Synergistic Effects of Fuel Components on Aromatics Formation in Combustion: A Review
Abstract
:1. Introduction
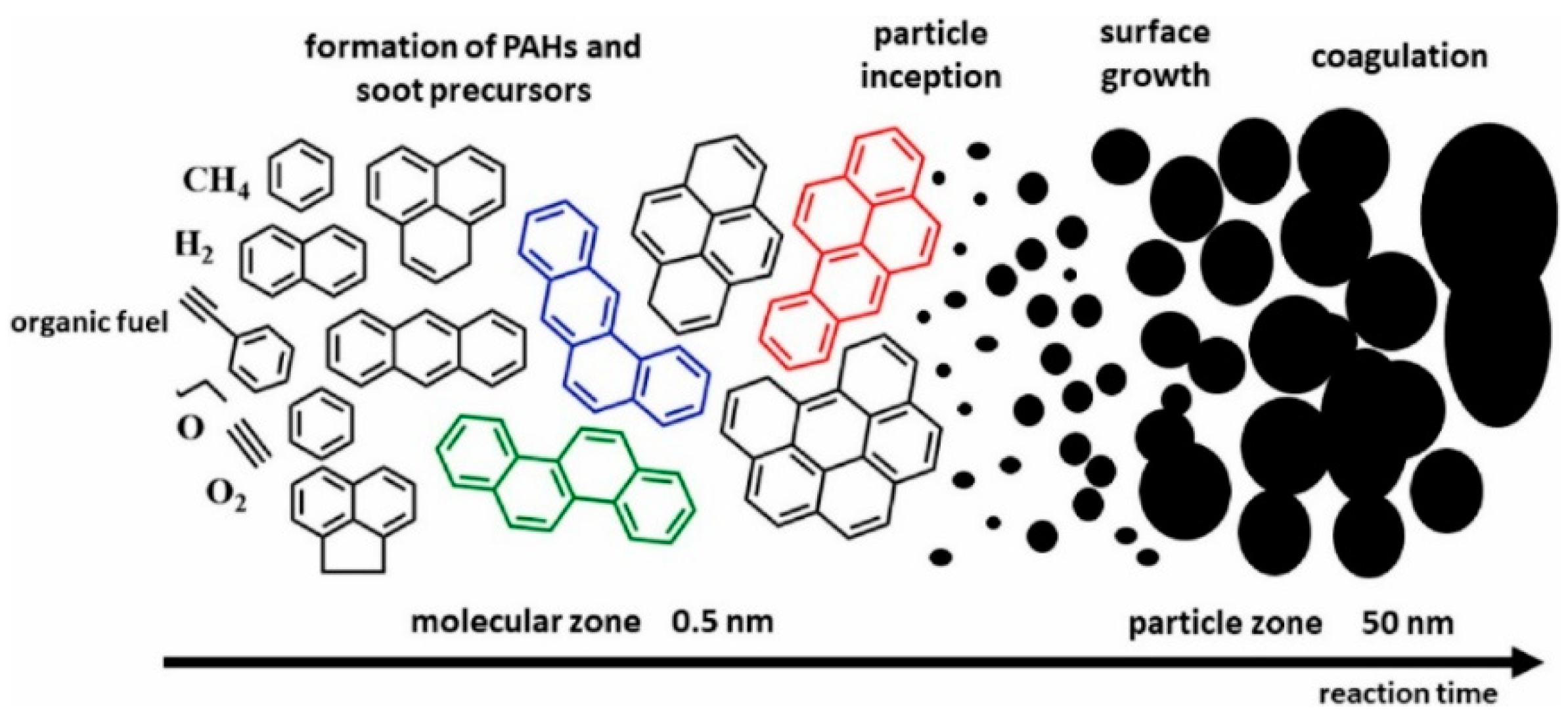
2. Formation Mechanisms of Benzene and PAHs
2.1. Formation Mechanisms of Benzene
2.2. Formation Mechanisms of PAHs
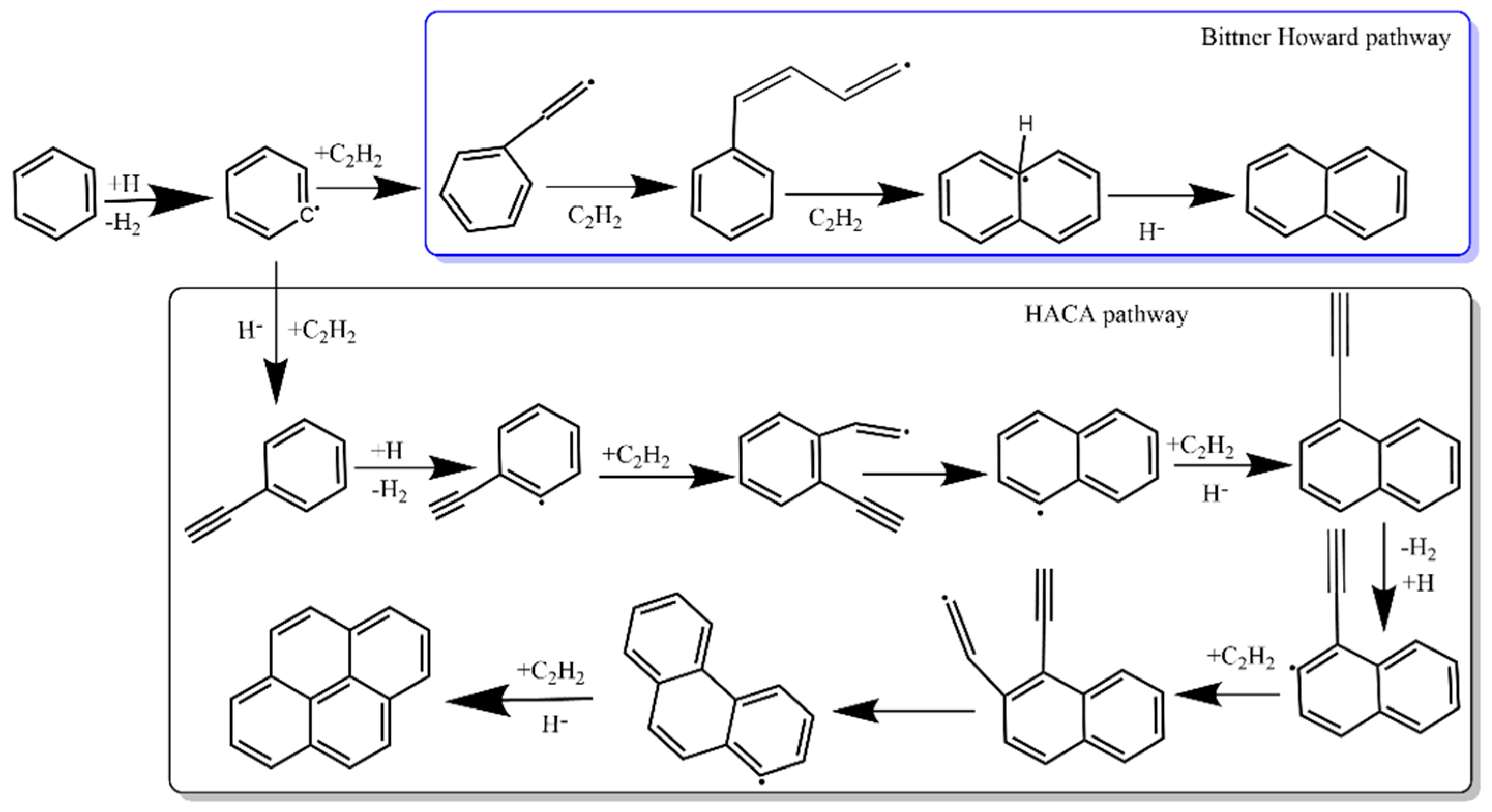
2.2.1. Hydrogen Abstraction and Acetylene Addition Mechanism
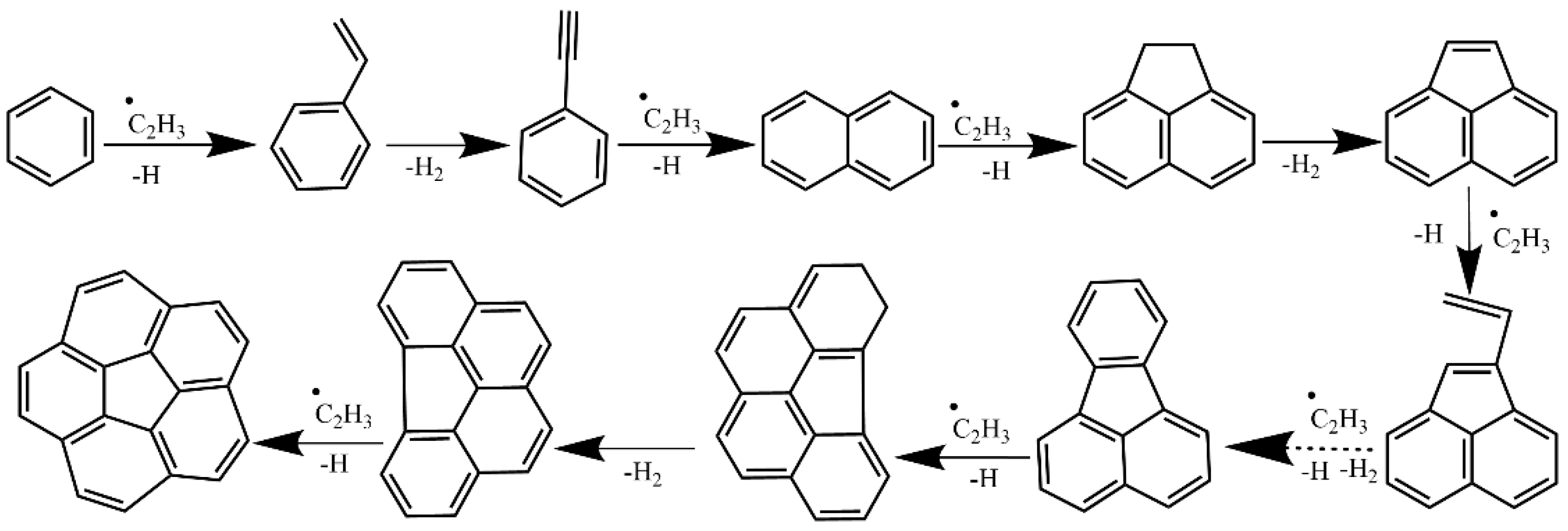
2.2.2. Hydrogen Abstraction and Vinylacetylene Addition Mechanism
2.2.3. Formation Mechanism with Radicals
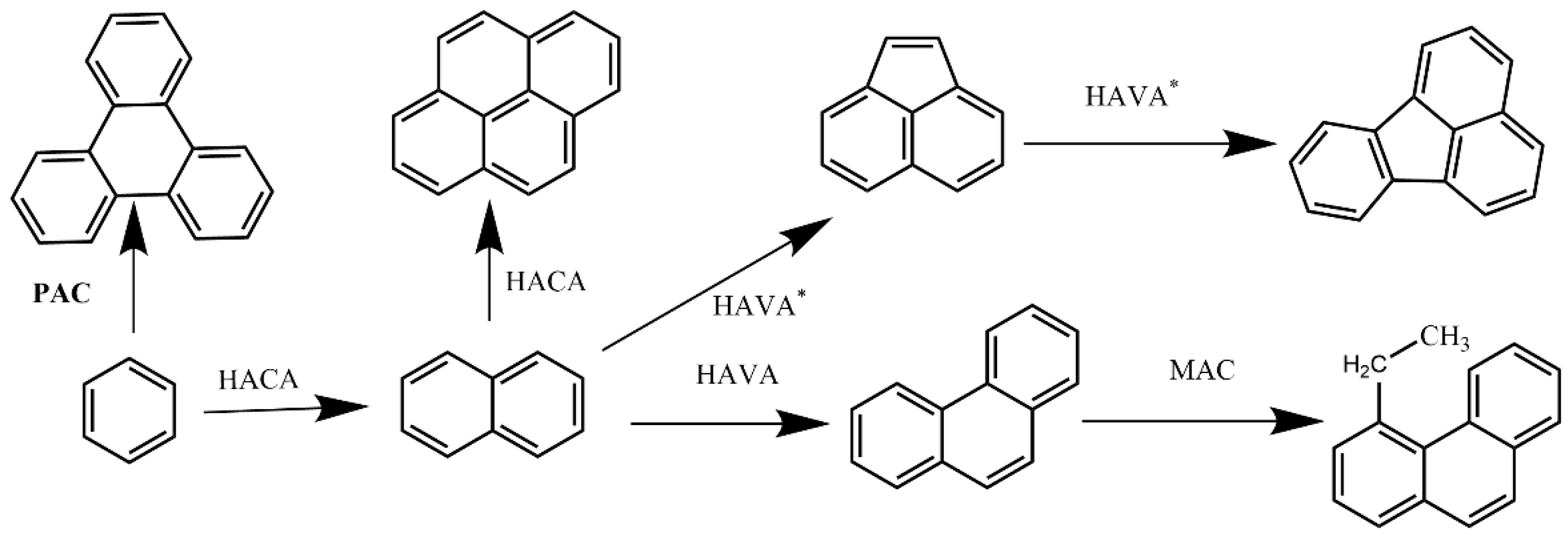
2.2.4. Hydrogen Abstraction Phenylacetylene Addition (HAPaA) Mechanism
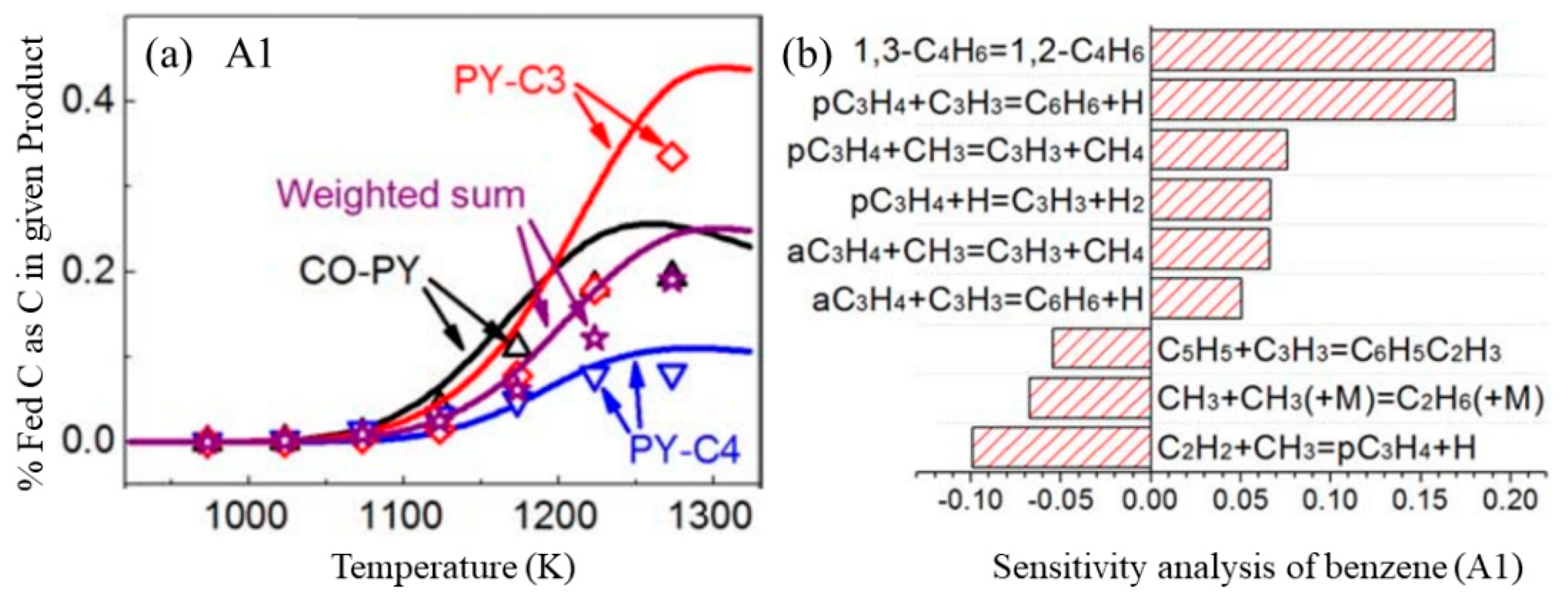
3. Synergistic Effects on Benzene Formation
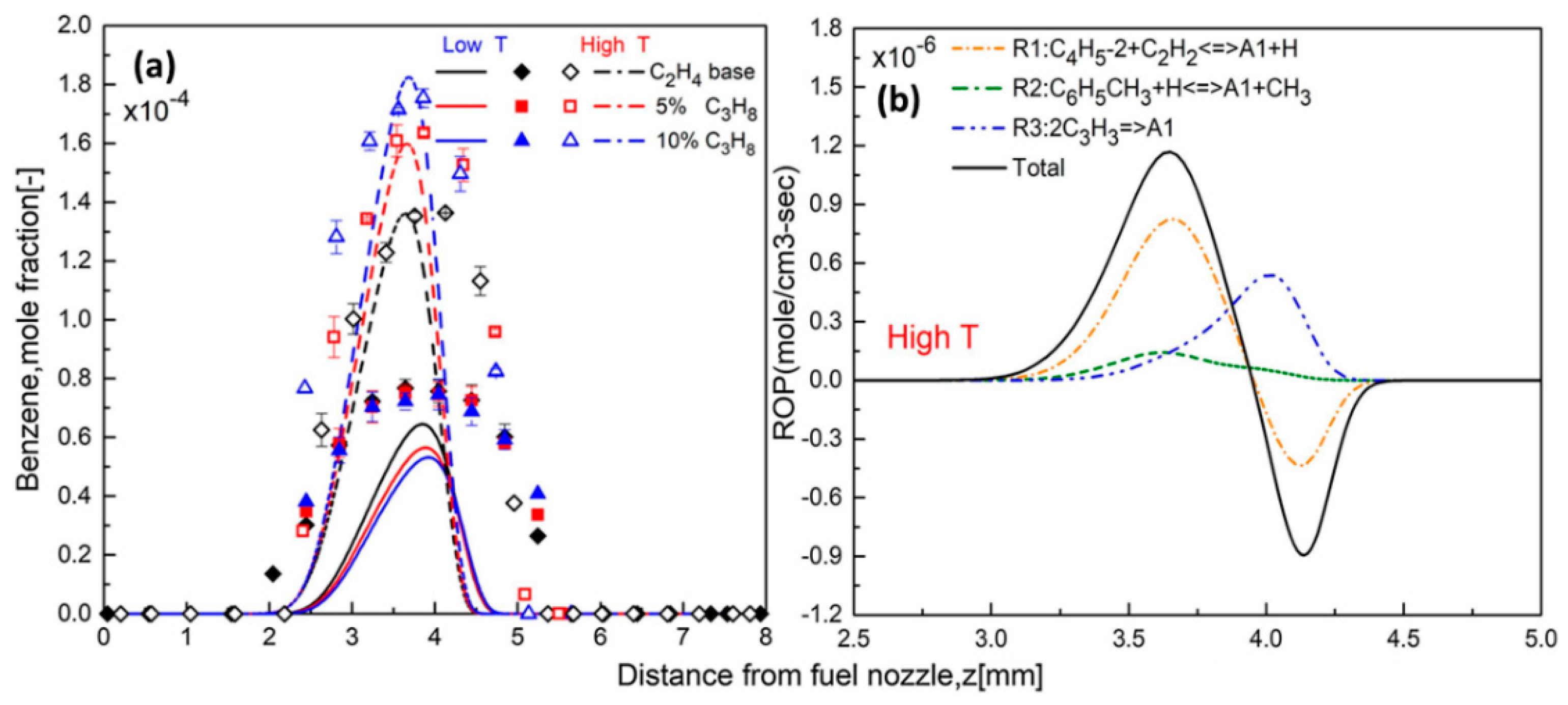
3.1. Synergistic Effects between Hydrocarbons
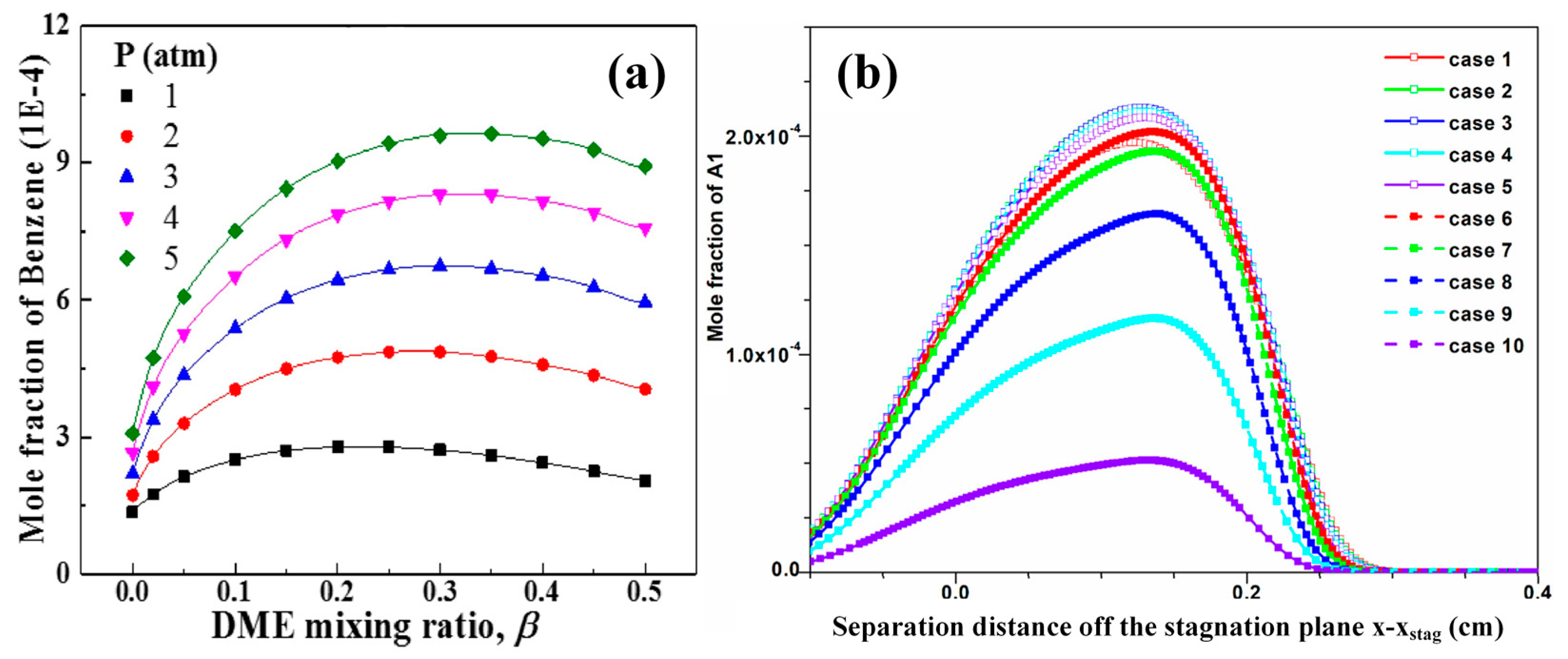
3.2. Synergistic Effects between Hydrocarbons and Oxygenated Fuels
4. Synergistic Effects on PAHs Formation
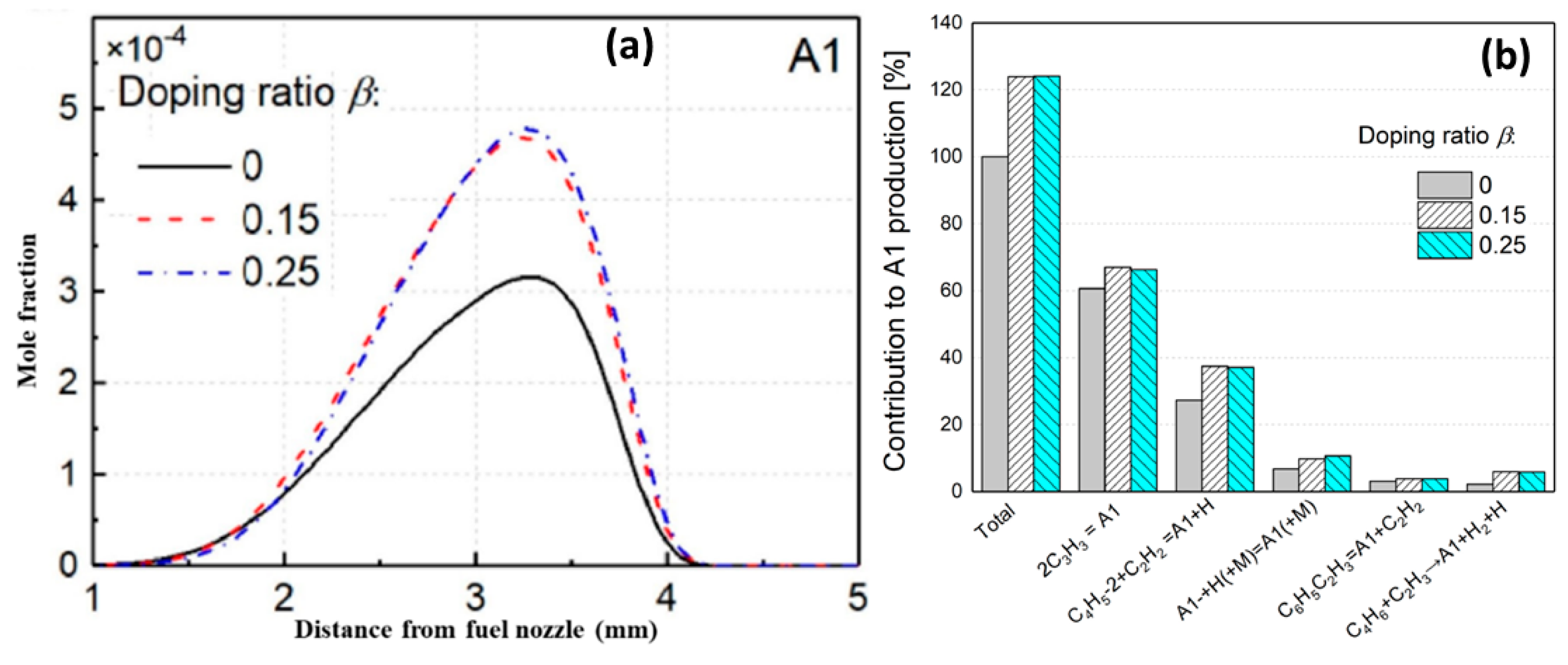
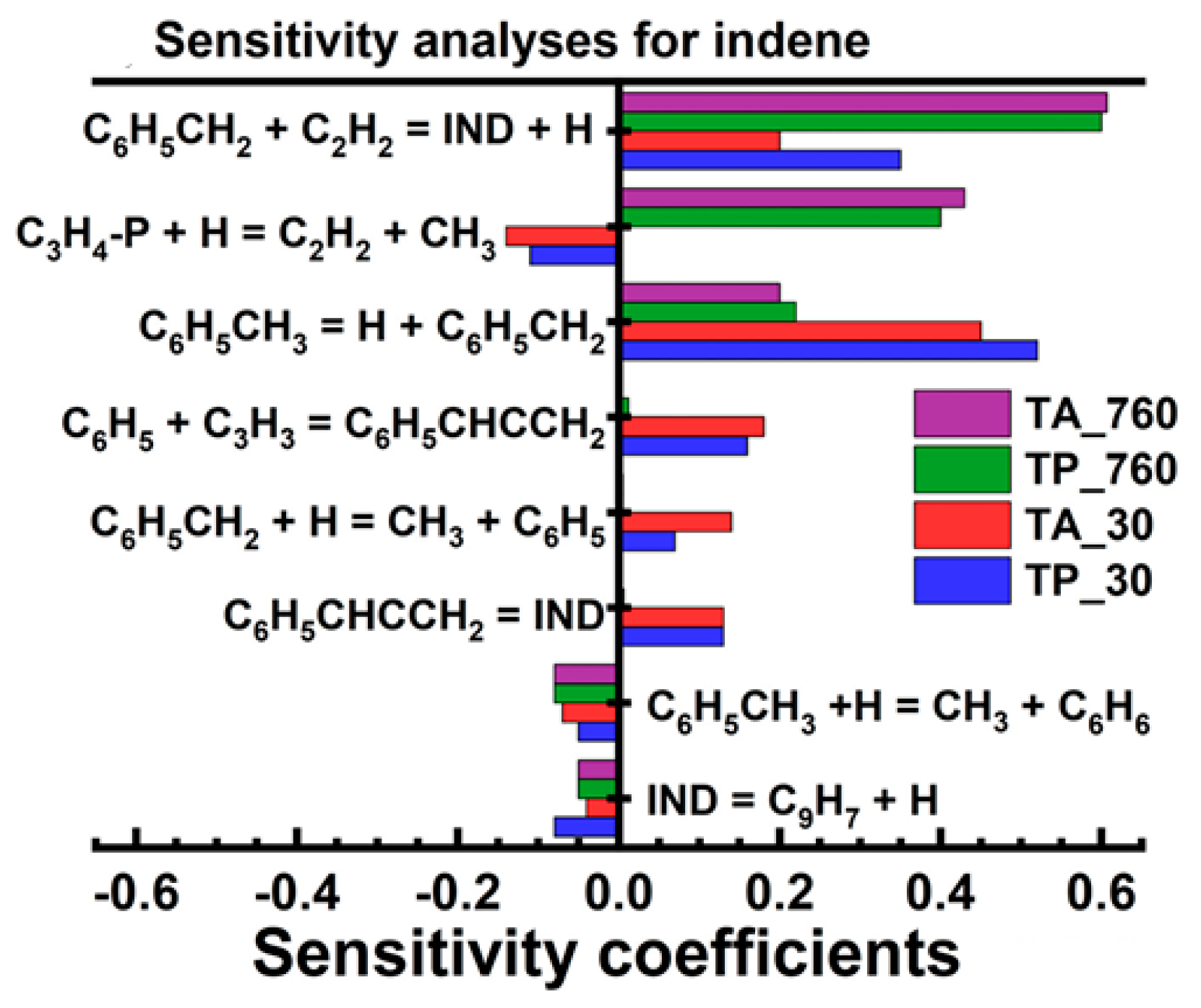
4.1. Synergistic Effect on Indene Formation
4.2. Synergistic Effect on Naphthalene Formation
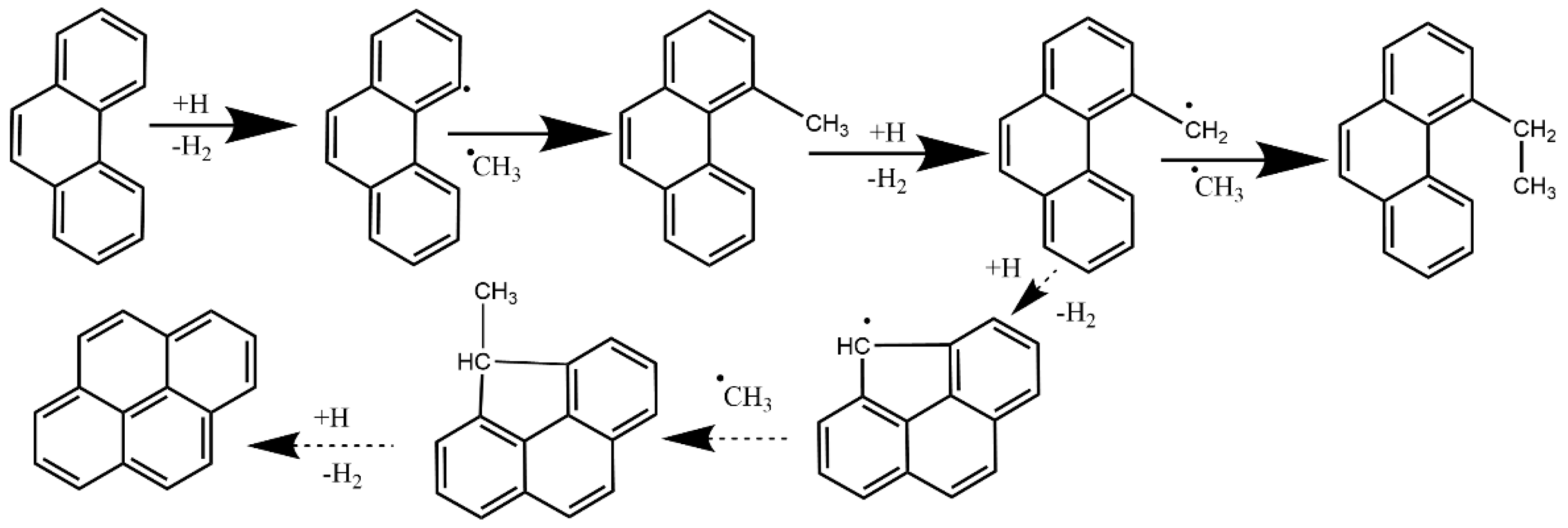
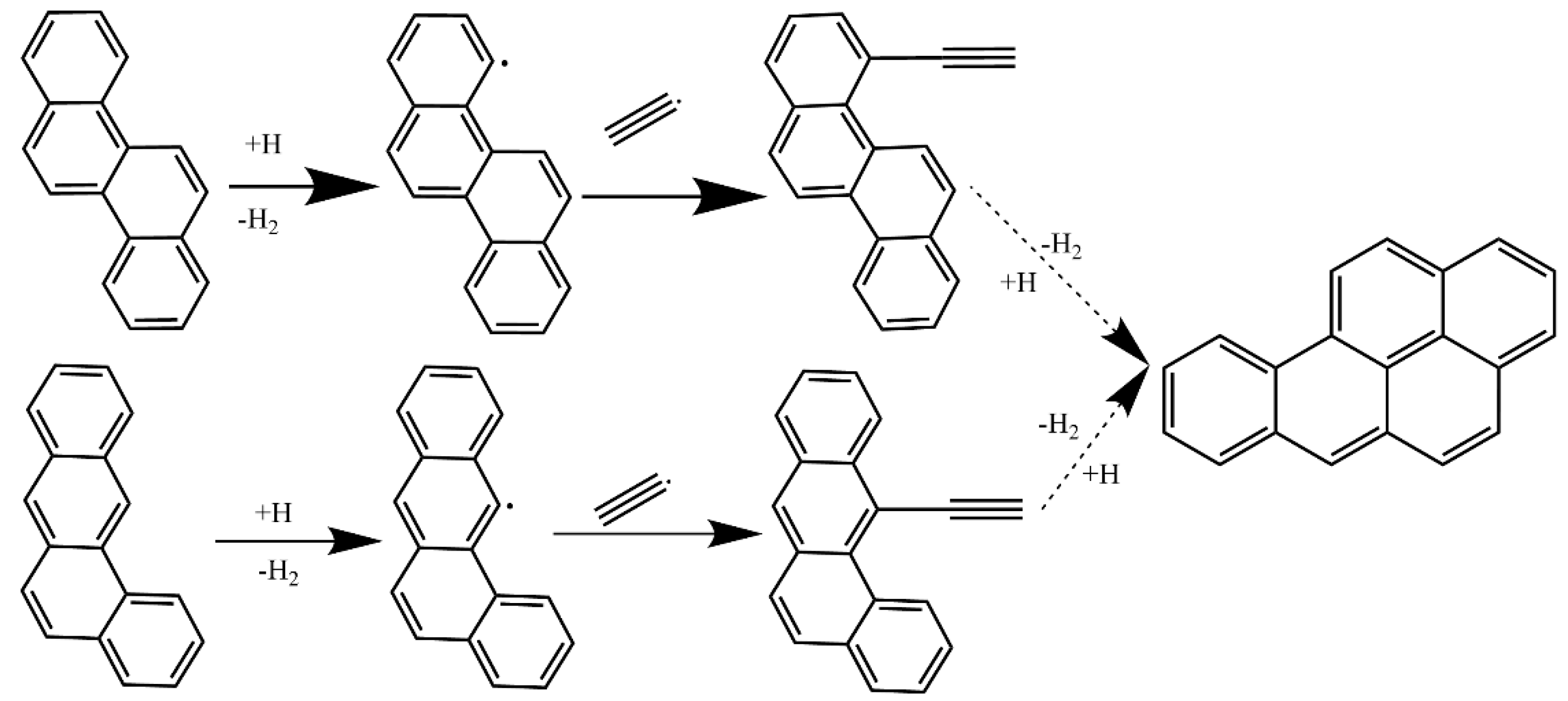
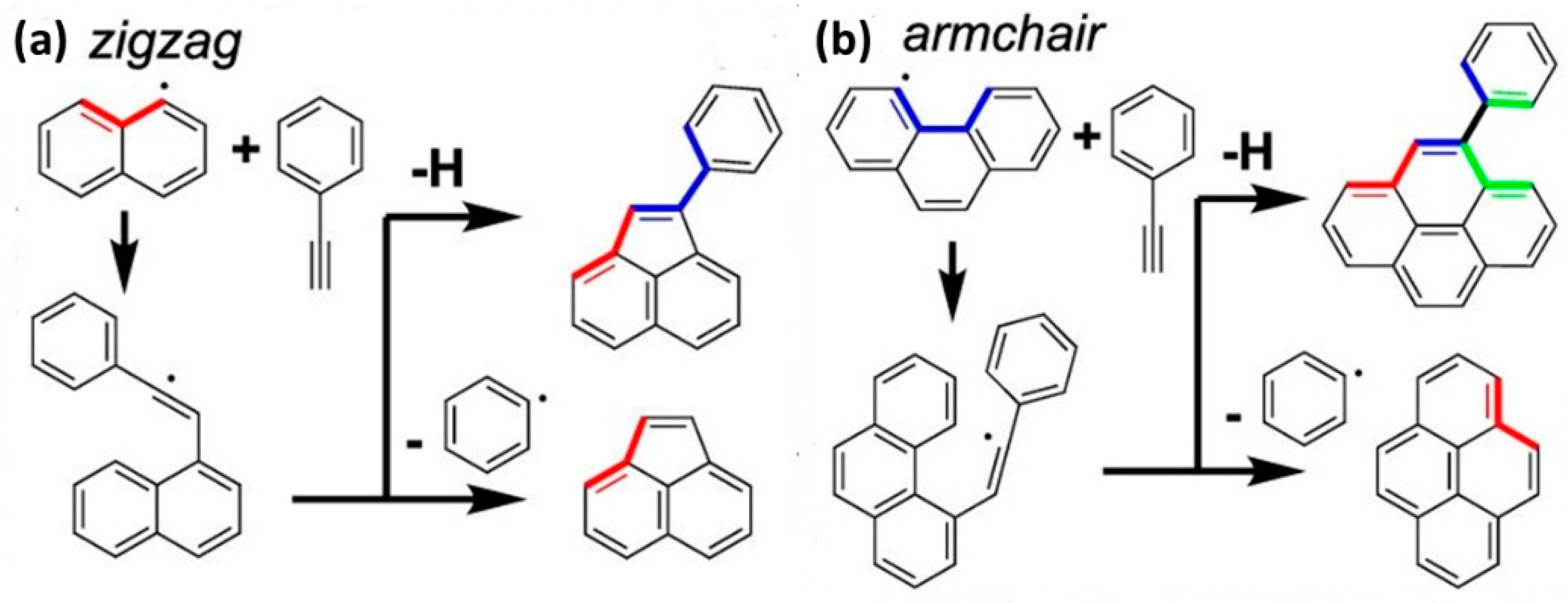
4.3. Synergistic Effect on Larger PAHs Formation
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, I.; Badran, G.; Verdin, A.; Ledoux, F.; Roumié, M.; Courcot, D.; Garçon, G. Polycyclic aromatic hydrocarbon derivatives in airborne particulate matter: Sources, analysis and toxicity. Environ. Chem. Lett. 2018, 16, 439–475. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.n.; Zhang, J.; Song, K.; Mu, G.; Sun, C.; Ju, H.; Ji, M. The relationship of chromophoric dissolved organic matter parallel factor analysis fluorescence and polycyclic aromatic hydrocarbons in natural surface waters. Environ. Sci. Pollut. Res. 2018, 25, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, A.; Sirignano, M. Detailed kinetic mechanisms of PAH and soot formation. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; Volume 45, pp. 647–672. [Google Scholar]
- Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Wei, Y.; Hu, M.; Zhao, L.; Toriba, A.; Hayakawa, K. Size distribution of particulate polycyclic aromatic hydrocarbons in fresh combustion smoke and ambient air: A review. J. Environ. Sci. 2020, 88, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2021, 291, 132793. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yan, F.; Dai, W.; Zhou, M.; Chung, S.H.; Wang, Y. Synergistic effects on soot formation in counterflow diffusion flames of acetylene-based binary mixture fuels. Combust. Flame 2020, 216, 24–28. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ashraf, M.A.; Steinmetz, S.A.; Dunn, M.J.; Masri, A.R. The role of DME addition on the evolution of soot and soot precursors in laminar ethylene jet flames. Proc. Combust. Inst. 2021, 38, 5319–5329. [Google Scholar] [CrossRef]
- Yang, S.S.; Gülder, Ö.L. Sooting characteristics of ethanol-ethylene blends in laminar coflow diffusion flames up to 10 bar. Combust. Flame 2021, 225, 39–47. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Yuan, W.; Cao, C.; Li, W.; Yang, J.; Li, Y. Unraveling synergistic effects on pyrolysis reactivity and indene formation in co-pyrolysis of toluene and acetylene. Proc. Combust. Inst. 2021, 38, 1413–1421. [Google Scholar] [CrossRef]
- Poddar, N.B.; Thomas, S.; Wornat, M.J. Polycyclic aromatic hydrocarbons from the co-pyrolysis of 1,3-butadiene and propyne. Proc. Combust. Inst. 2013, 34, 1775–1783. [Google Scholar] [CrossRef]
- Zhang, Y.; Somers, K.P.; Mehl, M.; Pitz, W.J.; Cracknell, R.F.; Curran, H.J. Probing the antagonistic effect of toluene as a component in surrogate fuel models at low temperatures and high pressures. A case study of toluene/dimethyl ether mixtures. Proc. Combust. Inst. 2017, 36, 413–421. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Shahanaghi, A.; Kaario, O.; Vuorinen, V.; Tripathi, R.; Sarjovaara, T. Effects of blending 2,5-dimethylfuran and dimethyl ether to toluene primary reference fuels: A chemical kinetic study. Fuel 2021, 304, 121401. [Google Scholar] [CrossRef]
