Genetic Variation Affects the Anti-Melanogenic Efficacy of Platycodon grandiflorus Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Extracts
2.2. Determination of Anti-Melanogenic Properties and Cytotoxicity
2.3. Expression Analysis Using Quantitative Real-Time PCR (qRT-PCR)
2.4. Saponin Analysis Using HPLC
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results and Discussion
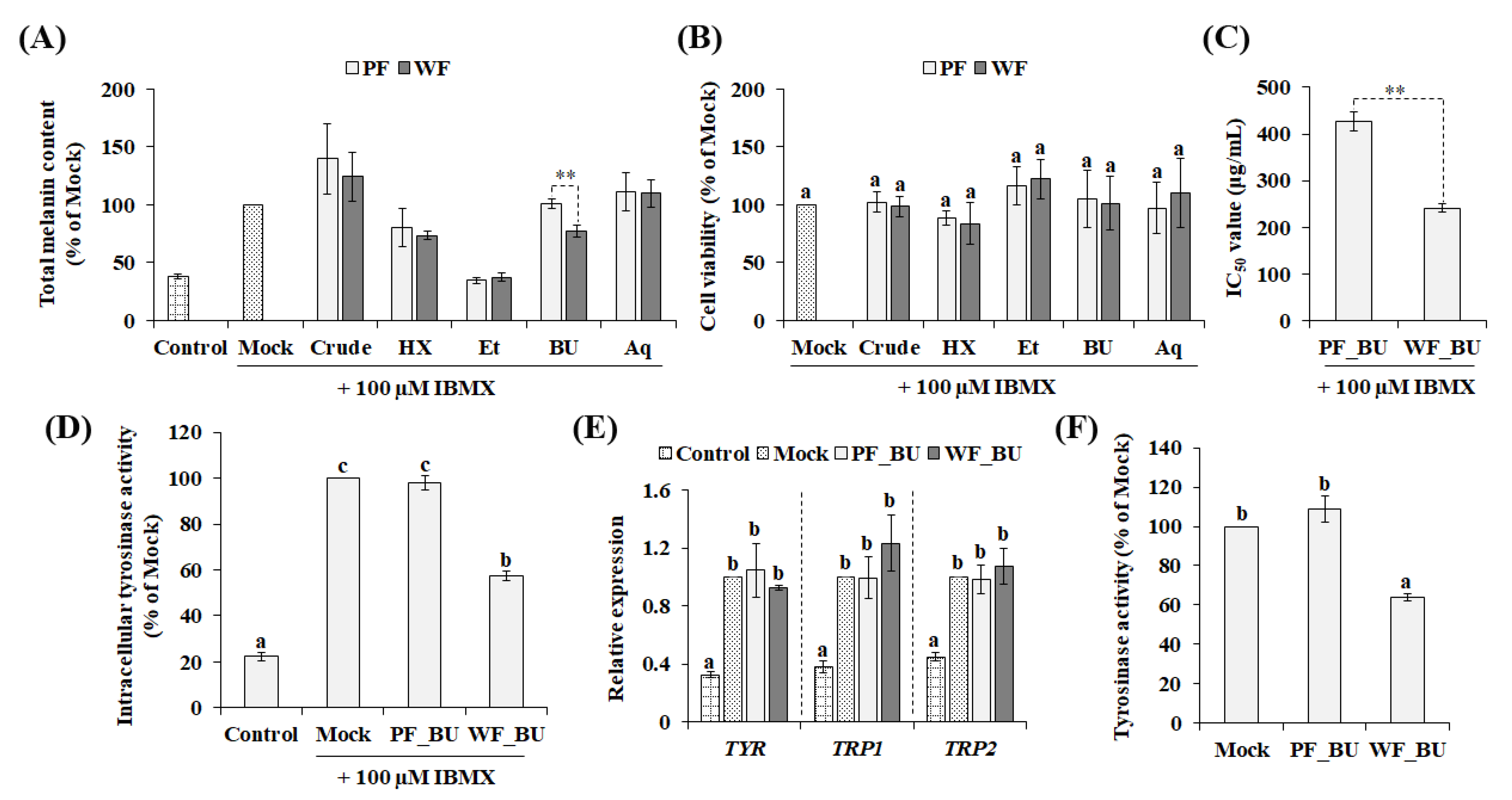
3.1. Comparative Evaluation of the Anti-Melanogenic Properties of P. grandiflorus Flower Extracts
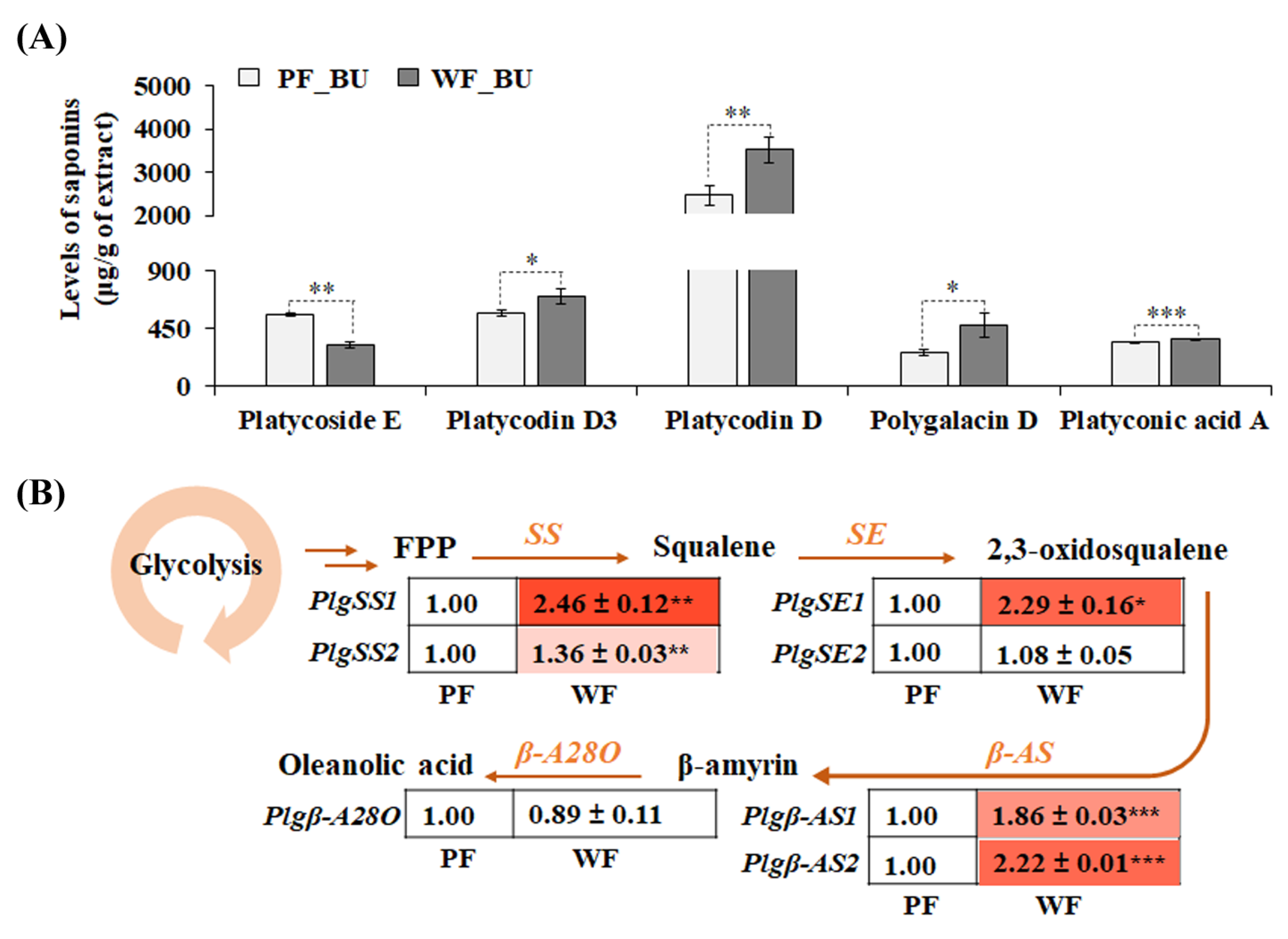
3.2. Variation in Saponin Content and Biosynthesis between Two Varieties
3.3. Molecular Docking Analysis of P. grandiflorus Saponins as Tyrosinase Inhibitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus—An Ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The Pharmacological Effects and Health Benefits of Platycodon grandiflorus—A Medicine Food Homology Species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, W.; Szulc, M.; Baraniak, J.; Derebecka, N.; Kania-Dobrowolska, M.; Piasecka, A.; Bogacz, A.; Karasiewicz, M.; Bartkowiak-Wieczorek, J.; Kujawski, R.; et al. The effect of different water extracts from Platycodon grandiflorum on selected factors associated with pathogenesis of chronic bronchitis in rats. Molecules 2020, 25, 5020. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhao, Y.-X.; Zheng, Y.; Li, X.-F. The pharmacology and mechanisms of platycodin D, an active triterpenoid saponin from Platycodon grandiflorus. Front. Pharmacol. 2023, 14, 1148853. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Plant By-Products and Food Industry Waste: A Source of Nutraceuticals and Biopolymers. In Food Bioconversion; Academic Press: Cambridge, MA, USA, 2017; pp. 279–315. [Google Scholar]
- Jeong, C.-H.; Choi, G.N.; Kim, J.H.; Kwak, J.H.; Kim, D.O.; Kim, Y.J.; Heo, H.J. Antioxidant activities from the aerial parts of Platycodon grandiflorum. Food Chem. 2010, 118, 278–282. [Google Scholar] [CrossRef]
