Preparation and Characterization of CPCM for Thermal Energy Storage Unit
Abstract
:1. Introduction
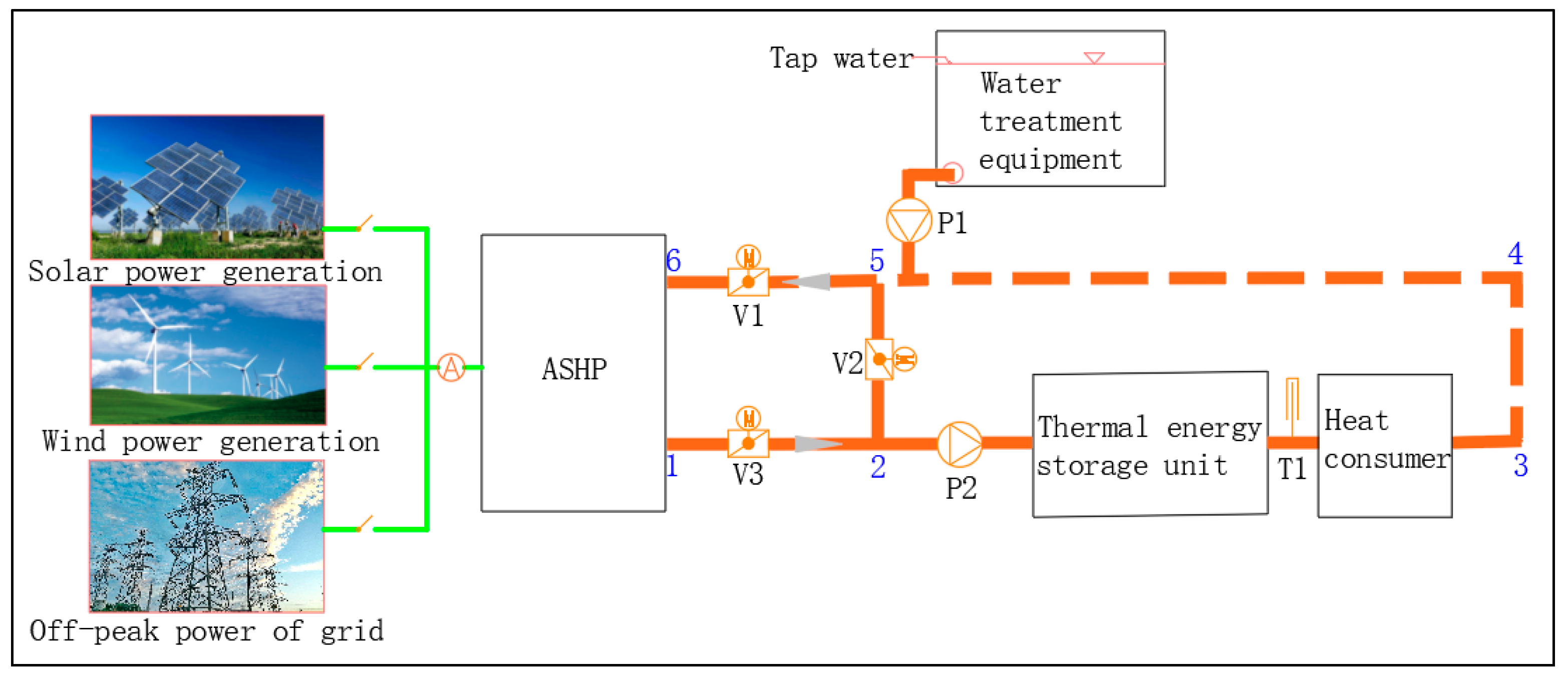
2. CPCM Preparation and Characterizing Instruments
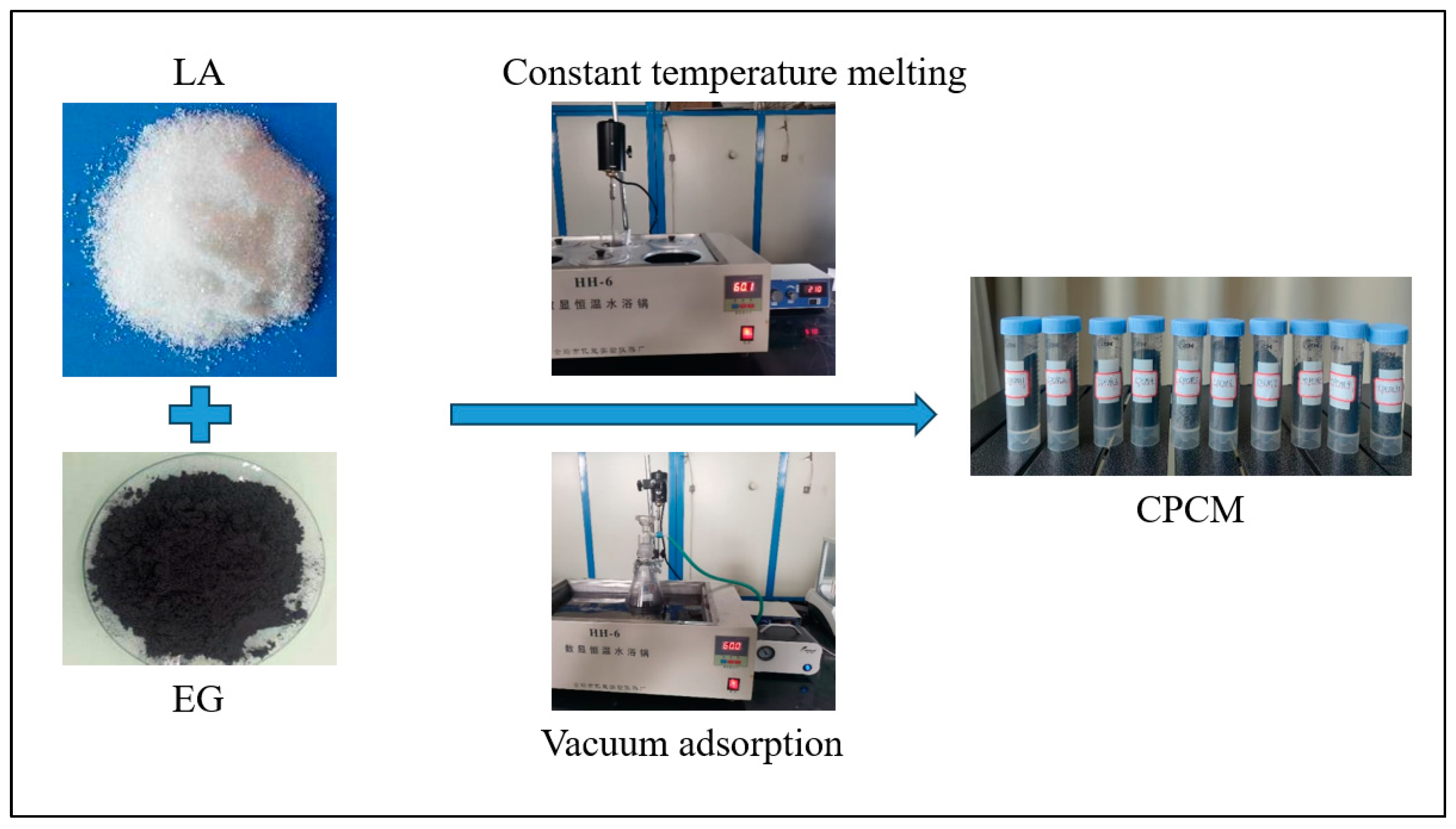
2.1. Preparation of LA/EG CPCMs
- (1)
- Weighing LA and EG by a HUAZHI 10,000-digit electronic balance (HUAZHI, Qingdao, China) with an accuracy of 0.0001 g and mixing them together.
- (2)
- Putting the mixture into a 60 °C constant temperature water bath (HH-6) and stirring it at a speed of 250 r/min for 5 min to melt evenly.
- (3)
- Transferring the mixture into a conical flask and adsorbing it for 10 min in the constant temperature water bath under a vacuum condition of −0.68 MPa.
2.2. Characterization Instruments
2.3. Uncertainty Analysis
3. Results and Discussion
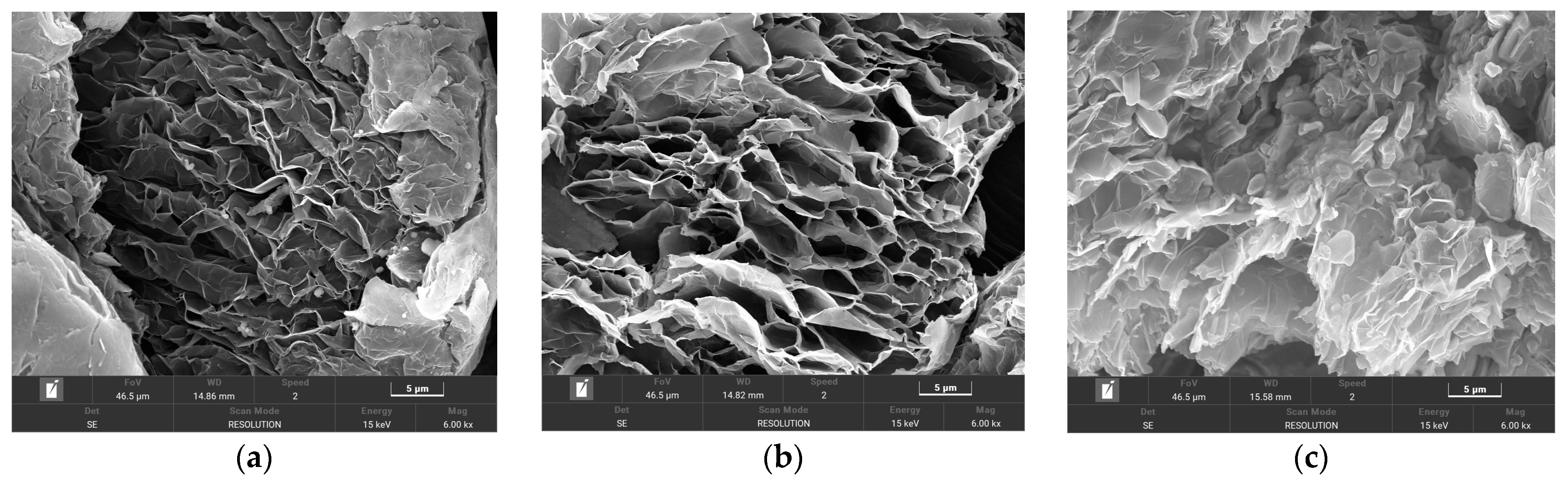
3.1. SEM Test Results
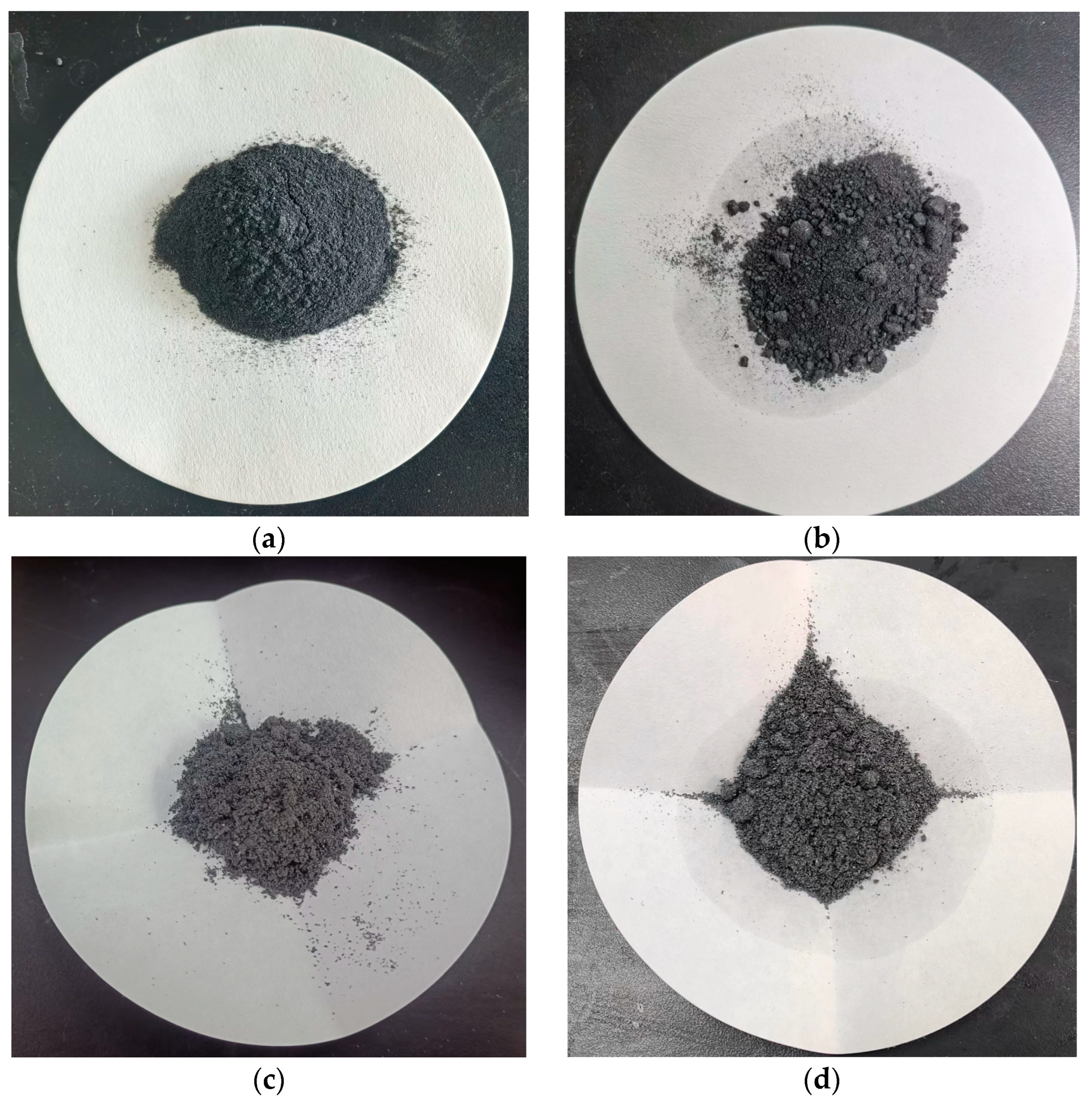
3.2. Maximum Content of LA
3.3. Thermal Conductivity
3.4. DSC Test Results
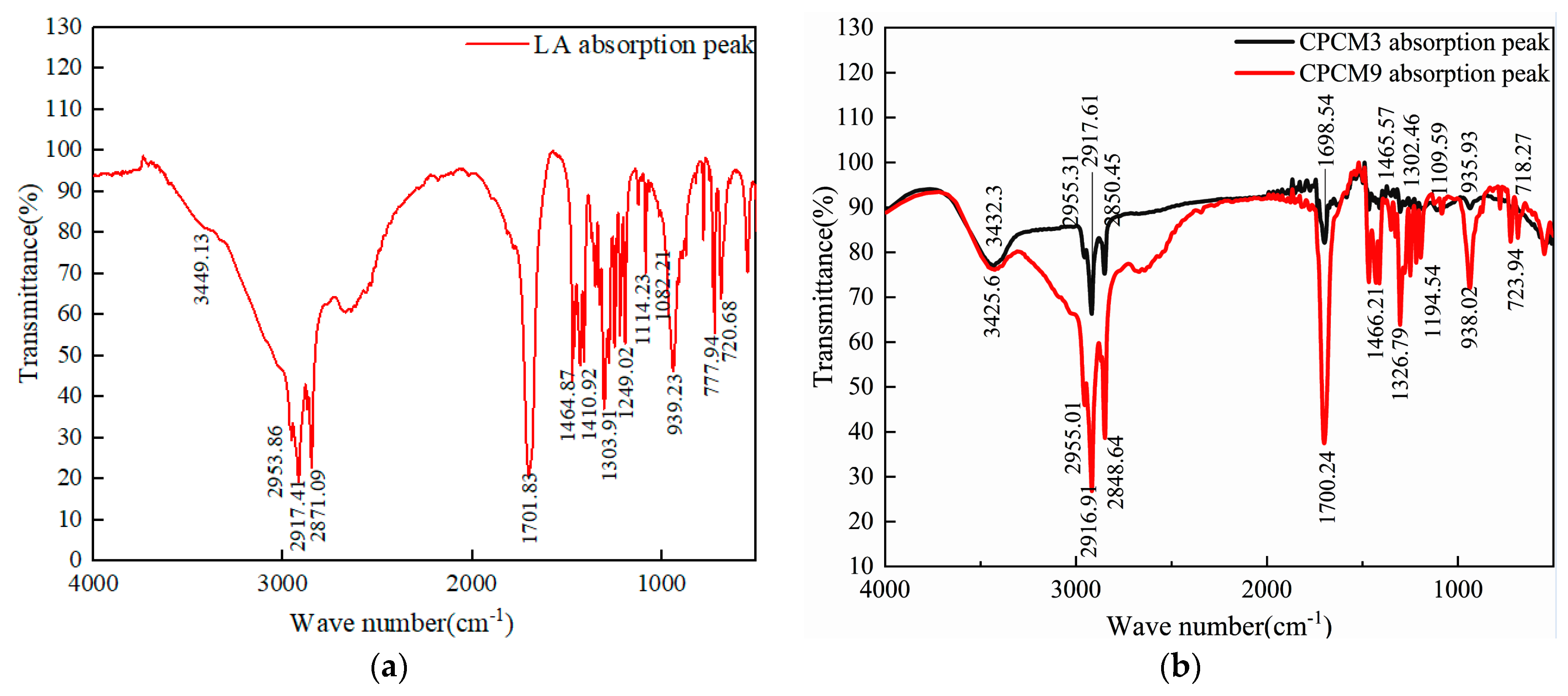
