Valorization of Grass Clipping Waste: A Sustainable Approach to Cellulose Extraction and Paper Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
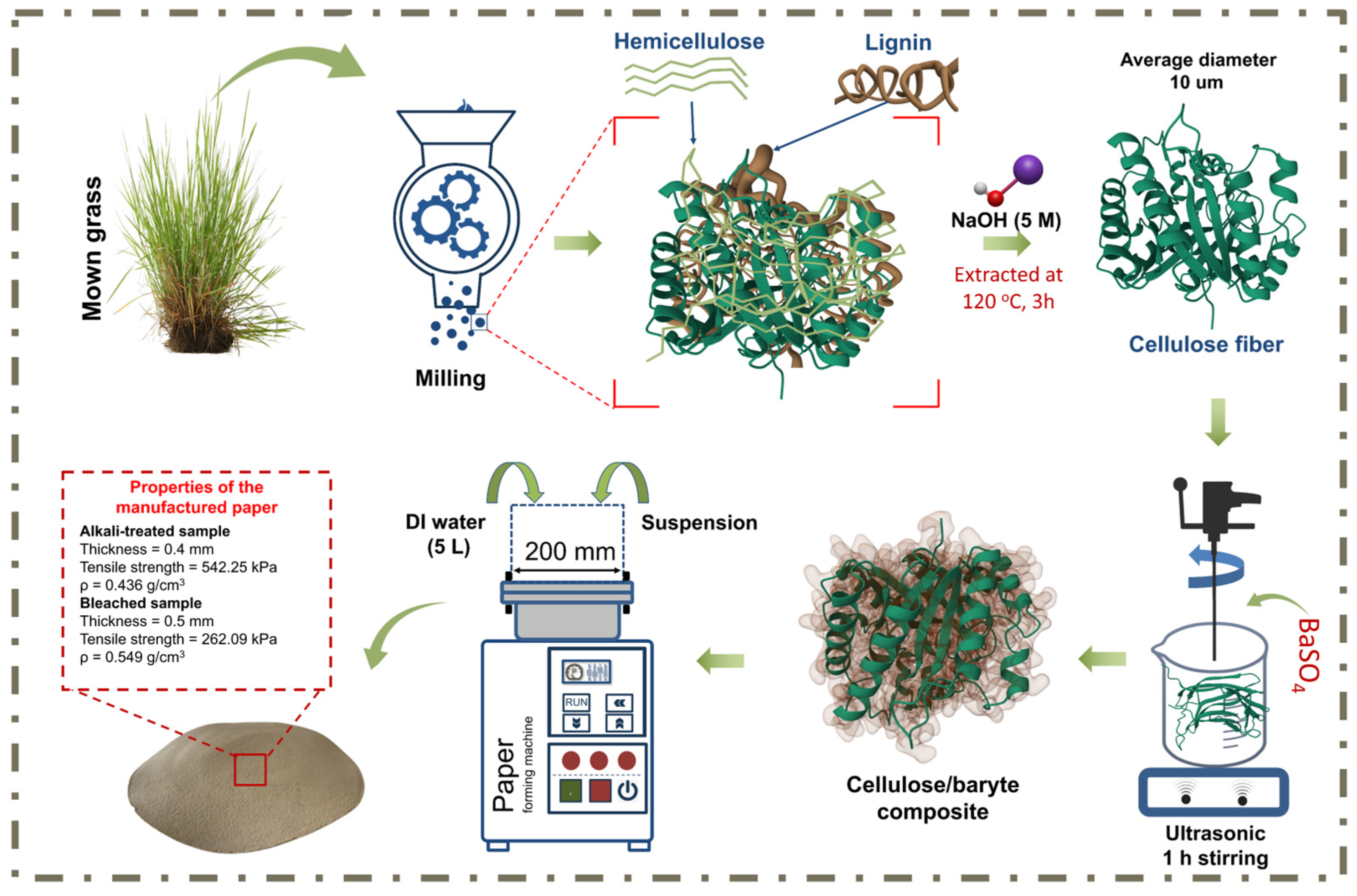
2.2. Cellulose Microfibril Extraction
2.2.1. Raw Material Pretreatment
2.2.2. Method 1: Alkaline Pulping Process
2.2.3. Method 2: Multistep Extraction Process with Bleaching
2.3. Preparation of Hand Sheet
2.4. Characterization Techniques
2.4.1. Compositional Analysis
2.4.2. Electron and Optical Microscopy
2.4.3. Structural Analysis
2.5. Characterization of Laboratory Samples of Paper Sheets
3. Results and Discussion
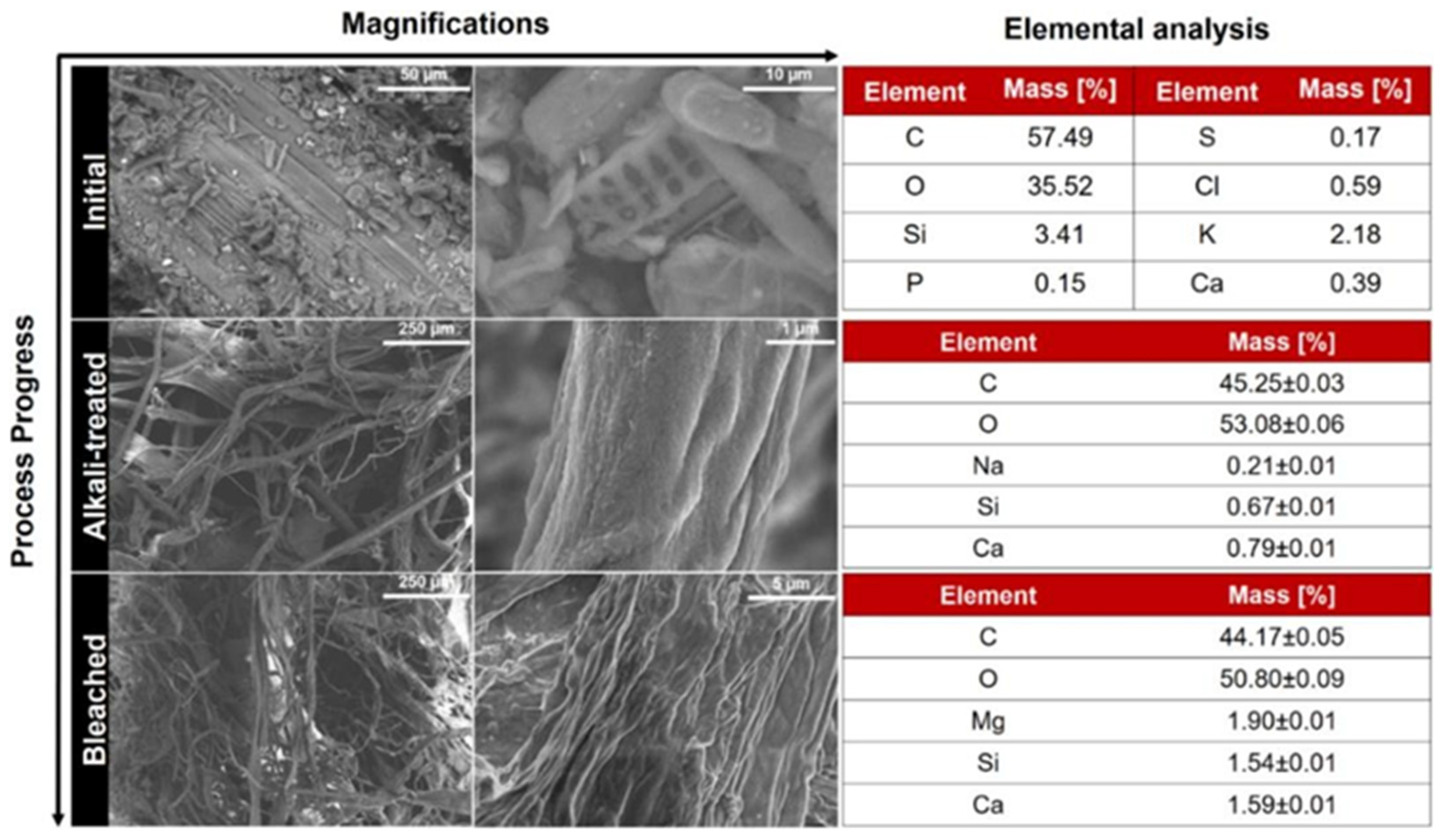
3.1. Characterization of Cellulose Fibers
3.1.1. Compositional Analysis
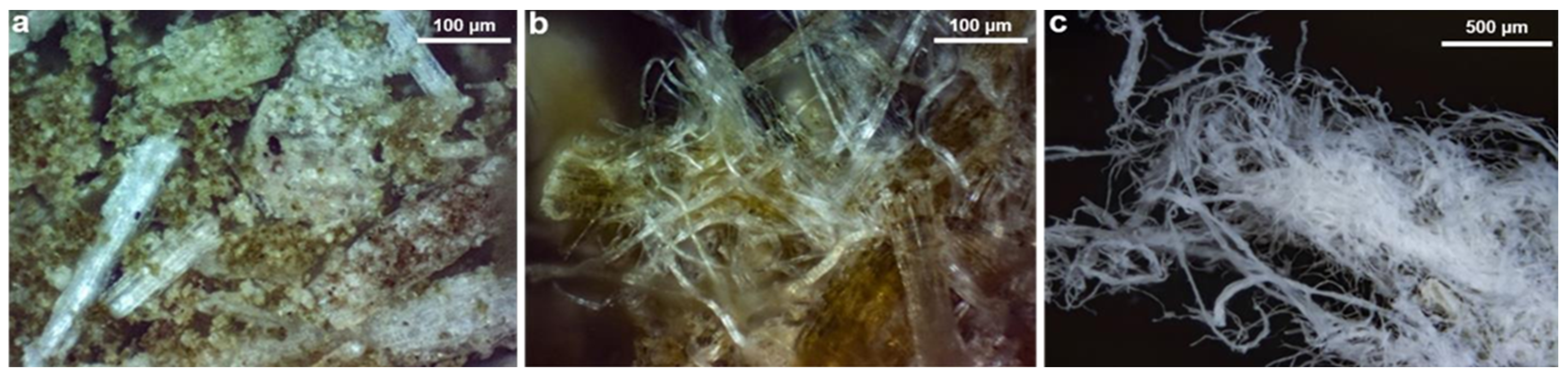
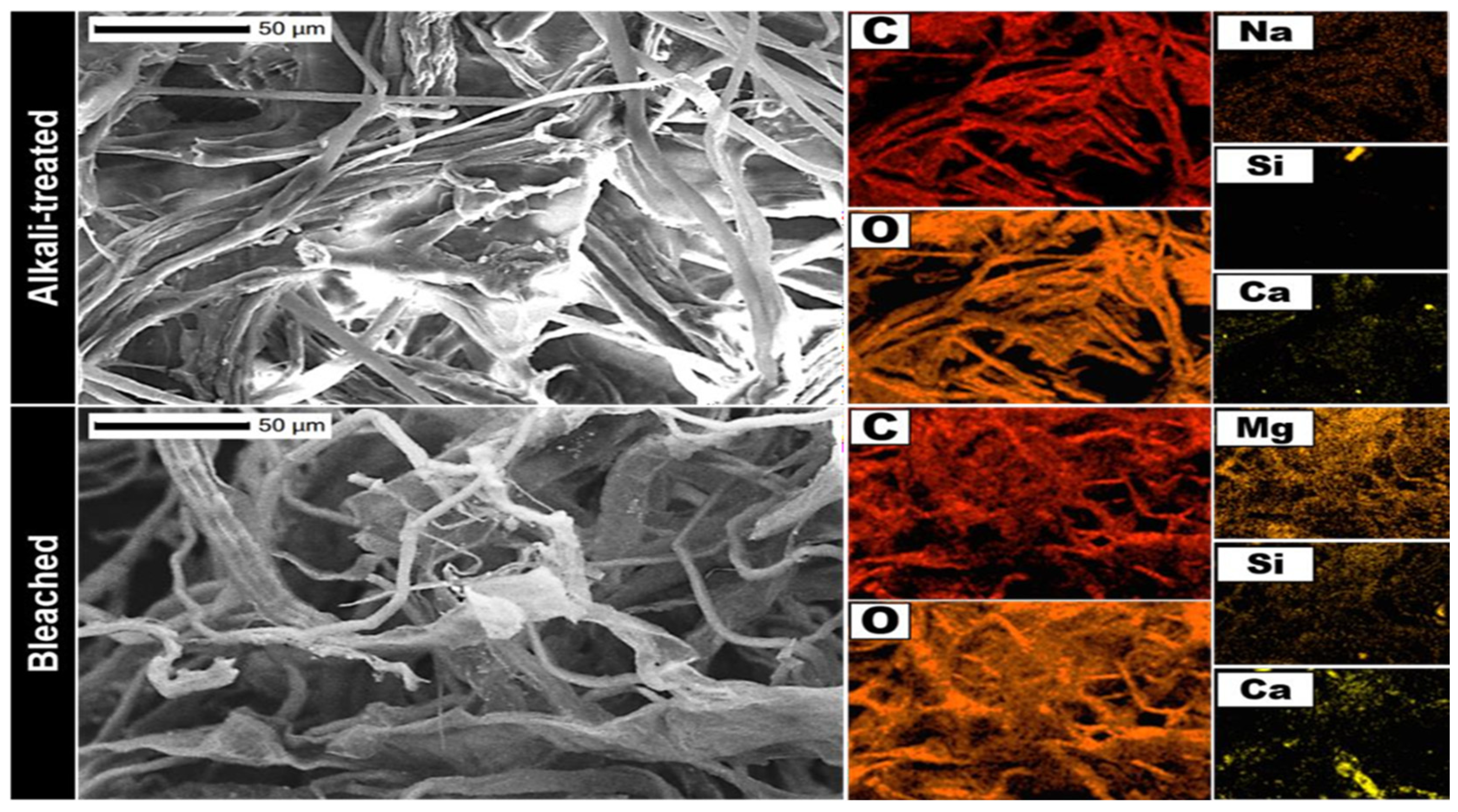
3.1.2. Morphological Analysis
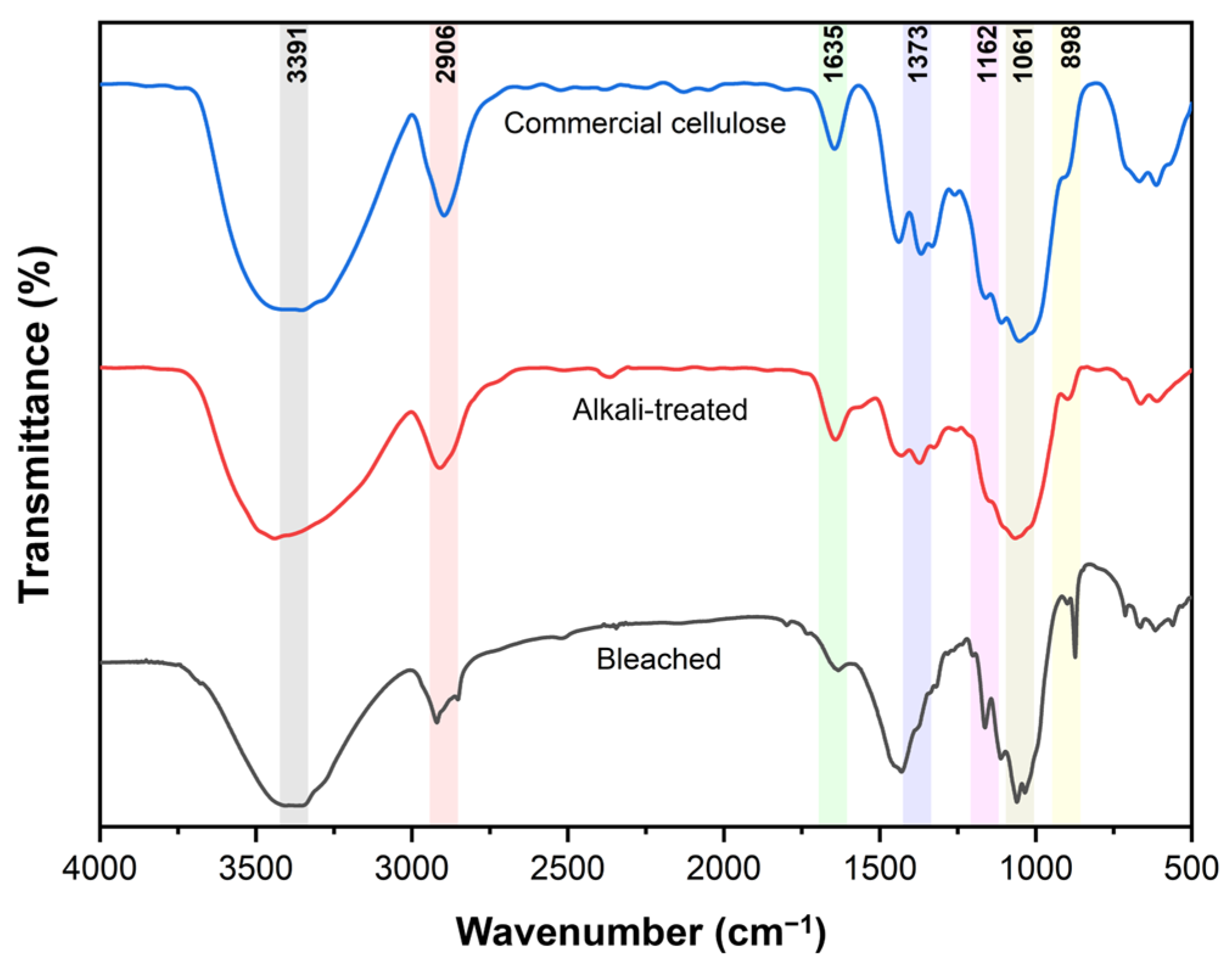
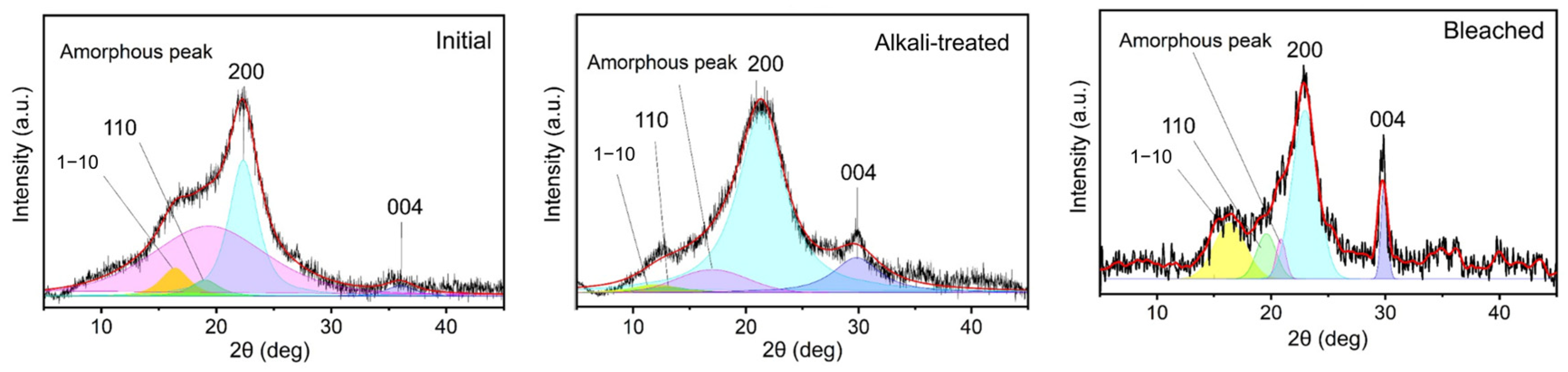
3.1.3. Structural Analysis
3.2. Handmade Paper Characterization
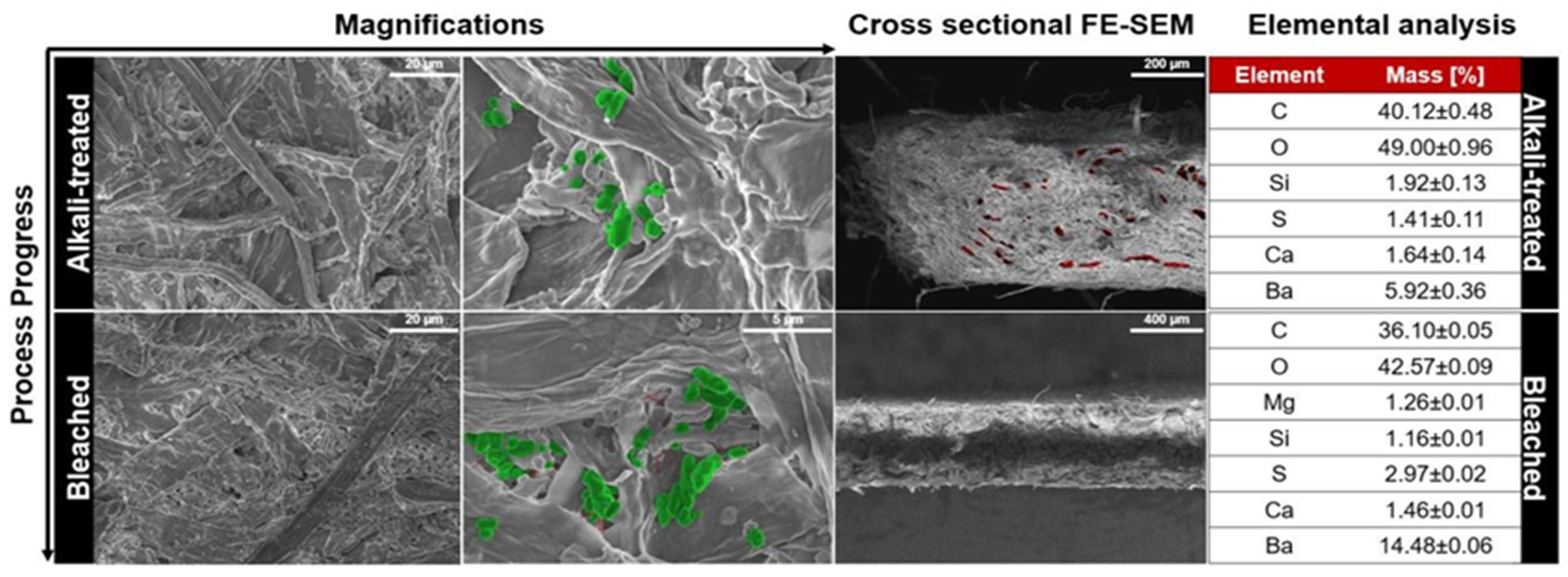
3.2.1. Morphological and Structural Analysis
- Barium’s high reflectivity contributes to increased whiteness, thereby improving the opacity and brightness of paper products.
- The high dispersion and stability of morphological parameters facilitate the uniform distribution of particles within the cellulose–mineral filler structure, leading to the high filling of interfibrillar pores.
- Products based on cellulose–barium sulfate composites demonstrate environmental safety during recycling processes.
3.2.2. Mechanical Characteristics of the Paper Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, P.K.; Keerthi, A.M.; Kallapur, S.S.; Choudhary, S. Overview of Cellulose Types and Applications. In Comprehensive Glycoscience; Elsevier: Amsterdam, The Netherlands, 2021; pp. 220–236. [Google Scholar] [CrossRef]
- Yun, T.; Tao, Y.; Li, Q.; Cheng, Y.; Lu, J.; Lv, Y.; Du, J.; Wang, H. Superhydrophobic Modification of Cellulosic Paper-Based Materials: Fabrication, Properties, and Versatile Applications. Carbohydr. Polym. 2023, 305, 120570. [Google Scholar] [CrossRef] [PubMed]
- Sayem, A.S.M.; Haider, J. An Overview on the Development of Natural Renewable Materials for Textile Applications. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 822–838. [Google Scholar] [CrossRef]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline Cellulose for Active Food Packaging Applications: A Review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Noroozi, R.; Hossain, M.; Ramakrishna, S.; Umer, R. 3D/4D Printing of Cellulose Nanocrystals-Based Biomaterials: Additives for Sustainable Applications. Int. J. Biol. Macromol. 2023, 251, 126287. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Ferdous, T.; Quaiyyum, M.A.; Salam, A.; Jahan, M.S. Pulping of Bagasse (Saccrarum officinarum), Kash (Saccharum spontaneum) and Corn Stalks (Zea Mays). Curr. Res. Green Sustain. Chem. 2020, 3, 100017. [Google Scholar] [CrossRef]
