Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Chitosan Hydrochloride
2.2. Extraction and Characterization of Plant-Derived Biosurfactants
2.3. Characterization of Biosurfactants in Plant Extracts Using Fourier Transform Infrared Spectroscopy
2.4. Determination of Surface Tension and Critical Micelle Concentration of Biosurfactants
2.5. Determination of Emulsification Activity
2.6. Preparation of Mouthwash Formulations
2.7. Determination of pH
2.8. Determination of Foaming Capacity
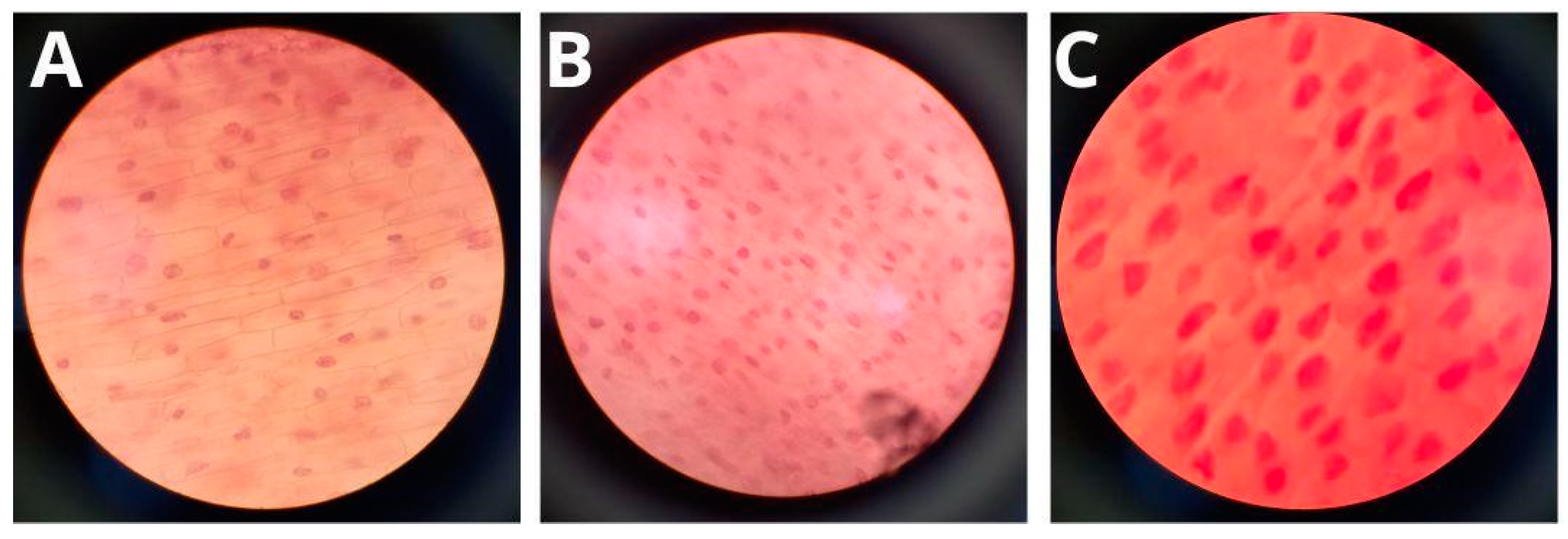
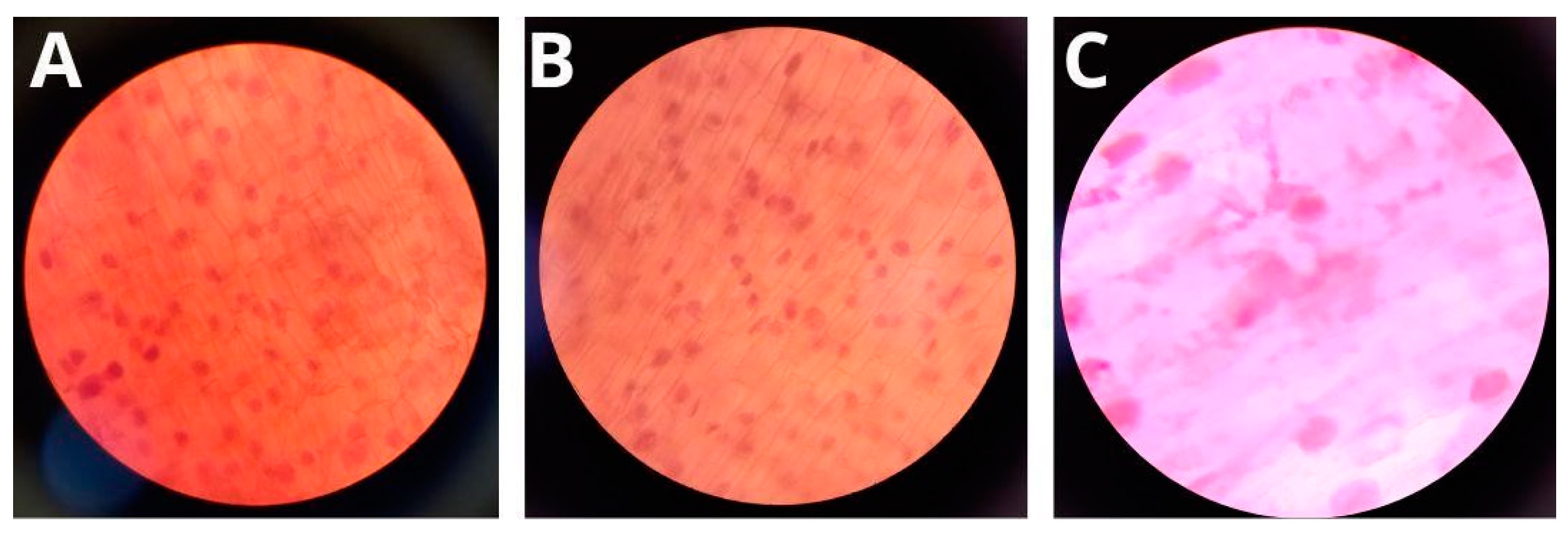
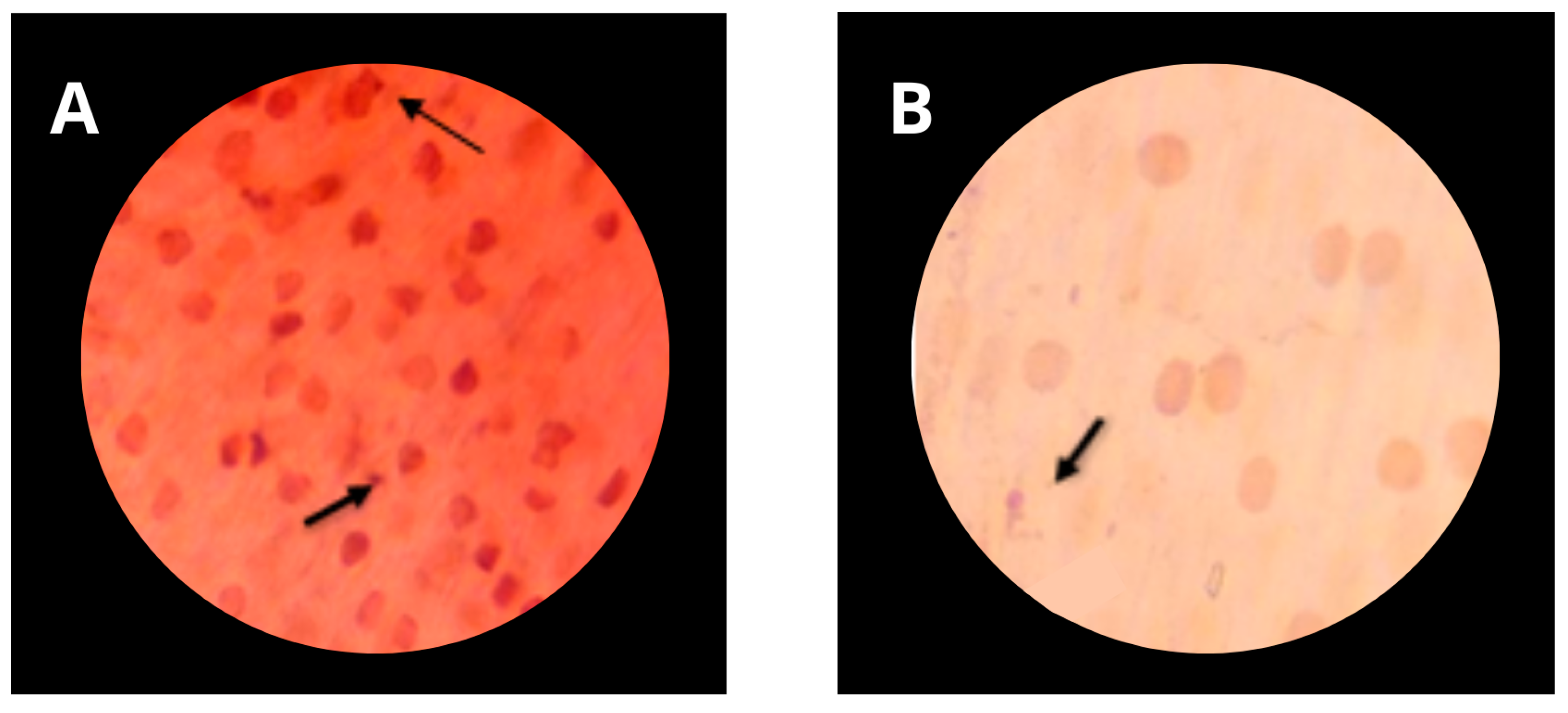
2.9. Determination of Genotoxicity of Formulations
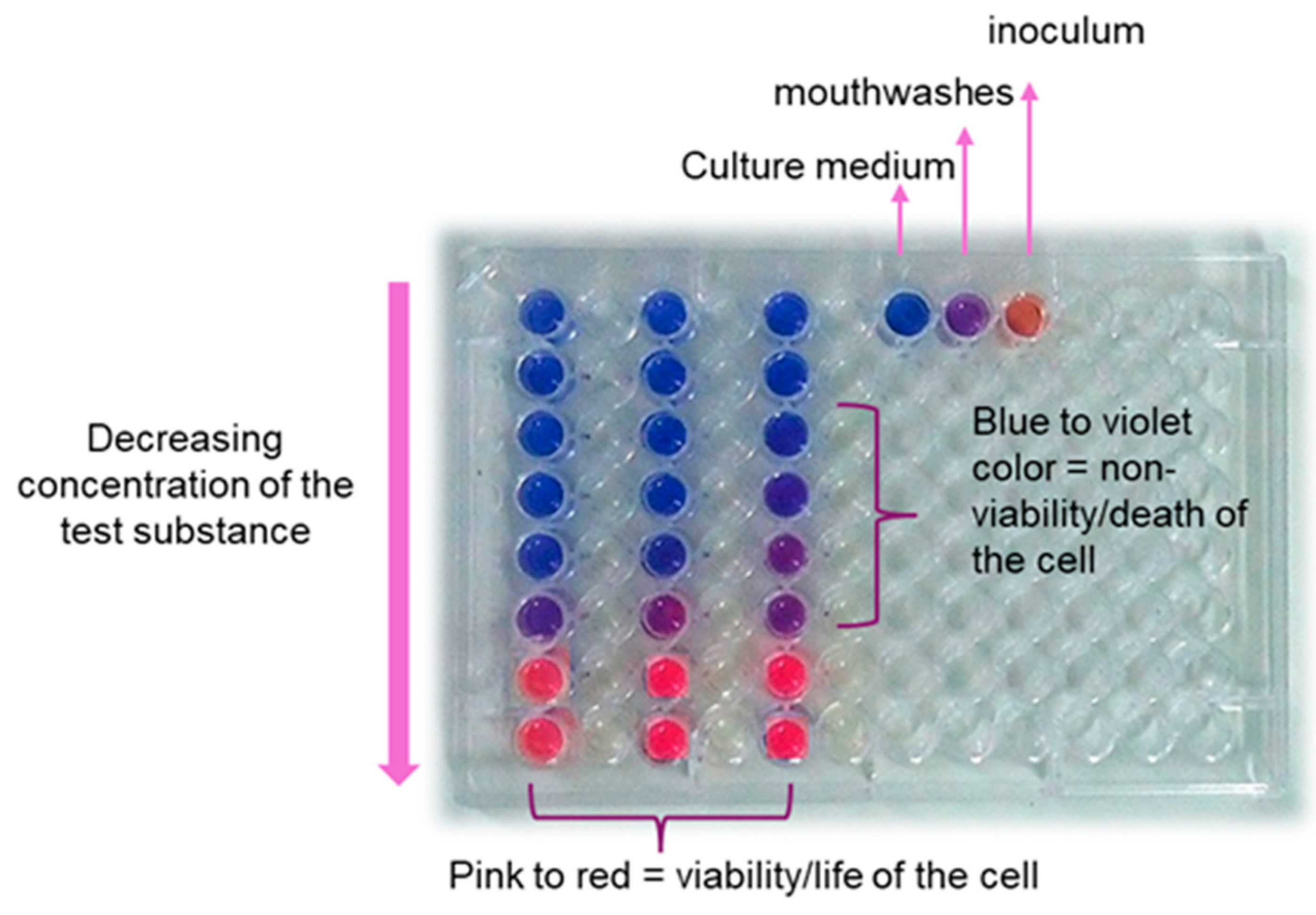
2.10. Determination of Antimicrobial Activity
2.11. Determination of Antibiofilm Activity
2.12. Statistical Analyses
3. Results
3.1. Characterization of Biosurfactants in Plant Extracts Using Fourier Transform Infrared Spectroscopy
3.2. Reduction in Water Surface Tension and Critical Micelle Concentration of Biosurfactants
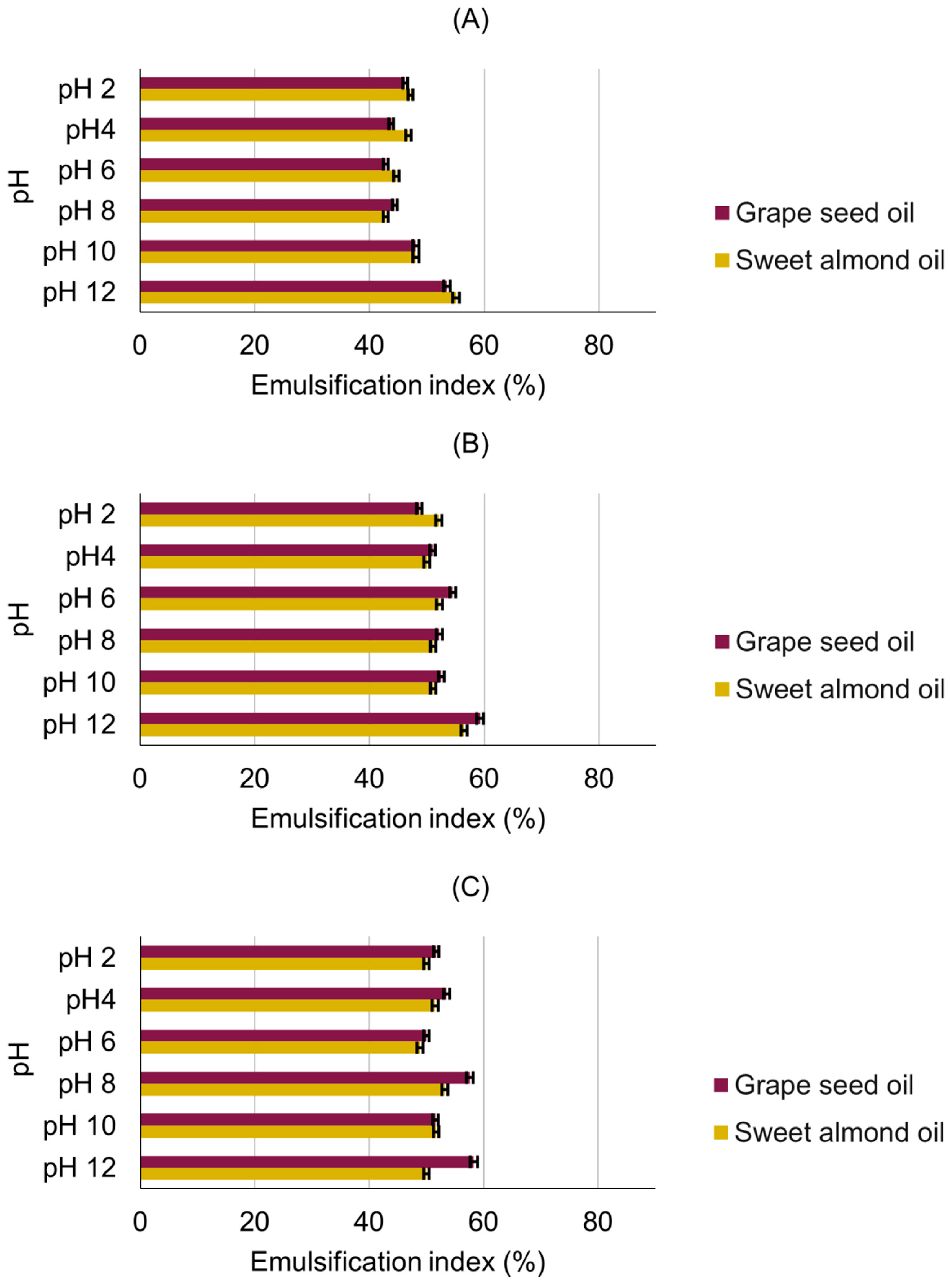
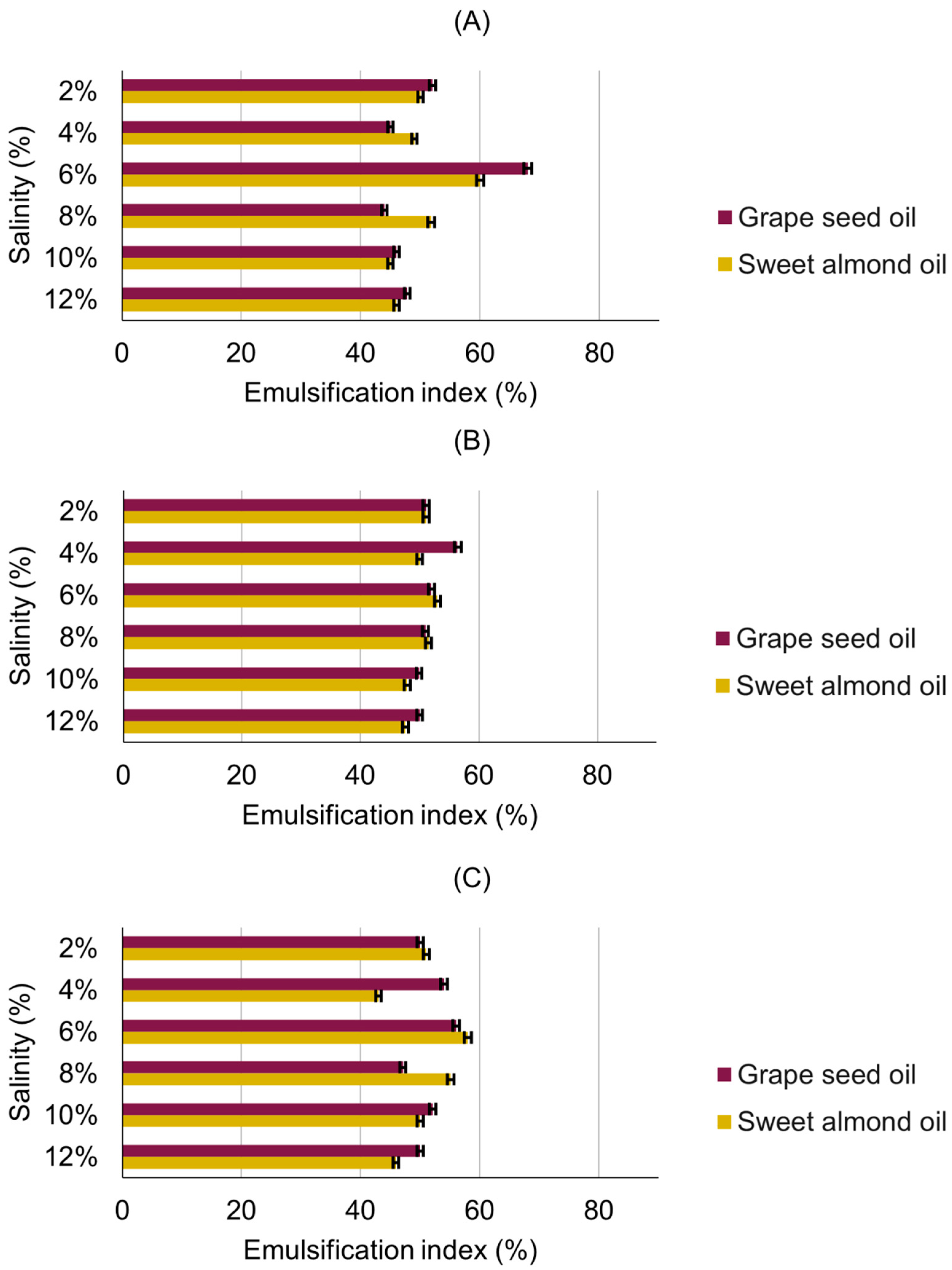
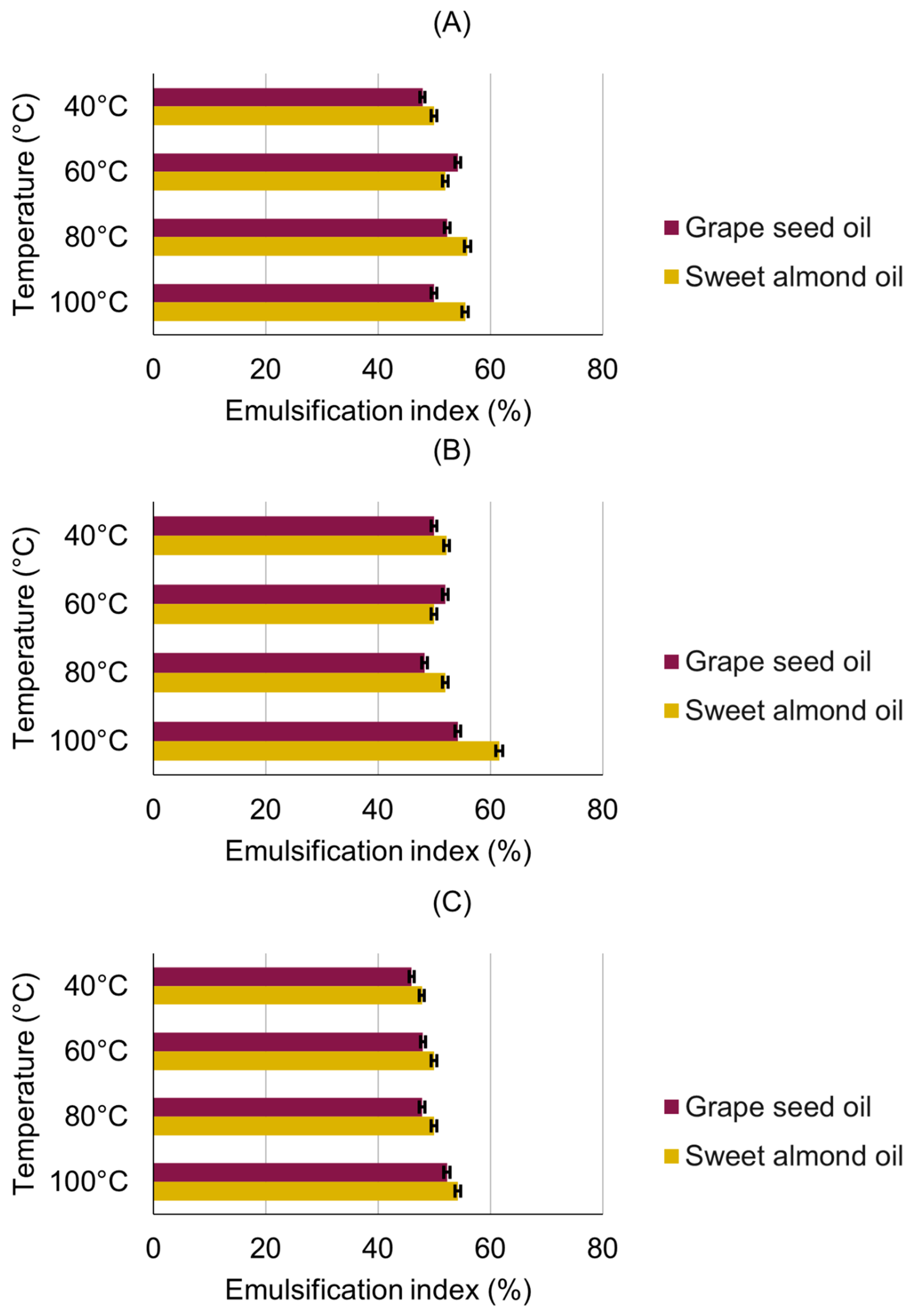
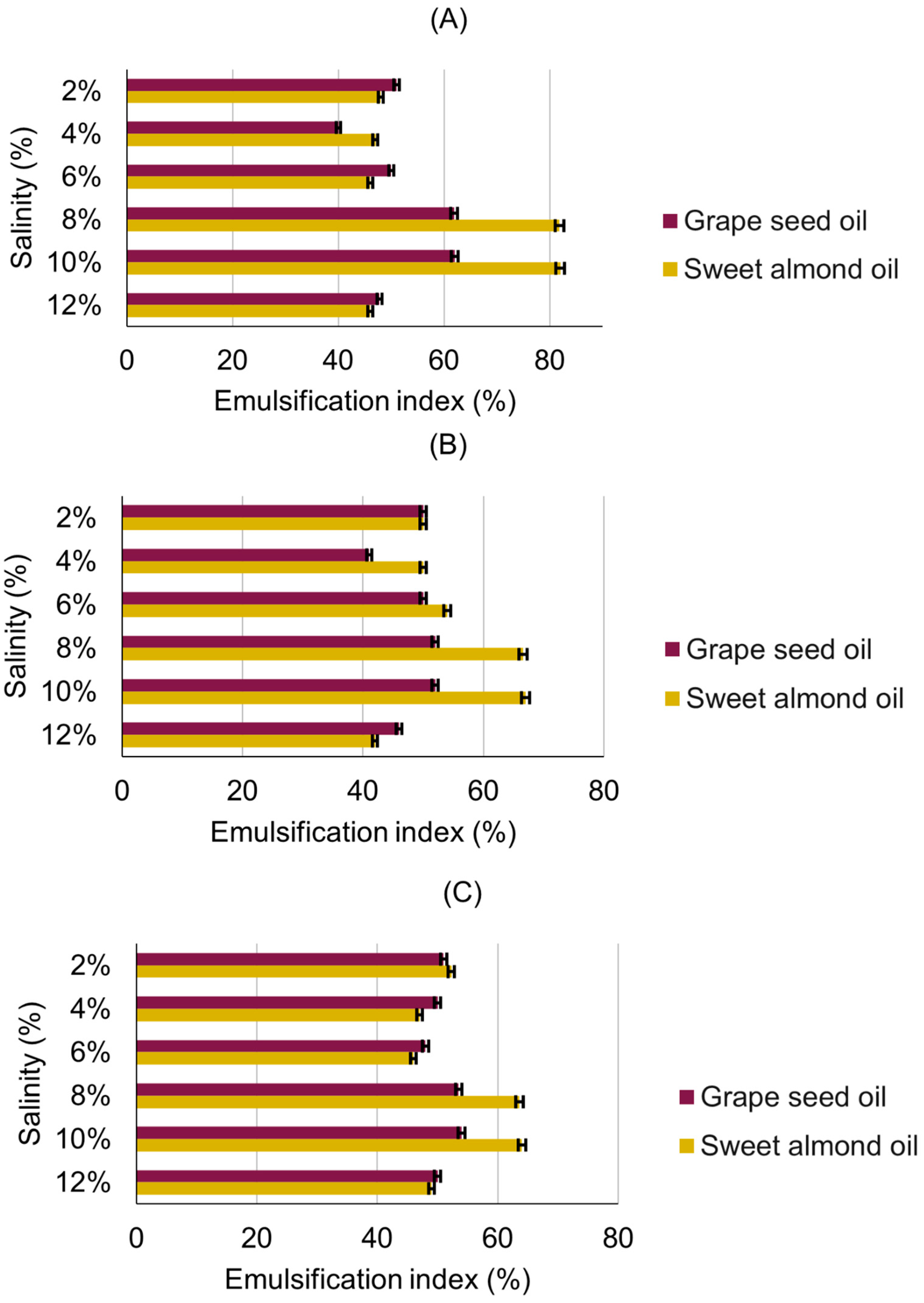
3.3. Emulsification Activity
3.4. Main Features of Mouthwash Formulations
3.5. Genotoxicity of Formulations
3.6. Antimicrobial Activity of Formulations
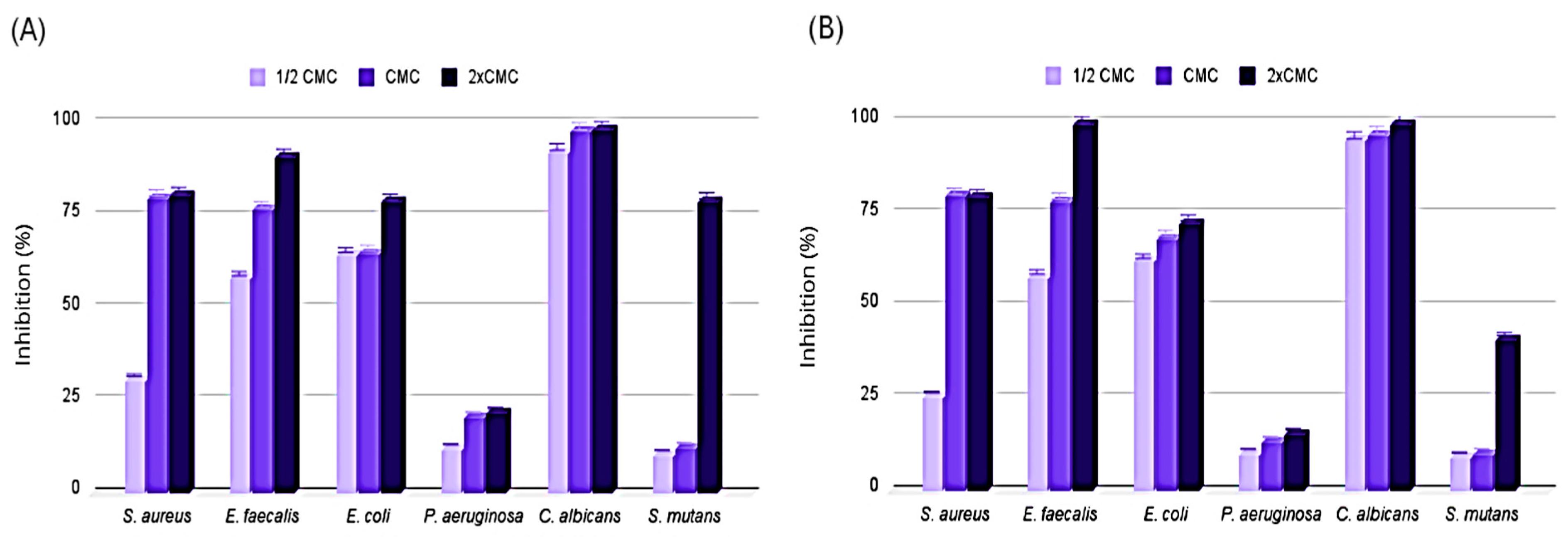
3.7. Antibiofilm Activity of Test Substances and Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, A.; Nagaiah, H.P.; Malligarjunan, N.; Pandian, S.K. Oral biofilms: Architecture and control. In Understanding Microbial Biofilms., 1st ed.; Das, S., Kungwani, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 29; pp. 485–507. [Google Scholar] [CrossRef]
