Comparative Study on the Responsiveness of Different Species to Coagulation and Complement for Hemocompatibility Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Blood Sampling and Preparation
2.3. Traditional Coagulative Parameters
2.4. Testing Methods: Enzyme-Linked Immunosorbent (ELISA) Assay
2.5. Statistics
3. Results
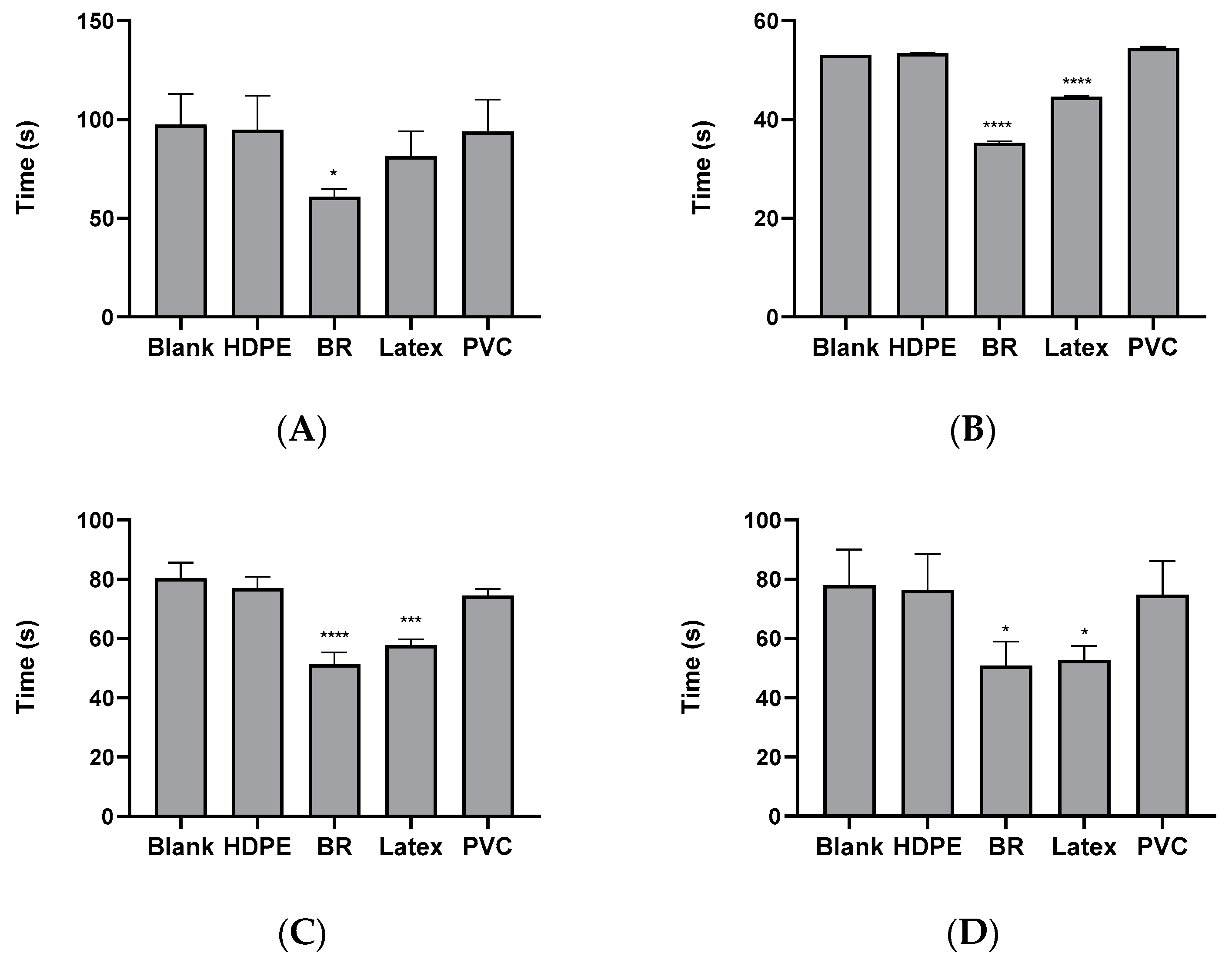
3.1. PTT of Polymeric Materials in Animal Plasma of Rabbit, Porcine, and Monkey
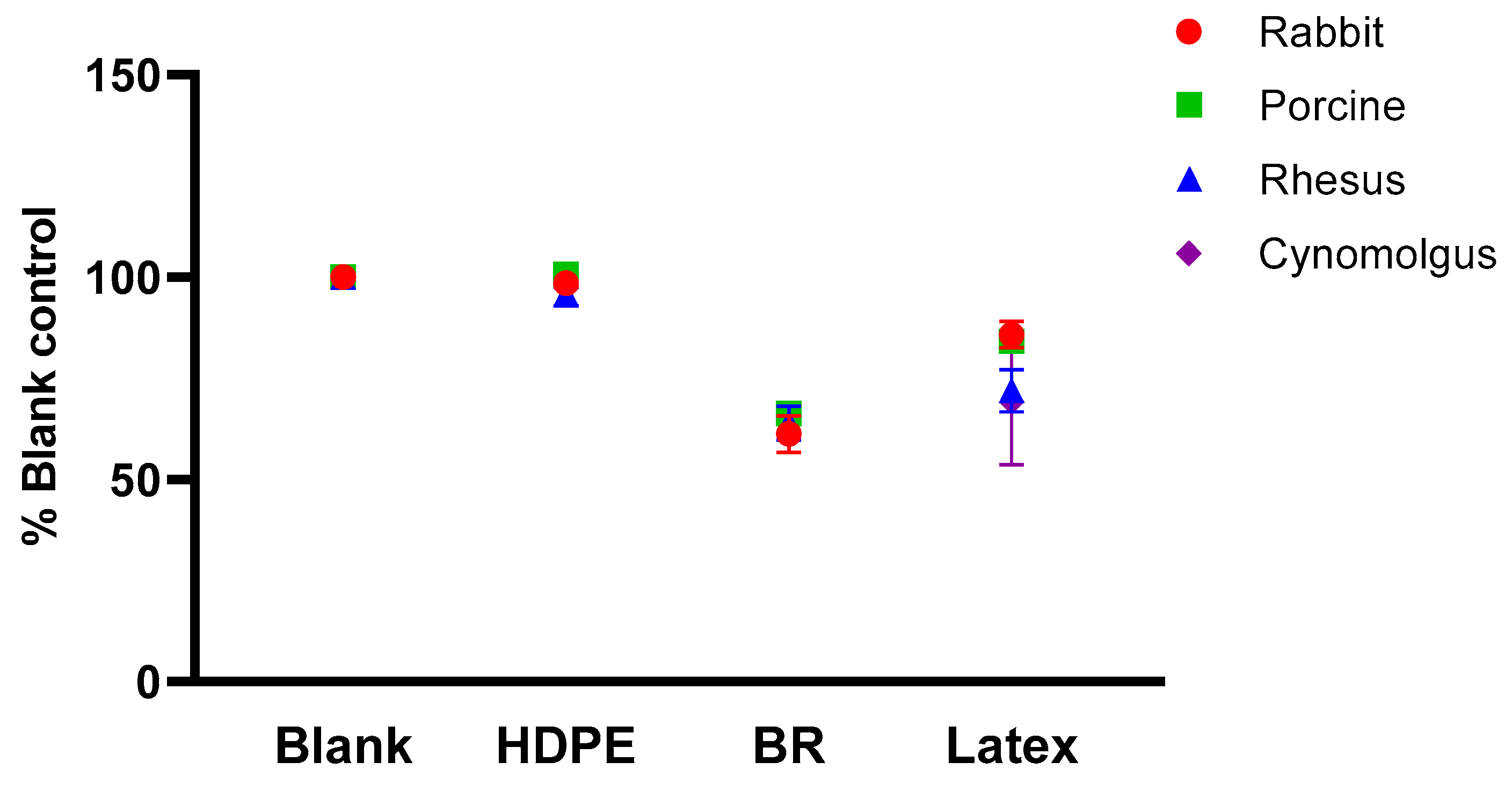
3.2. Comparison of Normalized PTT between Species
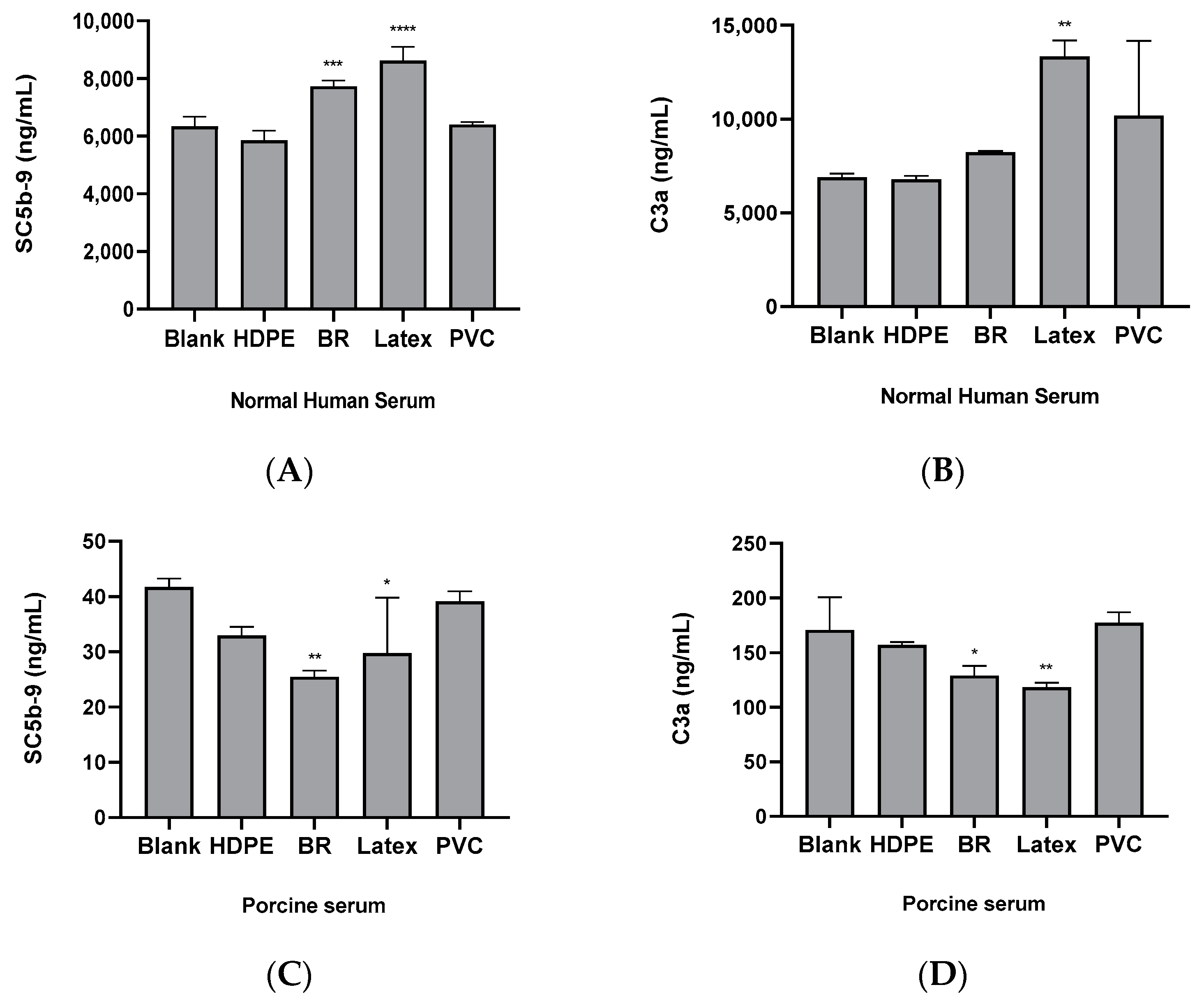
3.3. Comparison of the Complement System Activities by Substances in Human and Porcine Sera