- Wossine, S.E.; Thothadri, G.; Tufa, H.B. Extraction and Characterization of Cellulosic Fibers from Jenfokie and Doby Stems: Effect of Extraction Methods on Physicochemical, Mechanical, and Thermal Properties. J. Nat. Fibers 2023, 20, 2229518. [Google Scholar] [CrossRef]
- Pérez-Limiñana, M.A.; Pérez-Aguilar, H.; Ruzafa-Silvestre, C.; Orgilés-Calpena, E.; Arán-Ais, F. Effect of Processing Time of Steam-Explosion for the Extraction of Cellulose Fibers from Phoenix Canariensis Palm Leaves as Potential Renewable Feedstock for Materials. Polymers 2022, 14, 5206. [Google Scholar] [CrossRef] [PubMed]
- Navada, A.P.; Guna, V.; Paul, R.; Belino, N.; Tavares, M.; Reddy, N. Residues from Cajanus Cajan Plant Provide Natural Cellulose Fibers Similar to Flax. J. Nat. Fibers 2022, 19, 14539–14547. [Google Scholar] [CrossRef]
- Gordon-Falconí, C.; Iannone, M.F.; Zawoznik, M.S.; Debut, A.; Groppa, M.D. Discarded Yerba Mate as a Source of Cellulose Fibers with Promising Applications for Drinking Water Decontamination. Ind. Crops Prod. 2024, 211, 118253. [Google Scholar] [CrossRef]
- Suriaman, I.; Hendrarsakti, J.; Mardiyati, Y.; Pasek, A.D. Synthesis and Characterization of Air Filter Media Made from Cellulosic Ramie Fiber (Boehmeria Nivea). Carbohydr. Polym. Technol. Appl. 2022, 3, 100216. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Yu, Y.; Hao, X.; Xu, Y.; Yao, Q.; Guo, Y. Preparation of Pectin Lyase by Fermentation for Customized Extraction of Paper Pulp, Viscose Fiber, and Nanofibrillated Cellulose from Hemp Stalks. Ind. Crops Prod. 2023, 203, 117137. [Google Scholar] [CrossRef]
- Parida, P.K.; Pradhan, A.K.; Pandit, M.K. Characterization of Cellulose Fiber Extracted from Stems of Myriostachya Wightiana (MW) Plants: A Viable Reinforcement for Polymer Composite. Fibers Polym. 2023, 24, 489–503. [Google Scholar] [CrossRef]
- Gonzalez, M.; Pereira-Rojas, J.; Villanueva, I.; Agüero, B.; Silva, I.; Velasquez, I.; Delgado, B.; Hernandez, J.; Rodriguez, G.; Labrador, H.; et al. Preparation and Characterization of Cellulose Fibers from Meghatyrsus Maximus: Applications in Its Chemical Derivatives. Carbohydr. Polym. 2022, 296, 119918. [Google Scholar] [CrossRef]
- Lee, C.L.; Chin, K.L.; H’ng, P.S.; Hafizuddin, M.S.; Khoo, P.S. Valorisation of Underutilized Grass Fibre (Stem) as a Potential Material for Paper Production. Polymers 2022, 14, 5203. [Google Scholar] [CrossRef] [PubMed]
- Gundupalli, M.P.; Cheenkachorn, K.; Chuetor, S.; Kirdponpattara, S.; Gundupalli, S.P.; Show, P.-L.; Sriariyanun, M. Assessment of Pure, Mixed and Diluted Deep Eutectic Solvents on Napier Grass (Cenchrus purpureus): Compositional and Characterization Studies of Cellulose, Hemicellulose and Lignin. Carbohydr. Polym. 2023, 306, 120599. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.-C.; Huang, R.; Jiang, L.-Y.; Liu, G.-D.; Liu, P.-D.; Xu, W.-R. Facile Production of Cellulose Nanofibers from Raw Elephant Grass by an Aluminum Chloride-Enhanced Acidic Deep Eutectic Solvent. Int. J. Biol. Macromol. 2023, 246, 125687. [Google Scholar] [CrossRef] [PubMed]