- Verma, T.; Grover, N.; Mlhotra, S. Various applications of chlorhexidine as an antimicrobial agente in dentistry: A review. Int. Dent. J. Stud. Res. 2023, 22, 50–53. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for replacement by biosurfactants. Adv. Colloid Interface Sci. 2011, 288, 102–340. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A promising multifunctional cosmetic ingrediente for skin and hair care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Narayanan, A.; Kartik, R.; Sangeetha, E.; Dhamodharan, R. Super water absorbing polymeric gel from chitosan, citric acid and urea: Synthesis and mechanism of water absorption. Carbohydr. Polym. 2018, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Visnjar, T.; Jerman, U.D.; Veranic, P.; Kreft, M.E. Chitosan hydrochloride has no detrimental effect on bladder urothelial cancer cells. Toxicol. Vitro 2017, 44, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Xie, Q.T.; Zhu, J.; Pab, Y.; Meng, R.; Zhang, B.; Jin, Z.Y. Chitosan hydrochloride/carboxymethyl starch complex nanogels as novel pickering stabilizers: Physical stability and rheological properties. Food Hydrocoll. 2019, 93, 215–225. [Google Scholar] [CrossRef]
- Dias, K.B.; Silva, D.P.; Ferreira, L.A.; Fidelis, R.R.; Costa, J.L.; Silva, A.L.L.; Scheidt, G.N. Chitin and chitosan: Characteristics, uses and production current perspectives. J. Biotechnol. Biodivers. 2013, 4, 184–191. [Google Scholar] [CrossRef]
- Bezerra, K.G.O.; Silva, I.G.S.; Almeida, F.C.G.; Rufino, R.D.; Sarubbo, L.A. Plant-derived biosurfactants: Extraction, characteristics and properties for applications in cosmetics. Biocatal. Agric. Biotechnol. 2021, 34, 102036. [Google Scholar] [CrossRef]
- Zhou, T.; Ao, M.; Xu, G.; Liu, T.; Zhang, J. Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2013, 389, 175–181. [Google Scholar] [CrossRef]
