Enrichment of Bakery Products with Antioxidant and Dietary Fiber Ingredients Obtained from Spent Coffee Ground
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spent Coffee Ground Supply and Materials
2.2. Freeze Drying of Spent Coffee Ground
2.3. Extraction of Phenolic Compounds from Spent Coffee Ground
2.4. Total Phenolic Content (TPC) Determination (Folin–Ciocalteu)
2.5. HPLC-DAD Analyses of Phenolic Compounds
2.6. Total Dietary Fiber (TDF) Content Determination of Spent Coffee Ground
2.6.1. Defatting and Oil Content Determination of SCG
2.6.2. Enzymatic Digestion of Spent Coffee Ground
2.6.3. Ash Content Determination of Enzymatically Digested Spent Coffee Ground
2.6.4. Total Kjeldahl Nitrogen (TKN) Determination of Enzymatically Digested Spent Coffee Ground
2.7. Preparation of Cookies
2.8. Moisture Content of Cookies
2.9. Color Measurement of Cookies
2.10. Texture Analysis of Cookies
2.11. Sample Preparation of Cookies for Total Phenolic Content (TPC) and Peroxide Value (PV) Determination
2.12. Peroxide Value Determination
2.13. Sensory Evaluation
2.14. Data and Statistical Analysis
3. Results and Discussion
3.1. Spent Coffee Ground Characterization
3.2. Moisture Content of Cookies and SCG Concentration in Baked Products
3.3. Cookie Characterization
3.4. Shelf Life Analysis of Produced Cookies
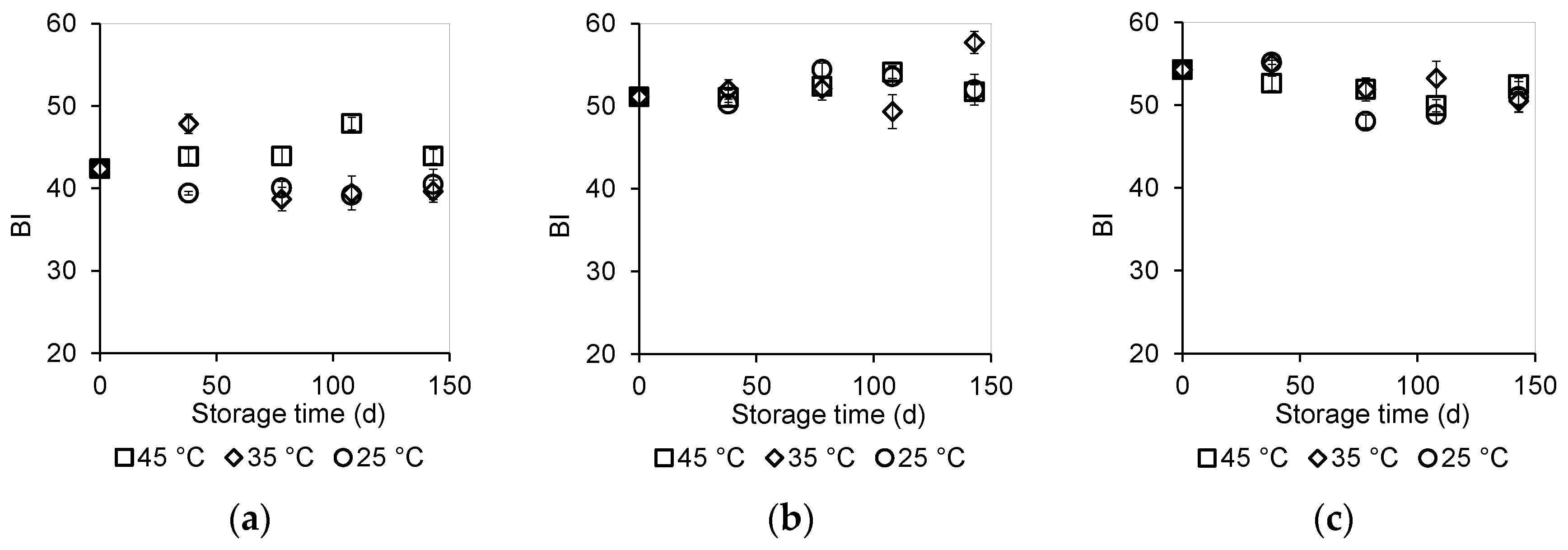
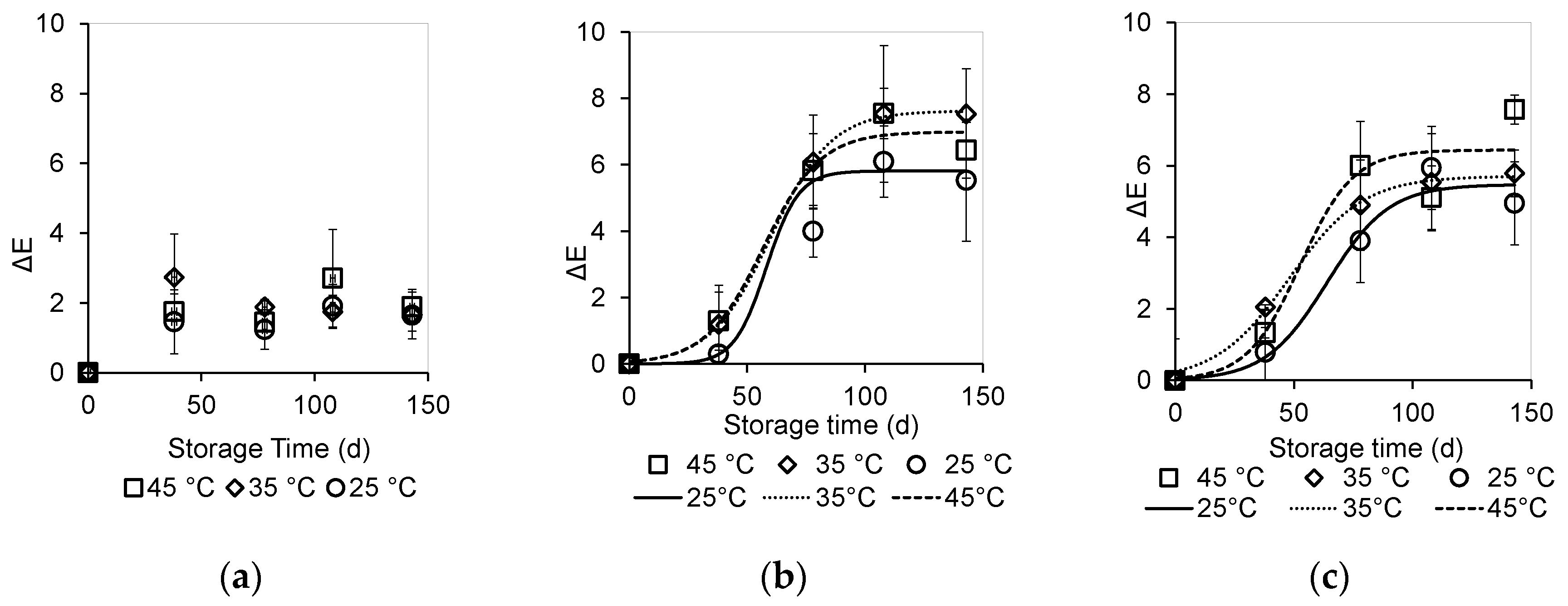
3.4.1. Color Measurement
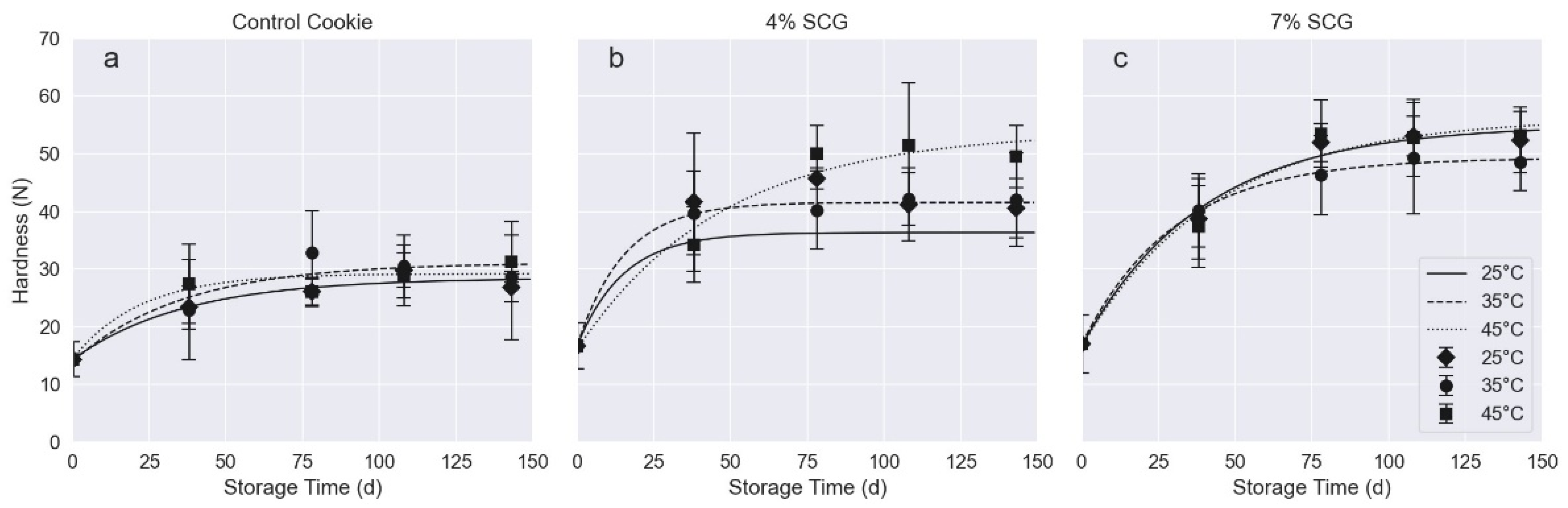
3.4.2. Texture Analysis
3.4.3. Peroxide Value
3.4.4. Total Phenolic Content
3.4.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc. Tech. 2014, 7, 3493–5203. [Google Scholar] [CrossRef]
- International Coffee Organization. International Coffee Organization—Trade Statistics Table. 2023. Available online: http://www.ico.org/trade_statistics.asp?section=Statistics (accessed on 2 June 2020).