3.5. FT-IR Analysis on CPCMs
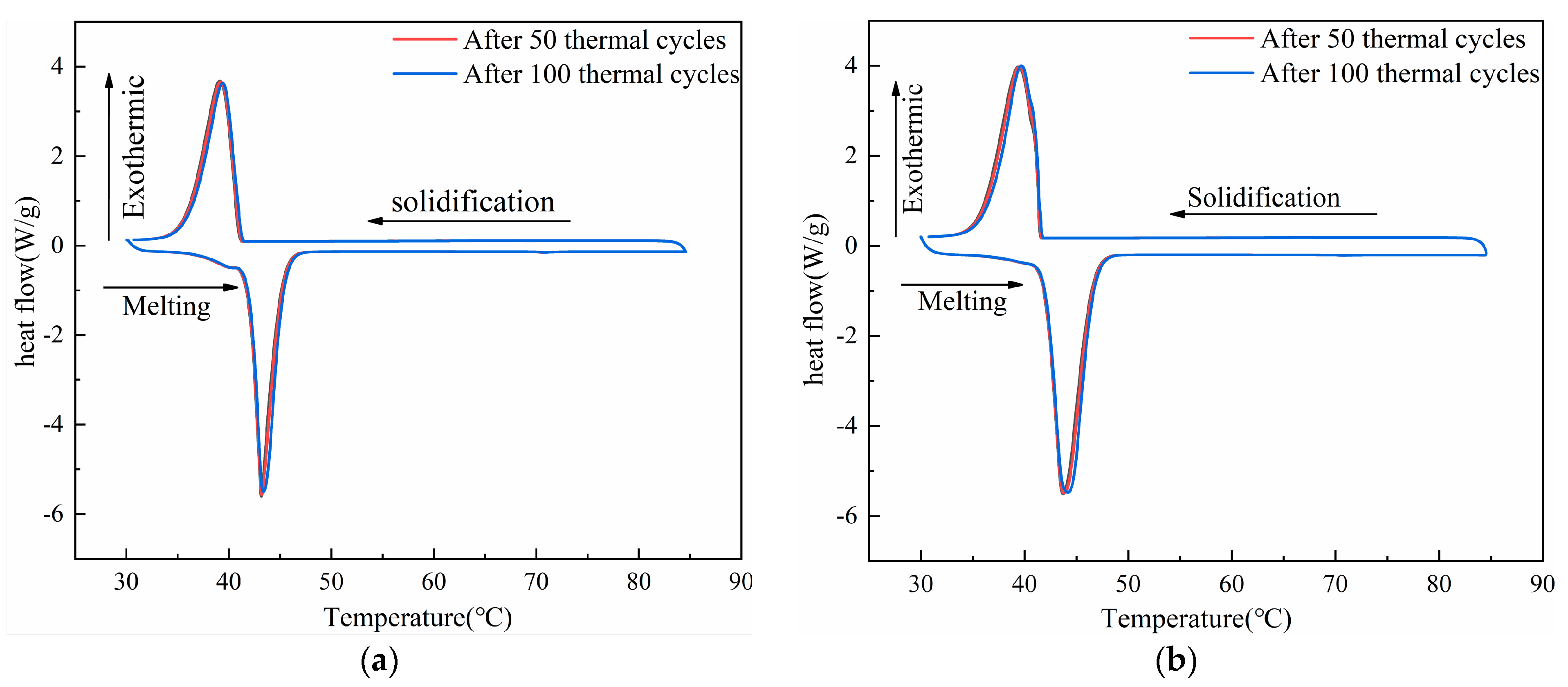
3.6. Thermal Stability of CPCMs
4. Conclusions
- The preparation of a CPCM is a physical process, and no chemical reaction happens. LA is effectively encapsulated in EG due to its capillary and surface tension, and the thermal stability of the CPCM is satisfied.