- Xu, H.; Yao, C.; Xu, G.; Wang, Z.; Jin, H. Experimental and modelling studies of the effects of methanol and ethanol addition on the laminar premixed low-pressure n-heptane/toluene flames. Combust. Flame 2013, 160, 1333–1344. [Google Scholar] [CrossRef]
- Lindstedt, R.; Skevis, G. Benene formation chemistry in premixed 1, 3-butadiene flames. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1996; pp. 703–709. [Google Scholar]
- Buras, Z.J.; Dames, E.E.; Merchant, S.S.; Liu, G.; Elsamra, R.M.I.; Green, W.H. Kinetics and Products of Vinyl + 1,3-Butadiene, a Potential Route to Benzene. J. Phys. Chem. A 2015, 119, 7325–7338. [Google Scholar] [CrossRef]
- Jursic, B.; Zdravkovski, Z. DFT study of the Diels–Alder reactions between ethylene with buta-1,3-diene and cyclopentadiene. J. Chem. Soc. Perkin Trans. 1995, 2, 1223–1226. [Google Scholar] [CrossRef]
- Woods, P.M.; Millar, T.; Zijlstra, A.; Herbst, E. The synthesis of benzene in the proto-planetary nebula CRL 618. Astrophys. J. 2002, 574, L167. [Google Scholar] [CrossRef]
- Wang, E.; Ding, J. Reaction between the i-C4H5 radical and propargyl radical (C3H3): A theoretical study. Chem. Phys. Lett. 2021, 768, 138407. [Google Scholar] [CrossRef]
- Zhang, F.; Jones, B.; Maksyutenko, P.; Kaiser, R.I.; Chin, C.; Kislov, V.V.; Mebel, A.M. Formation of the phenyl radical [C6H5 (X2A1)] under single collision conditions: A crossed molecular beam and ab initio study. J. Am. Chem. Soc. 2010, 132, 2672–2683. [Google Scholar] [CrossRef]
- Stein, S.E.; Walker, J.A.; Suryan, M.M.; Fahr, A. A new path to benzene in flames. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1991; pp. 85–90. [Google Scholar]
- Frenklach, M.; Clary, D.W.; Gardiner, W.C., Jr.; Stein, S.E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1985; pp. 887–901. [Google Scholar]
- Frenklach, M.; Clary, D.W.; Ramachandra, M. Shock Tube Study of the Fuel Structure Effects on the Chemical Kinetic Mechanisms Responsible for Soot Formation, Part 2. NAS 1.26:174880, 1 May 1985. [Google Scholar]
- Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1991; pp. 1559–1566. [Google Scholar]
- Moriarty, N.W.; Brown, N.J.; Frenklach, M. Hydrogen migration in the phenylethen-2-yl radical. J. Phys. Chem. A 1999, 103, 7127–7135. [Google Scholar] [CrossRef]
- Kislov, V.; Sadovnikov, A.; Mebel, A. Formation mechanism of polycyclic aromatic hydrocarbons beyond the second aromatic ring. J. Phys. Chem. A 2013, 117, 4794–4816. [Google Scholar] [CrossRef] [PubMed]
- Frenklach, M.; Singh, R.I.; Mebel, A.M. On the low-temperature limit of HACA. Proc. Combust. Inst. 2019, 37, 969–976. [Google Scholar] [CrossRef]
- Shukla, B.; Susa, A.; Miyoshi, A.; Koshi, M. Role of phenyl radicals in the growth of polycyclic aromatic hydrocarbons. J. Phys. Chem. A 2008, 112, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Kislov, V.; Islamova, N.; Kolker, A.; Lin, S.; Mebel, A. Hydrogen abstraction acetylene addition and Diels—Alder mechanisms of PAH formation: A detailed study using first principles calculations. J. Chem. Theory Comput. 2005, 1, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Xu, L.; Wang, Y.; Park, S.; Sarathy, S.M.; Chung, S.H. On the opposing effects of methanol and ethanol addition on PAH and soot formation in ethylene counterflow diffusion flames. Combust. Flame 2019, 202, 228–242. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Bittner, J.; Howard, J. Composition profiles and reaction mechanisms in a near-sooting premixed benzene/oxygen/argon flame. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1981; pp. 1105–1116. [Google Scholar]
- Raj, A.; Prada, I.D.C.; Amer, A.A.; Chung, S.H. A reaction mechanism for gasoline surrogate fuels for large polycyclic aromatic hydrocarbons. Combust. Flame 2012, 159, 500–515. [Google Scholar] [CrossRef]
- Mebel, A.M.; Georgievskii, Y.; Jasper, A.W.; Klippenstein, S.J. Temperature-and pressure-dependent rate coefficients for the HACA pathways from benzene to naphthalene. Proc. Combust. Inst. 2017, 36, 919–926. [Google Scholar] [CrossRef]
- Shinde, V.M.; Pradeep, P. Detailed gas-phase kinetics and reduced reaction mechanism for methane pyrolysis involved in CVD/CVI processes. J. Anal. Appl. Pyrolysis 2021, 154, 104998. [Google Scholar] [CrossRef]
