Comparative Analysis of Neuromuscular Activation Patterns Associated with Force between Semi-Professional Female Soccer Players with Previous Anterior Cruciate Ligament Surgery and Healthy Players in Thigh Musculature Related to Valgus Collapse
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
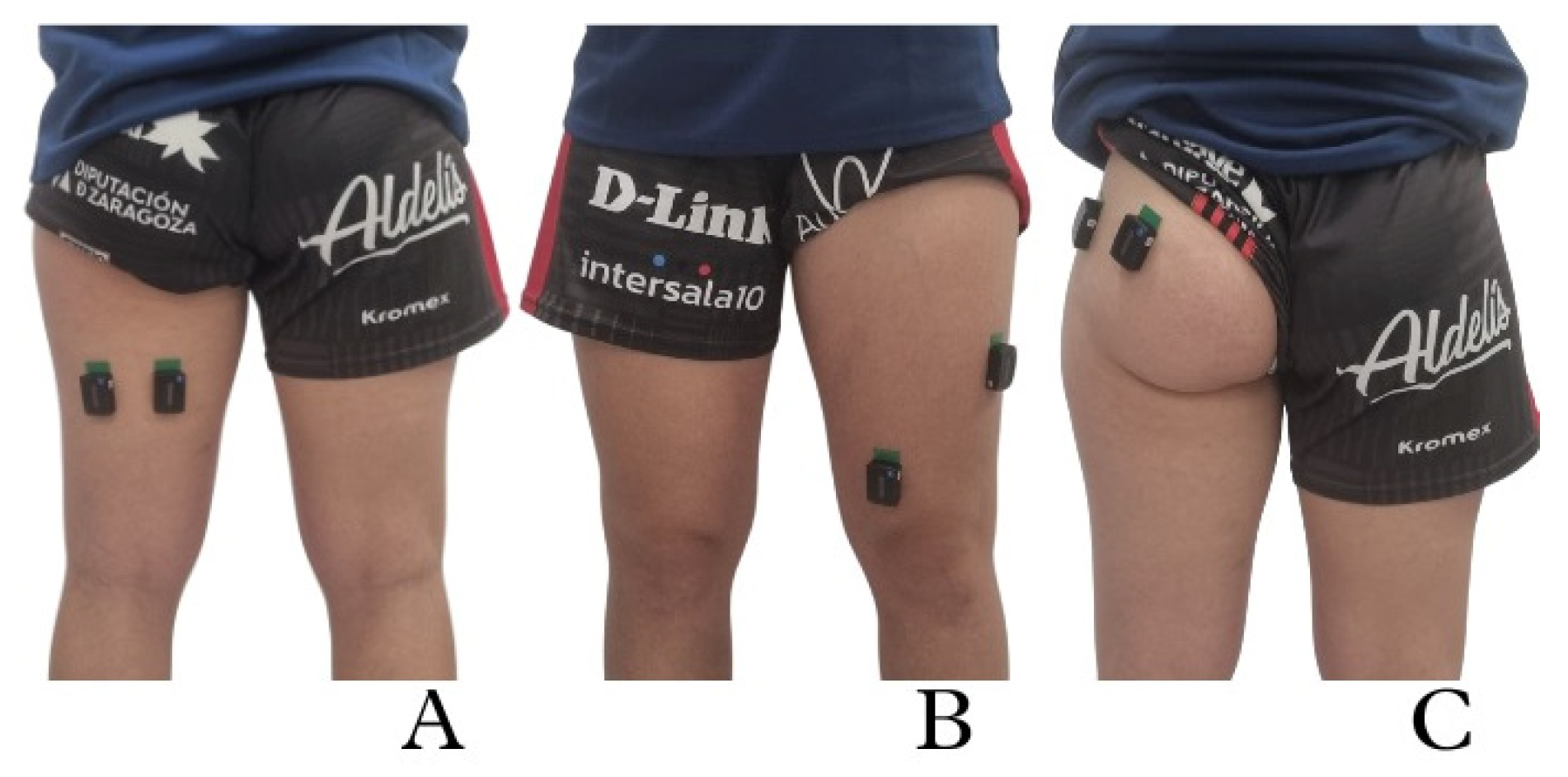
2.3. Procedure
2.4. Outcome Variables
2.4.1. EMG Outcome Variables
2.4.2. Force Outcome Variables
2.5. Sample Size
2.6. Data Analysis
3. Results
3.1. Comparative Analysis between Groups, ACL and NI, Considering DL or NDL
3.2. Analysis between Limbs in ACL and NI Groups Considering DL or NDL
4. Discussion
4.1. Limitations
4.2. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | anterior cruciate ligament |
| actEMG | electromyographical activation |
| NI | no injury |
| BF | biceps femoris |
| ST | semitendinosus |
| VL | vastus lateralis |
| VM | vastus medialis |
| GMax | gluteus maximus |
| Gm | gluteus medius |
| DL | dominant limb |
| NDL | no dominant limb |
| H | hamstrings |
| Q | quadriceps |
References
- Hallén, A.; Tomás, R.; Ekstrand, J.; Bengtsson, H.; Van den Steen, E.; Hägglund, M.; Waldén, M. UEFA Women’s Elite Club Injury Study: A prospective study on 1527 injuries over four consecutive seasons 2018/2019 to 2021/2022 reveals thigh muscle injuries to be most common and ACL injuries most burdensome. Br. J. Sports Med. 2024, 58, 128. [Google Scholar] [CrossRef]
- Chia, L.; De Oliveira Silva, D.; Whalan, M.; McKay, M.J.; Sullivan, J.; Fuller, C.W.; Pappas, E. Non-contact Anterior Cruciate Ligament Injury Epidemiology in Team-Ball Sports: A Systematic Review with Meta-analysis by Sex, Age, Sport, Participation Level, and Exposure Type. Sports Med. 2022, 52, 2447–2467. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, S.; Bragonzoni, L.; Della Villa, F.; Grassi, A.; Zaffagnini, S. Do healthy athletes exhibit at-risk biomechanics for anterior cruciate ligament injury during pivoting movements? Sports Biomech. 2022, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D. The future of in-field sports biomechanics: Wearables plus modelling compute real-time in vivo tissue loading to prevent and repair musculoskeletal injuries. Sports Biomech. 2021, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Lucarno, S.; Zago, M.; Buckthorpe, M.; Grassi, A.; Tosarelli, F.; Smith, R.; Della Villa, F. Systematic Video Analysis of Anterior Cruciate Ligament Injuries in Professional Female Soccer Players. Am. J. Sports Med. 2021, 49, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Horan, D.; Blake, C.; Hägglund, M.; Kelly, S.; Roe, M.; Delahunt, E. Injuries in elite-level women’s football-a two-year prospective study in the Irish Women’s National League. Scand. J. Med. Sci. Sports 2022, 32, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Collings, T.J.; Diamond, L.E.; Barrett, R.S.; Timmins, R.G.; Hickey, J.T.; DU Moulin, W.S.; Williams, M.D.; Beerworth, K.A.; Bourne, M.N. Strength and Biomechanical Risk Factors for Noncontact ACL Injury in Elite Female Footballers: A Prospective Study. Med. Sci. Sports Exerc. 2022, 54, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Schilaty, N.D.; McPherson, A.L.; Nagai, T.; Bates, N.A. Differences in psychological readiness for return to sport after anterior cruciate ligament injury is evident in thigh musculature motor unit characteristics. BMJ Open Sport Exerc. Med. 2023, 9, e001609. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.S.; Pierpoint, L.A.; Hellwinkel, J.E.; Berk, A.N.; Salandra, J.M.; Meade, J.D.; Piasecki, D.P.; Fleischli, J.E.; Ahmad, C.S.; Trofa, D.P.; et al. Clinical Outcomes After ACL Reconstruction in Soccer (Football, Futbol) Players: A Systematic Review and Meta-Analysis. Sports Health 2023, 15, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Mausehund, L.; Krosshaug, T. Knee Biomechanics During Cutting Maneuvers and Secondary ACL Injury Risk: A Prospective Cohort Study of Knee Biomechanics in 756 Female Elite Handball and Soccer Players. Am. J. Sports Med. 2024, 52, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Zebis, M.K.; Aagaard, P.; Andersen, L.L.; Hölmich, P.; Clausen, M.B.; Brandt, M.; Husted, R.S.; Lauridsen, H.B.; Curtis, D.J.; Bencke, J. First-time anterior cruciate ligament injury in adolescent female elite athletes: A prospective cohort study to identify modifiable risk factors. Knee Surgery Sports Traumatol. Arthrosc. 2022, 30, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Ueno, R.; Navacchia, A.; Schilaty, N.D.; Myer, G.D.; Hewett, T.E.; Bates, N.A. Anterior Cruciate Ligament Loading Increases With Pivot-Shift Mechanism During Asymmetrical Drop Vertical Jump in Female Athletes. Orthop. J. Sports Med. 2021, 9, 2325967121989095. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Arundale, A.; Silvers-Granelli, H.; Marmon, A.; Zarzycki, R.; Snyder-Mackler, L. Biomechanical measures during two sport-specific tasks differentiate between soccer players who go on to anterior cruciate ligament injury and those who do not: A prospective cohort analysis. Int. J. Sports Phys. Ther. 2020, 15, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Ferrández-Laliena, L.; Vicente-Pina, L.; Sánchez-Rodríguez, R.; Orantes-González, E.; Heredia-Jimenez, J.; Lucha-López, M.O.; Hidalgo-García, C.; Tricás-Moreno, J.M. Diagnostics Using the Change-of-Direction and Acceleration Test (CODAT) of the Biomechanical Patterns Associated with Knee Injury in Female Futsal Players: A Cross-Sectional Analytical Study. Diagnostics 2023, 13, 928. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pérez, I.; López-Valenciano, A.; Jiménez-Loaisa, A.; Elvira, J.L.L.; De Ste Croix, M.; Ayala, F. Injury incidence, characteristics and burden among female sub-elite futsal players: A prospective study with three-year follow-up. PeerJ 2019, 7, e7989. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, A.M.; Schneider, D.K.; Webster, K.E.; Yut, L.; Galloway, M.T.; Heidt, R.S.; Kaeding, C.C.; Kremcheck, T.E.; Magnussen, R.A.; Parikh, S.N.; et al. Anterior Cruciate Ligament Injury Risk in Sport: A Systematic Review and Meta-Analysis of Injury Incidence by Sex and Sport Classification. J. Athl. Train. 2019, 54, 472–482. [Google Scholar] [CrossRef]
- Arundale, A.J.H.; Silvers-Granelli, H.J.; Marmon, A.; Zarzycki, R.; Dix, C.; Snyder-Mackler, L. Changes in biomechanical knee injury risk factors across two collegiate soccer seasons using the 11+prevention program. Scand. J. Med. Sci. Sports 2018, 28, 2592–2603. [Google Scholar] [CrossRef]
- Smeets, A.; Malfait, B.; Dingenen, B.; Robinson, M.A.; Vanrenterghem, J.; Peers, K.; Nijs, S.; Vereecken, S.; Staes, F.; Verschueren, S. Is knee neuromuscular activity related to anterior cruciate ligament injury risk? A pilot study. Knee 2019, 26, 40–51. [Google Scholar] [CrossRef]