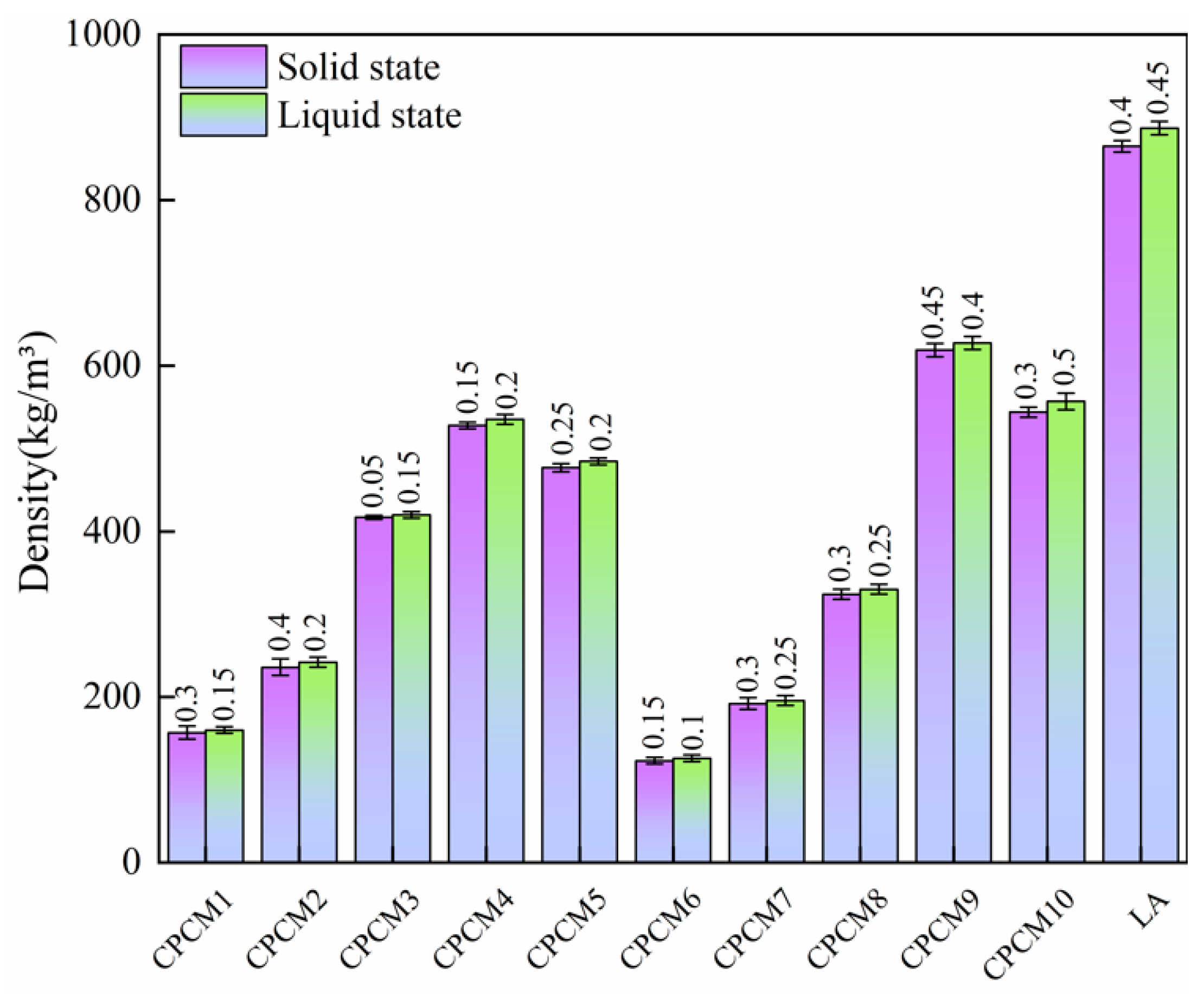
- Compared to LA, the phase change temperature of the CPCM changes little. Although the latent heat of the CPCM decreases due to the EG introduced, its thermal conductivity, supercooling degree, and volume expansion rate are significantly improved.