- Sun, W.; Hamadi, A.; Abid, S.; Chaumeix, N.; Comandini, A. An experimental and kinetic modeling study of phenylacetylene decomposition and the reactions with acetylene/ethylene under shock tube pyrolysis conditions. Combust. Flame 2020, 220, 257–271. [Google Scholar] [CrossRef]
- Naik, C.V.; Puduppakkam, K.V.; Modak, A.; Meeks, E.; Wang, Y.L.; Feng, Q.; Tsotsis, T.T. Detailed chemical kinetic mechanism for surrogates of alternative jet fuels. Combust. Flame 2011, 158, 434–445. [Google Scholar] [CrossRef]
- Li, T.; Mitra, T.; Chu, C.; Yuan, Y.; Thomson, M.J. Investigation of PAH and soot formation in a dimethyl ether (DME) laminar coflow diffusion flame. Combust. Flame 2021, 223, 437–449. [Google Scholar] [CrossRef]
- Slavinskaya, N.A.; Riedel, U.; Dworkin, S.B.; Thomson, M.J. Detailed numerical modeling of PAH formation and growth in non-premixed ethylene and ethane flames. Combust. Flame 2012, 159, 979–995. [Google Scholar] [CrossRef]
- Cassady, S.J.; Choudhary, R.; Pinkowski, N.H.; Shao, J.; Davidson, D.F.; Hanson, R.K. The thermal decomposition of ethane. Fuel 2020, 268, 117409. [Google Scholar] [CrossRef]
- Liu, P.; Li, Z.; Bennett, A.; Lin, H.; Sarathy, S.M.; Roberts, W.L. The site effect on PAHs formation in HACA-based mass growth process. Combust. Flame 2019, 199, 54–68. [Google Scholar] [CrossRef]
- Parker, D.S.; Kaiser, R.I.; Bandyopadhyay, B.; Kostko, O.; Troy, T.P.; Ahmed, M. Unexpected chemistry from the reaction of naphthyl and acetylene at combustion-like temperatures. Angew. Chem. Int. Ed. 2015, 54, 5421–5424. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. A highly efficient growth mechanism of polycyclic aromatic hydrocarbons. Phys. Chem. Chem. Phys. 2010, 12, 2427–2437. [Google Scholar] [CrossRef] [PubMed]
- Hamadi, A.; Sun, W.; Abid, S.; Chaumeix, N.; Comandini, A. An experimental and kinetic modeling study of benzene pyrolysis with C2−C3 unsaturated hydrocarbons. Combust. Flame 2022, 237, 111858. [Google Scholar] [CrossRef]
- Badger, G.; Buttery, R.; Kimber, R.; Lewis, G.; Moritz, A.; Napier, I. The formation of aromatic hydrocarbons at high temperatures. Part I. Introduction. J. Chem. Soc. 1958, 498, 2449–2452. [Google Scholar] [CrossRef]
- Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2 hydrocarbons. Combust. Flame 2000, 121, 122–136. [Google Scholar] [CrossRef]
- Mebel, A.M.; Landera, A.; Kaiser, R.I. Formation mechanisms of naphthalene and indene: From the interstellar medium to combustion flames. J. Phys. Chem. A 2017, 121, 901–926. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, L.; Yuan, W.; Qi, F.; Li, Y. Experimental and kinetic modeling investigation on laminar premixed benzene flames with various equivalence ratios. Proc. Combust. Inst. 2015, 35, 855–862. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Zagidullin, M.V.; Azyazov, V.N.; Howlader, A.H.; Wnuk, S.F.; Mebel, A.M. VUV photoionization study of the formation of the simplest polycyclic aromatic hydrocarbon: Naphthalene (C10H8). J. Phys. Chem. Lett. 2018, 9, 2620–2626. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.S.; Zhang, F.; Kim, Y.S.; Kaiser, R.I.; Landera, A.; Kislov, V.V.; Mebel, A.M.; Tielens, A.G. Low temperature formation of naphthalene and its role in the synthesis of PAHs (polycyclic aromatic hydrocarbons) in the interstellar medium. Proc. Natl. Acad. Sci. USA 2012, 109, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Li, Z.; Bennett, A.; Lin, H.; Sarathy, S.M.; Roberts, W.L. Computational study of polycyclic aromatic hydrocarbons growth by vinylacetylene addition. Combust. Flame 2019, 202, 276–291. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Evseev, M.M.; Bashkirov, E.K.; Azyazov, V.N.; Mebel, A.M. Low-temperature formation of polycyclic aromatic hydrocarbons in Titan’s atmosphere. Nat. Astron 2018, 2, 973–979. [Google Scholar] [CrossRef]
- Kim, Y.; Kaiser, R. An infrared spectroscopic study of amorphous and crystalline ices of vinylacetylene and implications for Saturn’s satellite Titan. Astrophys. J. Suppl. Ser. 2009, 181, 543. [Google Scholar] [CrossRef]