- Souza, A.C.; Silva, M.S.; Simões, L.A.; Fernandes, N.A.T.; Schwan, R.F.; Dias, D.R. Advantages of biosurfactants over petroleum-based surfactants. Indust. Appl. Biosurfactants Microorg. 2024, 1, 371–393. [Google Scholar] [CrossRef]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, remediation and green surfactants. Envirom. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Hartal, O.; Haddaji, C.; Anouzla, A.; Madinzi, A.; Souabi, S. Recent advances in physicochemical and biological techniques for the management of discharges loaded with surfactants. Chem. Biochem. Eng. 2023, 37, 1–16. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Jaysree, R.C.; Rajendran, N. A review on biosurfactants. Int. J. Res. Pharm. Sci. 2002, 23, 359–366. [Google Scholar] [CrossRef]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Bouzier, A.; Rojas, J.; Ibinga, S.K.K.; Lamarti, A.; Martin, P.; Morillo, M. The impact of saponins on health-review. Biointerface Res. Appl. Chem. 2023, 13, 362–382. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Y.; Yi, J.; Cao, Y.; Liu, F.; McClements, D.J. Comparison of natural and synthetic surfactants at forming and stabilizing nanoemulsions: Tea saponin, Quillaja saponin, and Tween 80. J. Colloid. Interface Sci. 2019, 536, 80–87. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Alhalafi, M.H.; Eman, E.M.; Ibrahim, H.; Mosaad, R.M. A review of chitosan and chitosan nanofiber: Preparation, characterization and its potential applications. Polymers 2023, 25, 2820. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.M.; Silva, S.; Madureira, A.R.; Cardelle-Cobas, A.; Tavaria, F.K.; Pintado, M.M. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr. Polym. 2014, 101, 1081–1086. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]