- Ndwandwa, N.; Ayaa, F.; Iwarere, S.A.; Daramola, M.O.; Kirabira, J.B. Extraction and Characterization of Cellulose Nanofibers From Yellow Thatching Grass (Hyparrhenia filipendula) Straws via Acid Hydrolysis. Waste Biomass Valorization 2023, 14, 2599–2608. [Google Scholar] [CrossRef]
- Scopel, E.; Camargos, C.H.M.; Pinto, L.O.; Trevisan, H.; Ferreira, E.S.; Rezende, C.A. Broadening the Product Portfolio with Cellulose and Lignin Nanoparticles in an Elephant Grass Biorefinery. Biofuels Bioprod. Biorefining 2023, 17, 859–872. [Google Scholar] [CrossRef]
- Doležal, J.; Kučerová, A.; Jandová, V.; Klimeš, A.; Říha, P.; Adamec, L.; Schweingruber, F.H. Anatomical Adaptations in Aquatic and Wetland Dicot Plants: Disentangling the Environmental, Morphological and Evolutionary Signals. Environ. Exp. Bot. 2021, 187, 104495. [Google Scholar] [CrossRef]
- Lewis, F.T. The Typical Shape of Polyhedral Cells in Vegetable Parenchyma and the Restoration of That Shape Following Cell Division. Proc. Am. Acad. Arts Sci. 1923, 58, 537. [Google Scholar] [CrossRef]
- Goossens, A.P. Notes on the anatomy of grass roots. Trans. R. Soc. S. Afr. 1935, 23, 1–21. [Google Scholar] [CrossRef]
- Grabber, J.H.; Jung, G.A. Isolation of Parenchyma and Sclerenchyma Cell Types from the Plant Parts of Grasses. Crop Sci. 1991, 31, 838–842. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Małachowska, E.; Dubowik, M.; Boruszewski, P.; Łojewska, J.; Przybysz, P. Influence of Lignin Content in Cellulose Pulp on Paper Durability. Sci. Rep. 2020, 10, 19998. [Google Scholar] [CrossRef]
- He, H.; An, F.; Wang, Y.; Wu, W.; Huang, Z.; Song, H. Effects of Pretreatment, NaOH Concentration, and Extraction Temperature on the Cellulose from Lophatherum Gracile Brongn. Int. J. Biol. Macromol. 2021, 190, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.; Ferraz, E.; Gamelas, J.A.F. Composites of Nanofibrillated Cellulose with Clay Minerals: A Review. Adv. Colloid Interface Sci. 2019, 272, 101994. [Google Scholar] [CrossRef]
- Ganapathy, V.; Muthukumaran, G.; Sudhagar, P.E.; Rashedi, A.; Norrrahim, M.N.F.; Ilyas, R.A.; Goh, K.L.; Jawaid, M.; Naveen, J. Mechanical Properties of Cellulose-based Multiscale Composites: A Review. Polym. Compos. 2023, 44, 734–756. [Google Scholar] [CrossRef]
- Nikkhah Dafchahi, M.; Resalati, H.; Zabihzadeh, S.M.; Nazarnezhad, N.; Asadpour, G.; Pirayesh, H. Novel Calcium Carbonate Filler for Cellulose Industry. Nord. Pulp Pap. Res. J. 2021, 36, 536–547. [Google Scholar] [CrossRef]
- Ghosh, I.; Sharma, C.; Tandon, R. Structural Evaluation of Chitosan-Modified Precipitated Calcium Carbonate Composite Fillers for Papermaking Applications. SN Appl. Sci. 2020, 2, 1577. [Google Scholar] [CrossRef]
- Fan, Z.; Li, Z.; Qi, W.; Zhao, S.; Zhou, B.; Liu, S.; Tian, Y. Preparation of In-Situ Modified Diatomite and Its Application in Papermaking. Colloids Surf. Physicochem. Eng. Asp. 2023, 657, 130582. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, G.; He, X.; Xu, L.; Zhang, X.; He, Z.; Zhang, L. Diatomite Modified with an Alkyl Ketene Dimer for Hydrophobicity of Cellulosic Paper. ACS Omega 2022, 7, 20129–20136. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.A.; Mohamed, S.A.A.; El-Samahy, M.A.; Abdel Kader, A.H. Novel Fire-Retardant Bagasse Papers Using Talc/Cyclodiphosphazane and Nanocellulose as Packaging Materials. Egypt. J. Pet. 2021, 30, 25–32. [Google Scholar] [CrossRef]