- Zhao, L.; Kaiser, R.I.; Lu, W.; Ahmed, M.; Evseev, M.M.; Bashkirov, E.K.; Azyazov, V.N.; Tönshoff, C.; Reicherter, F.; Bettinger, H.F. A free-radical prompted barrierless gas-phase synthesis of pentacene. Angew. Chem. Int. Ed. 2020, 59, 11334–11338. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.S.; Salama, F. Laboratory investigations of polycyclic aromatic hydrocarbon formation and destruction in the circumstellar outflows of carbon stars. Astrophys. J. Suppl. Ser. 2013, 208, 6. [Google Scholar] [CrossRef]
- Li, A. PAHs in Comets: An Overview; Springer: Berlin/Heidelberg, Germany, 2009; pp. 161–175. [Google Scholar]
- Yang, T.; Muzangwa, L.; Kaiser, R.I.; Jamal, A.; Morokuma, K. A combined crossed molecular beam and theoretical investigation of the reaction of the meta-tolyl radical with vinylacetylene—Toward the formation of methylnaphthalenes. Phys. Chem. Chem. Phys. 2015, 17, 21564–21575. [Google Scholar] [CrossRef]
- Couch, D.E.; Kukkadapu, G.; Zhang, A.J.; Jasper, A.W.; Taatjes, C.A.; Hansen, N. The role of radical-radical chain-propagating pathways in the phenyl + propargyl reaction. Proc. Combust. Inst. 2022, 39, 643–651. [Google Scholar] [CrossRef]
- Jin, H.; Guo, J.; Li, T.; Zhou, Z.; Im, H.G.; Farooq, A. Experimental and numerical study of polycyclic aromatic hydrocarbon formation in ethylene laminar co-flow diffusion flames. Fuel 2021, 289, 119931. [Google Scholar] [CrossRef]
- Georganta, E.; Rahman, R.K.; Raj, A.; Sinha, S. Growth of polycyclic aromatic hydrocarbons (PAHs) by methyl radicals: Pyrene formation from phenanthrene. Combust. Flame 2017, 185, 129–141. [Google Scholar] [CrossRef]
- Zhao, L.; Prendergast, M.B.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Sun, B.J.; Chen, Y.L.; Chang, A.H.; Mohamed, R.K. Synthesis of polycyclic aromatic hydrocarbons by phenyl addition–dehydrocyclization: The third way. Angew. Chem. 2019, 131, 17603–17611. [Google Scholar] [CrossRef]
- Vermeire, F.H.; De Bruycker, R.; Herbinet, O.; Carstensen, H.-H.; Battin-Leclerc, F.; Marin, G.B.; Van Geem, K.M. Experimental and kinetic modeling study of the pyrolysis and oxidation of 1,5-hexadiene: The reactivity of allylic radicals and their role in the formation of aromatics. Fuel 2017, 208, 779–790. [Google Scholar] [CrossRef]
- Roohi, H.; Moghadam, B. Decomposition mechanism of the phenylaminyl C6H5N H radical to propargyl and acetylene: A M06-2X, CBS-QB3 and G4 study. Chem. Phys. Lett. 2019, 730, 332–339. [Google Scholar] [CrossRef]
- Zhou, C.-W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Jin, H.; Xing, L.; Hao, J.; Yang, J.; Zhang, Y.; Cao, C.; Pan, Y.; Farooq, A. A chemical kinetic modeling study of indene pyrolysis. Combust. Flame 2019, 206, 1–20. [Google Scholar] [CrossRef]
- Shao, C.; Kukkadapu, G.; Wagnon, S.W.; Pitz, W.J.; Sarathy, S.M. PAH formation from jet stirred reactor pyrolysis of gasoline surrogates. Combust. Flame 2020, 219, 312–326. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, P.; Wang, J.; Kang, S.; Zhang, F.; Law, C.K.; Yang, B. Determination of rate constants for a thermoneutral H-abstraction reaction: Allylic hydrogen abstraction from 1,5-hexadiene by allyl radical. Proc. Combust. Inst. 2021, 38, 861–869. [Google Scholar] [CrossRef]
- Zhao, L.; Prendergast, M.B.; Kaiser, R.I.; Xu, B.; Lu, W.; Ablikim, U.; Ahmed, M.; Oleinikov, A.D.; Azyazov, V.N.; Mebel, A.M.; et al. Reactivity of the Indenyl Radical (C9H7) with Acetylene (C2H2) and Vinylacetylene (C4H4). ChemPhysChem 2019, 20, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Baroncelli, M.; Mao, Q.; Galle, S.; Hansen, N.; Pitsch, H. Role of ring-enlargement reactions in the formation of aromatic hydrocarbons. Phys. Chem. Chem. Phys. 2020, 22, 4699–4714. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.S.; Picca, F.; De Falco, G.; Commodo, M.; Minutolo, P.; Causà, M.; D′Anna, A. Soot inception: A DFT study of σ and π dimerization of resonantly stabilized aromatic radicals. Fuel 2020, 279, 118491. [Google Scholar] [CrossRef]
- Harding, L.B.; Klippenstein, S.J. Theoretical kinetic estimates for the recombination of hydrogen atoms with propargyl and allyl radicals. Proc. Combust. Inst. 2000, 28, 1503–1509. [Google Scholar] [CrossRef]
