Abstract
Indirect combustion with the chemical looping combustion (CLC) of solid oxygen carriers is one of the most promising technologies for capturing carbon dioxide (CO2) in energy production from fossil fuels since the separation of the generated CO2 is inherent to the process itself. Therefore, the cost associated with capturing this gas will be significantly reduced. This technology transfers oxygen from air to fuel through a metal oxide that acts as an oxygen carrier, avoiding direct contact between air and fuel. This oxygen carrier circulates in a fluidized bed reactor called a reduction reactor and an oxidation reactor. (1) This research work has focused on evaluating the behavior of oxygen carriers based on the original and improved manganese mineral (copper-impregnated mineral) named for this study, OXMN009 and OXMN009P, respectively. (2) Equilibrium experiments were carried out on a thermogravimetric balance (TGA) to evaluate the kinetic behavior of these oxygen transporters OXMN009 and OXMN009P, using the gases methane (CH4), carbon monoxide (CO), and hydrogen (H2). (3) The enhanced solid oxygen carrier OXMN009P exhibited good performance for the CLC process with gaseous fuels in terms of reactivity and combustion efficiency, having high reactivity and oxygen transfer properties due to copper impregnation. (4) The results show that OXMN009P has comparable reactivity to other manganese-based materials reported in the literature. It may be an effective option for carbon dioxide capture, as it uses metal oxides as the oxygen transporters (TO). (5) These oxygen transporters, OXMN009 and OXMN009P, are used in a cyclic process that prevents the formation of nitrogen oxides by keeping the air and fuel separate. (6) Thermogravimetric balance (TGA) experiments were conducted to evaluate the kinetic behavior of these copper-modified oxygen transporters. (7) It was found that OXMN009P improved the reactivity and oxygen transfer properties due to copper impregnation. The kinetic parameters obtained in the TGA indicate that the reaction is non-thermal and requires less energy to initiate. (8) The results show that OXMN009P has reactivity comparable to other manganese-based materials reported in the literature and can be an effective option for carbon dioxide capture.
1. Introduction
Indirect combustion with solid oxygen carriers (SOCs) (chemical looping combustion, CLC) is one of the most promising technologies for capturing CO2 in energy production from fossil fuels since the separation of the generated CO2 is inherent to the process itself. Therefore, the cost associated with capturing this gas will be significantly reduced. This technology transfers oxygen from the air to fuel through a metal oxide that acts as an oxygen carrier, avoiding direct contact between the air and fuel. The CLC process is a flexible and versatile technology that can be used with a wide range of fuels, both gaseous (methane, syngas, etc.), liquids (ethane, diesel, etc.), and solids (coal and biomass). In addition, this process is based on the transfer of oxygen from air to fuel through a solid oxygen carrier, usually a metal oxide. This conveyor constantly circulates between two interconnected fluidized bed reactors, a reduction reactor (RR) and an oxidation reactor (RO). This arrangement prevents direct contact between the fuel and air during combustion [1].
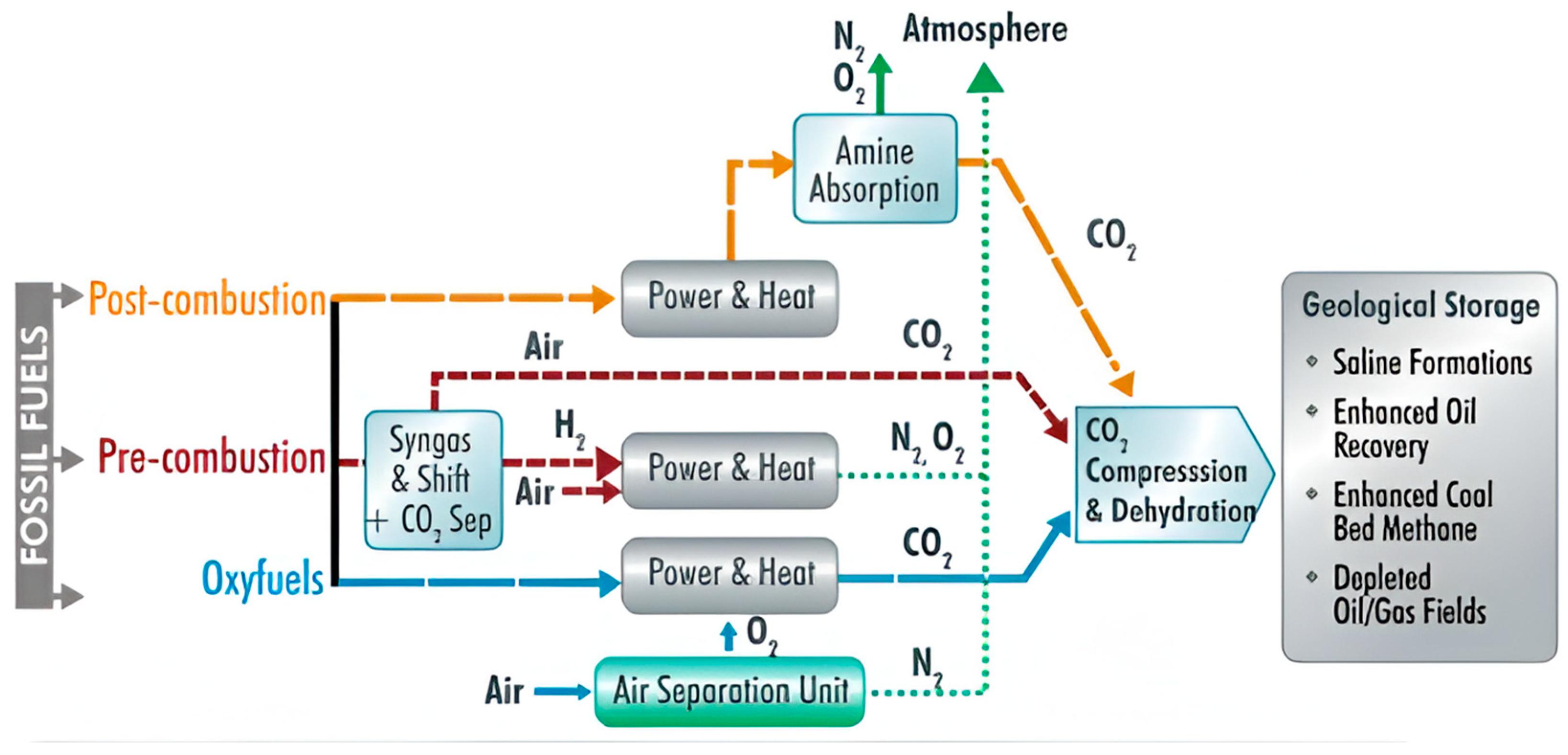
Compared to conventional fossil fuel combustion technologies, the CLC process has the potential to reduce greenhouse gas emissions and improve air quality significantly (see Figure 1).
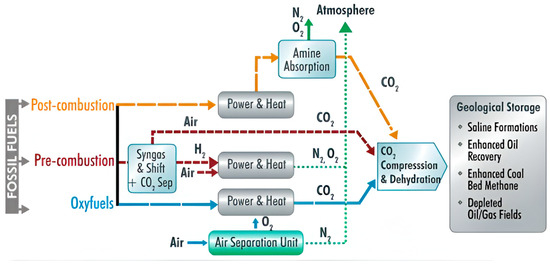
Figure 1.