- Grassino, A.N.; Jerković, I.; Pedisić, S.; Dent, M. Hydrodistillation fractions of coffee (green and roasted) and coffee by-product (silver skin and spent grounds) as a source of bioactive compounds. Sustain. Chem. Pharm. 2024, 39, 101592. [Google Scholar] [CrossRef]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products. Sustainable Applications; Galanakis, C.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–26. [Google Scholar]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Hu, F.; Miyakawa, T.; Tanokura, M. Complex mixture analysis of organic compounds in green coffee bean extract by two-dimensional NMR spectroscopy. Magn. Reson. Chem. 2010, 48, 857–865. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.A.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Rao, B.D.; Kulkarni, D.B.; Kavitha, C. Study on evaluation of starch, dietary fiber and mineral composition of cookies developed from 12 sorghum cultivars. Food Chem. 2018, 238, 82–86. [Google Scholar] [CrossRef]
- Sudha, M.L.; Vetrimani, R.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Beltrán-Medina, E.A.; Guatemala-Morales, G.M.; Padilla-Camberos, E.; Corona-González, R.I.; Mondragón-Cortez, P.M.; Arriola-Guevara, E. Evaluation of the use of a coffiee industry by-product in a cereal-based extruded food product. Foods 2020, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Pourfarzad, A.; Mahdavian-Mehr, H.; Sedaghat, N. Coffee silverskin as a source of dietary fiber in bread-making: Optimization of chemical treatment using response surface methodology. LWT-Food Sci. Technol. 2013, 50, 599–606. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Hoang, N.-H.; Do, H.H.; Dang, T.H.Y.; Ton, M.N.N.; Tran, T.T.T.; Le, V.V.M. Fiber-enriched biscuits prepared with enzyme-treated corncob powder: Nutritional composition, physical properties, and sensory acceptability. J. Food Proces. Preserv. 2022, 46, 16784. [Google Scholar] [CrossRef]