- Jin, H.; Xing, L.; Liu, D.; Hao, J.; Yang, J.; Farooq, A. First aromatic ring formation by the radical-chain reaction of vinylacetylene and propargyl. Combust. Flame 2021, 225, 524–534. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- Van Hoomissen, D.J.; Vyas, S. Impact of Conjugation and Hyperconjugation on the Radical Stability of Allylic and Benzylic Systems: A Theoretical Study. J. Org. Chem. 2017, 82, 5731–5742. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Hou, D.; Menon, A.; Pascazio, L.; Akroyd, J.; You, X.; Kraft, M. Reactivity of polycyclic aromatic hydrocarbon soot precursors: Implications of localized π-radicals on rim-based pentagonal rings. J. Phys. Chem. C 2019, 123, 26673–26682. [Google Scholar] [CrossRef]
- McEnally, C.S.; Pfefferle, L.D.; Atakan, B.; Kohse-Hoinghaus, K. Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap. Prog. Energy Combust. Sci. 2006, 32, 247–294. [Google Scholar] [CrossRef]
- Sinha, S.; Raj, A. Polycyclic aromatic hydrocarbon (PAH) formation from benzyl radicals: A reaction kinetics study. Phys. Chem. Chem. Phys. 2016, 18, 8120–8131. [Google Scholar] [CrossRef]
- Sinha, S.; Rahman, R.K.; Raj, A. On the role of resonantly stabilized radicals in polycyclic aromatic hydrocarbon (PAH) formation: Pyrene and fluoranthene formation from benzyl-indenyl addition. Phys. Chem. Chem. Phys. 2017, 19, 19262–19278. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xing, L.; Yang, J.; Zhou, Z.; Qi, F.; Farooq, A. Continuous Butadiyne Addition to Propargyl: A Radical-Efficient Pathway for Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. Lett. 2021, 12, 8109–8114. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Hörst, S.M.; Hicks, R.K.; Li, R.; de Gouw, J.A.; Tolbert, M.A. The role of benzene photolysis in Titan haze formation. Icarus 2014, 233, 233–241. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. A novel route for PAH growth in HACA based mechanisms. Combust. Flame 2012, 159, 3589–3596. [Google Scholar] [CrossRef]
- Weissman, M.; Benson, S.W. Pyrolysis of methyl chloride, a pathway in the chlorine-catalyzed polymerization of methane. Int. J. Chem. Kinet. 1984, 16, 307–333. [Google Scholar] [CrossRef]
- Gómez-Balderas, R.; Coote, M.L.; Henry, D.J.; Radom, L. Reliable theoretical procedures for calculating the rate of methyl radical addition to carbon−carbon double and triple bonds. J. Phys. Chem. A 2004, 108, 2874–2883. [Google Scholar] [CrossRef]
- Shukla, B.; Miyoshi, A.; Koshi, M. Role of methyl radicals in the growth of PAHs. J. Am. Soc. Mass Spectrom. 2010, 21, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.; Kutner, M.; Thaddeus, P. The ethynyl radical C2H-A new interstellar molecule. Astrophys. J. 1974, 193, L115–L119. [Google Scholar] [CrossRef]
- Mebel, A.M.; Kislov, V.V.; Kaiser, R.I. Photoinduced mechanism of formation and growth of polycyclic aromatic hydrocarbons in low-temperature environments via successive ethynyl radical additions. J. Am. Chem. Soc. 2008, 130, 13618–13629. [Google Scholar] [CrossRef]
- Marsh, N.D.; Wornat, M.J. Formation pathways of ethynyl-substituted and cyclopenta-fused polycyclic aromatic hydrocarbons. Proc. Combust. Inst. 2000, 28, 2585–2592. [Google Scholar] [CrossRef]
- Reizer, E.; Csizmadia, I.G.; Nehéz, K.; Viskolcz, B.; Fiser, B. Theoretical investigation of benzo (a) pyrene formation. Chem. Phys. Lett. 2021, 772, 138564. [Google Scholar] [CrossRef]
- Jin, H.; Ye, L.; Yang, J.; Jiang, Y.; Zhao, L.; Farooq, A. Inception of carbonaceous nanostructures via hydrogen-abstraction phenylacetylene-addition mechanism. J. Am. Chem. Soc. 2021, 143, 20710–20716. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, X.; Chen, H.; Yang, J.; Zhao, L.; Law, C.K.; Yang, B. Synergistic effects in toluene/C3H4 isomers co-pyrolysis: Formation of indene and naphthalene. Proc. Combust. Inst. 2022, 39, 989–997. [Google Scholar] [CrossRef]
- Sun, W.; Hamadi, A.; Abid, S.; Chaumeix, N.; Comandini, A. Influences of propylene/propyne addition on toluene pyrolysis in a single-pulse shock tube. Combust. Flame 2022, 236, 111799. [Google Scholar] [CrossRef]
- Sun, W.; Hamadi, A.; Abid, S.; Chaumeix, N.; Comandini, A. Detailed experimental and kinetic modeling study of toluene/C2 pyrolysis in a single-pulse shock tube. Combust. Flame 2021, 226, 129–142. [Google Scholar] [CrossRef]