- Farias, J.M.; Stamford, C.M.; Resende, A.H.M.; Aguiar, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing biosurfactant and chitosan: An eco-sustainable option for controlling cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Parvan, L.G.; Leite, T.G.; Freitas, T.B.; Pedrosa, P.A.A.; Calixto, J.S.; Agostinho, L.A. Bioensaio con Allium cepa revela genotoxicidade de herbicida com flumioxazin. Rev. Pan-Amaz. Saude 2020, 11, e202000544. [Google Scholar] [CrossRef]
- Driessche, F.V.; Rigole, P.; Brackman, G.; Coenye, T. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 2014, 98, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Amann, V.; Kissmann, A.; Kramer, M.; Krebs, I.; Perez-Erviti, J.A.; Otero-Gonzalez, A.J.; Vicente, F.M.; Rodríguez, A.; Standker, L.; Weil, T.; et al. Increased activities against biofilms of the pathogenic yeast Candida albicans of optimized Pom-1 derivatives. Polymers 2022, 14, 318. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.; Callow, M.E.; Bott, T.R. The structure of Pseudomonas fluorescens biofilms in contact with flowing systems. Biofouling 1991, 4, 319–336. [Google Scholar] [CrossRef]
- Le, B.; Do, D.T.; Nguyen, H.M.; Do, B.H.; Le, H.T. Preparation, characterization and anti-adhesive activity of sulfate polysaccharide from Caulerpa lentillifera against Helicobacter pylori. Polymers 2022, 14, 4993. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kaur, R.; Kumar, S.; Saini, R.K.; Sharma, S.; Pawde, S.V.; Kumar, V. Saponins: A concise review on food related aspects, applications and health implications. Food Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass spectrometry analysis of saponin. Mass Spectrom. Rev. 2023, 42, 954–983. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, K.G.O.; Meira, H.M.; Veras, B.O.; Stamford, T.C.M.; Fernandes, E.L.; Converti, A.; Rufino, R.D.; Sarubbo, L.A. Application of plants surfactants as cleaning agents in shampoo formulations. Processes 2023, 11, 879. [Google Scholar] [CrossRef]