- Of the 10 CPCMs, the thermal performance of CPCM3 is better and more suitable for the TESU combined with an ASHP heating system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Zhang, S.-H.; Xu, X.-F. Phase change energy storage in the application of heat pump system. J. Shanghai Energy Conserv. 2023, 11, 1656–1662. [Google Scholar] [CrossRef]
- Marsden, B.J.; Haverty, M.; Bodel, W.; Hall, G.N.; Jones, A.N.; Mummery, P.M.; Treifi, M. Dimensional change, irradiation creep and thermal/mechanical property changes in nuclear graphite. Int. Mater. Rev. 2016, 61, 155–182. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, M.; Chen, L. Preparation and properties of paraffin/alkali modified diatomite/expanded graphite composite phase change thermal storage materials. J. Inorg. Chem. 2018, 40, 533–543. [Google Scholar]
- Hou, J.; Yang, J.; Hao, J.; Hou, C.; Li, J.; Wang, Y. Preparation of octadecyl alcohol/ZIF-8 shaped CPCM and simulation of Thermal energy storage unit. J. Sol. Energy 2023, 44, 434–442. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Zhang, H.; Wu, Y. Heat transfer enhancement mechanism and heat storage and release performance of expanded graphite in double helix coils for phase change materials. Energy Storage Sci. Technol. 2023, 12, 3678–3689. [Google Scholar]
- Fang, G.; Zhao, M.; Sun, P. Preparation and Characterization of PCM energy storage Materials based on palmitic-stearic acid/Expanded graphite. Mater. Rev. 2019, 37, 186–192. [Google Scholar]
- Tang, X.; Zhu, B.; Xu, M.; Zhang, W.; Yang, Z.; Zhang, Y.; Yin, G.; He, D.; Wei, H.; Zhai, X. Shape-stabilized phase change materials based on fatty acid eutectics/expanded graphite composites for thermal storage. Energy Build. 2015, 109, 353–360. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, G.; Jia, Z.; Gao, Q.; Li, Y.; Gu, Z. Eutectic fatty acids phase change materials improved with expanded graphite. Materials 2022, 15, 6856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wei, J.; Li, K.; Zhu, J.; Cheng, X. Preparation and characterization of construction mortar with lauric acid-stearic acid/expanded vermiculite CPCM. Energy Storage Sci. Technol. 2019, 8, 92–97. [Google Scholar]
- Wang, S.; Sun, Z.; Li, J.; Li, C. Preparation and Properties of lauric acid/tetradecyl alcohol/silicon dioxide shaped phase change materials. Energy Storage Sci. Technol. 2020, 9, 1768–1774. [Google Scholar]
- Zeng, Y.; Yang, X.; Chen, Y. Preparation and Properties of lauric acid-tetradecanoic acid/nano-silica/activated carbon composite phase change energy storage materials. Chem. New Mater. 2022, 50, 262–266+269. [Google Scholar]
- Chen, Z.; Shan, F.; Cao, L.; Fang, G. Synthesis and thermal properties of shape-stabilized lauric acid/activated carbon composites as phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 102, 131–136. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Bai, R.; Zhang, J.; Dai, J. Preparation and properties of decanoic acid-lauric acid/expanded vermiculite composite phase change heat storage materials for building energy conservation. J. Liaoning Shihua Univ. 2019, 43, 34–40. [Google Scholar]
- Yang, X.; Yuan, Y.; Zhang, N.; Cao, X.; Liu, C. Preparation and properties of myristic–palmitic–stearic acid/expanded graphite composites as phase change materials for energy storage. Sol. Energy 2014, 99, 259–266. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; He, X. Research on the preparation and properties of lauric acid/expanded perlite phase change materials. Energy Build. 2016, 110, 108–111. [Google Scholar]