- Sirignano, M.; Ciajolo, A.; D’Anna, A.; Russo, C. Particle formation in premixed ethylene-benzene flames: An experimental and modeling study. Combust. Flame 2019, 200, 23–31. [Google Scholar] [CrossRef]
- Liu, F.; Hua, Y.; Wu, H.; Lee, C.-f. Effect of toluene addition on the PAH formation in laminar coflow diffusion flames of n-heptane and isooctane. Energy Fuels 2018, 32, 7142–7152. [Google Scholar] [CrossRef]
- Li, Z.; Amin, H.M.F.; Liu, P.; Wang, Y.; Chung, S.H.; Roberts, W.L. Effect of dimethyl ether (DME) addition on sooting limits in counterflow diffusion flames of ethylene at elevated pressures. Combust. Flame 2018, 197, 463–470. [Google Scholar] [CrossRef]
- Park, S.; Wang, Y.; Chung, S.H.; Sarathy, S.M. Compositional effects on PAH and soot formation in counterflow diffusion flames of gasoline surrogate fuels. Combust. Flame 2017, 178, 46–60. [Google Scholar] [CrossRef]
- Park, S.; Wang, Y.; Chung, S.H.; Sarathy, S.M. A PAH growth mechanism and effect of alcohol addition on PAH formation in counterflow ethylene diffusion flames. In Proceedings of the European Combustion Meeting, Budapest, Hungary, 30 March–2 April 2015; pp. 5–15, P5-15. [Google Scholar]
- Choi, S.K.; Choi, B.C.; Lee, S.M.; Choi, J.H. The effect of liquid fuel doping on PAH and soot formation in counterflow ethylene diffusion flames. Exp. Therm Fluid Sci. 2015, 60, 123–131. [Google Scholar] [CrossRef]
- Liu, F.; He, X.; Ma, X.; Zhang, Q.; Thomson, M.J.; Guo, H.; Smallwood, G.J.; Shuai, S.; Wang, J. An experimental and numerical study of the effects of dimethyl ether addition to fuel on polycyclic aromatic hydrocarbon and soot formation in laminar coflow ethylene/air diffusion flames. Combust. Flame 2011, 158, 547–563. [Google Scholar] [CrossRef]
- Choi, B.C.; Choi, S.K.; Chung, S.H. Soot formation characteristics of gasoline surrogate fuels in counterflow diffusion flames. Proc. Combust. Inst. 2011, 33, 609–616. [Google Scholar] [CrossRef]
- Poddar, N.B.; Thomas, S.; Wornat, M.J. Polycyclic aromatic hydrocarbons from the co-pyrolysis of catechol and propyne. Proc. Combust. Inst. 2011, 33, 541–548. [Google Scholar] [CrossRef]
- Yoon, S.S.; Anh, D.H.; Chung, S.H. Synergistic effect of mixing dimethyl ether with methane, ethane, propane, and ethylene fuels on polycyclic aromatic hydrocarbon and soot formation. Combust. Flame 2008, 154, 368–377. [Google Scholar] [CrossRef]
- Yoon, S.S.; Lee, S.M.; Chung, S.H. Effect of mixing methane, ethane, propane, and propene on the synergistic effect of PAH and soot formation in ethylene-base counterflow diffusion flames. Proc. Combust. Inst. 2005, 30, 1417–1424. [Google Scholar] [CrossRef]
- Lee, S.M.; Yoon, S.S.; Chung, S.H. Synergistic effect on soot formation in counterflow diffusion flames of ethylene-propane mixtures with benzene addition. Combust. Flame 2004, 136, 493–500. [Google Scholar] [CrossRef]
- Roesler, J.; Martinot, S.; McEnally, C.; Pfefferle, L.; Delfau, J.-L.; Vovelle, C. Investigating the role of methane on the growth of aromatic hydrocarbons and soot in fundamental combustion processes. Combust. Flame 2003, 134, 249–260. [Google Scholar] [CrossRef]
- Dong, X.; Chang, Y.; Niu, B.; Jia, M. Development of a practical reaction model of polycyclic aromatic hydrocarbon (PAH) formation and oxidation for diesel surrogate fuel. Fuel 2020, 267, 117159. [Google Scholar] [CrossRef]
- Zhang, P.; Kang, Y.; Wu, Z.; Lu, X.; Wang, Q.; Mei, L. Effect of dimethyl ether addition on soot formation dynamics of ethylene opposed-flow diffusion flames. Ind. Eng. Chem. Res. 2019, 58, 8370–8386. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Basu, S. An investigation into the heat release and emissions from counterflow diffusion flames of methane/dimethyl ether/hydrogen blends in air. Int. J. Hydrogen Energy 2019, 44, 22328–22346. [Google Scholar] [CrossRef]
- Kang, Y.; Sun, Y.; Lu, X.; Gou, X.; Sun, S.; Yan, J.; Song, Y.; Zhang, P.; Wang, Q.; Ji, X. Soot formation characteristics of ethylene premixed burner-stabilized stagnation flame with dimethyl ether addition. Energy 2018, 150, 709–721. [Google Scholar] [CrossRef]