- Nicole, T.Z.H.; Nichelle, T.S.; Elizabeth, T.E.; Yuliarti, O. Formulation of functional crackers enriched with fermented soybean (tempeh) paste: Rheological and microstructural properties. Future Foods 2021, 4, 100050. [Google Scholar] [CrossRef]
- Ateş, G.; Elmacı, Y. Physical, chemical and sensory characteristics of fiber-enriched cakes prepared with coffee silverskin as wheat flour substitution. J. Food Meas. Charact. 2019, 13, 755–763. [Google Scholar] [CrossRef]
- Dauber, C.; Romero, M.; Chaparro, C.; Ureta, C.; Ferrari, C.; Lans, R.; Frugoni, L.; Echeverry, M.V.; Calvo, B.S.; Trostchansky, A.; et al. Cookies enriched with coffee silverskin powder and coffee silverskin ultrasound extract to enhance fiber content and antioxidant properties. Appl. Food Res. 2024, 4, 100373. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Prot. Food Anal. Chem. 2002, 6, 1–8. [Google Scholar]
- Bertolino, M.; Barbosa-Pereira, L.; Ghirardello, D.; Botta, C.; Rolle, L.; Guglielmetti, A.; Dalla Vecchia, S.B.; Zeppa, G. Coffee silverskin as nutraceutical ingredient in yogurt: Its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 2019, 99, 4267–4275. [Google Scholar] [CrossRef]
- Nolasco, A.; Squillante, J.; Velotto, S.; D’Auria, G.; Ferranti, P.; Mamone, G.; Errico, M.E.; Avolio, R.; Castaldo, R.; Cirillo, T.; et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022, 370, 133520. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; Ullate, M.; Martin-Cabrejas, M.A.; Martorell, P.; Genovés, S.; Ramon, D.; del Castillo, M.D. A novel antioxidant beverage for body weight control based on coffee silver skin. Food Chem. 2014, 150, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Athanassiou, C.G.; Lalas, S.I. Utilization of spent coffee grounds as a feed additive for enhancing the nutritional value of Tenebrio molitor Larvae. Sustainability 2023, 15, 16224. [Google Scholar] [CrossRef]
