications. Miner. Metall. Process. 2015, 32, 102–110. [Google Scholar] [CrossRef]
- Kim, B.J.; Cho, K.H.; Lee, S.G.; Park, C.Y.; Choi, N.C.; Lee, S. Effective gold recovery from near-surface oxide zone using reductive microwave roasting and magnetic separation. Metals 2018, 8, 957. [Google Scholar] [CrossRef]
- Andji, J.Y.Y.; Toure, A.A.; Kra, G.; Jumas, J.C.; Yvon, J.; Blanchart, P. Iron role on mechanical properties of ceramics with clays from Ivory Coast. Ceram. Int. 2009, 35, 571–577. [Google Scholar] [CrossRef]
- Ferreira, D.P. Structural Iron Removal from Clays Used in Ceramic Industries. Master’s Thesis, University of Coimbra, Combra, Portugal, 2017. [Google Scholar]
- Cho, K.-H.; Kim, B.-J.; Choi, N.-C.; Park, C.-Y. The Mineralogical and Chemical Characteristics of Fe Impurities and the Efficiency of their Removal Using Microwave Heating and Magnetic Separation in the Pyrophyllite Ore. J. Mineral. Soc. Korea 2016, 29, 47–58. [Google Scholar] [CrossRef]
- Omran, M.; Fabritius, T.; Elmahdy, A.M.; Abdel-Khalek, N.A.; El-Aref, M.; Elmanawi, A.E.H. Effect of microwave pre-treatment on the magnetic properties of iron ore and its implications on magnetic separation. Sep. Purif. Technol. 2014, 136, 223–232. [Google Scholar] [CrossRef]
- Wiewióra, A.; Sánchez-Soto, P.J.; Avilés, M.A.; Justo, A.; Pérez-Rodríguez, J.L. Effect of dry grinding and leaching on polytypic structure of pyrophyllite. Appl. Clay Sci. 1993, 8, 261–282. [Google Scholar] [CrossRef]
- Bozkaya, Ö.; Yalçin, H.; Başibüyük, Z.; Bozkaya, G. Metamorphic-hosted pyrophyllite and dickite occurrences from the hydrous Al-silicate deposits of the Malatya-Pütürge region, central eastern Anatolia, Turkey. Clays Clay Miner. 2007, 55, 423–442. [Google Scholar] [CrossRef]
- Waanders, F.B.; Mohamed, W.; Wagner, N.J. Changes of pyrite and pyrrhotite in coal upon microwave treatment. J. Phys. Conf. Ser. 2010, 217, 012051. [Google Scholar] [CrossRef]
- Kingman, S.W. Recent developments in microwave processing of minerals. Int. Mater. Rev. 2006, 51, 1–12. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, H.A.M. Chemical and Mineralogical Characterization of Saudi-Pyrophyllite ore and its potential applications. Arab Gulf J. Sci. Res. 2022, 39, 1–18. [Google Scholar] [CrossRef]





| Constituent | SiO2 | Al2O3 | Fe2O3 | TiO2 | Na2O | K2O | CaO | Mg | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 67.72 | 25.04 | 1.4 | 0.36 | 0.14 | 0.16 | 0.31 | 0.14 | 0.13 | 4.2 |
| Feed Size (mm) | Products | Yield wt. % | Assay % | Recovery (%) | ||
|---|---|---|---|---|---|---|
| Fe2O3 | Al2O3 | Fe2O3 | Al2O3 | |||
| −0.250 + 0.045 | Conc. | 76.5 | 0.42 | 26.02 | 22.95 | 79.49 |
| Tail. | 23.5 | 4.59 | 21.85 | 77.05 | 20.51 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
| −0.125 + 0.045 | Conc. | 76.7 | 0.40 | 26.04 | 21.91 | 79.76 |
| Tail. | 23.3 | 4.69 | 21.75 | 78.09 | 20.24 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
| −0.250 + 0.075 | Conc. | 75.04 | 0.57 | 25.87 | 30.55 | 77.52 |
| Tail. | 24.96 | 3.90 | 22.55 | 69.45 | 22.48 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
| −0.125 + 0.075 | Conc. | 76.56 | 0.46 | 25.98 | 25.16 | 79.43 |
| Tail. | 23.44 | 4.47 | 21.97 | 74.84 | 20.57 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
| −0.075 + 0.045 | Conc. | 77 | 0.51 | 25.93 | 28.05 | 79.74 |
| Tail. | 23 | 4.38 | 22.06 | 71.95 | 20.26 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
| −0.045 | Conc. | 78 | 0.61 | 25.83 | 33.99 | 80.47 |
| Tail. | 22 | 4.2 | 22.23 | 66.01 | 19.53 | |
| Total | 100 | 1.40 | 25.04 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.A.M.; Khairy, N.; Ali, M.A. Iron Removal from Low-Grade Pyrophyllite Ore by Microwave Irradiation and Dry Magnetic Separation. Appl. Sci. 2024, 14, 6651. https://doi.org/10.3390/app14156651
Ahmed HAM, Khairy N, Ali MA. Iron Removal from Low-Grade Pyrophyllite Ore by Microwave Irradiation and Dry Magnetic Separation. Applied Sciences. 2024; 14(15):6651. https://doi.org/10.3390/app14156651
Chicago/Turabian StyleAhmed, Hussin A. M., Nesren Khairy, and Maaz A. Ali. 2024. "Iron Removal from Low-Grade Pyrophyllite Ore by Microwave Irradiation and Dry Magnetic Separation" Applied Sciences 14, no. 15: 6651. https://doi.org/10.3390/app14156651