- Consalvi, J.-L.; Liu, F.; Kashif, M.; Legros, G. Numerical study of soot formation in laminar coflow methane/air diffusion flames doped by n-heptane/toluene and iso-octane/toluene blends. Combust. Flame 2017, 180, 167–174. [Google Scholar] [CrossRef]
- Li, T.; Zou, J.-b.; Zhang, Y.; Cao, C.-c.; Li, W.; Yuan, W.-h. Numerical investigation on 1, 3-butadiene/propyne co-pyrolysis and insight into synergistic effect on aromatic hydrocarbon formation. Chin. J. Chem. Phys. 2017, 30, 287–294. [Google Scholar] [CrossRef]
- An, Y.-z.; Pei, Y.-q.; Qin, J.; Zhao, H.; Li, X. Kinetic modeling of polycyclic aromatic hydrocarbons formation process for gasoline surrogate fuels. Energy Convers. Manag. 2015, 100, 249–261. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. A computational tool for the detailed kinetic modeling of laminar flames: Application to C2H4/CH4 coflow flames. Combust. Flame 2013, 160, 870–886. [Google Scholar] [CrossRef]
- Wang, Y.; Raj, A.; Chung, S.H. A PAH growth mechanism and synergistic effect on PAH formation in counterflow diffusion flames. Combust. Flame 2013, 160, 1667–1676. [Google Scholar] [CrossRef]
- Guo, H.S.; Trottier, S.; Johnson, M.R.; Smallwood, G.J. A numerical investigation on soot formation from laminar diffusion flames of ethylene/methane mixture. In Proceedings of the 2008 ASME Summer Heat Transfer Conference, Jacksonville, FL, USA, 10–14 August 2008; pp. 103–109. [Google Scholar]
- Wang, Y.; Raj, A.; Chung, S.H. Soot modeling of counterflow diffusion flames of ethylene-based binary mixture fuels. Combust. Flame 2015, 162, 586–596. [Google Scholar]
- Wang, W.; Xu, L.; Yan, J.; Wang, Y. Temperature dependence of the fuel mixing effect on soot precursor formation in ethylene-based diffusion flames. Fuel 2020, 267, 117121. [Google Scholar]
- Chen, M.; Liu, D. Morphology and nanostructure transitions of soot with various dimethyl ether additions in nonpremixed ethylene flames at different scales. Energy Fuels 2020, 34, 16705–16719. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Zhang, P.; Wang, Y.; He, H.; Chung, S.H.; Roberts, W.L. Role of dimethyl ether in incipient soot formation in premixed ethylene flames. Combust. Flame 2020, 216, 271–279. [Google Scholar] [CrossRef]
- McEnally, C.S.; Pfefferle, L.D. The effects of dimethyl ether and ethanol on benzene and soot formation in ethylene nonpremixed flames. Proc. Combust. Inst. 2007, 31, 603–610. [Google Scholar] [CrossRef]
- Sirignano, M.; Salamanca, M.; D’Anna, A. The role of dimethyl ether as substituent to ethylene on particulate formation in premixed and counter-flow diffusion flames. Fuel 2014, 126, 256–262. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, P.; Zhan, R.; Huang, Z.; Lin, H. Investigation on the chemical effects of dimethyl ether and ethanol additions on PAH formation in laminar premixed ethylene flames. Fuel 2019, 256, 115809. [Google Scholar] [CrossRef]
- Esarte, C.; Millera, A.; Bilbao, R.; Alzueta, M.U. Effect of ethanol, dimethylether, and oxygen, when mixed with acetylene, on the formation of soot and gas products. Ind. Eng. Chem. Res. 2010, 49, 6772–6779. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, F.; Zhong, X.; Xu, L.; Wang, Y. Sooting characteristics of partially-premixed flames of ethanol and ethylene mixtures: Unravelling the opposing effects of ethanol addition on soot formation in non-premixed and premixed flames. Fuel 2021, 291, 120089. [Google Scholar] [CrossRef]
- Griffin, E.A.; Christensen, M.; Gülder, Ö.L. Effect of ethanol addition on soot formation in laminar methane diffusion flames at pressures above atmospheric. Combust. Flame 2018, 193, 306–312. [Google Scholar] [CrossRef]
- Parker, D.S.; Kaiser, R.I.; Kostko, O.; Ahmed, M. Selective formation of indene through the reaction of benzyl radicals with acetylene. ChemPhysChem 2015, 16, 2091–2093. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, H.; Atef, N.; Wang, Z.; Chen, B.; Almalki, M.; Zhang, Y.; Cao, C.; Yang, J.; Sarathy, S.M. Polycyclic aromatic hydrocarbons in pyrolysis of gasoline surrogates (n-heptane/iso-octane/toluene). Proc. Combust. Inst. 2019, 37, 993–1001. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Yue, Z.; Jia, M.; Reitz, R.D. A reduced toluene reference fuel chemical kinetic mechanism for combustion and polycyclic-aromatic hydrocarbon predictions. Combust. Flame 2015, 162, 2390–2404. [Google Scholar] [CrossRef]