- McPherson, A.L.; Bates, N.A.; Haider, C.R.; Nagai, T.; Hewett, T.E.; Schilaty, N.D. Thigh musculature stiffness during active muscle contraction after anterior cruciate ligament injury. BMC Musculoskelet. Disord. 2020, 21, 320. [Google Scholar] [CrossRef]
- Di Giminiani, R.; Marinelli, S.; La Greca, S.; Di Blasio, A.; Angelozzi, M.; Cacchio, A. Neuromuscular Characteristics of Unilateral and Bilateral Maximal Voluntary Isometric Contractions following ACL Reconstruction. Biology 2023, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.L.; Schilaty, N.D.; Anderson, S.; Nagai, T.; Bates, N.A. Arthrogenic muscle inhibition after anterior cruciate ligament injury: Injured and uninjured limb recovery over time. Front. Sports Act. Living 2023, 5, 1143376. [Google Scholar] [CrossRef] [PubMed]
- Lepley, A.S.; Grooms, D.R.; Burland, J.P.; Davi, S.M.; Kinsella-Shaw, J.M.; Lepley, L.K. Quadriceps muscle function following anterior cruciate ligament reconstruction: Systemic differences in neural and morphological characteristics. Exp. Brain Res. 2019, 237, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Sandon, A.; Engström, B.; Forssblad, M. High Risk of Further Anterior Cruciate Ligament Injury in a 10-Year Follow-up Study of Anterior Cruciate Ligament-Reconstructed Soccer Players in the Swedish National Knee Ligament Registry. Arthroscopy 2020, 36, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Glattke, K.E.; Tummala, S.V.; Chhabra, A. Anterior Cruciate Ligament Reconstruction Recovery and Rehabilitation: A Systematic Review. J. Bone Jt. Surg. 2022, 104, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Brinlee, A.W.; Dickenson, S.B.; Hunter-Giordano, A.; Snyder-Mackler, L. ACL Reconstruction Rehabilitation: Clinical Data, Biologic Healing, and Criterion-Based Milestones to Inform a Return-to-Sport Guideline. Sports Health 2022, 14, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E.; Sahinis, C.; Baltzopoulos, V. Is hamstrings-to-quadriceps torque ratio useful for predicting anterior cruciate ligament and hamstring injuries? A systematic and critical review. J. Sport Health Sci. 2023, 12, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Bencke, J.; Zebis, M.K. The influence of gender on neuromuscular pre-activity during side-cutting. J. Electromyogr. Kinesiol. 2011, 21, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Heidt, R.S., Jr.; Colosimo, A.J.; McLean, S.G.; Van Den Bogert, A.J.; Paterno, M.V.; Succop, P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. Am. J. Sports Med. 2005, 33, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.M.; Padua, D.A.; Blackburn, J.T.; Prentice, W.E.; Hirth, C.J. Muscle Activation During Side-Step Cutting Maneuvers in Male and Female Soccer Athletes. J. Athl. Train. 2008, 43, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Bencke, J.; Aagaard, P.; Zebis, M.K. Muscle Activation During ACL Injury Risk Movements in Young Female Athletes: A Narrative Review. Front. Physiol. 2018, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Dedinsky, R.; Baker, L.; Imbus, S.; Bowman, M.; Murray, L. Exercises that facilitate optimal hamstring and quadriceps co-activation to help decrease acl injury risk in healthy females: A systematic review of the literature. Int. J. Sport Phys. Ther. 2017, 12, 3–15. [Google Scholar]