Post-combustion capture, carbon utilization, and storage technologies.
CLC transfers oxygen from the air to fuel via an oxygen transporter to prevent contact between the air and fuel.
Oxygen transport is achieved by continuously circulating the oxygen transporter between two interconnected fluidized bed reactors (fuel and air). Fuel reactors oxidize fuel to carbon dioxide (CO2) and water vapor (H2O) primarily by reducing metal oxides.
Traditionally, the CLC process has been widely studied for the combustion of gaseous fuels. However, there has been significant interest in exploring the CLC process for burning solid fuels, such as coal, due to their abundance and low cost in the last decade [2]. Not only is the combustion of coal through the CLC process attractive, but there is also the possibility of using other solid fuels, such as biomass, due to its potential to mitigate associated CO2 emissions [3,4,5].
There are two approaches to approaching the CLC process with solid fuels. The first involves pre-gasifying the coal using oxygen and water vapor to produce syngas, similar to the first stage of pre-combustion capture technology (Shen et al., 2010) [6]. The resulting synthesis gas is introduced as fuel into the reduction reactor, following an approach similar to the CLC process with gases. Within this strategy of direct coal feeding into the reduction reactor, two options have been proposed: in situ coal gasification (iG-CLC) and combustion with oxygen decoupling in the gas phase (CLOU) [6].
Decoupling gaseous oxygen with coal, commonly known as chemical looping with oxygen uncoupling (CLOU), is characterized by using an oxygen carrier to release gaseous oxygen. This allows the direct combustion of coal without requiring a prior gasification stage.
2. Materials and Methods
Combustion with solid oxygen carriers (CLC) is the most suitable technology for CO2 capture. The primary function of these systems is to separate the CO2 produced into a gas stream with a high CO2 concentration.
There are a variety of studies in the referenced literature on solid oxygen carrier (SOC) preparation methods, including mechanical mixing [7], coprecipitation [4,8], mediated synthesis of surfactant [9,10], granulation by freezing [11,12,13], and impregnation [2,14]. The SOC particles used in these methods have a porous structure with a large surface area, facilitating the diffusion of reaction gases to metal oxide. However, any study preparing large-scale SOCs is scientifically novel [13].
Given the high cost of producing synthetic SOCs, low-cost materials such as ilmenite, manganese, and iron ores are sought. Like iron, manganese metal oxides are becoming increasingly important because they are cheap, non-toxic, and have a higher transport capacity than iron.
The highest oxidation state of Mn is MnO2, which decomposes at 500 °C. However, at temperatures above 800 °C, only Mn3O4 is a stable material. For this reason, only the conversion between Mn3O4 and MnO is considered for its application in CLC. Mn2O3 can be used as an alternative to the CLOU process.
Recently, many studies have been conducted on materials formed by the mixture of manganese oxides, which have shown appropriate suitability [14,15,16]; however, in this type of material, the decoupling of oxygen is highly conditioned by the conditions required for its previous oxidation. In addition, these solids have always been characterized by their low mechanical resistance, which has not allowed more than four hours of combustion with gases in a unit in continuous compound stirring. This aspect has been a vital conditioning factor in obtaining a manganese-based conveyor, not only for the coal removal process but for the process in general [7]. Therefore, this research developed an oxygen transporter based on this oxide with improved characteristics that increased combustion efficiency using thermogravimetry. For this, it was necessary to propose and select the most suitable material for the prepared solid based on the positive variables (oxygen transport capacity, mechanical resistance, and reaction speed), which were reported with excellent results, as indicated in (1).
Identifying solid compounds that can transfer oxygen in the CLC system is essential. Therefore, a compound must have a high fuel conversion to CO2 and H2O. Jerndal et al. (2006) presented an extensive thermodynamic analysis of several systems considered to be CLC. It recorded Cu, Ni, Co, Fe, and Mn oxides with favorable thermodynamics to react with CH4, H2, and CO, with equilibrium constant at the temperatures and pressures required in the CLC process [17,18].
2.1. Preparation of Materials
From the project “Combustion with CO2 capture using solid oxygen carriers (Chemical Looping Combustion)” to find potential low-cost SOCs in the country, 16 natural minerals of different types were characterized by X-ray fluorescence (XRF) analysis. In their regions of origin, these materials are mining designer minerals. Among them is the manganese-based mineral called OXMN009 in the present study. To improve this material, it is first impregnated with copper, obtaining a manganese-based mineral enhanced with copper called 0XMN009P. Data, such as a breaking strength of 5.7, were also obtained. These two minerals are solid oxygen carriers tested on a thermogravimetric balance (TGA) using CH4, CO, and H2 as the fuel. To achieve the particle size required to perform the tests on the thermogravimetric balance, a mill with a blade accessory was used, and the ground sample was passed through a No. 70 sieve (Tyler series), which guaranteed a diameter between 100 and 300 µm.
The screening and sufficiency process of the size of the manganese-based oxygen transporter was carried out. Calcination was performed on the 0XMN009 solid conveyor to increase its mechanical strength and obtain a modified manganese 0XMN009P using the incipient impregnation method for Cu processing of the selected sample, adding a 5 M solution volume of copper nitrate trihydrate (Cu(NO3)2·3H2O) corresponding to the pore volume of the material, which allowed contact between the ore and the impregnant. The copper nitrate was decomposed into copper oxide, the sample was calcined at a temperature of 550 °C, and finally, a second calcination was performed at 850 °C to stabilize the sample.
The available materials (original and modified solid oxygen carriers) were characterized by X-ray fluorescence (XRF) to quantify the concentration of manganese-based materials, followed by thermogravimetric analysis. Scanning electron microscopy (SEM) characterization and X-ray diffraction (XRD) were performed to identify the crystalline phases in oxygen-carrying particles.
For TSO’s XRF analysis, a PANalytical AXIOS max sequential wavelength dispersive X-ray fluorescence spectrometer (WDXRF) was equipped with a rhodium tube with a maximum power of 4.0 KW. It used a primary hybrid monochromator that produces a parallel beam, ideal for studying grazing samples (thin layers) to quantify elements present in the sample and help read the diffraction pattern information obtained with XRD.
For the XRD analysis of the TSOs, Panalytical Empyrean equipment with a cobalt X-ray source was used to perform a sweep between the 20° and 100° angles of the 2θθ range to identify the crystalline phases of the materials.
The SEM analysis was performed using a JEOL-JSM 6490 LV Scanning Electron Microscope (JEOL Ltd., Tokyo, Japan). This microscope uses electrons instead of light to form the image. To achieve this, the device has a filament that generates a beam of electrons to illuminate the sample. If the sample is not conductive, it must be covered with a thin layer of gold. The images produced by this method can be used to determine the morphology of SOC.
EDS analysis was used for elemental identification by chemical mapping using the same electron microscopy described above, which helped identify the Cu deposited in the immersion-treated SOC.
2.2. Thermogravimetric Balance Analysis
The assembly and commissioning of the thermogravimetric analysis (TGA) system for the CLC process was carried out. TGA analysis was performed on the solid oxygen (OXMN009) manganese-transporting material with particle diameters D to determine conversion at four different temperatures (between 650 and 950 °C), using CH4, CO, and H2 as the fuel, where the experimental design is indicated for three factors, CH4, CO, and H2; therefore, the number of experiments was 24. The experimental design included the type of material, temperature, and constant fuel concentration.