- Ma, X.; Shao, S.; Xiao, F.; Zhang, H.; Zhang, R.; Wang, M.; Li, G.; Yan, M. Platycodon grandiflorum extract: Chemical composition and whitening, antioxidant, and anti-inflammatory effects. RSC Adv. 2021, 11, 10814–10826. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, B.; Huang, W.; Amrouche, A.T.; Maurizio, B.; Simal-Gandara, J.; Tundis, R.; Xiao, J.; Zou, L.; Lu, B. Edible flowers as functional raw materials: A review on anti-aging properties. Trends Food Sci. Technol. 2020, 106, 30–47. [Google Scholar] [CrossRef]
- Kim, E.; Hyun, T.K. PlgMYB1, an R2R3-MYB transcription factor, plays as a negative regulator of anthocyanin biosynthesis in Platycodon grandiflorus. 3 Biotech 2023, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Choi, B.-R.; Lee, J.W.; Um, Y.; Yoon, D.; Kim, H.-G.; Lee, Y.-S.; Kim, G.-S.; Lee, Y.-H.; Baek, N.-I. Simultaneous determination of various platycosides in four Platycodon grandiflorum cultivars by UPLC-QTOF/MS. Appl. Biol. Chem. 2019, 62, 47. [Google Scholar] [CrossRef]
- Jung, E.; Hwang, W.; Kim, S.; Kim, Y.-S.; Kim, Y.-S.; Lee, J.; Park, D. Depigmenting action of platycodin D depends on the cAMP/Rho-dependent signalling pathway. Exp. Dermatol. 2011, 20, 986–991. [Google Scholar] [CrossRef]
- Jin, S.; Hyun, T.K. Ectopic expression of production of Anthocyanin Pigment 1 (PAP1) improves the antioxidant and anti-melanogenic properties of Ginseng (Panax ginseng C.A. Meyer) Hairy Roots. Antioxidants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Efferth, T. Interaction of antihistaminic drugs with human Translationally Controlled Tumor Protein (TCTP) as novel approach for differentiation therapy. Oncotarget 2016, 7, 16818–16839. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, C.; Liu, C.; Yu, M.; Qi, Y.; Li, S. Saponins from Panax Bipinnatifidus Seem.: New strategy of extraction, isolation, and evaluation of tyrosinase inhibitory activity based on mathematical calculations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1039, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Guillen Quispe, Y.N.; Hwang, S.H.; Wang, Z.; Lim, S.S. Screening of peruvian medicinal plants for tyrosinase inhibitory properties: Identification of tyrosinase inhibitors in Hypericum laricifolium Juss. Molecules 2017, 22, 402. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, S.; Hachiya, A.; Shimotoyodome, Y.; Kameyama, A.; Miyauchi, Y.; Higuchi, K.; Fujimori, T.; Ohuchi, A.; Shibuya, Y.; Kitahara, T. The inhibitory effect of a Platycodon root extract on ultraviolet B-induced pigmentation due to a decrease in kit expression. J. Nat. Med. 2014, 68, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.A.; Lee, J.; Hyun, T.K. Histone deacetylase inhibitor, sodium nutyrate-induced metabolic modulation in Platycodon grandiflorus roots enhances anti-melanogenic properties. Int. J. Mol. Sci. 2023, 24, 11804. [Google Scholar] [CrossRef] [PubMed]
- Jirasripongpun, K.; Jirakanjanakit, N.; Pola, S.; Obsuwan, K. Antioxidation and anti-melanogenesis of three colored flowers of Dendrobium hybrids. Sci. Eng. Health Stud. 2022, 16, 22030006. [Google Scholar]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Granata, G.; Napoli, E. Effect of petal color, water status, and extraction method on qualitative characteristics of Rosa Rugosa Liqueur. Plants 2022, 11, 1859. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Kai, T.; Tsuchiya, Y.; Tamaki, M. Flower colour and antioxidant activity of Violas (Viola × Wittrockiana) as edible flowers. J. Hortic. Sci. Biotechnol. 2023, 98, 678–684. [Google Scholar] [CrossRef]
- Joo, S.S.; Kim, Y.B.; Lee, D.I. Antimicrobial and antioxidant properties of secondary metabolites from white rose flower. The Plant Pathol. J. 2010, 26, 57–62. [Google Scholar] [CrossRef]
- Huang, H.C.; Ho, Y.C.; Lim, J.M.; Chang, T.Y.; Ho, C.L.; Chang, T.M. Investigation of the anti-melanogenic and antioxidant characteristics of Eucalyptus camaldulensis flower essential oil and determination of its chemical composition. Int. J. Mol. Sci. 2015, 16, 10470–10490. [Google Scholar] [CrossRef]
- Jani, R.; Nag, S.; Setty, S. Visualization of intracellular tyrosinase activity in vitro. Bio-Protocol 2016, 6, e1794. [Google Scholar] [CrossRef]