- Olsen, O.-E.; Myklebust, G.; Engebretsen, L.; Bahr, R. Injury mechanisms for anterior cruciate ligament injuries in team handball: A systematic video analysis. Am. J. Sports Med. 2004, 32, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Arundale, A.; Silvers-Granelli, H.; Marmon, A.; Zarzycki, R.; Arch, E.; Snyder-Mackler, L. Descriptive trunk kinematics in healthy collegiate women’s soccer players indicate trunk center of mass is laterally positioned prior to decelerating and cutting. J. ISAKOS 2022, 7, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Smeets, A.; Willems, M.; Gilson, L.; Verschueren, S.; Staes, F.; Vandenneucker, H.; Claes, S.; Vanrenterghem, J. Neuromuscular and biomechanical landing alterations persist in athletes returning to sport after anterior cruciate ligament reconstruction. Knee 2021, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Mau-Moeller, A.; Wassermann, F.; Bruhn, S. Effect of Fatigue on Hamstring Reflex Responses and Posterior-Anterior Tibial Translation in Men and Women. PLoS ONE 2013, 8, e56988. [Google Scholar] [CrossRef] [PubMed]
- Zebis, M.K.; Andersen, L.L.; Bencke, J.; Kjær, M.; Aagaard, P. Identification of athletes at future risk of anterior cruciate ligament ruptures by neuromuscular screening. Am. J. Sports Med. 2009, 37, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Serpell, B.G.; Scarvell, J.M.; Pickering, M.R.; Ball, N.B.; Newman, P.; Perriman, D.; Warmenhoven, J.; Smith, P.N. Medial and lateral hamstrings and quadriceps co-activation affects knee joint kinematics and ACL elongation: A pilot study Rehabilitation, physical therapy and occupational health. BMC Musculoskelet. Disord. 2015, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Husted, R.S.; Bencke, J.; Andersen, L.L.; Myklebust, G.; Kallemose, T.; Lauridsen, H.B.; Hölmich, P.; Aagaard, P.; Zebis, M.K. A comparison of hamstring muscle activity during different screening tests for non-contact ACL injury. Knee 2016, 23, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Maniar, N.; Schache, A.G.; Sritharan, P.; Opar, D.A. Non-knee-spanning muscles contribute to tibiofemoral shear as well as valgus and rotational joint reaction moments during unanticipated sidestep cutting. Sci. Rep. 2018, 8, 2501. [Google Scholar] [CrossRef] [PubMed]
- DeLang, M.D.; Salamh, P.A.; Farooq, A.; Tabben, M.; Whiteley, R.; van Dyk, N.; Chamari, K. The dominant leg is more likely to get injured in soccer players: Systematic review and meta-analysis. Biol. Sport 2021, 38, 397–435. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Ethical principles for medical research involving human subjects. Eur. J. Emerg. Med. 2001, 8, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Risberg, M.A.; Steffen, K.; Nilstad, A.; Myklebust, G.; Kristianslund, E.; Moltubakk, M.M.; Krosshaug, T. Normative Quadriceps and Hamstring Muscle Strength Values for Female, Healthy, Elite Handball and Football Players. J. Strength Cond. Res. 2018, 32, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, G.; Gawda, P. Surface Electromyography in Dentistry—Past, Present and Future. J. Clin. Med. 2024, 13, 1328. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. The ABC of EMG A Practical Introduction to Kinesiological Electromyography; Noraxon USA Inc.: Scottsdale, AZ, USA, 2005; pp. 1–61. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- van Melick, N.; van der Weegen, W.; van der Horst, N. Quadriceps and Hamstrings Strength Reference Values for Athletes with and without Anterior Cruciate Ligament Reconstruction Who Play Popular Pivoting Sports, including Soccer, Basketball, and Handball: A Scoping Review. J. Orthop. Sports Phys. Ther. 2022, 52, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Hedt, C.; Lambert, B.; Jackson, J.A.; Brager, E.; Forbes, G.; McCulloch, P.; Jackson, J.A.; Ankersen, J.P. Electromyography (EMG) Analysis of Multi-Regional Lower Extremity and Trunk Musculature During Sidelying Hip Abduction With Frontal Plane Stabilization. Cureus 2023, 15, e43523. [Google Scholar] [CrossRef] [PubMed]
- Geisler, S.; Havers, T.; Isenmann, E.; Schulze, J.; Lourens, L.K.; Nowak, J.; Held, S.; Haff, G.G. Effects of Expertise on Muscle Activity during the Hang Power Clean and Hang Power Snatch Compared to Snatch and Clean Pulls—An Explorative Analysis. J. Sports Sci. Med. 2023, 22, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Zaki, S.; Alam, F.; Sharma, S.; Aysha, T.; Khiyami, A.T.; Althobaiti, A.J.; Alnefaie, H.A.; Nuhmani, S. Effects of facilitatory and inhibitory Kinesio taping on lateral gastrocnemius muscle activity, motor neuron excitability, and countermovement jump height in university athletes from multiple sports: A randomized controlled trial. Heliyon 2023, 9, e23230. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.; Selfe, J.; Sinclair, J.; May, K.; Thomas, G. The effect of different decline angles on the biomechanics of double limb squats and the implications to clinical and training practice. J. Hum. Kinet. 2016, 52, 125–138. [Google Scholar] [CrossRef][Green Version]
- Sawilowsky, S.S. Very large and huge effect sizes. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Steiner, M.; Baur, H.; Blasimann, A. Sex-specific differences in neuromuscular activation of the knee stabilizing muscles in adults—A systematic review. Arch. Physiother. 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, T.E.; Smith, A.J.J.; Benoit, D.L. Sex-related differences in neuromuscular control: Implications for injury mechanisms or healthy stabilisation strategies? J. Orthop. Res. 2013, 32, 310–317. [Google Scholar] [CrossRef]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J.; Hostage, E.C. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J. Neurophysiol. 2010, 104, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Blasimann, A.; Busch, A.; Henle, P.; Bruhn, S.; Vissers, D.; Baur, H. Neuromuscular control in males and females 1 year after an anterior cruciate ligament rupture or reconstruction during stair descent and artificial tibial translation. Sci. Rep. 2023, 13, 15316. [Google Scholar] [CrossRef] [PubMed]
- Blasimann, A.; Busch, A.; Henle, P.; Bruhn, S.; Vissers, D.; Baur, H. Bilateral neuromuscular control in patients one year after unilateral ACL rupture or reconstruction. A cross-sectional study. Heliyon 2024, 10, e24364. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, H.; Yeo, I. Anatomical study of the sacrotuberous ligament and the hamstring muscles: A histomorphological analysis. Clin. Anat. 2024, 37, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Chu, V.W.S.; Yung, P.S.H.; Fong, D.T.P. Effects of Gluteus Medius and Biceps Femoris Stimulation on Reduction of Knee Abduction Moment during a Landing Task. J. Appl. Biomech. 2023, 39, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, D.; van den Bogert, A.J.; Mössner, M.; Nachbauer, W. Model-based estimation of muscle and ACL forces during turning maneuvers in alpine skiing. Sci. Rep. 2023, 13, 9026. [Google Scholar] [CrossRef] [PubMed]
