Performance Evaluation and Mechanism Study of Solid Waste-Based Cementitious Materials for Solidifying Marine Soft Soil under Seawater Mixing and Erosion Action
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Trial Soil
2.1.2. Solid Waste-Based Cementitious Materials
2.2. Proportions
2.3. Experimental Methodology
2.3.1. Preparation of Seawater Solutions
2.3.2. Preparation and Basic Performance Testing of Stabilized Soft Soil Samples
- (1)
- Preparation of stabilized soil and cement soil samples
- (2)
- Compressive strength test
- (3)
- Water resistance test
- (4)
- Seawater erosion resistance test
2.3.3. Micro Analysis Test
- (1)
- X-ray Diffraction (XRD)
- (2)
- Scanning Electron Microscopy (SEM)
- (3)
- Thermogravimetric Analysis (TG)
- (4)
- Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Optimization of Soft Soil Stabilization Material Mix Proportions
3.2. Influence of Seawater Mixing on Stabilized Soft Soil
3.3. Test on the Durability of Stabilized Soft Soil
3.3.1. Water Resistance Test
3.3.2. Seawater Erosion Resistance Test
3.4. Analysis of the Mechanism of Curing Soft Soil
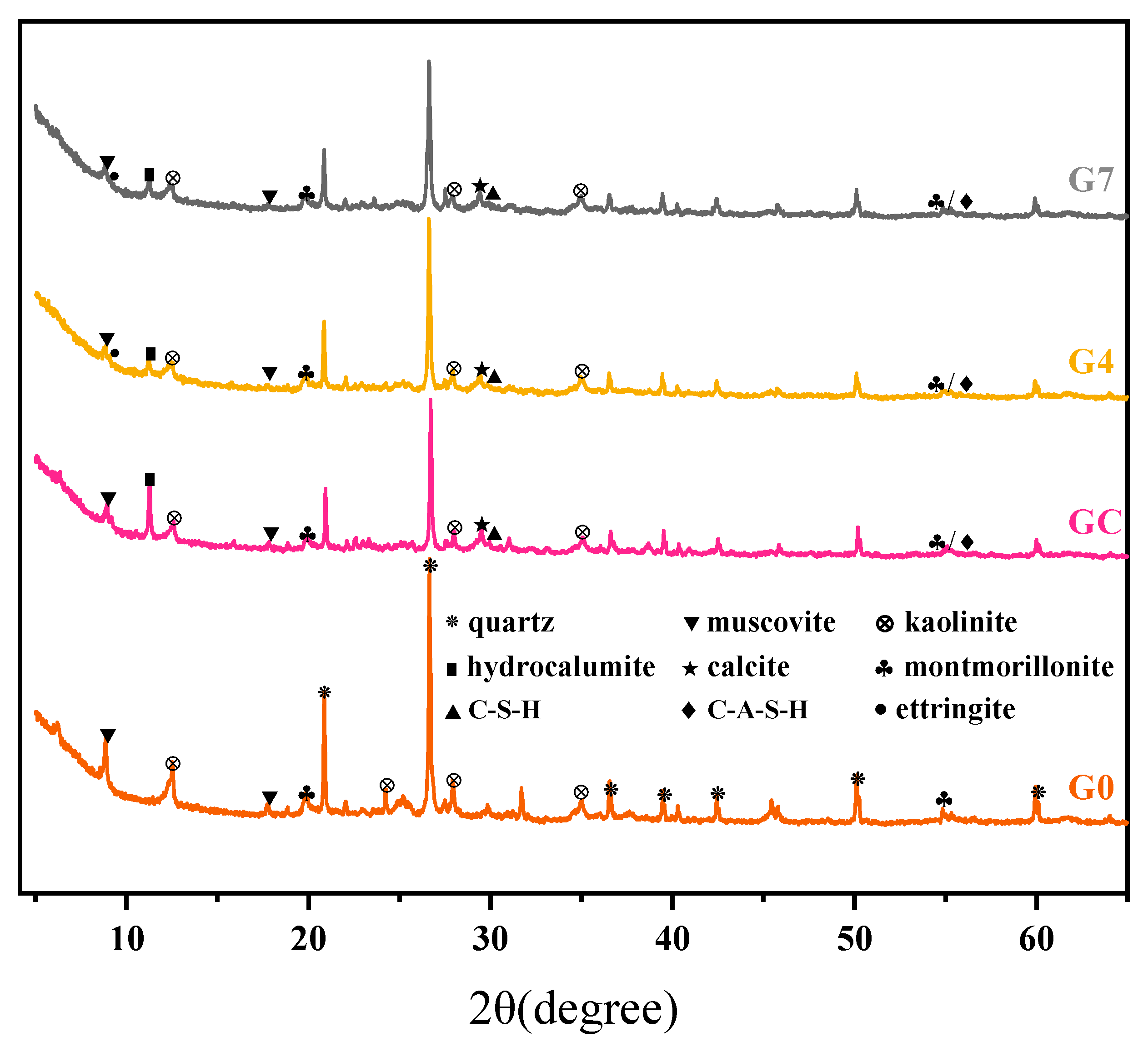
3.4.1. XRD Analysis
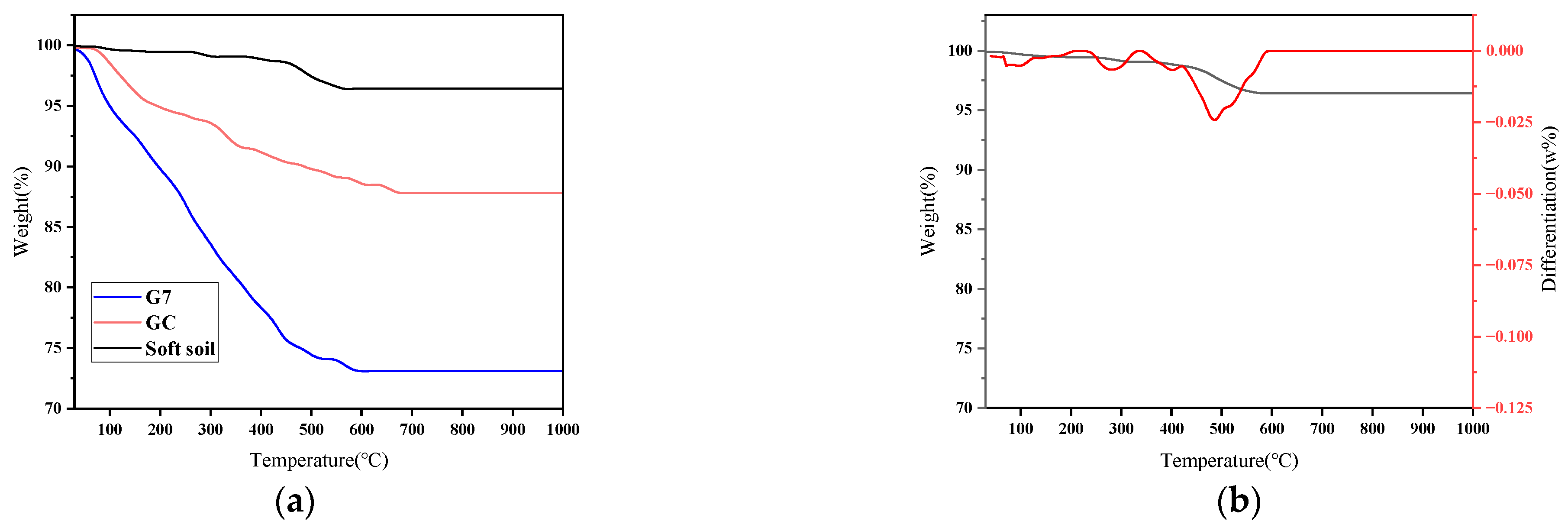