- Wu, R.; Tian, M.; Shu, C.; Zhou, C.; Guan, W. Determination of the critical micelle concentration of surfactants using fluorescence strategies. Soft Matter 2022, 18, 8920–8930. [Google Scholar] [CrossRef]
- Unal, D.N.; Yildririm, S.; Kurbanoglu, S.; Uslu, B. Current trends and roles of surfactants for chromatographic and electrochemical sensing. Trend Anal. Chem. 2021, 144, 116418. [Google Scholar] [CrossRef]
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377. [Google Scholar] [CrossRef]
- Ralla, T.; Herz, E.; Salminen, H.; Edelmann, M.; Dawid, C.; Hofmann, T.; Weiss, J. Emulsifying properties of natural extracts from Panax ginseng L. Food Biophys. 2017, 12, 479–490. [Google Scholar] [CrossRef]
- Ferreira, D.S. Application of Infrared Spectroscopy and Multivariate Analysis to Predict Quality Parameters in Soybean and Quinoa. PhD Thesis, State University of Campinas, Campinas, Brazil, 2013. [Google Scholar] [CrossRef]
- Patil, A.; Bhide, S.; Bookwala, M.; Soneta, B.; Shankar, V.; Almotairy, A.; Almutairi, M.; Murthy, S.N. Stability of organoleptic agentes in pharmaceuticals and cosmetics. AAPS Pharm. SciTech. 2017, 19, 36–47. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, K.; Wang, W.; Ge, Y.; Yu, Z.; Peng, Z.; Gong, J. Effect of oil-soluble/water-soluble surfactants on the stability of water-in-oil systems, an atomic force microscopy study. Langmuir 2023, 39, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Saengsorn, K.; Jimtaisong, A. Determination of hydrophilic–lipophilic balance value and emulsion properties of sacha inchi oil. Asian Pac. J. Trop. Biomed. 2017, 7, 1092–1096. [Google Scholar] [CrossRef]