- Naijian, F.; Rudi, H.; Resalati, H.; Torshizi, H.J. Application of Bio-Based Modified Kaolin Clay Engineered as Papermaking Additive for Improving the Properties of Filled Recycled Papers. Appl. Clay Sci. 2019, 182, 105258. [Google Scholar] [CrossRef]
- Liang, Y.; Ding, H. Mineral-TiO2 Composites:Preparation and Application in Papermaking, Paints and Plastics. J. Alloys Compd. 2020, 844, 156139. [Google Scholar] [CrossRef]
- Feng, Y.; Cölfen, H.; Xiong, R. Organized Mineralized Cellulose Nanostructures for Biomedical Applications. J. Mater. Chem. B 2023, 11, 5321–5349. [Google Scholar] [CrossRef]
- Mungpayaban, H.; Rueanngoen, A.; Rindhatayathon, P.; Ninlaphruk, S.; Ekgasit, S.; Pengprecha, S. Barium Sulphate–Amorphous Cellulose Composite as Innovative X-Ray Shielding Substrate. Mater. Res. Innov. 2023, 27, 45–54. [Google Scholar] [CrossRef]
- Luo, J.; Liu, J.; Guo, X.; Liu, Y.; Jin, H.; Fan, D. Preparation, Characterisation, and Application of Bifunctional BaSO4 Sheets. Materials 2020, 13, 2903. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Xie, X.; Zhou, X.; Bai, Z.-W. Novel Composite Materials for Chiral Separation from Cellulose and Barium Sulfate. Int. J. Polym. Sci. 2013, 2013, 312615. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, X.; Chang, C.; Liu, S.; Luo, X. X-ray Shielding Structural and Properties Design for the Porous Transparent BaSO4/Cellulose Nanocomposite Membranes. Int. J. Biol. Macromol. 2019, 139, 793–800. [Google Scholar] [CrossRef]
- Boyarko, G.Y.; Bolsunovskaya, L.M. World’s Barite Resources as Critical Raw Material. Gorn. Nauk. Tekhnologii Min. Sci. Technol. Russ. 2023, 8, 264–277. [Google Scholar] [CrossRef]
- Netramai, S.; Kijchavengkul, T.; Kittipinyovath, P. Pulp and Paper Production. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780081005965032017. [Google Scholar] [CrossRef]
- Hafemann, E.; Battisti, R.; Bresolin, D.; Marangoni, C.; Machado, R.A.F. Enhancing Chlorine-Free Purification Routes of Rice Husk Biomass Waste to Obtain Cellulose Nanocrystals. Waste Biomass Valorization 2020, 11, 6595–6611. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry (TAPPI). Alpha-, Beta- and Gamma-Cellulose in Pulp. TAPPI T203 cm-09. 2009. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T203.aspx (accessed on 1 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C. TAPPI T211 om-02. 2002. Available online: https://www.tappi.org/content/sarg/t211.pdf (accessed on 1 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Moisture in Pulp, Paper and Paperboard. TAPPI T412 om-02. 2002. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T412.aspx (accessed on 1 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Preparation of Laboratory Sheets for Physical Testing of Pulp. TAPPI T264 cm-07. 