- Baek, S.H.; Lee, S.H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the Intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Bukhori, M.F.M.; Rahmat, M.H.; Rahmat, A. Assessment and comparison of phytochemical constituents and biological activities of bitter bean (Parkia speciosa Hassk.) collected from different locations in Malaysia. Chem. Cent. J. 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.J.; Kim, K.C.; Kim, H.; Kim, J.S.; Hyun, T.K. Variability of polyphenolic compounds and biological activities among Perilla frutescens Var. Crispa genotypes. Horticulturae 2021, 7, 404. [Google Scholar] [CrossRef]
- Ajayi, O.E.; Yoon, S.Y.; Moon, S.; Kim, K.H.; Kim, J.H.; Chung, J.W.; Jang, K., II; Hyun, T.K. Variability in phytochemical contents and biological activities among Adenophora triphylla genotypes. Appl. Sci. 2023, 13, 11184. [Google Scholar] [CrossRef]
- Villareal, M.O.; Chaochaiphat, T.; Makbal, R.; Gadhi, C.; Isoda, H. Molecular analysis of the melanogenesis inhibitory effect of saponins-rich fraction of Argania spinosa leaves extract. Molecules 2022, 27, 6762. [Google Scholar] [CrossRef]
- Kooltheat, N.; Tedasen, A.; Yamasaki, K.; Chatatikun, M. Melanogenesis inhibitory activity, chemical components and molecular docking studies of Prunus cerasoides Buch.-Ham. D. Don. flowers. J. Evidence-Based Integr. Med. 2023, 28, 2515690X231152928. [Google Scholar] [CrossRef]
- Wei, G.; Yang, F.; Wei, F.; Zhang, L.; Gao, Y.; Qian, J.; Chen, Z.; Jia, Z.; Wang, Y.; Su, H.; et al. Metabolomes and transcriptomes revealed the saponin distribution in root tissues of Panax quinquefolius and Panax notoginseng. J. Ginseng Res. 2020, 44, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Huang, C.; Yao, W.; Niu, T.; Yang, X.; Wang, R. Full-length transcriptome, proteomics and metabolite analysis reveal candidate genes involved triterpenoid saponin biosynthesis in Dipsacus asperoides. Front. Plant Sci. 2023, 14, 1134352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Tang, Y.; Zhang, M.; Huang, M. Regulation of transcriptome networks that mediate ginsenoside biosynthesis by essential ecological factors. PLoS ONE 2023, 18, e0290163. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, Y.; Han, L.; Wang, Z.; Cao, M.; Wu, L.; Jiang, W.; Meng, F.; Guo, X.; Yu, N.; et al. Candidate gene identified in converting platycoside E to platycodin D from Platycodon grandiflorus by transcriptome and main Metabolites analysis. Sci. Rep. 2021, 11, 9810. [Google Scholar] [CrossRef] [PubMed]