- Santos, D.S.F.; Peralta-Mamani, M.; Brandão, F.S.; Andrade, F.B.; Cruvinel, T.; Santos, P.S.S. Could polyhexanide and chlorine dioxide be used as an alternative to chlorhexidine? A systematic review. Sao Paulo Med. J. 2022, 140, 42–55. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Jan, C.C. How fluoride protects dental enamel from demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134–141. [Google Scholar] [CrossRef]
- Stamford Arnaud, T.M.S.; Barros Neto, B.; Diniz, F.B. Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J. Dent. 2010, 38, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, M.; Fourati, N.; Krichen, F.; Zouari, R.; Ellouz-Chaabouani, S.; Ghribi, D. Potential application of Bacillus subtilis SPB1 lipopeptides in toothpaste. J. Adv. Res. 2017, 8, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Kuruppuarachchi, S.U.; Jayawardena, U.A.; Gunathilake, V.K. Use of the Allium cepa model to assess the cytogenotoxicity of Luffariella herdmani marine sponge extract. Altern. Lab. Anim. 2023, 51, 175–187. [Google Scholar] [CrossRef]
- Postay, L.F.; Cabral, D.S.; Heringer, O.A.; Vieira, L.V.; de Moraes, L.R.; Freitas, G.; Gomes, L.C. The effectiveness of surfactants applied with essential oil of Lippia alba in the anesthesia of Nile tilapia (Oreochromis niloticus) and their toxicity assessment for fish and mammals. Environ. Sci. Pollut. Res. Int. 2021, 28, 10224–10233. [Google Scholar] [CrossRef] [PubMed]
- Cmikova, N.; Galovicovva, L.; Schwarzova, M.; Kacaniova, M. use of Mentha spicata essential oil for prolonging postharvest life of fresh vegetables. Acta Hortic. Regiotect. 2023, 26, 35–42. [Google Scholar] [CrossRef]