TGA C I Electronics
C I Electronics provided the thermogravimetric balance, which monitors the changes in mass that a sample undergoes when exposed to controlled atmospheres for a given period. This equipment has an accuracy of 0.1 μm and can work at high temperatures and with atmospheres of different compositions.
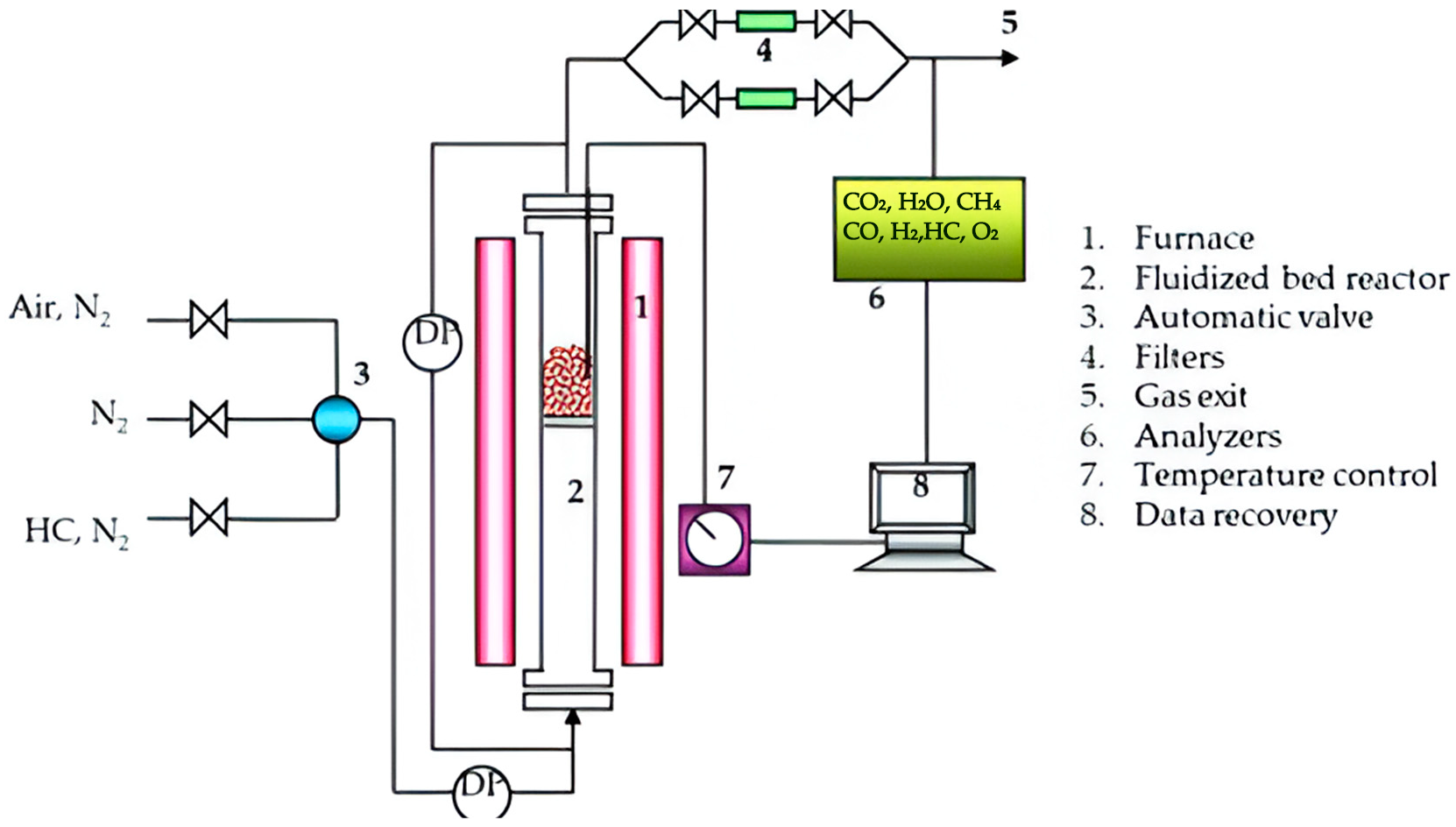
The advantage offered by this equipment is the diversity of working atmospheres comprising CO, CH4, and H2 needed in the present study. The equipment is composed of a furnace, a data collection system called LabWeight, a reactor, a head where the most sensitive part of the equipment is located, the balance, with which the weight variations that the sample undergoes when exposed to the study conditions are determined, valves, meters, controllers, etc. A disbal system, temperature controller, and a bubbler allow the injection of steam into the reactor (see Figure 2).
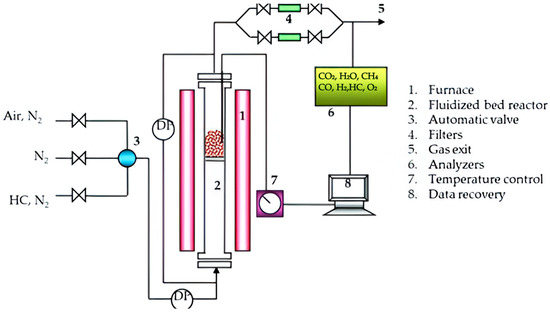
Figure 2.
LCC thermogravimetric balance (TGA); schematic of C I Electronics.
The method used for the thermogravimetric analysis procedure consisted of adding 50 mg ± 1 mg of solid oxygen carrier (SOC) to the basket, which was then placed at one end of the thermogravimetric balance and heated to the working temperature in an air atmosphere. Once the operation temperature was reached, the SOC maintained a constant mass and began analysis. The SOC went through cycles of reduction and oxidation, alternating gas lines, and maintaining a constant volumetric flow of 25 LN/h. The record obtained on the thermobalance was the change in weight or loss of mass and the solid carrier oxidation or reduction reaction time after the N2 purge to ensure an inert environment after reduction, resulting in the complete regeneration of the solid carrier by a gain in weight in the oxidation-reduction stage.
3. Results
Among Mn minerals, OXMN009 had the lowest reactivity with CH4 in TGA; however, in the literature, Mattisson, Johansson, and Lyngfelt (2006) reported that Mn-based SOC had a low reactivity with CH4 and high reactivity with H2 and CO. Therefore, the material of the present study OXMN009 was brought to a particle size between 100 and 300 µm [11,12].
Orrego (2017) investigated the effect of the initial impregnation of Cu in Fe and Mn TSO. The study variables were the type of fuel (CH4 and H2) and the percentage of Cu impregnated (1.5%, 3.5%, and 5%); it was identified that using H2 as the reducing gas and a Cu impregnation percentage between 3.5% and 5%, the improvement in SOC reactivity was more pronounced. For the above reasons, and considering that the detection limit of the XRD equipment used to identify the crystalline phases in the material is 4%, it was decided to impregnate the SOC for this study with 5% Cu [19].
The impregnation procedure was carried out to obtain OXMN009 with five wt.% Cu, as indicated above. Although multiple calculations were required, the particle size remained constant in the TSO, indicating no significant particle accumulation when subjected to temperatures up to 1050 °C in an oxidizing atmosphere.