- Hussain, B.; Fang, Q.; Fang, J.; Zhang, Y.; Li, W.; Li, Y. Experimental and kinetic modeling investigation on the co-pyrolysis of benzene, acetylene, and dimethyl ether: Insights into the fuel component interactions. J. Anal. Appl. Pyrolysis 2024, 178, 106418. [Google Scholar] [CrossRef]




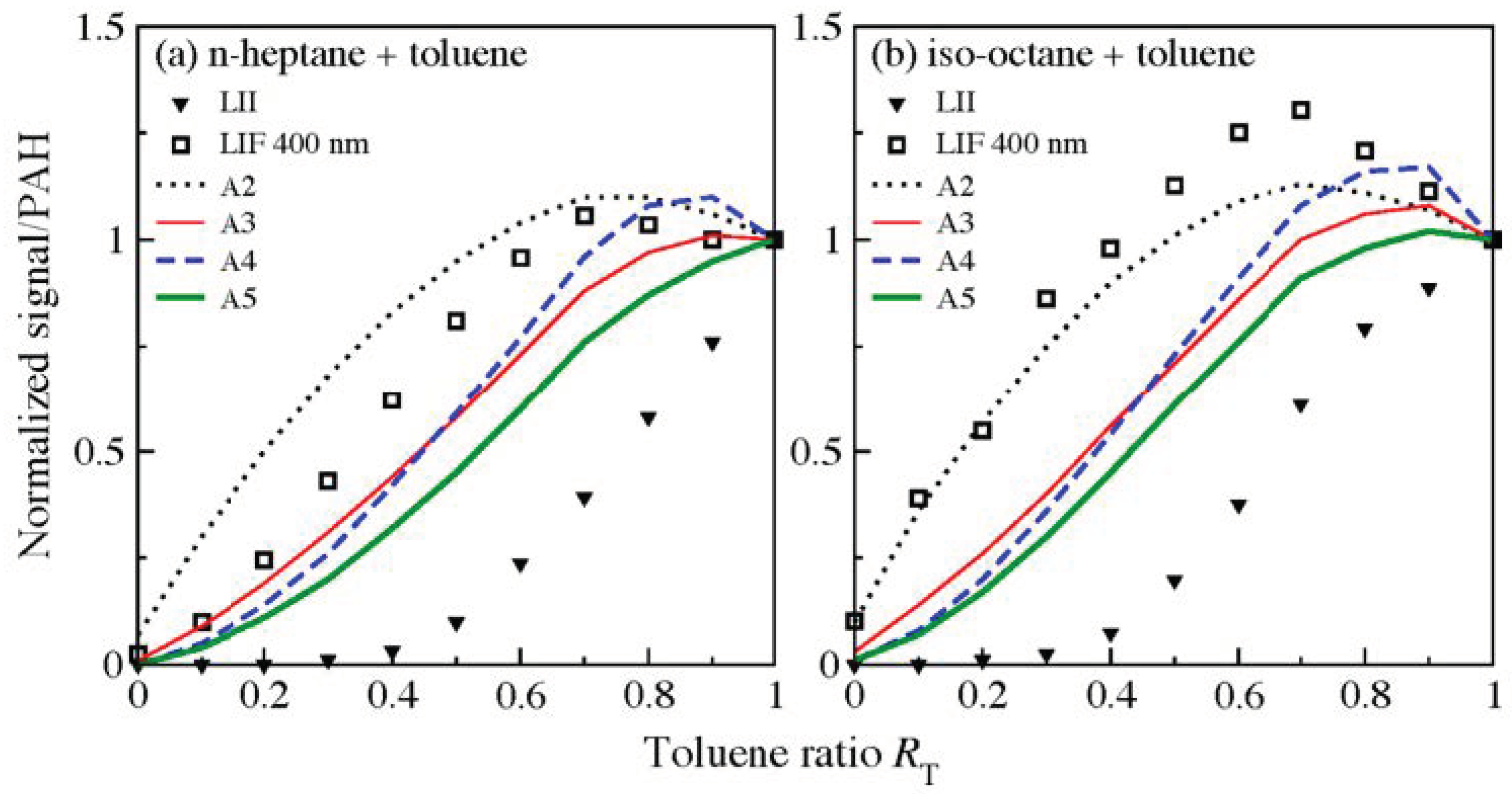
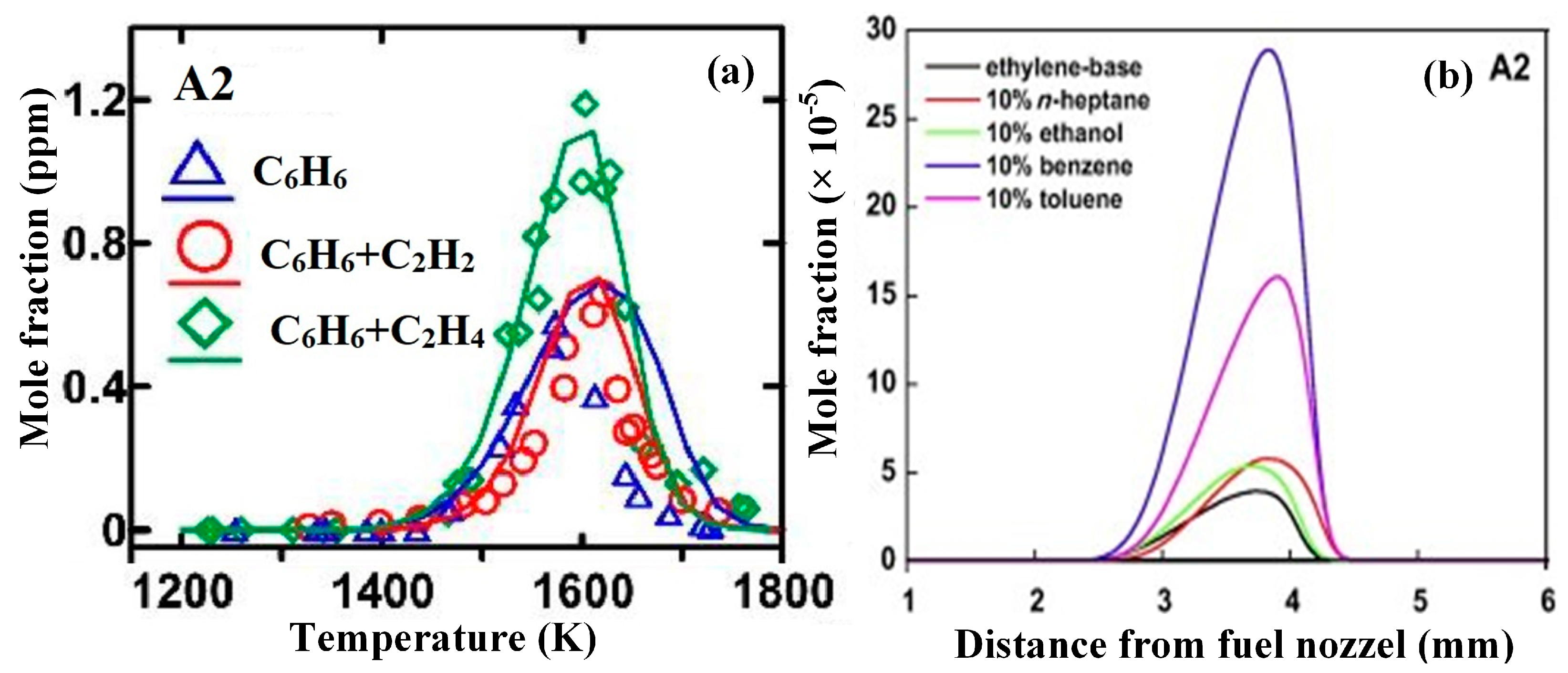
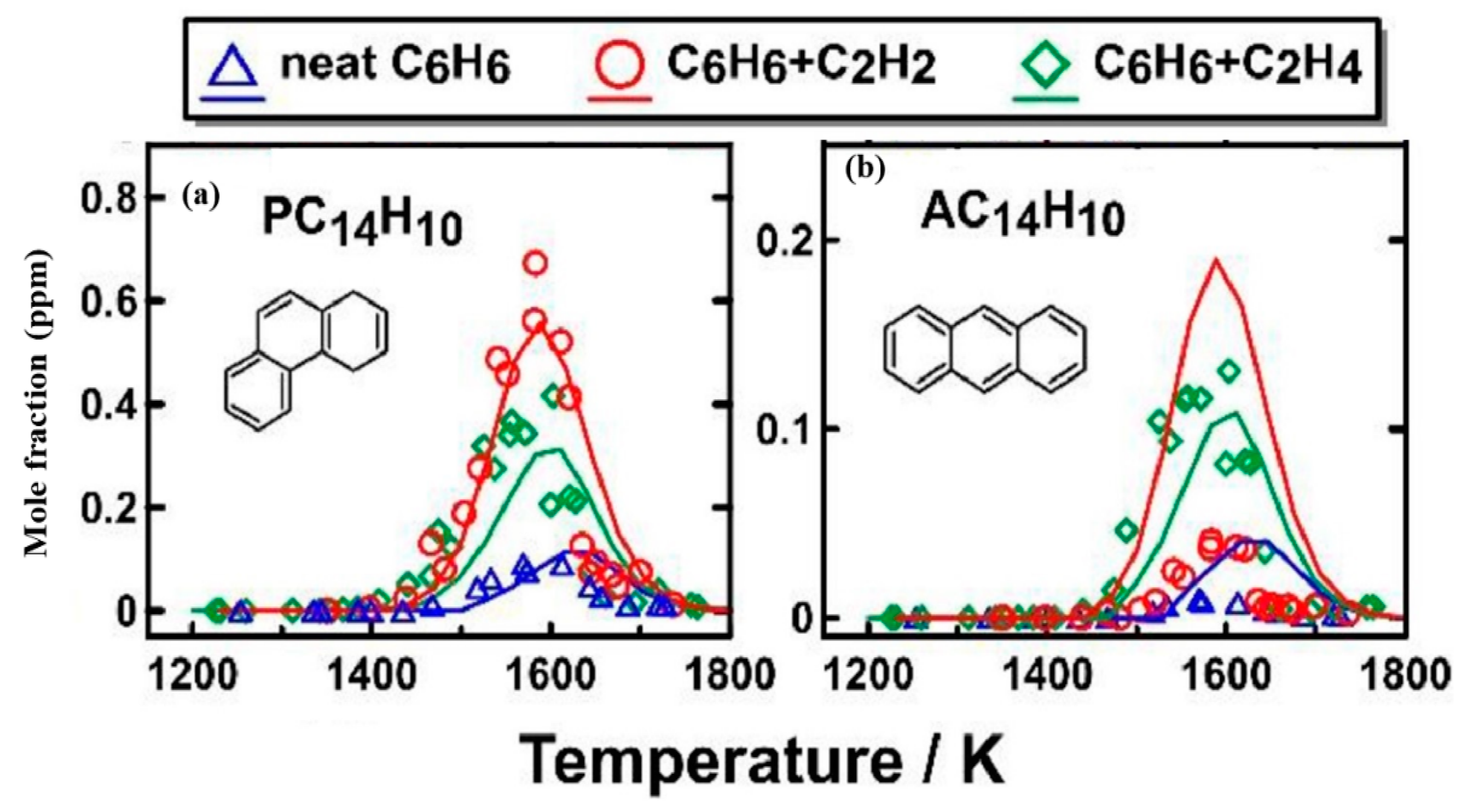
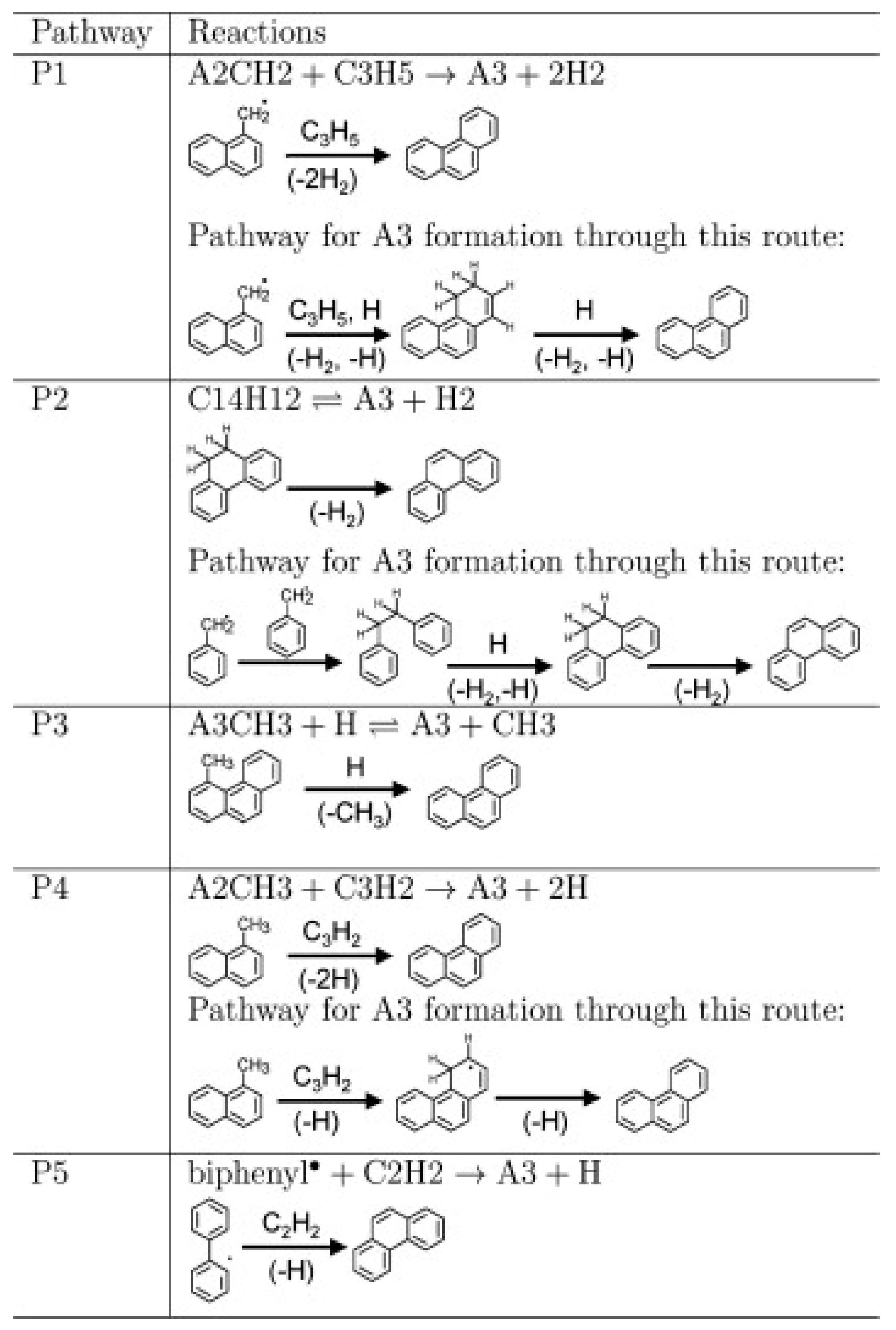

| Literature | Facility | Methods | Fuels |
|---|---|---|---|
| Liu et al. [91] | Flow reactor | SVUV-PIMS a | toluene/propyne; toluene/allenes |
| Sun et al. [92] | Shock tube | GC b/GC–MS | toluene/propylene; toluene/propyne |
| Sun et al. [93] | Shock tube | GC/GC–MS | toluene/ethylene; toluene/acetylene |
| Li et al. [11] | Flow reactor | SVUV-PIMS | toluene/acetylene |
| Hamadi et al. [93] | Shock tube | GC/GC–MS | benzene/ethylene; benzene/acetylene; benzene/propyne; benzene/allenes |
| Shao et al. [67] | JSR | GC/GC–MS | toluene/n-heptane |
| Yan et al. [31] | Counterflow flames | LII c, LIF d | ethylene/ethanol |
| Sirignano et al. [94] | Laminar premixed flames | LII, LIF | benzene/ethylene |
| Liu et al. [95] | Laminar diffusion flame | PLIF e | toluene/n-heptane; toluene/iso-octane |
| Li et al. [96] | Counterflow flames | LS f | ethylene/dimethyl ether |
| Park et al. [97] | Counterflow flame | LII, LIF | toluene/n-heptane; toluene/iso-octane |
| Park et al. [98] | Counterflow flames | LII | ethylene/propane |
| Choi et al. [99] | Counterflow flames | LIF | n-heptane/ethylene, ethanol/ethylene, benzene/ethylene, toluene/ethylene |
| Poddar et al. [12] | Flow reactor | GC | 1,3 butadiene/propyne |
| Liu et al. [100] | Co-flow flames | LII | ethylene/dimethyl ether |
| Choi et al. [101] | Counterflow flame | LII, LIF | toluene/n-heptane; toluene/iso-octane |
| Poddar et al. [102] | Flow reactor | HPLC g, GC | catechol/propyne |
| Yoon et al. [103] | Counterflow flames | PLII, LIF | ethylene/dimethyl ether |
| Yoon et al. [104] | Counterflow flames | LII, LIF | ethane/ethylene |
| Lee et al. [105] | Counterflow flames | PLII, LIF | ethylene/propane/benzene |
| Roesler et al. [106] | Flow reactor, Laminar co-flow flame, Premixed laminar flame | FTIR h, GC, LII | methane/ethylene |
| Xue et al. [107] | Premixed laminar flames, JSR | Numerical studies | toluene/n-heptane; toluene/iso-octane |
| Zhang et al. [108] | Counterflow flames | Numerical studies | ethylene/dimethyl ether |
| Bhattacharya et al. [109] | Counterflow flames | Numerical studies | methane/dimethyl ether |
| Kang et al. [110] | Premixed laminar flames | Numerical studies | ethylene/dimethyl ether |
| Consalvi et al. [111] | Co-flow flames | Numerical studies | toluene/n-heptane; toluene/iso-octane |
| Li et al. [112] | Flow reactor | Numerical studies | 1,3 butadiene/propyne |
| An et al. [113] | Counterflow flames | Numerical studies | toluene/n-heptane; toluene/iso-octane |
| Cuoci et al. [114] | Laminar flames | Numerical studies | ethylene/methane |
| Wang et al. [115] | Counterflow flames | Numerical studies | ethylene/propane/benzene |
| Raj et al. [34] | Premixed laminar flames | Numerical studies | toluene/n-heptane; toluene/iso-octane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, B.; Li, W.; Fang, Q.; Li, Y. Synergistic Effects of Fuel Components on Aromatics Formation in Combustion: A Review. Appl. Sci. 2024, 14, 6720. https://doi.org/10.3390/app14156720
Hussain B, Li W, Fang Q, Li Y. Synergistic Effects of Fuel Components on Aromatics Formation in Combustion: A Review. Applied Sciences. 2024; 14(15):6720. https://doi.org/10.3390/app14156720
Chicago/Turabian StyleHussain, Bilal, Wei Li, Qilong Fang, and Yuyang Li. 2024. "Synergistic Effects of Fuel Components on Aromatics Formation in Combustion: A Review" Applied Sciences 14, no. 15: 6720. https://doi.org/10.3390/app14156720
APA StyleHussain, B., Li, W., Fang, Q., & Li, Y. (2024). Synergistic Effects of Fuel Components on Aromatics Formation in Combustion: A Review. Applied Sciences, 14(15), 6720. https://doi.org/10.3390/app14156720