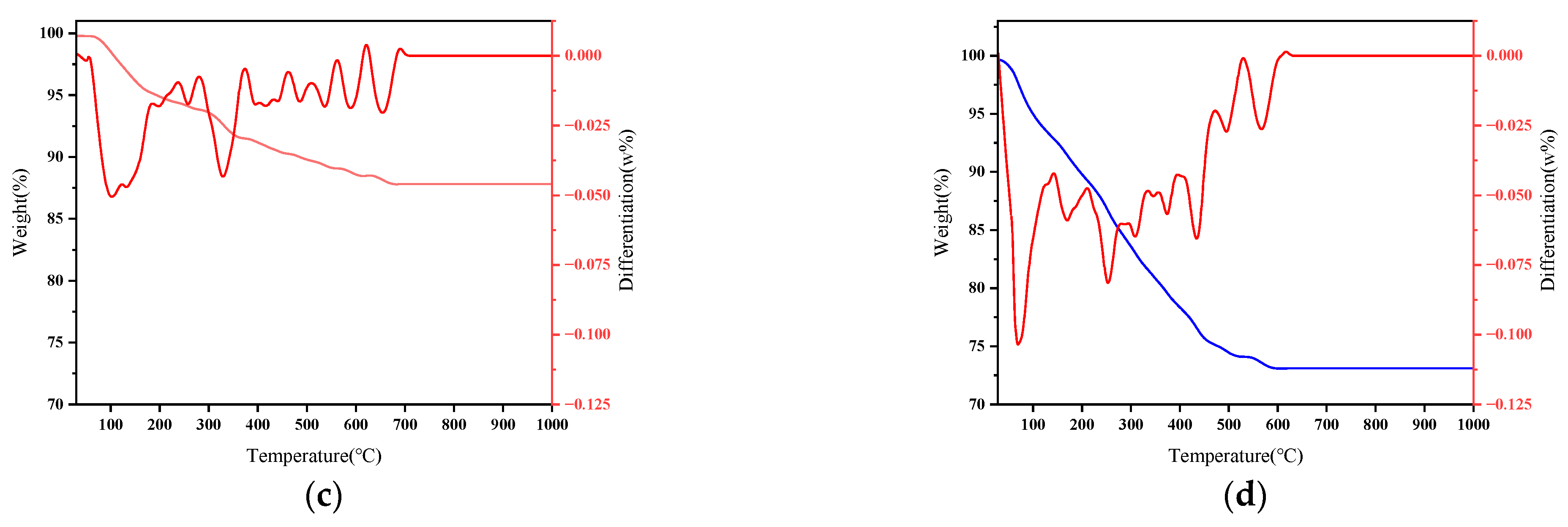
3.4.2. Thermogravimetric Analysis
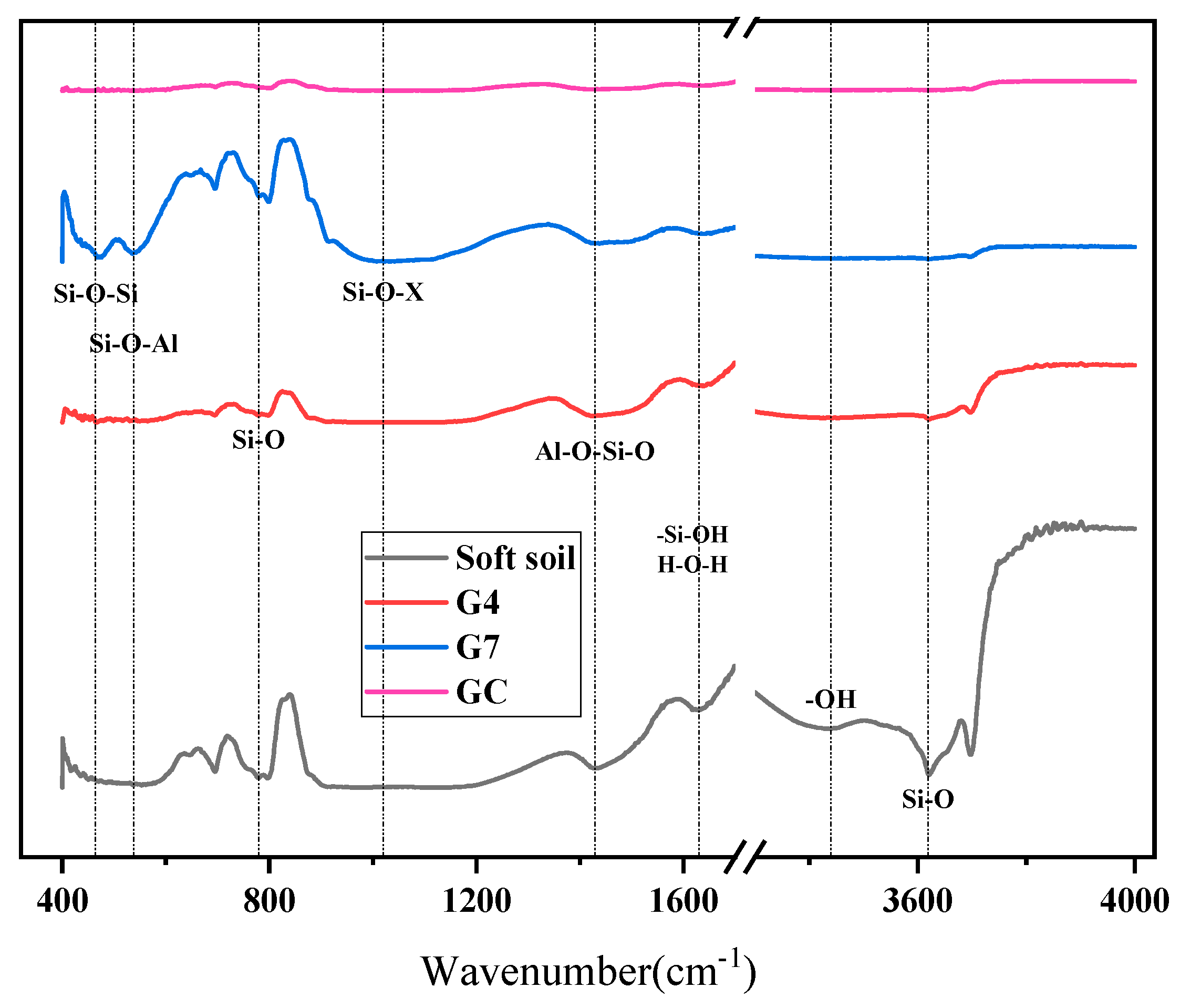
3.4.3. FTIR Analysis
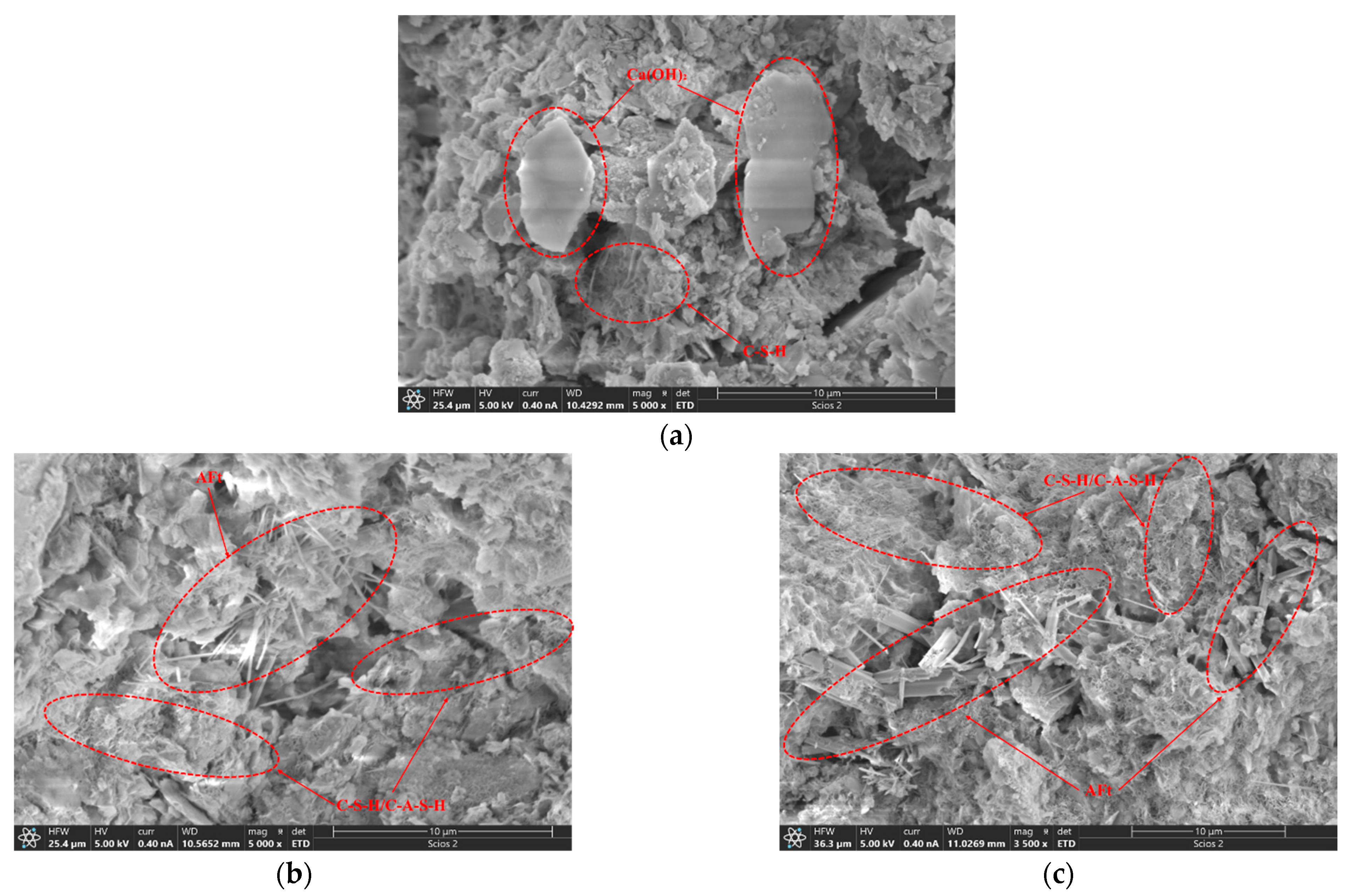
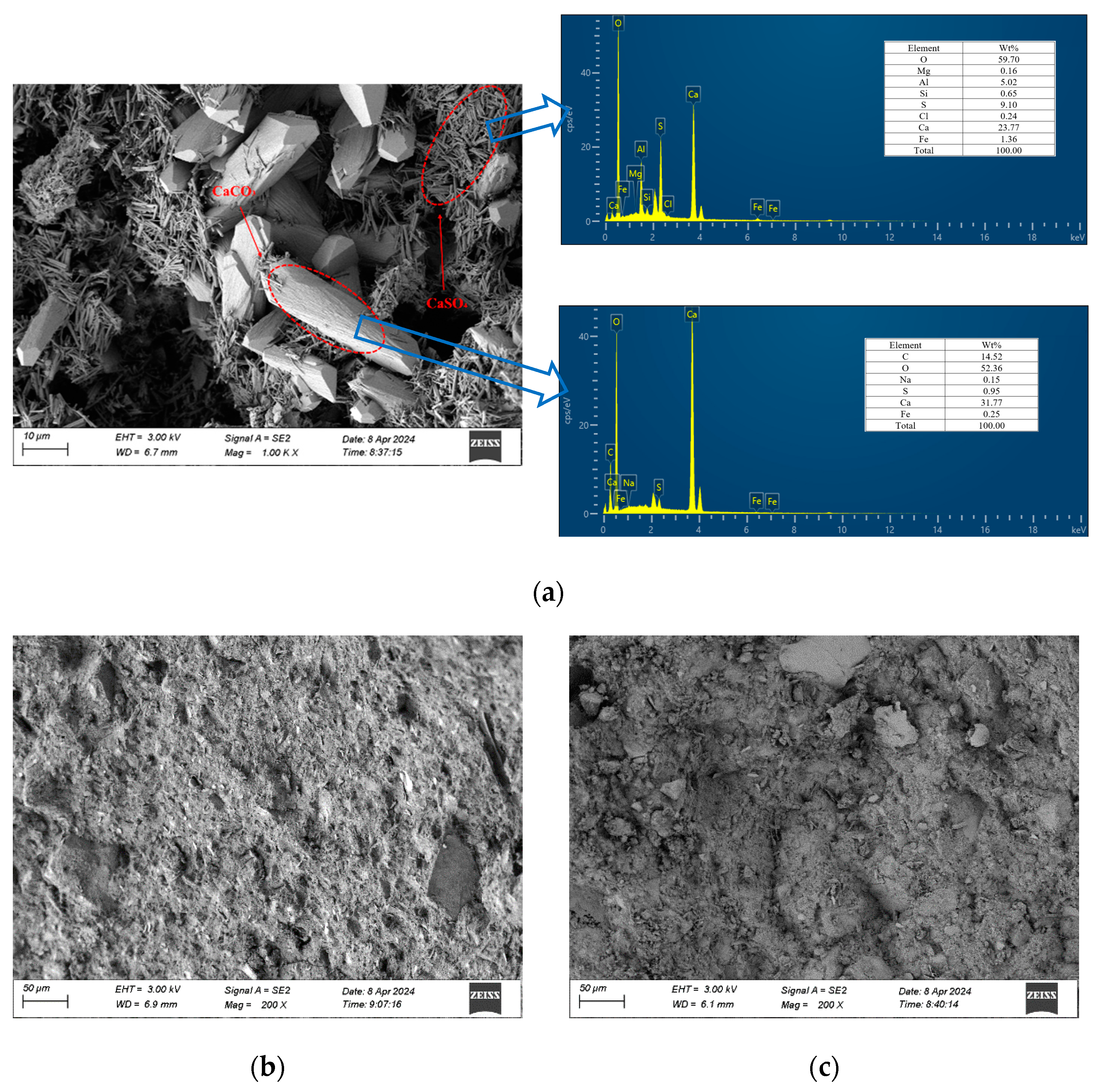
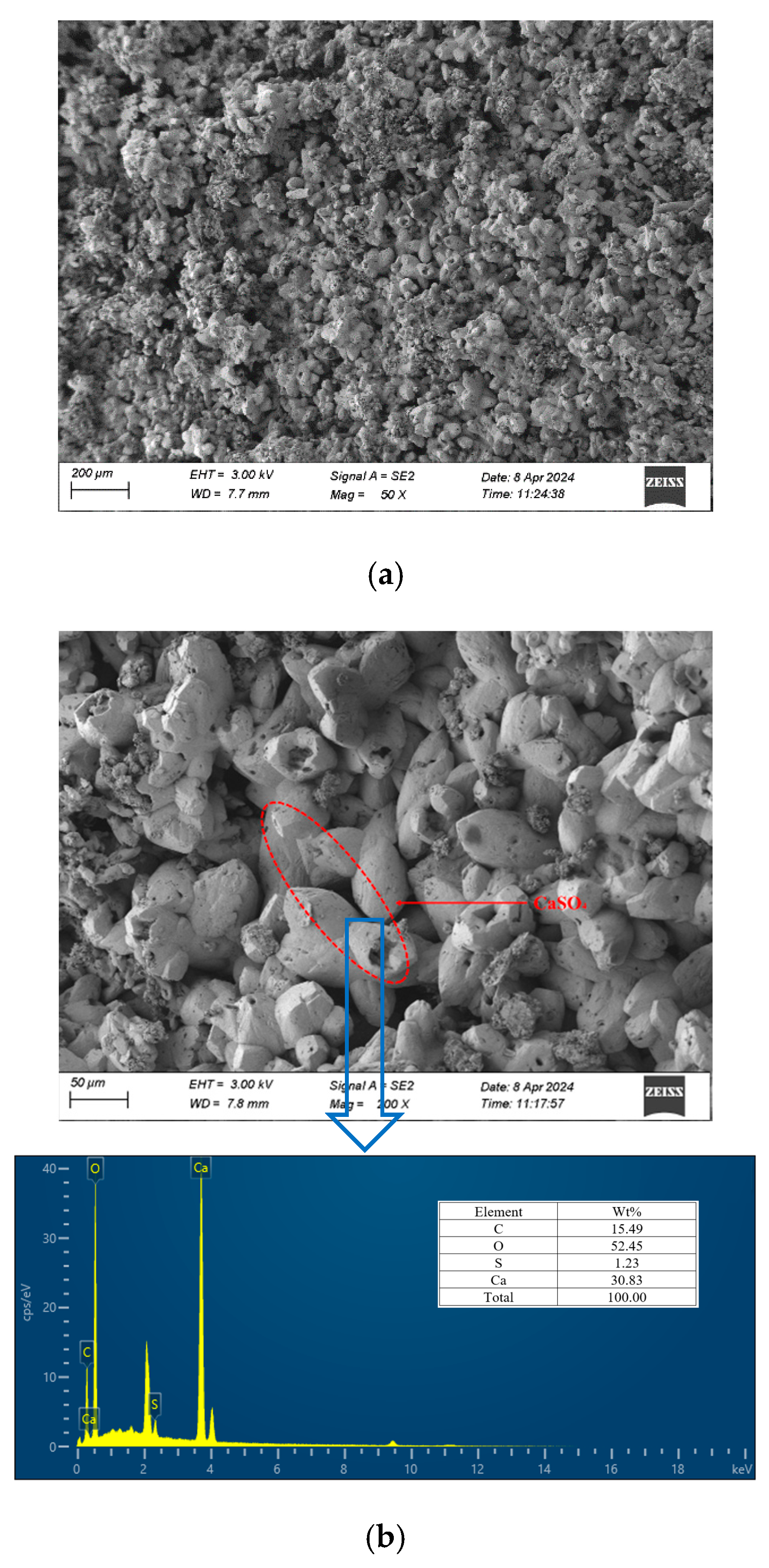
3.4.4. SEM/EDS Analysis
4. Conclusions
- (1)
- The optimized formulations G4 and G7 had higher early and late compressive strengths compared to GC. G4 showed rapid early strength, with a 7-day compressive strength of 4.92 MPa, while G7 had superior late strength, reaching 5.97 MPa at 28 days. At 30‰ salinity, the 28-day