- Kumalo, H.; Bhakat, S.; Soliman, M. Theory and applications of covalent docking in drug discovery: Merits and Pitfalls. Molecules 2015, 20, 1984–2000. [Google Scholar] [CrossRef]
- Goldfeder, M.; Kanteev, M.; Adir, N.; Fishman, A. Influencing the monophenolase/diphenolase activity ratio in tyrosinase. Biochim. Biophys. Acta 2013, 1834, 629–633. [Google Scholar] [CrossRef]
| Compounds | Lowest Binding Energy (kcal/mol) | Mean Binding Energy (kcal/mol) | Residues Involved in Hydrogen Bond Interaction | Residues Involved in Hydrophobic Interaction with Ligand | Pki (μM) * |
|---|---|---|---|---|---|
| PE | −4.90 ± 1.85 | −4.00 ± 1.14 | Lys 4 | Lys 4, Val 7, Ile 237, Val 240, Ile 243, Val 285, Tyr 286 | 42.94 ± 7.66 |
| PD3 | −9.39 ± 0.11 | −8.72 ± 1.26 | Asn 57, Arg 209 | Asp 55, Asp 57, Glu 158, Asp 183, Met 184, Thr 185, Val 218 | 26.99 ± 19.91 |
| PD | −8.76 ± 2.22 | −8.21 ± 1.87 | Gln 142, Lys 150, Gln 214 | Gly 46, Lys 47, Asn 57, Asp 123, Ile 125, Asp 140, Glu 141, Gln 142, Gly 148, Leu 149, Lys 150, Gln 214, Val 217, Val 218, Pro 219 | 3.84 ± 1.00 |
| PgD | −6.33 ± 0.81 | −5.62 ± 0.05 | Lys 157, Arg 209 | His 49, Glu 141, Gln 142, Ala 155, Thr 156, Lys 157, Arg 209, Gly 216, Val 218, Pro 219 | 73.78 ± 6.15 |
| PcA | −5.86 ± 0.01 | −5.86 ± 0.01 | Lys 47, Gln 142, Val 218 | Gly 46, Lys 47,Gly 53, Glu 141, Gln 142, Asn 144, Val 218, Pro 219 | 51.01 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Hyun, T.K. Genetic Variation Affects the Anti-Melanogenic Efficacy of Platycodon grandiflorus Flowers. Appl. Sci. 2024, 14, 6867. https://doi.org/10.3390/app14166867
Kim E, Hyun TK. Genetic Variation Affects the Anti-Melanogenic Efficacy of Platycodon grandiflorus Flowers. Applied Sciences. 2024; 14(16):6867. https://doi.org/10.3390/app14166867
Chicago/Turabian StyleKim, Eunhui, and Tae Kyung Hyun. 2024. "Genetic Variation Affects the Anti-Melanogenic Efficacy of Platycodon grandiflorus Flowers" Applied Sciences 14, no. 16: 6867. https://doi.org/10.3390/app14166867