The XRF analysis reflects the characterization of the OXMN009 and OXMN009P materials (93.15% and 89.32%), as indicated in Table 1. It was carried out to determine the presence of Mn elements associated with low-cost TO, so the presence of these elements in high concentrations indicates the mineral’s potential as TO [20,21,22,23].

Table 1.
XRF X-ray fluorescence (XRF) analysis.
Samples OXMN009 and OXMN009P have a high Mn content, which makes Mn oxide the main active phase in these materials; the Si content is low, so the presence of mixed oxides of Mn and Si as the active phase is negligible. A variety of metals in low proportions in XRF analysis is a common characteristic of natural minerals. Only active phases that can form with most elements are considered to facilitate subsequent calculations.
The results reported in Table 2 correspond to the crystalline phases detected in the diffractograms obtained. The many low-intensity peaks found and the overlap of peaks at closed 2θ angles, which usually occur in natural minerals, indicate that the crystalline phases reported in Table 3 are not all identified. There are probably also unidentified phases, as shown in Figure 3. XRD analysis detected thermite (CuO) in the Cu-treated SOC, consistent with an initial impregnation process in which the sample was calcined at 850 °C in an oxidizing atmosphere to decompose the added copper nitrate and form copper oxide.

Table 2.
Reduction and oxidation reactions of the active phases found in SOC.

Table 3.
X-ray disfraction.
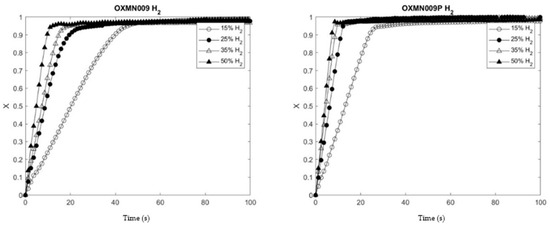
Figure 3.
Comparison between OXMN009 and OXMN009P. Red: 25% H2 and 75% N2; Ox: 100% air; T: 950 °C.
In the samples OXMN009 and OXMN009P, two oxidation states of manganese oxide were detected: Manganese III oxide (Mn2O3) and Manganese II oxide (Mn3O4). Refs. [14,24] studied the reduction and oxidation behavior of Mn oxides and found that once Mn2O3 was reduced to Mn3O4, its reoxidation was not detectable at high temperatures (800 °C) because, on the other hand, they discovered that Mn3O4 can be decreased to MnO and is easily reoxidized to Mn3O4 in air. Therefore, only the Mn3O4/MnO system in CLC is considered [16].
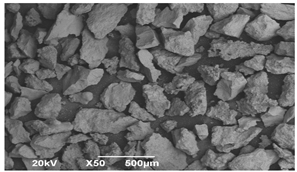
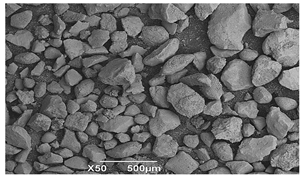
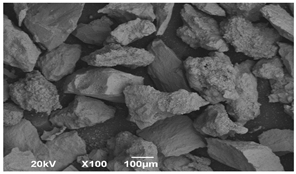
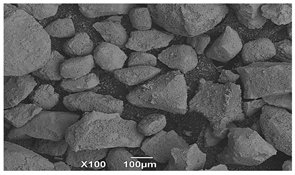
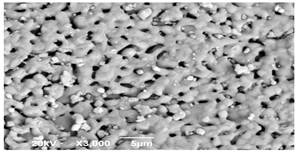
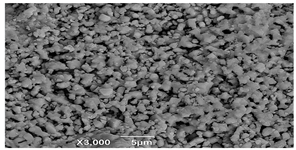
SEM microscopy of the SOC was taken in its native state and processed at magnifications of 50, 100, and 3000 to understand the morphology, size distribution, and possible differences caused by Cu treatment. The SEM microscopy of samples OXMN009 and OXMN009P are shown in Table 4. No difference was observed between the two solids at 50× and 100× magnification. Both have an irregular size distribution, flat particle shape, and rough surface. At 3000× magnification, many pores can be seen on the surface of sample OXMN009, but in sample OXMN009P, these pores appear occupied, possibly by impregnated CuO particles.

Table 4.
SEM microscopies of OXMN009 and OXMN009P at 50, 100, and 3000× magnification.
The EDS analysis was performed on the SOCs in their raw and processed states. To create a representative distribution of the elements on the samples’ surfaces, nine points were chosen to detect dispersive energy in the microscopies at 100× magnification of each SOC (see Table 4). Table 5 lists the elements found in each solid oxygen carrier in their maximum and minimum concentrations.

Table 5.
EDS analysis results for TSOs.
Finding high Mn contents in OXMN009 and OXMN009P, the elemental composition results in Table 5 support the findings in XFR (see Table 1) and XRD (see Table 2). Cu was discovered in the processed SOC at each elemental detection point, indicating that the element is well distributed within the SOC particles. It should also be noted that the EDS results were only used to determine the presence of the impregnated phase and its distribution in the SOC particles because they do not represent the total elemental composition of the samples.
The understudied SOCs OXMN009 and 0XMN009P were analyzed in TGA, which allowed the determination of the materials’ oxygen transport capacities, reaction rate indices, and kinetic parameters with the best behavior.