compressive strengths were 2.64 MPa for GC, 5.91 MPa for G4, and 5.97 MPa for G7. G4 and G7 consistently outperformed GC, indicating the feasibility of seawater mixing.
- (2)
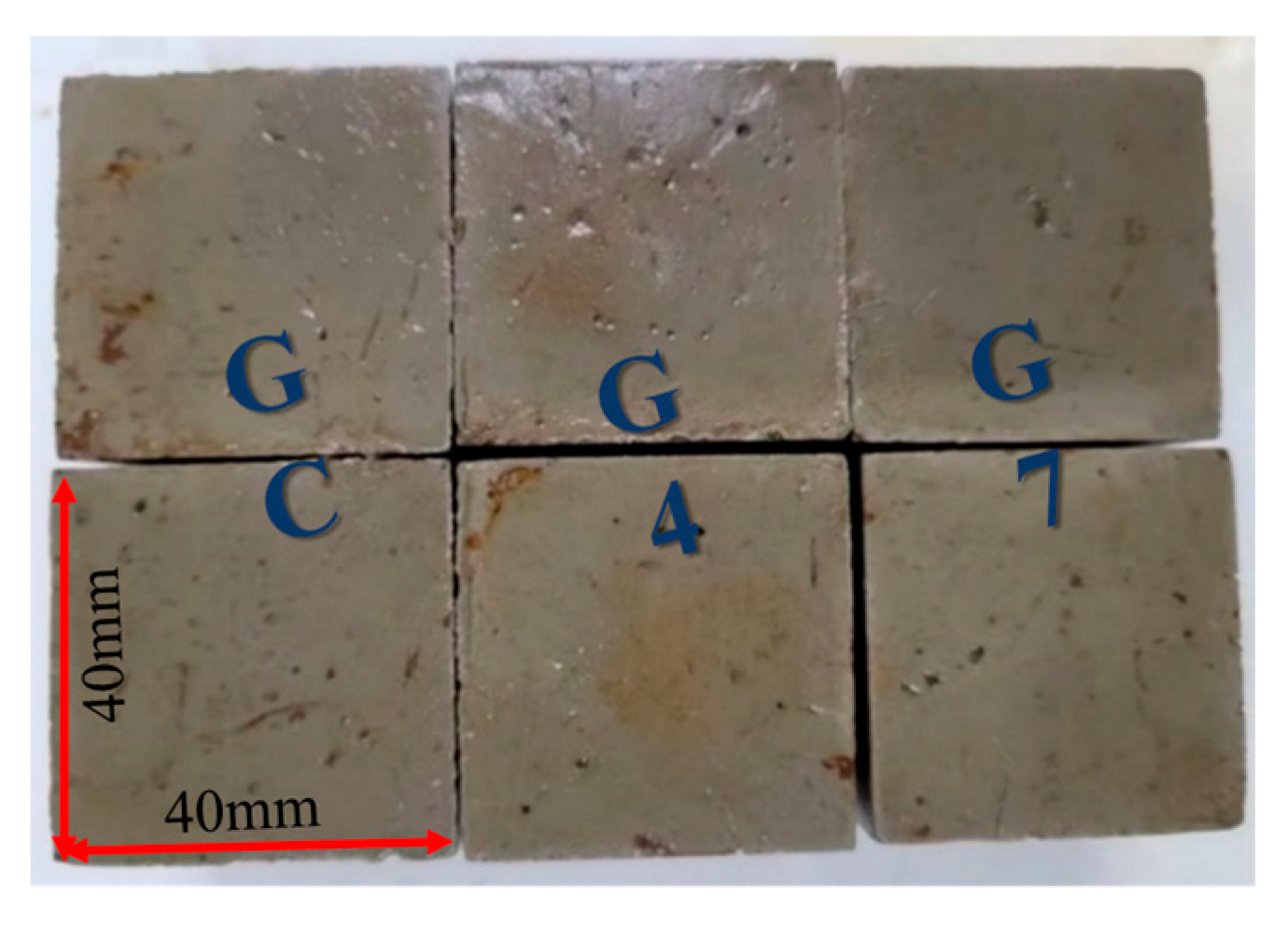
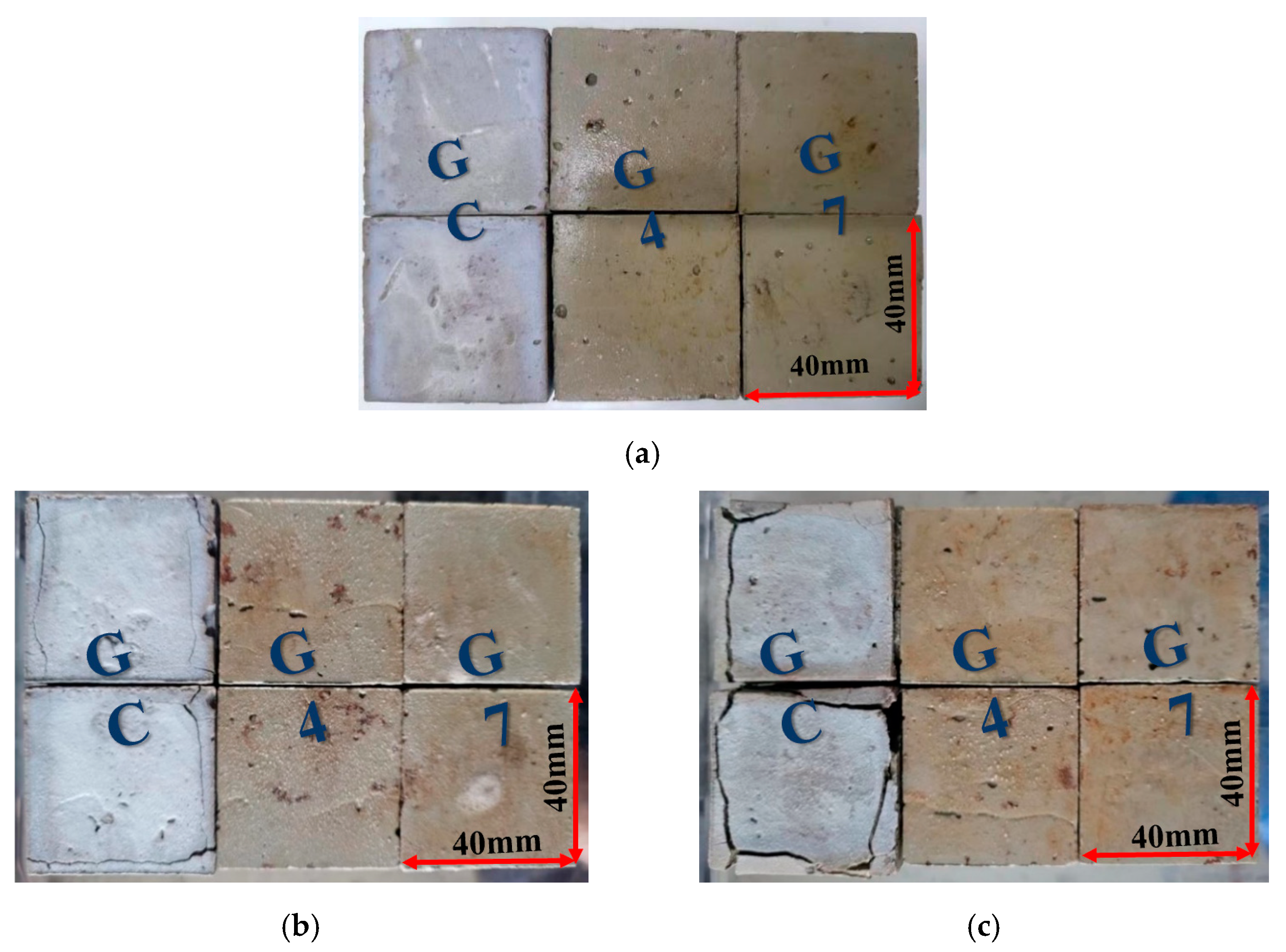
- When GC, G4, and G7 were immersed in pure water and seawater, it was observed that the stability of cement soil and stabilized soil in pure water was good. However, the resistance of cement soil GC to seawater corrosion was significantly worse than that of G4 and G7, with corrosion resistance coefficients of 66.97%, 90.48%, and 91.41% after 60 days of immersion, respectively. The surface of GC was covered with white crystals and exhibited many cracks, while the stabilized soil samples remained intact. This indicates that the stabilized soil has better resistance to seawater corrosion.
- (3)
- The hydration products in cement soil with seawater as mixing water were primarily calcium hydroxide (CH) and a small amount of C-S-H gel, with some Friedel salts present, which have low gel-forming capability. Existing studies indicate that CH and Friedel salts contribute little to the strength of the soil, which explains the low strength of cement soil.
- (4)
- In the stabilized soil, a significant amount of C-A-S-H, which has gel-forming properties, and AFt, which has a filling effect, were observed. Compared to the small amount of C-S-H in cement soil, the stabilized soil contained a substantial amount of C-A-S-H. It is generally accepted that C-A-S-H has a higher adsorption capacity for Cl− and SO42− than C-S-H, which reduces the concentration of Cl− and SO42− in the reaction solution. This effectively slows down the inhibition of the hydration reaction and the structural damage caused by crystal precipitation.