- Roslan, Ν.A.; Sukri, S.A.M.; Wei, L.S.; Shahjahan, M.; Rohani, M.F.; Yea, C.S.; Kabir, M.A.; Guru, A.; Goh, K.W.; Kallem, P.; et al. Replacement of fishmeal by fermented spent coffee ground: Effects on growth performance, feed stability, blood biochemistry, liver, and intestinal morphology of African catfish (Clarias gariepinus). Aquac. Res. 2024, 36, 102073. [Google Scholar] [CrossRef]
- Cantele, C.; Tedesco, M.; Ghirardello, D.; Zeppa, G.; Bertolino, M. Coffee Silverskin as a Functional Ingredient in Vegan Biscuits: Physicochemical and Sensory Properties and in Vitro Bioaccessibility of Bioactive Compounds. Foods 2022, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Tsevdou, M.; Ntzimani, A.; Katsouli, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Comparative Study of Microwave, Pulsed Electric Fields, and High-Pressure Processing on the Extraction of Antioxidants from Olive Pomace. Molecules 2024, 29, 2303. [Google Scholar] [CrossRef] [PubMed]
- AOCS (American Oil Chemists’ Society). Official Method Cd Peroxide Value. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; AOCS: Champaign, IL, USA, 1998; pp. 8–53. [Google Scholar]
- AOAC. Method 985.29. Total Dietary Fiber in Foods. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović-Šeremešić, M. Sugar beet and apple fibres coupled with hydroxypropylmethylcellulose as functional ingredients in gluten-free formulations: Rheological, technological and sensory aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef] [PubMed]
- ICC Standard 104/1 Determination of Ash in Cereals and Cereal Products. Available online: https://icc.or.at/icc-standards/standards-overview/104-1-standard-method (accessed on 2 June 2020).