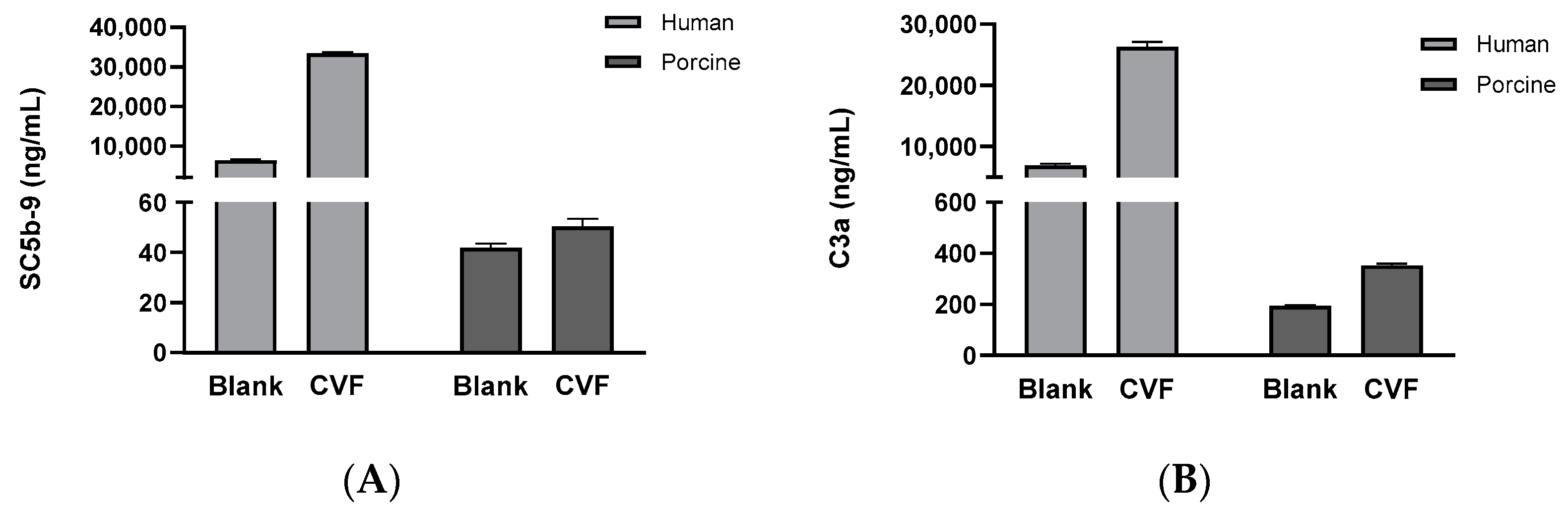
3.4. Comparison of Complement System Activity in Human and Porcine Sera Reacted with the CVF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 10993-4; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. The International Organization for Standardization (ISO): Arlington, TA, USA, 2017.