- (5)
- The analysis of the cement soil and stabilized soil samples after seawater immersion confirmed the presence of calcium carbonate and calcium sulfate on the surface and inside the cement soil sample, leading to the expansion and cracking of the structure. In contrast, no such issues were observed in the stabilized soil samples.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jia, M.; Wang, Z.; Mao, D.; Ren, C.; Wang, C.; Wang, Y. Rapid, robust, and automated mapping of tidal flats in China using time series Sentinel-2 images and Google Earth Engine. Remote Sens. Environ. 2021, 255, 112285. [Google Scholar] [CrossRef]
- Lei, H.Y.; Wang, L.; Zhang, W.D.; Jiang, M.J.; Bo, Y.; Song, W.F.; Cao, Q.G. Geotechnical properties of the South China Sea soft soil: A comparative study with the soils from Bohai Sea and Yellow Sea. Bull. Eng. Geol. Environ. 2023, 82, 13. [Google Scholar] [CrossRef]
- Hu, H.; Han, L. Evaluation of Land Carrying Capacity of 31 Provinces in China Based on a Natural–Societal-Supply–Demand Framework. Sustainability 2023, 15, 1037. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, Q.; Chen, S.; Dai, R.; Gao, J.; Li, P. Research progress of soft soil foundation treatment technology. Earth Environ. Sci. 2020, 455, 012081. [Google Scholar] [CrossRef]
- Ren, Q. Comparison and Cost Analysis of Soft Soil Foundation Treatment Schemes in Port Construction. Adv. Civ. Eng. 2022, 2022, 6442750. [Google Scholar] [CrossRef]
- Heo, K.K.; Ahn, B.C.; Min, B.U. Analysis on the Safety of Structure and Economics of Replacement Method Using Rock Debris in the Soft Ground—Case Study of Miho Stream Crossing Road in Cheongju City. J. Korean Soc. Civ. Eng. 2016, 36, 705–713. [Google Scholar]
- Hassan, I.; Mohamedelhassan, E. Improving the Characteristics of a Lean Clay by Electrokinetic Treatment. Int. J. Civ. Eng. 2021, 19, 911–922. [Google Scholar] [CrossRef]
- Cristovao, A.; Figueres, M.; Pinto, A.; Rosa, P. Case Study: Preliminary Field Testing as a Basis of Design for Ground Improvement Using Vibrocompaction at Lomé Container Terminal—Togo. Procedia Eng. 2016, 143, 1451–1459. [Google Scholar] [CrossRef]
- Chen, F.; Li, H.T. Construction technology of cement deep mixing piles in Huanghua Port region. Chin. J. Geotech. Eng. 2015, 37, 156–160. [Google Scholar]
- Zhang, Y.P.; Wang, Z.L.; Zheng, Y.F. Research and Application of Soil Solidifying Agent. E3S Web Conf. 2020, 165, 03026. [Google Scholar]
- Xu, J.; Chen, X.; Yang, G.; Niu, X.; Chang, F.; Lacidogna, G. Review of research on micromechanical properties of cement-based materials based on molecular dynamics simulation. Constr. Build. Mater. 2021, 312, 125389. [Google Scholar] [CrossRef]
- Solihu, H. Cement Soil Stabilization as an Improvement Technique for Rail Track Subgrade, and Highway Subbase and Base Courses: A Review. J. Civ. Environ. Eng. 2020, 10, 2020. [Google Scholar] [CrossRef]
- James, J.; Pandian, P.K. Industrial Wastes as Auxiliary Additives to Cement/Lime Stabilization of Soils. Adv. Civ. Eng. 2016, 2016, 1267391. [Google Scholar] [CrossRef]
- Verma, H.; Ray, A.; Rai, R.; Gupta, T.; Mehta, N. Ground improvement using chemical methods: A review. Heliyon 2021, 7, E07678. [Google Scholar] [CrossRef] [PubMed]
- Murmu, A.L.; Jain, A.; Patel, A. Mechanical Properties of Alkali Activated Fly Ash Geopolymer Stabilized Expansive Clay. KSCE J. Civ. Eng. 2019, 23, 3875–3888. [Google Scholar] [CrossRef]
- Wang, X.S.; Kim, S.; Wu, Y.P.; Liu, Y.; Liu, T.Y.; Wang, Y.M. Study on the optimization and performance of GFC soil stabilizer based on response surface methodology in soft soil stabilization. Soils Found. 2023, 63, 13. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.J.; Liu, M.Y.; Cai, L.; Wei, N.; Liu, Y.J. Microanalytical characterizations, mechanical strength and water resistance performance of solidified dredged sludge with industrial solid waste and architecture residue soil. Case Stud. Constr. Mater. 2022, 17, 13. [Google Scholar] [CrossRef]
- Yao, J.L.; Qiu, H.J.; He, H.; Chen, X.; Hao, G.Y. Application of a Soft Soil Stabilized by Composite Geopolymer. J. Perform. Constr. Facil. 2021, 35, 11. [Google Scholar] [CrossRef]
- Huang, Q.H.; Yang, G.H.; Li, C.Z.; Guo, M.Z.; Wang, T.; Jiang, L.H. Use of Alkali-Activated Slag as an Environment-Friendly Agent for High-Performance Stabilized Soil. Materials 2023, 16, 16. [Google Scholar] [CrossRef]
- Jiang, N.J.; Du, Y.J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]
- Li, H.; Yang, M. Study on unconfined compressive strength and deformation characteristics of chlorine saline soil. Sci. Rep. 2024, 14, 1478. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chai, S.; Guo, Q.; Wang, P.; Li, F. Mechanical properties and stabilizing mechanism of stabilized saline soils with four stabilizers. Bull. Eng. Geol. Environ. 2020, 79, 5341–5354. [Google Scholar] [CrossRef]
- Su, W. Experimental Study on Fluid Solidified Saline Soil of Cement-AASF Osite Iron Tailings Sand; Shenyang University of Technology: Shenyang, China, 2023. [Google Scholar]
- Jun, W. Interaction between Hydration Products and Clay Minerals and Constituent Design Methodology of IBPs-Based Cementitious Materials in Stabilized Clay. Ph.D. Thesis, Southeast University, Nanjing, China, 2022. [Google Scholar]