- AOAC. Method 979.09. Protein in Grain. Official Methods of Analysis of AOAC International, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- AOAC. Method 23.003. Official Methods of Analysis of AOAC International, 11th ed.; AOAC: Washington, DC, USA, 1999. [Google Scholar]
- Wei, F.; Furihata, K.; Koda, M.; Hu, F.; Miyakawa, T.; Tanokura, M. Roasting process of coffee beans as studied by nuclear magnetic resonance: Time course of changes in composition. J. Agric. Food Chem. 2012, 60, 1005–1012. [Google Scholar] [CrossRef]
- Rover, M.R.; Brown, R.C. Quantification of total phenols in bio-oil using the Folin–Ciocalteu method. J. Anal. Appl. Pyrolysis. 2013, 104, 366–371. [Google Scholar] [CrossRef]
- Ikawa, M.; Dollard, C.A.; Schaper, T.D. Reaction of Folin-Ciocalteau phenol reagent with purines, pyrimidines, and pteridines and its relationship to structure. J. Agric. Food Chem. 1988, 36, 309–311. [Google Scholar] [CrossRef]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef]
- Toledo, P.R.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K.; Kim, Y.; Liu, S.X. Antioxidant activity of spent coffee ground extracts toward soybean oil and fish oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800372. [Google Scholar] [CrossRef]
- Loyao, A.S., Jr.; Villasica, S.L.G.; Peña, P.L.L.D.; Go, A.W. Extraction of lipids from spent coffee grounds with non-polar renewable solvents as alternative. Ind. Crops Prod. 2018, 119, 152–161. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council on Nutrition and Health Claims Made on Foods; European Parliament, Council of the European Union: Strasbourg, France, 2006. [Google Scholar]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef]
- Adams, A.; De Kimpe, N.; van Boekel, M.A.J.S. Modification of casein by the lipid oxidation product malondialdehyde. J. Agric. Food Chem. 2008, 56, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Ngo, H.B.G.; Phu, M.L.; Tran, T.T.T.; Ton, N.M.N.; Nguyen, T.Q.N.; Man Le, V.V. Dietary fiber-and antioxidant-enriched cookies prepared by using jackfruit rind powder and ascorbic acid. Heliyon 2024, 10, e30884. [Google Scholar] [CrossRef]
- Dachana, K.B.; Rajiv, J.; Indrani, D.; Prakash, J. Effect of dried moringa (Moringa oleifera lam) leaves on rheological, microstructural, nutritional, textural and organoleptic characteristics of cookies. J. Food Qual. 2010, 33, 660–677. [Google Scholar] [CrossRef]
- Iwansyah, A.C.; Melanie, D.; Cahyadi, W.; Indraningsih, A.W.; Khasanah, Y.; Indriati, A.; Andriansyah, R.C.E.; Hamid, H.A.; Yahya, I.H. Shelf-life evaluation of formulated cookies from Hanjeli (Coix lacryma-jobi L.) and Moringa leaf flour (Moringa oleifera). Food Biosci. 2022, 47, 101787. [Google Scholar] [CrossRef]
- de Morais, M.P.; Caliari, M.; Nabeshima, E.H.; Batista, J.E.R.; Campos, M.R.H.; Soares Junior, M.S. Storage stability of sweet biscuit elaborated with recovered potato starch from effluent of fries industry. Food Sci. Technol. 2018, 38, 216–222. [Google Scholar] [CrossRef]