- ASTM F2888-19; Standard Practice for Platelet Leukocyte Count-An In-Vitro Measure for Hemocompatibility Assessment of Cardiovascular Materials. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 7198:2016; Cardiovascular Implants and Extracorporeal Systems—Vascular Prostheses—Tubular Vascular Grafts and Vascular Patches. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 7199:2016; Cardiovascular Implants and Artificial Organs—Blood-Gas Exchangers (Oxygenators). International Organization for Standardization: Geneva, Switzerland, 2016.
- Mason, R.G.; Read, M.S. Some species differences in fibrinolysis and blood coagulation. J. Biomed. Mater. Res. 1971, 5, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Todd, M.E.; McDevitt, E.; Goldsmith, E.I. Blood-clotting mechanisms of nonhuman primates. Choice of the baboon model to simulate man. J. Med. Primatol. 1972, 1, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Lechner, R.; Helm, M.; Müller, M.; Wille, T.; Riesner, H.J.; Friemert, B. In-vitro study of species-specific coagulation differences in animals and humans using rotational thromboelastometry (ROTEM). J. R. Army Med. Corps. 2019, 165, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Hofferbert, B.V.; Koo, G.; Malinauskas, R.A. In vitro shear stress-induced platelet activation: Sensitivity of human and bovine blood. Artif. Organs. 2013, 37, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.H.; Pieper, I.L.; Robinson, C.R.; Friedmann, Y.; Kanamarlapudi, V.; Thornton, C.A. Shear stress-induced total blood trauma in multiple species. Artif. Organs. 2017, 41, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.L. Sheep, pig, and human platelet-material interactions with model cardiovascular biomaterials. J. Biomed. Mater. Res. 1999, 45, 240–250. [Google Scholar] [CrossRef]
- Pelagalli, A.; Belisario, M.A.; Tafuri, S.; Lombardi, P.; D’Angelo, D.; Avallone, L.; Staiano, N. Adhesive properties of platelets from different animal species. J. Comp. Pathol. 2003, 128, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Siller-Matula, J.M.; Plasenzotti, R.; Spiel, A.; Quehenberger, P.; Jilma, B. Interspecies differences in coagulation profile. Thromb. Haemost. 2008, 100, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Pelagalli, A.; Lombardi, P.; D’Angelo, D.; Della Morte, R.; Avallone, L.; Staiano, N. Species variability in platelet aggregation response to different agonists. J. Comp. Pathol. 2002, 127, 126–132. [Google Scholar] [CrossRef]
- Kessler, U.; Grau, T.; Gronchi, F.; Berger, S.; Brandt, S.; Bracht, H.; Marcucci, C.; Zachariou, Z.; Jakob, S.M. Comparison of porcine and human coagulation by thrombelastometry. Thromb. Res. 2011, 128, 477–482. [Google Scholar] [CrossRef]
- ASTM F2382-18; Standard Test Method for Assessment of Circulating Blood-Contacting Medical Device Materials on Partial Thromboplastin Time (PTT). ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. The International Organization for Standardization (ISO): Arlington, VA, USA, 2021.