- Fowler, P.W.; Wright, C.; Spiers, H.; Zhu, T.; Barten, E.M.L.; Hoosdally, S.W.; Gilbertoni-Cruz, A.L.; Roohi, A.; Kouchaki, S.; Walker, T.M.; et al. A crowd of BashTheBug volunteers reproducibly and accurately measure the minimum inhibitory concentrations of 13 antitubercular drugs from photographs of 96-well broth microdilution plates. Microbiol. Infect. Dis. 2023, 11, e75046. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.B.; Reinert, L.H.; Meneguello, J.E.; Ieque, A.L.; Siqueira, V.L.S.; Lima Scodro, R.B.; Caleffi-Ferracioli, K.R. Advantage of the use of oxyreductive dye and automated reading in the determination of pharmaceutical activity in bacteria of medical importance. Rev. Uningá 2023, 60, eUJ4398. [Google Scholar] [CrossRef]
- Monteiro, M.C.; De La Cruz, M.; Cantizani, J.; Moreno, C.; Tormo, J.R.; Mellado, E.; De Lucas, J.R.; Asensio, F.; Valiante, V.; Brakhage, A.A.; et al. A new approach to drug Discovery: High-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J. Biomol. Screen. 2012, 17, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Montoro, E.; Lemus, D.; Echemendia, M.; Martin, A.; Portaels, F.; Palomino, J.C. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microlitre ssay for drug susceptibility testing of clinical isolates of Mycobacteruim turbeculosis. J. Antimicrob. Chemother. 2005, 55, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.H.; Nazni, W.A.; Nurulhusna, A.H.; Norazah, A.; Lee, H.L. Determination of antibacterial activity nd minimum inhibitory concentration of larval extract of fly via resazurin-based turbidometric assay. BMC Microbiol. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf. B 2011, 84, 1–5. [Google Scholar] [CrossRef]



| Component | Function | Proportion (%, w/w) |
|---|---|---|
| Chitosan hydrochloride | Active principle | 1.0 |
| Mentha spicata essential oil | Refreshment and flavor | 0.1 |