- Mrabet, A.; Hamdi, A.; Rodríguez-Arcos, R.; Guillen-Bejarano, R.; Jimenez-Araujo, A. Date seed by-products as source of bioactive ingredient for healthy cookies. Food Biosci. 2024, 61, 104543. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. Advances in instrumental methods to determine food quality deterioration. In Food and Beverage Stability and Shelf Life. Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 381–404. [Google Scholar]
- Bee, S.; Brando, C.H.J.; Brumen, G.; Carvalhaes, N.; Kolling-Speer, I.; Speer, K.; Suggi Liverani, F.; Texeira, A.A.; Thomaziello, R.A.; Viani, R.; et al. The Raw Bean. In Espresso Coffee: The Science of Quality, 2nd ed.; Illy, A., Viani, R., Eds.; Elsevier Academic Press: London, UK, 2005; pp. 87–178. [Google Scholar]
- Koay, H.Y.; Azman, A.T.; Zin, Z.M.; Portman, K.L.; Rusli, N.D.; Hasmadi, M.; Rusli, N.D.; Aidat, O.; Zainol, M.K. Assessing the impact of spent coffee ground (SCG) concentrations on shortbread: A study of physicochemical attributes and sensory acceptance. Future Foods 2023, 8, 100245. [Google Scholar] [CrossRef]
- Bekedam, E.K.; Schols, H.A.; van Boekel, M.A.J.S.; Smit, G. Incorporation of chlorogenic acids in coffee brew melanoidins. J. Agric. Food Chem. 2008, 56, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food. Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Jaric, A.M.; Pasquino, M.N.; Seremet, D.; Pudic, R.E.; Vojvodic, A.; Komes, D. The assesement of bioactive potential and sensory acceptability of coffee and its byproducts-cascara and silverskin. Croat. J. Food Technol. 2021, 16, 35–40. [Google Scholar]
- Sakac, M.; Pestoric, M.; Mandic, A.; Misan, A.; Nedeljkovic, N.; Jambrec, D.; Jovanov, P.; Lazic, V.; Pezo, L.; Sedej, I. Shelf-life prediction of gluten-free rice-buckwheat cookies. J. Cereal Sci. 2016, 69, 336–343. [Google Scholar] [CrossRef]
- Gebreselassie, E.; Clifford, H. Oxidative stability and shelf life of crackers, cookies, and biscuits. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; Academic Press: Cambridge, MA, USA; AOCS Press: Champaign, IL, USA, 2016; Volume 1904, pp. 461–478. [Google Scholar]




| Ingredients (%) | Control | Cookie Formula A | Cookie Formula B |
|---|---|---|---|
| All-purpose flour | 47.4 | 43.96 | 41.45 |
| Baking powder | 0.4 | 0.4 | 0.4 |
| Salt | 0.2 | 0.2 | 0.2 |
| Sugar | 26 | 26 | 26 |
| Butter | 21 | 21 | 21 |
| Eggs | 5 | 5 | 5 |
| SCG 1 | 0 | 3.44 | 5.95 |
| Total | 100 | 100 | 100 |
| Contents of Dried SCG | Concentration |
|---|---|
| Total Phenolic Content (mg GAE, 1 per g dry SCG) | 21.6 ± 0.7 |
| Recovered by H2O | 11.0 ± 0.6 |
| Recovered by MeOH:H2O (1:1) | 5.0 ± 0.2 |
| Recovered by acetone:H2O (1:1) | 2.2 ± 0.1 |
| Recovered by acetone | 3.3 ± 0.1 |
| Oil Content (% on dry SCG) | 12.2 ± 0.5 |
| Total Dietary Fiber (% on dry SCG) | 66 ± 5 |
| Total Kjeldahl Nitrogen (% on dry SCG) | 2.0 ± 0.1 |
| Ash (% on dry SCG) | 2.0 ± 0.1 |
| Control Cookie | 4% SCG-Added | 7% SCG-Added | ||
|---|---|---|---|---|
| Moisture Content (%) | 7.00 ± 0.01 | 6.5 ± 0.4 | 4.0 ± 0.4 | |
| Color | Lo | 79 ± 1 | 40 ± 1 | 46 ± 1 |
| ao | −0.70 ± 0.01 | 5.0 ± 0.1 | 5.0 ± 0.2 | |
| bo | 28 ± 3 | 15 ± 0.4 | 17 ± 1 | |
| Hardness, Ho (N) | 14.2 ± 0.2 | 16.5 ± 0.3 | 17.0 ± 0.3 | |
| Total Phenolic Content (mgGAE/kgcookies) | 68 ± 2 | 588 ± 24 | 1017 ± 5 | |