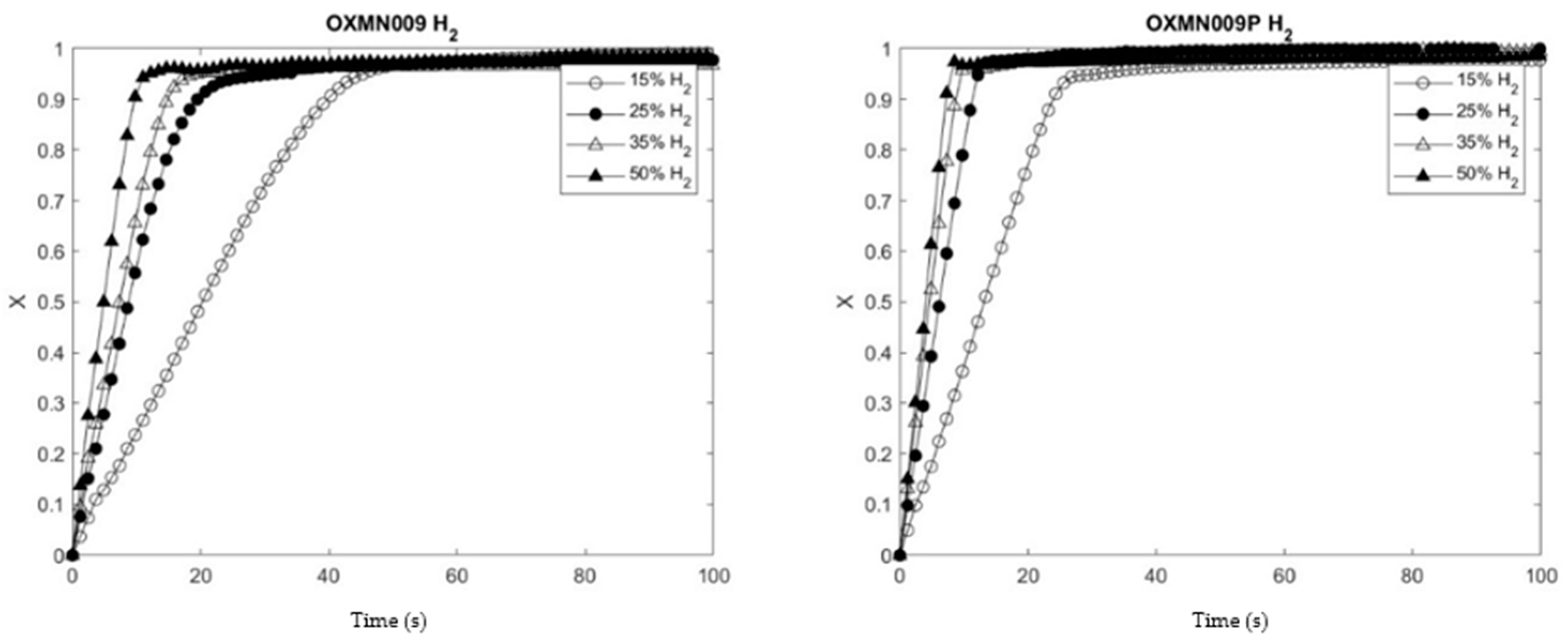
To determine the effect of copper treatment on the studied SOCs, the reduction and oxidation conversion curves of the native and treated SOCs were compared under the same operating conditions using the thermogravimetric balance (TGA). The operating temperature of 950 °C was chosen for these preliminary tests to show agglomeration problems, which did not occur in any of the samples studied. The analysis of the samples in an H2 atmosphere gave positive results in terms of reactivity; in addition, the samples treated with Cu showed higher reduction and oxidation conversions at 30 seconds. On the other hand, the analysis in a CO atmosphere showed similar results in OXMN009P for the reduction of Mn SOC and more excellent oxidation conversion at 30 seconds. However, this result is insufficient since the impregnation of Cu reactivity would have improved TSO with CO as fuel.
From the TGA data, reduction (Mred) and oxidation (Mox) conversion curves as a function of time can be generated using Equations (1) and (2), where Xred = reduce conversion; Xox = oxidation conversion; mox = rusty mass; m = mass at an instant in time t. With these data, the conversions of TO in oxidation and reduction can be quantified with the weights of the TGA analysis because the other parameters are known.
Mattison et al. (2004) determined the reaction rate index (RI) as a variable in solid oxygen carriers and established Equations (3) and (4).
where (dX/dt) is the derivative of the TSO conversion to time; pref is the reference partial pressure of the fuel gas; p TGA is the partial pressure of the fuel in TGA experiments; and the subscript norm is used to express the product p ref/p_TGA × (dX/dt) [11].
Table 6 shows the reaction rate indices (RI) obtained for the preliminary analyses carried out on each sample, which were calculated according to Equation (1), facilitating the comparison of the reaction rate between different SOCS, indicated in Equation (1), in the conversion delta between 0 and 0.15 when the reaction is faster, and the Pr was set at 0.15 atm, which is commonly used in the literature [11,15]. OXMN009P has a higher RI than OXMN009, and both materials present RI in the range reported in the literature for other Mn materials (see Table 7).

Table 6.
RI (%/min) in TGA tests at 950 °C of the Mn minerals reported in the literature.

Table 7.
Reaction order.
The activation energies (Ea) obtained for OXMN009 and OXMN009P using CO, H2, and CH4 as reactive gases are between 5 and 18.7 kJ/mol, a similar range to that found in the literature for Mn-based SOC (10.2 to 19.5 kJ/mol) [24] and lower than that reported for the synthetic SOC of Fe, Cu, Ni, and Mn (14 to 33 kJ/mol) [8] and other materials of natural origin, such as activated Ilmenite (25.5 to 80.7 kJ/mol) [26]. The Ea reported for OXMN009P are lower than those of OXMN009 in the reduction reactions, indicating that OXMN009P presents a greater reactivity concerning OXMN009 and a lower energy barrier for the reaction to take place [1], according to the kinetic results in Table 8 [27]. However, the oxidation of a lower Ea was obtained in SOC OXMN009.

Table 8.
Kinetics of materials 0XMN009 and OXMN009P.
The kinetic parameters (K) found for OXMN009 and OXMN009P are similar in order of magnitude in all the reactions studied and are close to those reported in the literature [23] for the oxidation of MnO to Mn3O4 m/s(mol/m3)n.
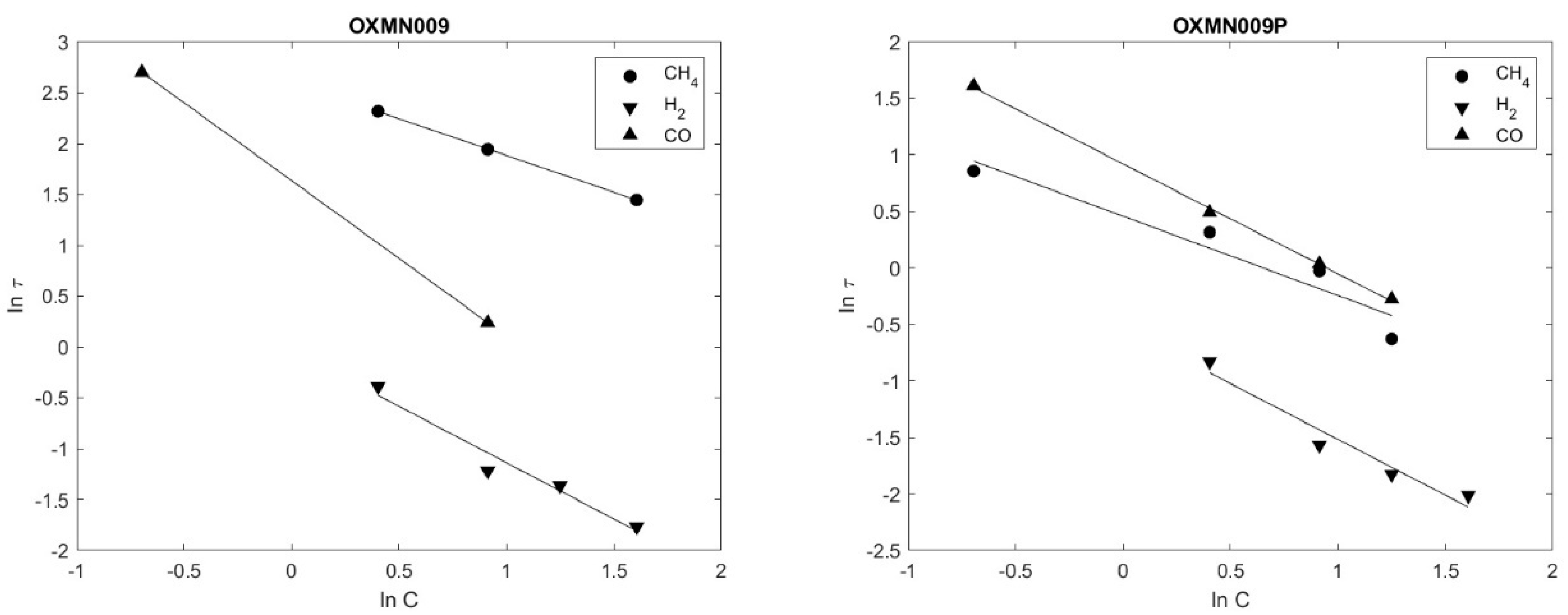
Figure 3 shows that the experimental data fit linearly to the grain SCM model for OXMN009 and OXMN009P for all gases studied.