- Lv, S.; Zhu, N.; Feng, G. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar]
- Fan, Z.; Zhao, Y.; Liu, X.; Shi, Y.; Jiang, D. Thermal properties and reliabilities of lauric acid-based binary eutectic fatty acid as a phase change material for building energy conservation. ACS Omega 2022, 7, 16097–16108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yan, H.; Wang, H. Preparation and thermal properties of lauric acid/expanded perlite composite phase change materials. Mater. Rev. 2017, 31, 107–111+131. [Google Scholar]
- Wang, B.; Zhou, W.; Wu, J.; Sun, L.; Wang, M.; Zhang, W. A phase change thermal storage material and its performance for floor electric heating system. J. Energy Storage 2023, 59, 106518. [Google Scholar] [CrossRef]
- Celzard, A.; Schneider, S.; Marêché, J.F. Densification of expanded graphite. Carbon 2002, 40, 2185–2191. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and characterization of myristic acid/expanded graphite composite phase change materials for thermal energy storage. Sci. Rep. 2020, 10, 10889. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Du, W.; He, Q.; Gu, Q.; Wang, L. Study of phase-transition characteristics of new composite phase change materials of capric acid–palmitic acid/expanded graphite. ACS Omega 2020, 5, 27522–27529. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Wang, H.; Yu, R.; Zhao, S. Preparation and properties of lauric acid CPCM. New Chem. Mater. 2016, 44, 64–66. [Google Scholar]
- Sabbah, R.; An, X.W.; Chickos, J.S.; Leitaō, M.L.P.; Roux, M.V.; Torres, L.A. Reference materials for calorimetry and differential thermal analysis. Thermochim. Acta 1999, 331, 93–204. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; White, M.A. Thermal property determination for phase change materials. J. Chem. Thermodyn. 2021, 160, 106439. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Fu, S.; Zhang, H. Study of a novel ceramsite-based shape-stabilized composite phase change material (PCM) for energy conservation in buildings. Constr. Build. Mater. 2020, 246, 118479. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Kumar Shukla, S. Improvement in thermal properties of PCM/Expanded vermiculite/expanded graphite shape stabilized composite PCM for building energy applications. Renew. Energy 2021, 176, 295–304. [Google Scholar] [CrossRef]
- He, Q.; Fei, H.; Du, W.; Zhang, Z. Expanded graphite base phase change materials thermal conductivity of pore structure and the research progress of. Chem. New Mater. 2021, 49, 11–15+20. [Google Scholar]
- Fei, H.; Du, W.; Gu, Q.; Wang, L.; He, Q.; Pan, Y. The phase change characteristics of Capric acid-based binary low eutectic mixtures adsorbed in expanded graphite. Energy Fuels 2020, 34, 14893–14901. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Z. Preparation and characteristics of microencapsulated lauric acid as composite thermal energy storage materials. Mater. Sci. 2020, 26, 88–93. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.S.; Singh, J.K. pH-controlled synthesis of sustainable lauric acid/SiO2 phase change material for scalable thermal energy storage. Sci. Rep. 2021, 11, 15012. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Munyalo, J.M.; Tian, Z.; Ji, J. Preparation and thermophysical properties of low temperature CPCM octanoic-lauric acid/expanded graphite. J. Mol. Liq. 2019, 277, 577–583. [Google Scholar] [CrossRef]