2007. Available online: https://webstore.ansi.org/standards/tappi/tappi264cm07 (accessed on 16 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Kappa Number of Pulp. TAPPI T236 cm-85. 1985. Available online: https://www.scribd.com/document/87301017/T236 (accessed on 1 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Folding Endurance of Paper (Schopper Type). TAPPI T 511 om-20. 2020. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T511.aspx (accessed on 16 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Tensile Breaking Strength of Paper and Paperboard (Using Constant Rate of Elongation Apparatus). TAPPI T403 om-15. 2015. Available online: https://www.normsplash.com/TAPPI/185225749/TAPPI-T-403-om?src=spdf (accessed on 22 April 2024).
- Technical Association of the Pulp and Paper Industry (TAPPI). Grammage of Paper and Paperboard (Weight per Unit Area). TAPPI T 410 om-08. 2008. Available online: https://www.tappi.org/content/tag/sarg/t410.pdf (accessed on 22 April 2024).
- GOST 13525.19-91; Pulp and Paper. Test Methods for Determining Moisture Content. Interstate Standard (GOST): Moscow, Russia, 1991. Available online: https://files.stroyinf.ru/Data2/1/4294852/4294852296.pdf (accessed on 22 April 2024).
- ISO 1762:2019; Paper, Board and Pulps—Determination of Ash. International Organization for Standardization (ISO): Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/73414.html (accessed on 22 April 2024).
- Tan, R.; Li, K.; Sun, Y.; Fan, X.; Shen, Z.; Tang, L. Sustainable Management of Campus Fallen Leaves through Low-Temperature Pyrolysis and Application in Pb Immobilization. J. Environ. Sci. 2024, 139, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Thi Thuy Van, N.; Gaspillo, P.; Thanh, H.G.T.; Nhi, N.H.T.; Long, H.N.; Tri, N.; Thi Truc Van, N.; Nguyen, T.-T.; Ky Phuong Ha, H. Cellulose from the Banana Stem: Optimization of Extraction by Response Surface Methodology (RSM) and Charaterization. Heliyon 2022, 8, e11845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Y.; Tan, Z.; Zhou, T. Cellulose Extraction from Rice Straw Waste for Biodegradable Ethyl Cellulose Films Preparation Using Green Chemical Technology. J. Clean. Prod. 2024, 439, 140839. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Liu, K.; Yang, H.; Lin, Q.; Xu, T.; Song, X.; Du, H.; Bai, L.; Yao, S.; et al. Sustainable Preparation of Lignocellulosic Nanofibrils and Cellulose Nanopaper from Poplar Sawdust. J. Clean. Prod. 2023, 384, 135582. [Google Scholar] [CrossRef]
- Inkoua, S.; Li, C.; Gao, G.; Zhang, L.; Zhang, S.; Tang, Y.; Wang, D.; Leng, C.; Hu, X. Thermal Treatment of Wood, Bark and Leaves of Same Poplar Tree through Hydrothermal Carbonization and Activation: Roles of Aliphatic Structures in Pore Development. Biomass Bioenergy 2024, 182, 107101. [Google Scholar] [CrossRef]
- Liu, Y. Chemical Composition and Characterization of Cotton Fibers. In Cotton Fiber: Physics, Chemistry and Biology; Fang, D.D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 75–94. [Google Scholar] [CrossRef]