| Total Dietary Fiber (g/100 g cookies) | 0.87 ± 0.04 | 2.65 ± 0.18 | 4.64 ± 0.32 | |
| Nutritional claim | N/A | N/A | Source of dietary fiber [28] | |
| Sample | Temperature (°C) | ΔΕmax | k (Days−1) | to (Days) | R2 |
|---|---|---|---|---|---|
| Control | 25 °C | - | - | - | - |
| 35 °C | - | - | - | - | |
| 45 °C | - | - | - | - | |
| 4% SCG | 25 °C | 6.0 ± 0.5 a | 13 ± 5 a | 64 ± 8 a | 0.9729 |
| 35 °C | 6.0 ± 0.2 a | 16 ± 2 a | 48 ± 2 b | 0.9966 | |
| 45 °C | 6.4 ± 1.0 a | 11 ± 9 a | 53 ± 15 ab | 0.9279 | |
| 7% SCG | 25 °C | 6.0 ± 0.2 a | 7 ± 2 a | 58 ± 5 a | 0.9957 |
| 35 °C | 7.6 ± 0.1 b | 13 ± 1 b | 60 ± 1 a | 0.9996 | |
| 45 °C | 7.0 ± 0.5 b | 12 ± 4 b | 57 ± 7 a | 0.9833 |
| Cookie Sample | Storage Temp | R2 | Ho (N) | a (N) | b (Days−1) | Hinf (N) |
|---|---|---|---|---|---|---|
| Control | 25 °C | 0.9535 | 14.3 ± 1.8 aA | 14.2 ± 2.4 aA | 0.03 ± 0.013 aA | 28.47 ± 3.01 aA |
| 35 °C | 0.8941 | 14.0 ± 4.0 bA | 17 ± 5 bA | 0.03 ± 0.021 aA | 31.12 ± 5.66 aA | |
| 45 °C | 0.9265 | 14.4 ± 2.5 bA | 14.8 ± 3.0 aA | 0.05 ± 0.032 aA | 29.21 ± 3.95 aA | |
| 4% SCG-added | 25 °C | 0.9868 | 17.0 ± 1.4 aB | 19.6 ± 2.0 aB | 0.07 ± 0.028 aB | 36.35 ± 2.17 aA |
| 35 °C | 0.9959 | 16.7 ± 1.0 aB | 25 ± 1 bB | 0.07 ± 0.015 aB | 41.58 ± 1.53 bB | |
| 45 °C | 0.9688 | 16.0 ± 3.7 aB | 37.8 ± 5.8 cB | 0.02 ± 0.009 bA | 53.90 ± 6.87 bB | |
| 7% SCG-added | 25 °C | 0.9887 | 16.7 ± 2.3 aB | 38.3 ± 3.2 aC | 0.03 ± 0.006 aA | 54.99 ± 9.98 aC |
| 35 °C | 0.9984 | 17.0 ± 0.7 aB | 32.2 ± 1.0 bC | 0.03 ± 0.003 aA | 49.28 ± 1.24 bC | |
| 45 °C | 0.9774 | 16.6 ± 3.0 aB | 40.0 ± 5 aB | 0.02 ± 0.008 aA | 56.25 ± 5.96 aB |
| Storage Temp. (°C) | Control Cookie | 4% SCG-Added Cookie | 7% SCG-Added Cookie | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensory Deterioration Rate | R2 | Shelf Life (d) ** | Sensory Deterioration Rate | R2 | Shelf Life (d) ** | Sensory Deterioration Rate | R2 | Shelf Life (d) ** | |
| 25 | 0.010 ± 0.001 aA | 0.8879 | 359 ± 2 | 0.0069 ± 0.0001 aB | 0.9898 | 435 | 0.0068 ± 0.001 aB | 0.9651 | 471 |
| 35 | 0.010 ± 0.001 aA | 0.9194 | 0.0103 ± 0.0002 bAB | 0.9529 | 291 | 0.0110 ± 0.001 bB | 0.8557 | 291 | |
| 45 | 0.010 ± 0.001 aA | 0.9377 | 0.0209 ± 0.0002 cB | 0.9898 | (144) | 0.0307 ± 0.001 cC | 0.9836 | (104) | |
| _ | _ | 43.77 ± 0.21 | 0.9806 | 59.59 ± 0.23 | 0.9654 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, C.; Dermesonlouoglou, E.; Tsimogiannis, D.; Taoukis, P. Enrichment of Bakery Products with Antioxidant and Dietary Fiber Ingredients Obtained from Spent Coffee Ground. Appl. Sci. 2024, 14, 6863. https://doi.org/10.3390/app14166863
Papageorgiou C, Dermesonlouoglou E, Tsimogiannis D, Taoukis P. Enrichment of Bakery Products with Antioxidant and Dietary Fiber Ingredients Obtained from Spent Coffee Ground. Applied Sciences. 2024; 14(16):6863. https://doi.org/10.3390/app14166863
Chicago/Turabian StylePapageorgiou, Christos, Efimia Dermesonlouoglou, Dimitrios Tsimogiannis, and Petros Taoukis. 2024. "Enrichment of Bakery Products with Antioxidant and Dietary Fiber Ingredients Obtained from Spent Coffee Ground" Applied Sciences 14, no. 16: 6863. https://doi.org/10.3390/app14166863