The calculated reaction orders of OXMN009 and OXMN009P with CH4 (0.7; 0.7), H2 (1.11; 1.09), and CO (1.5; 1.0), respectively, as shown in Table 8, are within the ranges reported for synthetic Mn with CH4 (1.0) and O2 (0.65), and within the ranges reported for synthetic Cu-based minerals with CH4 (0.4), H2 (0.6), CO (0.8), and O2 (1.0); Fe with CH4 (1.3), H2 (0.8), CO (1.0), and O2 (1.0); Nor with CH4 (0.4), H2 (0.6), CO (0.8), and O2 (1.0) [28]. In general, the reaction order also depends on the fuel used. Regarding CH4, the reaction order obtained presents the same order of magnitude based on what was previously reported by Zafar and collaborators [23]. The results indicate that the reaction rate for the transporters studied follows first-order kinetics on average. The complete conversion times τ found are shown in Table 8. The correlation coefficients obtained from the linear regression X vs. time range of 0.9594 to 0.9999 reflect that the conversion varies linearly with time and fits the SCM model proposed following what was stated by Arango (2016) [16].
4. Discussion
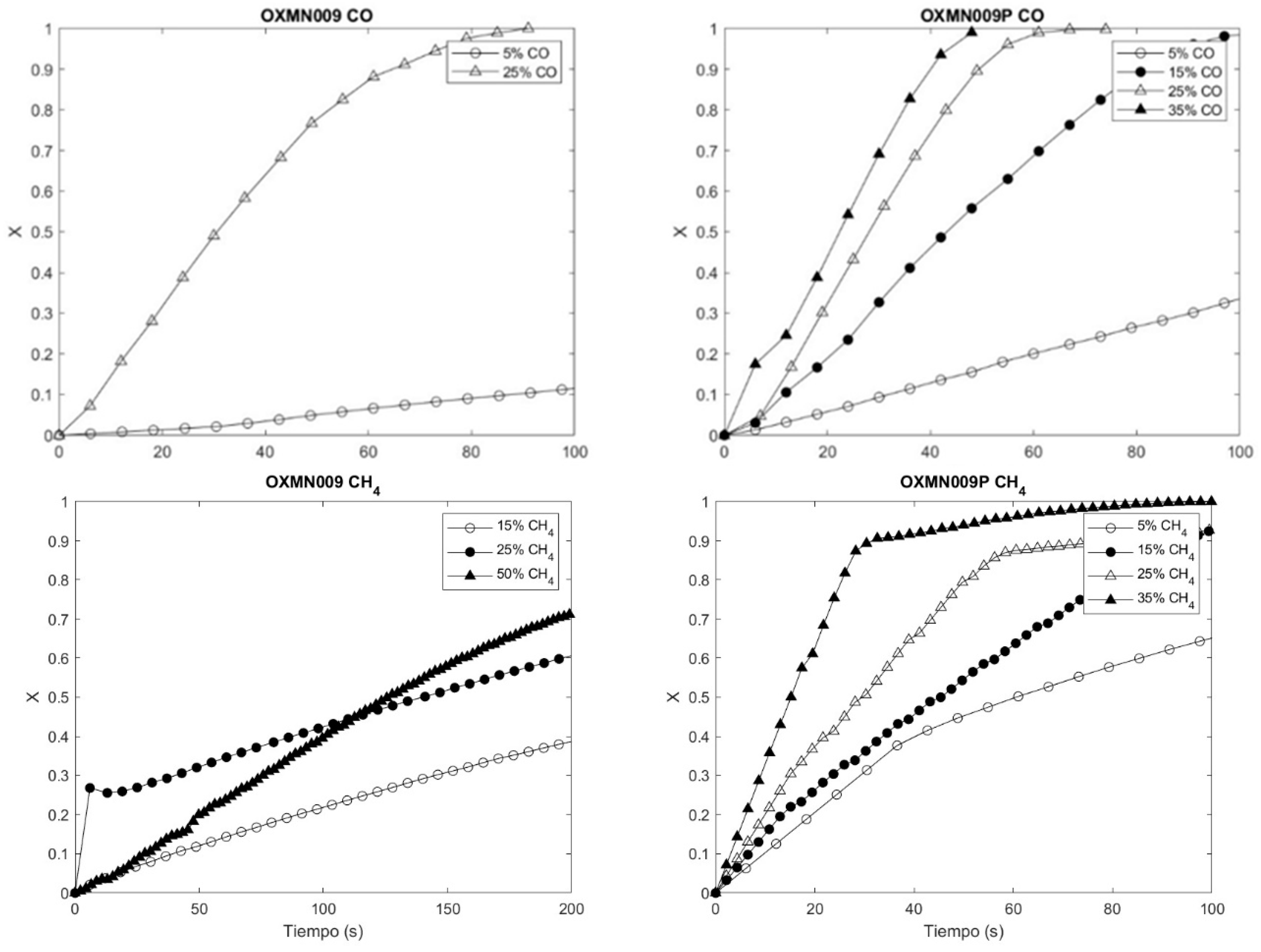
Figure 3 and Figure 4 of the concentration profiles show the compliance of the reaction rate increase with the concentration increase for the reducing agents CO, H2, and CH4 when they interact with OXMN009P. However, the same does not happen for the interaction of CH4 with OXMN009, since in a range of 0 to 100 s, the reaction rate is more significant at a concentration of 25% than at 50%, which could indicate the formation of product deposits on the surface of the conveyor constituting a diffusional resistance not considered in the proposed kinetic model.
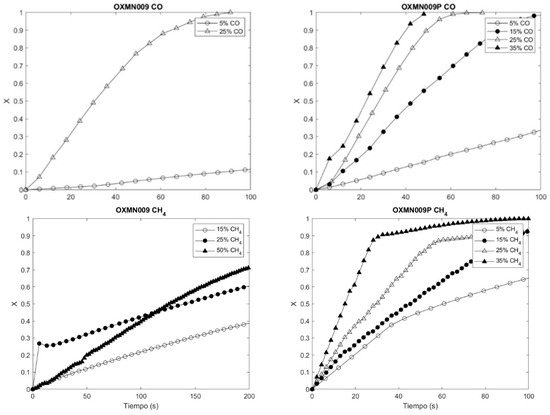
Figure 4.
Comparison OXMN009 and OXMN009P. Red: 25%CO and 75% N2; Ox:100% air; T: 950 °C.
Figure 5 shows that the experimental data fit linearly to the grain SCM model for OXMN009 and OXMN009P for all gases studied.
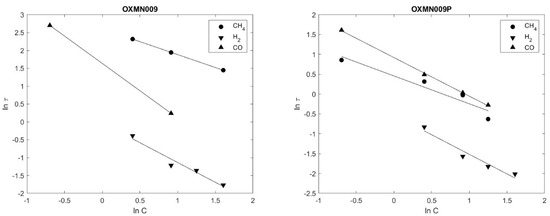
Figure 5.