- Roshal, M.; Gil, M.R. Activated Partial Thromboplastin Time. In Transfusion Medicine and Hemostasis, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 779–781. [Google Scholar] [CrossRef]
- Groth, T.; Synowitz, J.; Malsch, G.; Richau, K.; Albrecht, W.; Lange, K.P.; Paul, D. Contact activation of plasmatic coagulation on polymeric membranes measured by the activity of kallikrein in heparinized plasma. J. Biomater. Sci. Polym. Ed. 1997, 8, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Gando, S.; Nanzaki, S.; Sasaki, S.; Kemmotsu, O. Significant correlations between tissue factor and thrombin markers in trauma and septic patients with disseminated intravascular coagulation. Thromb. Haemost. 1998, 79, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Pliszczak-Król, A.; Rząsa, A.; Gemra, M.; Król, J.; Łuczak, G.; Zyzak, A.; Zalewski, D.; Iwaszko-Simonik, A.; Graczyk, S. Age-related changes of platelet and plasma coagulation parameters in young pigs. J. Vet. Diagn. Investig. 2016, 28, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Gentry, P.A. Comparative aspects of blood coagulation. Vet. J. 2004, 168, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Counts, R.B.; Haisch, C.; Simon, T.L.; Maxwell, N.G.; Heimbach, D.M.; Carrico, C.J. Hemostasis in massively transfused trauma patients. Ann. Surg. 1979, 190, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. Second of two parts. N. Engl. J. Med. 2001, 344, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Harboe, M.; Thorgersen, E.B.; Mollnes, T.E. Advances in assay of complement function and activation. Adv. Drug Deliv. Rev. 2011, 63, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, J.; Felber, E.; Reiter, C.; Riethmüller, G.; Feucht, H.E. Deposition of complement activation products on plastic-adsorbed immunoglobulins. A simple ELISA technique for the detection of defined complement deficiencies. J. Immunol. Methods 1989, 124, 121–129. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Overview of complement activation and regulation. Semin. Nephrol. 2013, 33, 479–492. [Google Scholar] [CrossRef]
- Schaapherder, A.F.M.; Gooszen, H.G.; te Bulte, M.T.J.W.; Daha, M.R. Human complement activation via the alternative pathway on porcine endothelium initiated by IgA antibodies. Transplantation 1995, 60, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Kitano, E.; Hatanaka, M.; Lo, P.; Matsuura, R.; Deguchi, K.; Eguchi, H.; Maeda, A.; Watanabe, M.; Matsunari, H.; et al. Studies of Pig Complement: Measurement of Pig CH50, ACH50, and Components. Transplant. Proc. 2016, 48, 1282–1284. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Bawa, R. Human clinical relevance of the porcine model of pseudoallergic infusion reactions. Biomedicines 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, M.K.; Kapadvanjwala, M.; Sanchez, A.; Elson, R.; Serafini, A.N.; Zilleruelo, G.E.; Sfakianakis, G.N. Quantitation of comparative thrombogenicity of dog, pig, and human platelets in a hemodialyzer. ASAIO J. 1992, 38, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Didisheim, P.; Dewanjee, M.K.; Frisk, C.S.; Kaye, M.P.; Fass, D.N. Animal models for predicting clinical performance of biomaterials for cardiovascular use. In Contemporary Biomaterials: Material and Host Response, Clinical Applications, New Technology, and Legal Aspects; Noyes Publications: Saddle River, NJ, USA, 1984; pp. 132–179. [Google Scholar]
| Parameters | Conditions |
|---|---|
| Species (strain, sex, age) | Rabbit (Oryctolagus cuniculus, Female, 16 weeks), Monkey (Rhesus macaque and Cynomolgus macaque, Male, 5 months) Porcine (Sus scrofa, Female, 3 months) |
| Anticoagulant (final ratio with blood) | 3.2% sodium citrate (blood: sodium citrate solution volume ratio = 9:1) |
| Test materials | HDPE, Black rubber, Latex rubber, PVC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, G.; Lim, J.; Kim, S.; Eom, J.; Kim, T. Comparative Study on the Responsiveness of Different Species to Coagulation and Complement for Hemocompatibility Evaluation. Appl. Sci. 2024, 14, 6721. https://doi.org/10.3390/app14156721
Kim J, Kim G, Lim J, Kim S, Eom J, Kim T. Comparative Study on the Responsiveness of Different Species to Coagulation and Complement for Hemocompatibility Evaluation. Applied Sciences. 2024; 14(15):6721. https://doi.org/10.3390/app14156721
Chicago/Turabian StyleKim, Jeonghwa, Geonyong Kim, Jiyoung Lim, Sekyung Kim, Joonho Eom, and Taewon Kim. 2024. "Comparative Study on the Responsiveness of Different Species to Coagulation and Complement for Hemocompatibility Evaluation" Applied Sciences 14, no. 15: 6721. https://doi.org/10.3390/app14156721