- Dehabadi, L.; Karoyo, A.H.; Soleimani, M.; Alabi, W.O.; Simonson, C.J.; Wilson, L.D. Flax Biomass Conversion via Controlled Oxidation: Facile Tuning of Physicochemical Properties. Bioengineering 2020, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Sapuan, S.M.; Ainun, Z.M.A.; Zakiah, S.; Nazrin, A.; Ilyas, R.A. Introduction to Nonwood Plant Fibers for Pulp and Papermaking Production. In Pulping and Papermaking of Nonwood Plant Fibers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–15. [Google Scholar] [CrossRef]
- Kumar, A.; Dutta, D.; Kalita, D.; Majumdar, B.; Saikia, S.P.; Banik, D. Extraction, Physicochemical and Structural Characterisation of Palm Grass Leaf Fibres for Sustainable and Cleaner Production of Textile and Allied Cellulosic Applications. J. Clean. Prod. 2024, 448, 141733. [Google Scholar] [CrossRef]
- Danial, W.H.; Mohd Taib, R.; Abu Samah, M.A.; Mohd Salim, R.; Abdul Majid, Z. The Valorization of Municipal Grass Waste for the Extraction of Cellulose Nanocrystals. RSC Adv. 2020, 10, 42400–42407. [Google Scholar] [CrossRef]
- Danielewicz, D.; Surma-Ślusarska, B.; Żurek, G.; Martyniak, D.; Kmiotek, M.; Dybka, K. Selected Grass Plants as Biomass Fuels and Raw Materials for Papermaking, Part II. Pulp and Paper Properties. BioResources 2015, 10, 8552–8564. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Estimation of Hydrogen Peroxide Effectivity during Bleaching Using the Kappa Number. Chem. Pap. 2021, 75, 5749–5758. [Google Scholar] [CrossRef]
- Aryal, G.M.; Kandel, K.P.; Bhattarai, R.K.; Giri, B.; Adhikari, M.; Ware, A.; Han, S.; George, G.; Luo, Z.; Gautam, B.R.; et al. Material Properties of Traditional Handmade Paper Samples Fabricated from Cellulosic Fiber of Lokta Bushes. ACS Omega 2022, 7, 32717–32726. [Google Scholar] [CrossRef]
- Young, R.A. Comparison of the Properties of Chemical Cellulose Pulps. Cellulose 1994, 1, 107–130. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical Isolation and Characterization of Different Cellulose Nanofibers from Cotton Stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of Cellulose Nanocrystals and Carboxylated Cellulose Nanocrystals from Borer Powder of Bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Guo, X.; Liu, L.; Wu, J.; Fan, J.; Wu, Y. Qualitatively and Quantitatively Characterizing Water Adsorption of a Cellulose Nanofiber Film Using Micro-FTIR Spectroscopy. RSC Adv. 2018, 8, 4214–4220. [Google Scholar] [CrossRef]
- Aryasena, R.; Kusmono; Umami, N. Production of Cellulose Nanocrystals Extracted from Pennisetum Purpureum Fibers and Its Application as a Lubricating Additive in Engine Oil. Heliyon 2022, 8, e11315. [Google Scholar] [CrossRef] [PubMed]
- Gril, J. (Ed.) Wood Science for Conservation of Cultural Heritage-Braga 2008: Proceedings of the International Conference Held by COST Action IE0601 in Braga (Portugal), 5–7 November: Firenze Univ. Press, Firenze. 2010. Available online: https://books.fupress.com/catalogue/wood-science-for-conservation-of-cultural-heritage--braga-2008/2150 (accessed on 26 June 2024).