- Gu, X.; Liu, P.; Bian, L.; He, H. Enhanced thermal conductivity of palmitic acid/mullite phase change composite with graphite powder for thermal energy storage. Renew. Energy 2019, 138, 833–841. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Bao, J.; Zou, D.; Zhu, S.; Ma, Q.; Wang, Y.; Hu, Y. A medium-temperature, metal-based, microencapsulated phase change material with a void for thermal expansion. Chem. Eng. J. 2021, 415, 128965. [Google Scholar] [CrossRef]
- Janghel, D.; Karagadde, S.; Saha, S.K. Thermal performance analysis of phase change material based thermal storage system using a generalized numerical model with volumetric expansion and shrinkage. Appl. Therm. Eng. 2020, 180, 115826. [Google Scholar] [CrossRef]

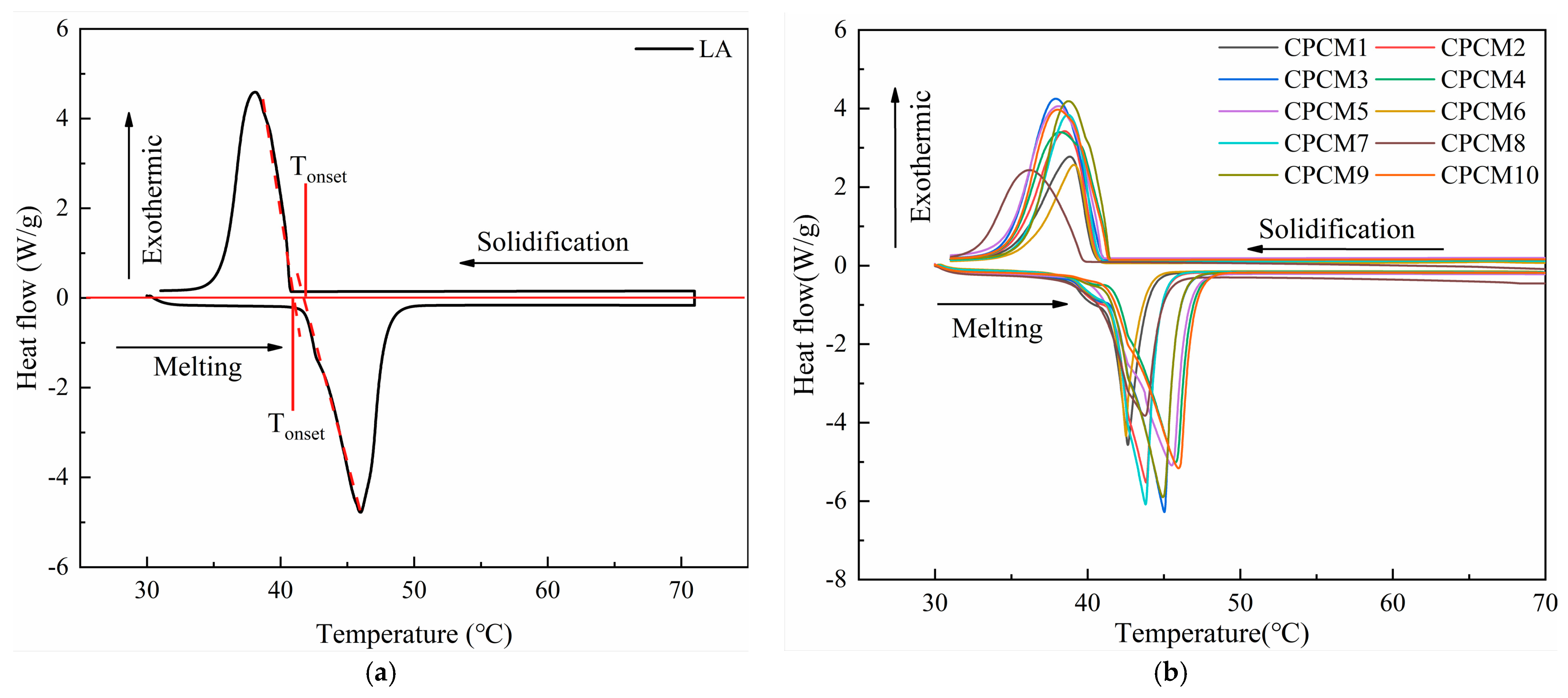

| Sample Name | M(LA)/M(EG) | EG (Mesh) | Melting Process | Solidifying Process | Degree of Supercooling | ||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Latent Heat (kJ/kg) | Temperature (°C) | Latent Heat (kJ/kg) | (°C) | |||
| CPCM1 | 6:4 | 100 | 41.38 | 107.04 | 40.32 | 102.25 | 1.06 |
| CPCM2 | 7:3 | 100 | 41.71 | 134.67 | 40.53 | 130.62 | 1.18 |
| CPCM3 | 8:2 | 100 | 41.94 | 168.28 | 40.98 | 164.34 | 0.96 |
| CPCM4 | 9:1 | 100 | 42.00 | 168.13 | 41.64 | 164.56 | 0.36 |
| CPCM5 | 9.5:0.5 | 100 | 41.96 | 173.08 | 41.57 | 168.77 | 0.39 |
| CPCM6 | 6:4 | 200 | 41.68 | 86.56 | 40.43 | 83.01 | 1.25 |
| CPCM7 | 7:3 | 200 | 41.85 | 144.60 | 40.62 | 140.60 | 1.23 |
| CPCM8 | 8:2 | 200 | 40.41 | 160.83 | 39.4 | 157.76 | 1.01 |
| CPCM9 | 9:1 | 200 | 41.80 | 170.65 | 41.23 | 167.70 | 0.57 |
| CPCM10 | 9.5:0.5 | 200 | 41.75 | 178.49 | 40.8 | 175.34 | 0.95 |
| LA | / | / | 42.52 | 180.40 | 40.73 | 175.04 | 1.79 |
| Relative error (%) | / | 0.1% | 0.15% | 0.1% | 0.15% | 0.14% | |
| Chemical Groups | Absorption Peaks (cm−1) |
|---|---|
| Hydroxy-oh stretching vibration peak | 3449.13, 3432.30, 3425.60 |
| Methylene C-H antisymmetric stretching vibration and symmetric stretching vibration peaks | 2953.86, 2917.41, 2871.09, 2955.31, 2917.61, 2955.01, 2916.91, 2850.45, 2848.64 |
| Carbonyl C=O stretching vibration peak | 1701.83, 1698.54, 1700.24 |
| C-H bending vibration peak of saturated alkyl group | 1464.87, 1410.92, 1303.91, 1249.02, 1465.57, 1302.46, 1466.21, 1326.79 |
| C-O stretching vibration peak | 1114.23, 1082.21, 1109.59, 1194.54 |
| Unsaturated hydrocarbon group =C-H out-of-plane rocking vibration peak | 939.23, 935.90, 938.02 |
| C-H out-of-plane bending vibration peak | 777.94, 720.68, 718.27, 723.94 |
| Name | CPCM Source | Base Material | Latent Heat (kJ/kg) | Thermal Conductivity (W/(m·K)) | Phase Transition Temperature (°C) | Density (kg/m³) | Degree of Supercooling (°C) |
|---|---|---|---|---|---|---|---|
| CPCM3 | This paper | LA+EG | 164.34–168.28 | 8.15–8.33 | 40.98–41.94 | 417–420 | 0.96 |
| PCM-A-42 | Dongguan Donglin | PA | 198–200 | 0.2–0.25 | 42–43.5 | 780–850 | 1.5 |
| PCM-M-40 | Hebei Ruosen | n-Docosane + PMMA | 134.3–136.5 | 0.2–0.21 | 39.17–42.97 | 770–800 | 3.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Cao, H.; Zhao, Y.; Zhang, W.; Shen, W. Preparation and Characterization of CPCM for Thermal Energy Storage Unit. Appl. Sci. 2024, 14, 6724. https://doi.org/10.3390/app14156724
Zhou W, Cao H, Zhao Y, Zhang W, Shen W. Preparation and Characterization of CPCM for Thermal Energy Storage Unit. Applied Sciences. 2024; 14(15):6724. https://doi.org/10.3390/app14156724
Chicago/Turabian StyleZhou, Wenhe, Hailan Cao, Yun Zhao, Wenxiang Zhang, and Wei Shen. 2024. "Preparation and Characterization of CPCM for Thermal Energy Storage Unit" Applied Sciences 14, no. 15: 6724. https://doi.org/10.3390/app14156724