Ln(τ) vs. Ln(C) OXMN009; OXMN009P (reductor gas: H2, CO, and CH4 between 5 and 50%, balance con N2. Air 100%. T = 950 °C.
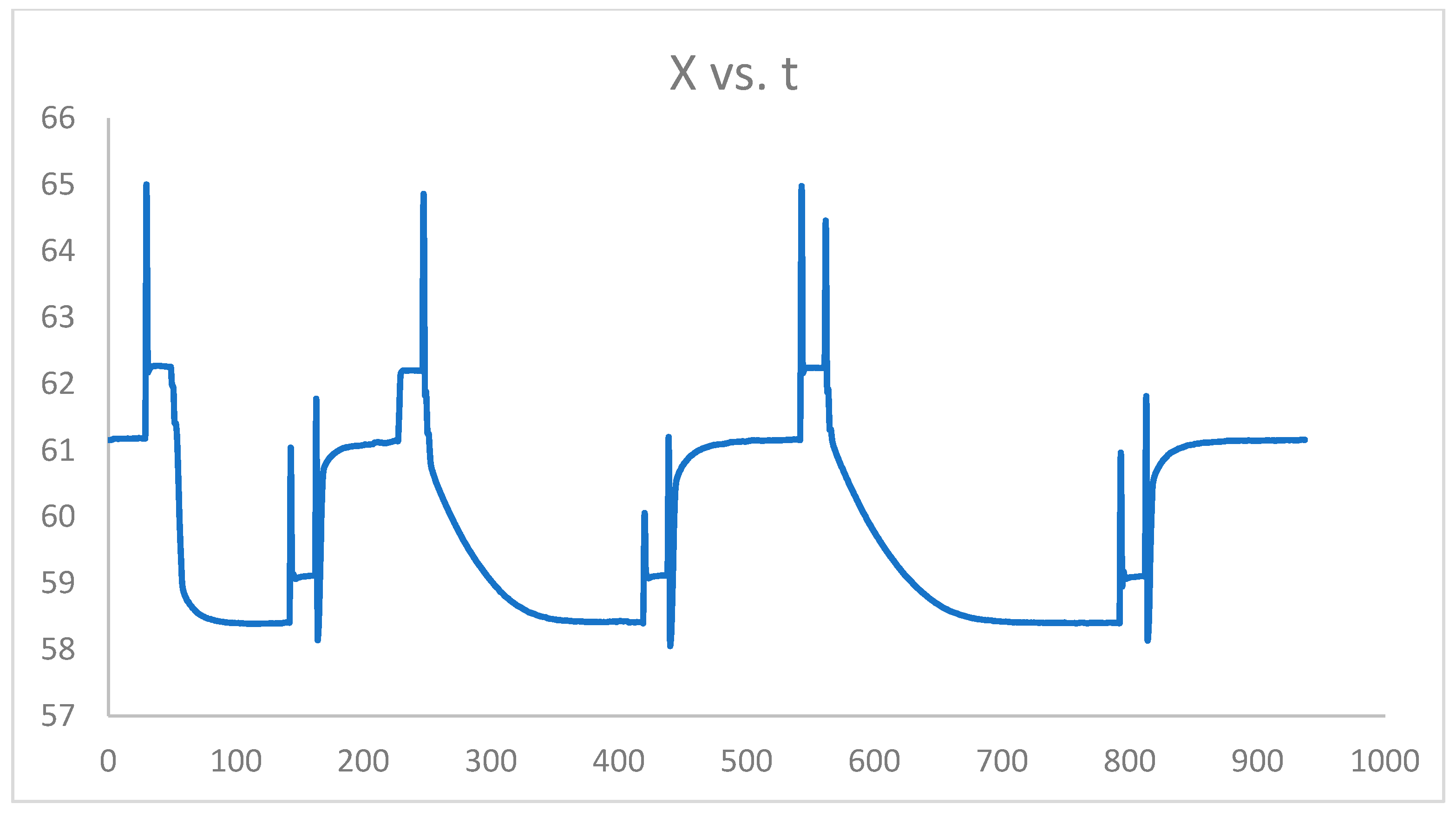
Figure 6 reflects the thermobalance (TGA) scheme used to perform TGA analysis in CLC.
Figure 6.
X vs. t graph of the thermogravimetric balance (TGA) results.
The calculated reaction orders of OXMN009 and OXMN009P with CH4 (0.7; 0.7), H2 (1.11; 1.09), and CO (1.5; 1.0) are within the ranges reported for synthetic Mn with CH4 (1.0) and O2 (0.65), and within the ranges reported for synthetic Cu-based minerals with CH4 (0.4), H2 (0.6), CO (0.8), and O2 (1.0); Fe with CH4 (1.3), H2 (0.8), CO (1.0), and O2 (1.0); Nor with CH4 (0.4), H2 (0.6), CO (0.8), and O2 (1.0) [23,29]. In general, the reaction order also depends on the fuel used. Regarding CH4, the reaction order obtained presents the same order of magnitude based on what was previously reported by Zafar and collaborators [23]. The results indicate that the reaction rate for the transporters studied follows, on average, first-order kinetics. Data is found in supplementary data
5. Conclusions
In this study, a manganese mineral (OXMN009) was modified with Cu using the incipient impregnation method and related to its use in CLC technology. In its natural state, it was found that OXMN009 has a much lower reactivity with CO and H2 than OXMN009P and did not suffer improvements when processed. Based on the above, the research called the mineral (OXMN009) natural state and the mineral (OXMN009P) processed or improved.
This research evaluated the behavior of the minerals OXMN009 and OXMN009P when evaluated using thermogravimetric balance analyses containing CO, H2, and CH4. These analyses gave reaction products with RI (Equation (3)) and reaction rates (7.9–20.1%/min) comparable to other manganese-based materials reported in the literature. In addition, improvements in the reactivity and oxygen transfer properties related to copper impregnation were obtained for the mineral OXMN009P.
The tests using the thermogravimetric balance (TGA) available in the Fuel Combustion Laboratory of the Universidad del Valle, CO, CH4, and H2 fuels were required. The equipment of both the TGA and the LFd was conditioned with their respective complements for operation.
The TGA products provided the kinetic parameters for the reduction of OXMN009 and OXMN009P (with CO and H2) with activation energies ranging between 11.0 and 28.0 kJ/mol, demonstrating that the reaction is non-thermal at 650–950 °C, independent, and requires less energy to start. The studied data’s dependence on the reagent’s concentration is approx. 1 ± 0.1, which is related to the publications on Mn materials.
Supplementary Materials
The following supporting information can be downloaded at: https://ugye-my.sharepoint.com/:f:/g/personal/sandra_penam_ug_edu_ec/EknhTWpSP2RIuU1GOL4eDHYBNBvDWcr0BVYPii_6so5g7Q?e=uzvgY (accessed on 1 January 2024).