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Vydrina, I.; Malkov, A.; Vashukova, K.; Tyshkunova, I.; Mayer, L.; Faleva, A.; Shestakov, S.; Novozhilov, E.; Chukhchin, D. A New Method for Determination of Lignocellulose Crystallinity from XRD Data Using NMR Calibration. Carbohydr. Polym. Technol. Appl. 2023, 5, 100305. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved Cellulose X-Ray Diffraction Analysis Using Fourier Series Modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding Changes in Cellulose Crystalline Structure of Lignocellulosic Biomass during Ionic Liquid Pretreatment by XRD. Bioresour. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cui, S.; Wang, S. Preparation and Crystalline Analysis of High-grade Bamboo Dissolving Pulp for Cellulose Acetate. J. Appl. Polym. Sci. 2008, 107, 1029–1038. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Z.; Xu, N.; Chen, J.; Gao, Y. Influencing Factors for Determining the Crystallinity of Native Cellulose by X-ray Diffraction. Anal. Sci. 2020, 36, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Divakaran, D.; Suyambulingam, I.; Sanjay, M.R.; Raghunathan, V.; Ayyappan, V.; Siengchin, S. Isolation and Characterization of Microcrystalline Cellulose from an Agro-Waste Tamarind (Tamarindus Indica) Seeds and Its Suitability Investigation for Biofilm Formulation. Int. J. Biol. Macromol. 2024, 254, 127687. [Google Scholar] [CrossRef] [PubMed]
- Chavez, B.K.; Garces-Porras, K.; Parada, D.C.; Flores, E. Thermochemical Isolation and Characterization of Nanofibrillated Cellulose from Stipa Obtusa Fibers. Carbohydr. Polym. Technol. Appl. 2023, 6, 100344. [Google Scholar] [CrossRef]
- Zendrato, H.M.; Masruchin, N.; Nikmatin, S.; Wistara, N.J. Effective Cellulose Isolation from Torch Ginger Stem by Alkaline Hydrogen Peroxide–Peracetic Acid System. J. Ind. Eng. Chem. 2024, 131, 376–387. [Google Scholar] [CrossRef]
- Mirzaee, N.; Nikzad, M.; Battisti, R.; Araghi, A. Isolation of Cellulose Nanofibers from Rapeseed Straw via Chlorine-Free Purification Method and Its Application as Reinforcing Agent in Carboxymethyl Cellulose-Based Films. Int. J. Biol. Macromol. 2023, 251, 126405. [Google Scholar] [CrossRef]
- Qu, L.; Tian, M.; Zhang, X.; Guo, X.; Zhu, S.; Han, G.; Li, C. Barium Sulfate/Regenerated Cellulose Composite Fiber with X-Ray Radiation Resistance. J. Ind. Text. 2015, 45, 352–367. [Google Scholar] [CrossRef]
- Grothe, S.; Fritzen, P.; Winkler, J.; Rohe, B.; Bittmann, B.; Haupert, F. Barium Sulfate-Containing Composite. CA2661509A1; Canada, 28 February 2008. Available online: https://patents.google.com/patent/CA2661509A1/en (accessed on 11 March 2024).
- Fukuoka, M.; Nakatani, T.; Oishi, M.; Goto, S. Composite of Barium Sulfate and Filament and Production Method Thereof. JP2017066578A; Japan, 24 June 2020. Available online: https://patents.google.com/patent/JP2017066578A/en?oq=JP2017066578A (accessed on 11 March 2024).