- Jiang, X. . Microscopic Mechanism and Nonlinear Mechanical Behaviors of Inorganic Composite Stabilized Soils with Rice Husk Ash; Zhejiang University: Zhejiang, China, 2024. [Google Scholar]
- Durdziński, P.T. Hydration of Multi-Component Cements Containing Cement Clinker, Slag, Calcareous Fly Ash and Limestone; EPFL: Lausanne, Switzerland, 2016. [Google Scholar]
- Jian, G.; Haiming, Y.; Liwei, M. Effect of attack of sodium sulfate solution on the stability of bounded chloride ions. J. Build. Mater. 2015, 18, 919–925. [Google Scholar]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part II: Effect of Al2O3. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Yang, X. Research on Properties of Alkali-Activated Coal Gangue-Based Geopolymerand Solidified Saline Soil; Inner Mongolia Agricultural University: Hohhot, China, 2023. [Google Scholar]
- Fang, J. Study on Mechanical Properties and Frost Resistance of Soft Soil Solidified with Wollaston-Calcium Carbide Slag; Shaoxing College of Arts and Sciences: Shaoxing, China, 2023. [Google Scholar]
- Tian, X.; Zhang, D.; Hou, H.; Yang, Z.; Liu, H. Microstructure of weak soil stabilization slag cementing material. J. Chin. Ceram. Soc. 2006, 5, 636–640. [Google Scholar]
- Liu, Z.; Ong, D.E.L.; Wang, S.; Oh, E.; Liu, Y.X. Mineralogical and Microstructural Characterization of Cement-Stabilized Soft Soils Based on Quantitative Analyses. J. Mater. Civ. Eng. 2023, 35, 04022487. [Google Scholar] [CrossRef]
- Han, C.Z.; Shen, W.G.; Ji, X.; Wang, Z.W.; Ding, Q.J.; Xu, G.L.; Lv, Z.J.; Tang, X.L. Behavior of high performance concrete pastes with different mineral admixtures in simulated seawater environment. Constr. Build. Mater. 2018, 187, 426–438. [Google Scholar] [CrossRef]
- Feng, P.; Miao, C.W.; Bullard, J.W. Factors Influencing the Stability of AFm and AFt in the Ca-Al-S-O-H System at 25 °C. J. Am. Ceram. Soc. 2016, 99, 1031–1041. [Google Scholar] [CrossRef]







| γ (g/cm3) | LL (%) | PL (%) | W (%) | Sc (g/kg) | Oc (g/kg) | ESP (%) | e (--) | a (MPa−1) |
|---|---|---|---|---|---|---|---|---|
| 16.40 | 49.70 | 31.20 | 60.60 | 30.23 | 1.84 | 28.80 | 1.56 | 1.28 |
| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | K2O2 | MgO | Na2O | TiO2 | Other |
|---|---|---|---|---|---|---|---|---|---|
| Soft soil | 58.06 | 15.75 | 5.83 | 4.33 | 2.52 | 2.27 | 0.86 | 0.86 | 8.44 |
| Composition | SiO2 | CaO | Al2O3 | SO3 | MgO | Fe2O3 | Na2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| GGBS | 29.37 | 40.82 | 14.12 | 0.20 | 1.32 | 8.02 | 0.46 | 0.36 | 1.12 |
| ACP | 21.35 | 57.03 | 6.45 | 3.69 | 3.10 | 4.01 | 0.32 | 0.75 | 0.28 |
| DA | 8.03 | 42.94 | 4.80 | 18.43 | 0.22 | 0.49 | 0.06 | 0.25 | 16.56 |
| OPC | 27.61 | 48.98 | 4.93 | 1.85 | 2.32 | 3.39 | 0.21 | 0.47 | 3.66 |
| Sample | Cementitious Materials | Admixtures | W/C | C/S | ||||
|---|---|---|---|---|---|---|---|---|
| ACP | GGBS | DA | OPC | NS | SA | |||
| G1 | 12.5 | 62.5 | 25.0 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G2 | 25.0 | 62.5 | 12.5 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G3 | 0 | 75.0 | 25.0 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G4 | 25.0 | 75.0 | 0 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G5 | 12.5 | 75.0 | 12.5 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G6 | 25.0 | 50.0 | 25.0 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| G7 | 16.7 | 66.6 | 16.7 | / | 0.5 | 0.05 | 0.6 | 0.2 |
| GC | / | / | / | 100 | 0.5 | 0.05 | 0.6 | 0.2 |
| Sample | Compressive Strength | ||
|---|---|---|---|
| 3 d/MPa | 7 d/MPa | 28 d/MPa | |
| G1 | 1.07 | 3.06 | 4.18 |
| G2 | 2.01 | 4.03 | 5.75 |
| G3 | 0.48 | 2.93 | 5.50 |
| G4 | 3.96 | 4.92 | 5.91 |
| G5 | 2.68 | 4.38 | 4.79 |
| G6 | 1.25 | 2.85 | 4.22 |
| G7 | 2.71 | 4.61 | 5.97 |
| GC | 0.89 | 1.68 | 2.64 |
| Sample | Coefficient of Water Stability | ||
|---|---|---|---|
| Soaking 14 d | Submerged for 28 d | Submerged for 60 d | |
| GC | 105.02% | 103.88% | 114.61% |
| G4 | 113.64% | 107.32% | 119.80% |
| G7 | 108.91% | 101.72% | 112.79% |
| Sample | Coefficient of Resistance to Seawater Erosion | ||
|---|---|---|---|
| Seawater Immersion for 14 d | Seawater Immersion for 28 d | Seawater Immersion for 60 d | |
| GC | 87.39% | 86.79% | 66.97% |
| G4 | 92.47% | 92.01% | 90.48% |
| G7 | 95.34% | 94.76% | 91.41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Guan, C.; Hua, S.; Zhang, Y.; Zhang, D.; Bao, Y.; Yuan, Z. Performance Evaluation and Mechanism Study of Solid Waste-Based Cementitious Materials for Solidifying Marine Soft Soil under Seawater Mixing and Erosion Action. Appl. Sci. 2024, 14, 6666. https://doi.org/10.3390/app14156666
Zhang Z, Guan C, Hua S, Zhang Y, Zhang D, Bao Y, Yuan Z. Performance Evaluation and Mechanism Study of Solid Waste-Based Cementitious Materials for Solidifying Marine Soft Soil under Seawater Mixing and Erosion Action. Applied Sciences. 2024; 14(15):6666. https://doi.org/10.3390/app14156666
Chicago/Turabian StyleZhang, Zheng, Cheng Guan, Sudong Hua, Yanan Zhang, Dongrui Zhang, Youzhi Bao, and Zhizhou Yuan. 2024. "Performance Evaluation and Mechanism Study of Solid Waste-Based Cementitious Materials for Solidifying Marine Soft Soil under Seawater Mixing and Erosion Action" Applied Sciences 14, no. 15: 6666. https://doi.org/10.3390/app14156666