| Biosurfactant | Active ingredient and surfactant | 0.1 |
| Sodium benzoate | Preservative | 0.1 |
| Sodium saccharin | Sweetener | 0.2 |
| Sorbitol | Sweetener | 3.0 |
| Distilled water | Solvent | Up to 100 mL |
| Microorganism | Minimum Inhibitory Concentration of Mouthwash (g/L) | ||||||
|---|---|---|---|---|---|---|---|
| CMCC | CMM | CMCD | Chenopodium quinoa | Glycine max | |||
| Quinoa | CHC | Soy | CHC | ||||
| Staphylococcus aureus ATCC 6538 | 0.2 | 0.168 | 0.36 | 2.1 | 3.0 | 2.4 | 3.0 |
| Enterococcus faecalis ATCC 29212 | 0.2 | 0.126 | 0.24 | 1.05 | 1.5 | 1.6 | 2.0 |
| Escherichia coli ATCC 25922 | 0.15 | 0.126 | 0.24 | 1.4 | 2.0 | 1.6 | 2.0 |
| Pseudomonas aeruginosa ATCC 9027 | 0.2 | 0.168 | 0.36 | 2.1 | 3.0 | 1.6 | 2.0 |
| Candida albicans URM 14053 | 0.25 | 0.168 | 0.48 | 2.8 | 4.0 | 2.4 | 3.0 |
| Streptococcus mutans ATCC 25175 | 0.15 | 0.126 | 0.24 | 0.7 | 1.0 | 0.8 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, I.R.; Bezerra, K.G.O.; Oliveira, C.L.; Meira, H.M.; Stamford, T.C.M.; Converti, A.; Sarubbo, L.A.; Rufino, R.D. Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity. Appl. Sci. 2024, 14, 6711. https://doi.org/10.3390/app14156711
Souza IR, Bezerra KGO, Oliveira CL, Meira HM, Stamford TCM, Converti A, Sarubbo LA, Rufino RD. Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity. Applied Sciences. 2024; 14(15):6711. https://doi.org/10.3390/app14156711
Chicago/Turabian StyleSouza, Izabelle R., Káren G. O. Bezerra, Camila L. Oliveira, Hugo M. Meira, Thayza C. M. Stamford, Attilio Converti, Leonie A. Sarubbo, and Raquel D. Rufino. 2024. "Mouthwash Containing Plant-Derived Biosurfactant and Chitosan Hydrochloride: Assessment of Antimicrobial Activity, Antibiofilm Activity, and Genotoxicity" Applied Sciences 14, no. 15: 6711. https://doi.org/10.3390/app14156711