- Tripathi, S.K.; Bhardwaj, N.K. Pulping and Papermaking of Cornstalk. In Pulping and Papermaking of Nonwood Plant Fibers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 63–99. [Google Scholar] [CrossRef]
- Hirn, U.; Schennach, R. Comprehensive Analysis of Individual Pulp Fiber Bonds Quantifies the Mechanisms of Fiber Bonding in Paper. Sci. Rep. 2015, 5, 10503. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef]
- Williams, J.C.; Krasow, M.R. Folding Endurance and Tensile Strength of Paper. Int. Inst. Conserv. Hist. Artist. Work. 1973, 14, 25–41. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Chen, J.; Ji, X.; Wang, Q. Production and Characterization of Cellulose Nanofibrils from Different Chemical and Mechanical Pulps. J. Wood Chem. Technol. 2018, 38, 149–158. [Google Scholar] [CrossRef]
- Chen, X.; Qian, X.; An, X. Using Calcium Carbonate Whiskers as Papermaking Filler. BioResources 2011, 6, 2435–2447. [Google Scholar] [CrossRef]
- Ferraz, A.P.A.; Ventorim, G. A study of the physico-mechanical properties in short bleaching sequences. Rev. Árvore 2018, 42, e420505. [Google Scholar] [CrossRef]
- Barhoum, A.; Deshmukh, K.; García-Betancourt, M.-L.; Alibakhshi, S.; Mousavi, S.M.; Meftahi, A.; Sabery, M.S.K.; Samyn, P. Nanocelluloses as Sustainable Membrane Materials for Separation and Filtration Technologies: Principles, Opportunities, and Challenges. Carbohydr. Polym. 2023, 317, 121057. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Singh, S.; Bhardwaj, S.; Gupta, S.; Nandi, U.; Chandra, R.; Maji, P.K. Recent Advancements in Nanocellulose-Based Supercapacitors for Energy Storage Devices: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100416. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Ren, H.; Jin, J.; He, H.; Jin, P.; Wu, Z.; Zheng, Y. Research Progress of Nanocellulose-Based Food Packaging. Trends Food Sci. Technol. 2024, 143, 104289. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Gao, M.; Guo, Y.; Xu, T.; Jiang, H.; Zhang, Z.; Ji, X.; Si, C. Nanocellulose-Based Functional Materials for Physical, Chemical, and Biological Sensing: A Review of Materials, Properties, and Perspectives. Ind. Crops Prod. 2024, 212, 118326. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Tian, Z.; Zhang, C.; Han, X.; Jiang, S.; Liu, K.; Duan, G. Preparation of Nanocellulose and Its Applications in Wound Dressing: A Review. Int. J. Biol. Macromol. 2024, 254, 127997. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Dutta, S.D.; Randhawa, A.; Patil, T.V.; Ganguly, K.; Acharya, R.; Lee, J.; Park, H.; Lim, K.-T. Recent Advances and Biomedical Application of 3D Printed Nanocellulose-Based Adhesive Hydrogels: A Review. Int. J. Biol. Macromol. 2024, 264, 130732. [Google Scholar] [CrossRef] [PubMed]
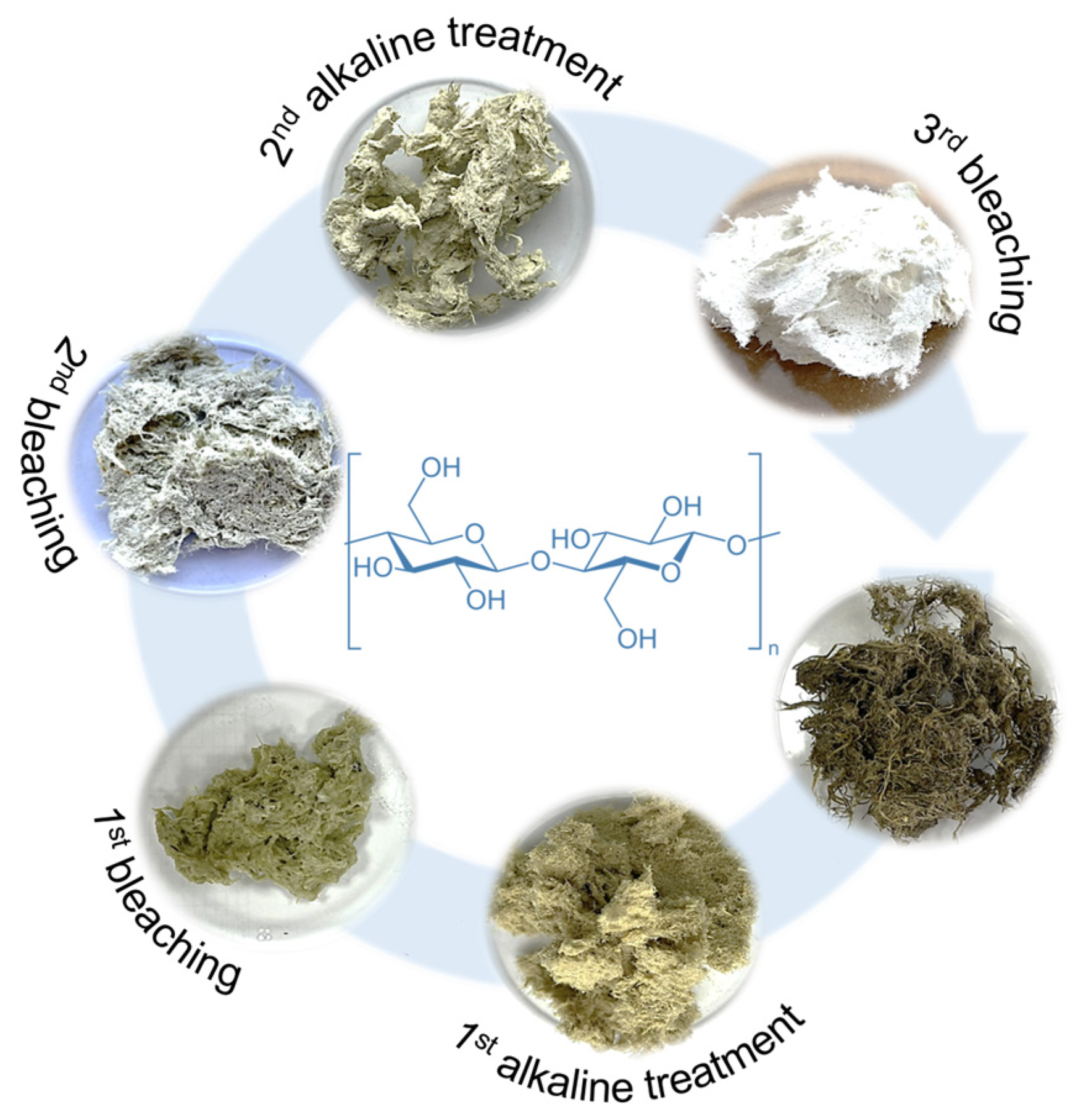

| Treatment | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Untreated grass clippings | 14.06 | 46.95 | 24.94 |
| Alkali-treated grass clippings | 47.61 | 21.87 | 12.68 |
| Bleached grass clippings | 65.39 | 12.70 | 7.50 |
| Sample | ρ, g/cm3 | W/Moisture Content, % | X, % | Thickness, mm | Fracture Strength | Tensile Strength, kPa |
|---|---|---|---|---|---|---|
| Alkali-treated | 0.436 | 5.6 | 38.87 | 0.41 | 40 | 542.25 |
| Bleached | 0.549 | 2.51 | 37.38 | 0.5 | 3 | 262.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taurbekov, A.; Kaidar, B.; Baltabay, A.; Imash, A.; Ko, W.-B.; Ko, J.-W.; Atamanov, M.; Mansurov, Z.; Smagulova, G. Valorization of Grass Clipping Waste: A Sustainable Approach to Cellulose Extraction and Paper Manufacturing. Appl. Sci. 2024, 14, 6680. https://doi.org/10.3390/app14156680
Taurbekov A, Kaidar B, Baltabay A, Imash A, Ko W-B, Ko J-W, Atamanov M, Mansurov Z, Smagulova G. Valorization of Grass Clipping Waste: A Sustainable Approach to Cellulose Extraction and Paper Manufacturing. Applied Sciences. 2024; 14(15):6680. https://doi.org/10.3390/app14156680
Chicago/Turabian StyleTaurbekov, Azamat, Bayan Kaidar, Akniyet Baltabay, Aigerim Imash, Weon-Bae Ko, Jeong-Won Ko, Meiram Atamanov, Zulkhair Mansurov, and Gaukhar Smagulova. 2024. "Valorization of Grass Clipping Waste: A Sustainable Approach to Cellulose Extraction and Paper Manufacturing" Applied Sciences 14, no. 15: 6680. https://doi.org/10.3390/app14156680