Author Contributions
Conceptualization and methodology, S.P.M.; validation, E.A.; investigation, resources, S.P.M.; writing—original draft preparation, S.P.M.; writing—review and editing, C.F. and F.V.-S.; visualization, supervision, project administration, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
The Colombia Scientific Program provided financial support through the call Ecosistema Científico (Contract No. FP44842-218-2018) and the Coal Science and Technology Laboratory of the Universidad del Valle, Cali-Colombia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors express gratitude for the support from the Petroleum Laboratory of the Faculty and Career of Chemical Engineering of the University of Guayaquil, Guayaquil-Ecuador.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, J.; Zhang, Z. Carbon Capture, Utilization and Storage (CCUS). Appl. Energy 2019, 235, 1289–1299. [Google Scholar] [CrossRef]
- de Diego, L. Estado actual del proceso de combustión con transportadores sólidos de oxígeno. Bol. Grupo Español Carbón Estado 2015, 35, 21–25. [Google Scholar]
- Benavides, H. Información Técnica Sobre Gases de Efecto Invernadero y el Cambio Climático; Ideam: Bogotá, Colombia, 2007; pp. 1–102. [Google Scholar]
- Adánez, I.; Abad, A.; Gayán, P.; De Diego, L.; García, F.; Adánez, J. Biomass combustion with CO2 capture by chemical looping with oxygen uncoupling (CLOU). Fuel Process. Technol. 2014, 124, 104–114. [Google Scholar] [CrossRef]
- Mendiara, T.; Abad, A.; de Diego, L.; García, F.; Gayán, P.; Adánez, J. Biomass combustion in a CLC system using an iron ore as an oxygen carrier. Int. J. Greenh. Gas Control 2013, 19, 322–330. [Google Scholar] [CrossRef]
- Cabello, A.; Abad, A.; Gayán, P.; De Diego, L.F.; García-Labiano, F.; Adánez, J. Effect of operating conditions and H2S presence on the performance of CaMg0.1Mn0.9O3-δ perovskite material in chemical looping combustion (CLC). Energy Fuels 2014, 28, 1262–1274. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Development of (Mn0.77Fe0.23)2O3 particles as an oxygen carrier for coal combustion with CO2 capture via in-situ gasification chemical looping combustion (iG-CLC) aided by oxygen uncoupling (CLOU). Fuel Process. Technol. 2017, 164, 69–79. [Google Scholar] [CrossRef]
- Adánez, J.; Dueso, C.; De Diego, L.; García-Labiano, F.; Gayán, P.; Abad, A. Methane combustion in a 500 Wth chemical-looping combustion system using an impregnated ni-based oxygen carrier. Energy Fuels 2009, 23, 130–142. [Google Scholar] [CrossRef]
- Song, B.; Forsyth, J.A. Carbon Capture and Hydrogen Production with Membranes. Energy Procedia 2013, 37, 1050–1059. [Google Scholar] [CrossRef]
- Xu, C.; Hu, S.; Xiang, J.; Yang, H.; Sun, L.; Su, S.; Wang, B.; Chen, Q.; He, L. Kinetic models comparison for steam gasification of coal/biomass blend chars. Bioresour. Technol. 2014, 171, 253–259. [Google Scholar] [CrossRef]
- Mattisson, T. Materials for Chemical-Looping with Oxygen Uncoupling. ISRN Chem. Eng. 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Keller, M.; Arjmand, M.; Leion, H.; Mattisson, T. Interaction of coal mineral matter with oxygen carriers in chemical-looping combustion (CLC). Chem. Eng. Res. Des. 2014, 92, 1753–1770. [Google Scholar] [CrossRef]
- Linderholm, C.; Schmitz, M.; Knutsson, P.; Lyngfelt, A. Chemical-looping combustion in a 100-kW unit using a mixture of ilmenite and manganese ore as an oxygen carrier. Fuel 2016, 166, 533–542. [Google Scholar] [CrossRef]
- Arango, E.; Forero, C.; Velasco-Sarria, F. Use of a Low-Cost Colombian Manganese Mineral as a Solid Oxygen Carrier in Chemical Looping Combustion Technology. Energy Fuels 2021, 35, 12252–12259. [Google Scholar] [CrossRef]
- Mei, D.; Mendiara, T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J.; Zhao, H. Manganese Minerals as Oxygen Carriers for Chemical Looping Combustion of Coal. Ind. Eng. Chem. Res. 2016, 55, 6539–6546. [Google Scholar] [CrossRef]
- Arango, E.; Vasquez, F. Determinación de los Parámetros Cinéticos para la Combustión Usando Minerales del Suroccidente Colombiano Como Transportadores Sólidos de Oxígeno. 2016. Available online: https://bibliotecadigital.univalle.edu.co/handle/10893/16893 (accessed on 26 September 2021).
- Snijkers, F.; Jerndal, E.; Thijs, I.; Mattisson, T.; Lyngfelt, A.; Frans, S. Prepare oxygen carriers for chemical looping combustion by industrial spray drying method. In Proceedings of the 1st International Conference on Chemical Looping, Lyon, France, 17–19 March 2010; pp. 1–10. [Google Scholar]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal analysis of chemical-looping combustion. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Orrego, A. Preparación y Caracterización de Transportadores Sólidos de Oxígeno Basados en fe y mn Modificados con cuo para Combustión con Captura de CO2. 2017. Available online: https://bibliotecadigital.univalle.edu.co/handle/10893/14517 (accessed on 26 September 2021).
- Adánez-Rubio, I.; Pérez-Astray, A.; Mendiara, T.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Chemical looping combustion of biomass: CLOU experiments with a Cu-Mn mixed oxide. Fuel Process. Technol. 2018, 172, 179–186. [Google Scholar] [CrossRef]
- Forero, C.R.; Adánez Elorza, J.; Gayán Sanz, P.; de Diego Poza, L.F.; García Labiano, F.; Abad Secades, A. Captura de CO2 mediante transportadores sólidos. Rev. Colomb. Química 2010, 39, 271–285. [Google Scholar]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Zafar, Q.; Abad, A.; Mattisson, T.; Gevert, B.; Strand, M. Reduction and oxidation kinetics of Mn3O4/Mg–ZrO2 oxygen carrier particles for chemical-looping combustion. Chem. Eng. Sci. 2007, 62, 6556–6567. [Google Scholar] [CrossRef]
- Velasco-Sarria, F.; Forero, C.; Adánez-Rubio, I.; Abad, A.; Adánez, J. Assessment of low-cost oxygen carrier in South-western Colombia, and its use in the in-situ gasification chemical looping combustion technology. Fuel 2018, 218, 417–424. [Google Scholar] [CrossRef]
- Mendiara, T.; Jensen, A.; Glarborg, P. Chemical Looping Reforming of Generator Gas. 2010. Available online: https://orbit.dtu.dk/en/publications/chemical-loop-reforming-of-generator-gas-interim-report (accessed on 1 January 2024).
- Forero, C.; Adánez, J.; Gayán, P.; De Diego, L.; Francisco, G.-L.; Abad, A. CO2 Capture by Chemical Looping Combustion. Rev. Colomb. Química 2010, 39, 271–285. [Google Scholar]
- Levenspiel, O. Ingeniería de las Reacciones; Reverte: Barcelona, Spain, 1993. [Google Scholar]
- Abad, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Relevance of plant design on CLC process performance using a Cu-based oxygen carrier. Fuel Process. Technol. 2018, 171, 78–88. [Google Scholar] [CrossRef]
- Abad, A.; de las Obras-Loscertales, M.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. In situ gasification Chemical-Looping Combustion of coal using limestone as oxygen carrier precursor and sulfur sorbent. Chem. Eng. J. 2017, 310, 226–239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).