- Lehecka, B.J.; Smith, B.S.; Rundell, T.; Cappaert, T.A.; Hakansson, N.A. The Reliability and Validity of Gluteal Endurance Measures (GEMs). Int. J. Sports Phys. Ther. 2021, 16, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.; Grip, H.; Arumugam, A.; Boraxbekk, C.-J.; Selling, J.; Häger, C.K. Right hemisphere brain lateralization for knee proprioception among right-limb dominant individuals. Front. Hum. Neurosci. 2023, 17, 969101. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.K.; Lee, J.H.; Rhim, H.C.; Cho, I.-Y.; Han, S.-B.; Jang, K.-M. Comparison of muscle strength and neuromuscular control up to 1 year after anterior cruciate ligament reconstruction between patients with dominant leg and non-dominant leg injuries. Knee 2021, 29, 15–25. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, F.T.; Castillo-Rodríguez, A.; Rodríguez-García, L.; Clemente, F.M.; Silva, A.F. A Data Analytics Approach to Assess the Functional and Physical Performance of Female Soccer Players: A Cohort Design. Int. J. Environ. Res. Public Health 2022, 19, 8941. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, T.; Isberg, J.; Nilsson, J.; Carlsson, M. Influence of Task Conditions on Side Foot-Kick Accuracy among Swedish First League Women’s Soccer Players. J. Sports Sci. Med. 2018, 17, 74–81. [Google Scholar] [PubMed]


| ACL (n = 11) | NI (n = 11) | p-Value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (years) | 22.36 ± 3.56 | 20.63 ± 3.58 | 0.287 |
| Weight (kg) | 59.72 ± 5.80 | 61.99 ± 6.54 | 0.408 |
| Height (cm) | 162.68 ± 7.05 | 163.17 ± 6.09 | 0.869 |
| DOMINANT LIMB (DL) | NO DOMINANT LIMB (NDL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| BF_peak actEMG (%) | 85.81 ± 85.81 | 90.06 ± 5.05 | 0.157 | 0.73 | (−10.33 to 1.83) | 91.22 ± 5.59 | 93.61 ± 5.45 | 0.464 | 0.43 | (−9.29 to 4.52) |
| ST_peak actEMG (%) | 83.79 ± 83.79 | 89.38 ± 7.65 | 0.320 | 0.49 | (−17.29 to 6.11) | 91.34 ± 6.98 | 86.44 ± 5.96 | 0.204 | 0.75 | (−3.07 to 12.86) |
| VL_peak actEMG (%) | 87.04 ± 87.04 | 89.18 ± 8.69 | 0.647 | 0.23 | (−11.86 to 7.59) | 86.14 ± 8.59 | 89.39 ± 5.16 | 0.407 | 0.46 | (−11.55 to 5.05) |
| VM_peak actEMG (%) | 82.68 ± 82.68 | 87.44 ± 10.10 | 0.347 | 0.47 | (−15.19 to 5.68) | 78.84 ± 7.34 | 86.78 ± 7.43 | 0.087 | 1.07 | (−17.23 to 1.35) |
| GMax_peak actEMG (%) | 87.26 ± 87.26 | 90.08 ± 6.39 | 0.509 | 0.34 | (−11.76 to 6.11) | 94.37 ± 4.67 | 88.09 ± 6.38 | 0.085 | 1.12 | (−1.01 to 13.58) |
| Gm_peak actEMG (%) | 89.68 ± 89.68 | 88.04 ± 6.16 | 0.611 | 0.26 | (−5.11 to 8.39) | 91.59 ± 4.86 | 91.08 ± 6.16 | 0.879 | 0.09 | (−6.67 to 7.69) |
| DOMINANT LIMB (DL) | NO DOMINANT LIMB (NDL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| BF_average actEMG (%) | 57.64 ± 57.64 | 63.60 ± 13.94 | 0.315 | 0.50 | (−18.17 to 6.25) | 49.49 ± 10.01 | 60.56 ± 5.28 | 0.023 * | 1.38 | (−20.30 to −1.84) |
| ST_average actEMG (%) | 48.57 ± 48.57 | 60.21 ± 15.26 | 0.145 | 0.75 | (−27.76 to 4.49) | 54.77 ± 10.99 | 53.55 ± 6.38 | 0.803 | 0.14 | (−9.27 to 11.70) |
| VL_average actEMG (%) | 58.95 ± 58.95 | 55.97 ± 12.32 | 0.543 | 0.30 | (−7.26 to 13.23) | 55.57 ± 8.25 | 57.92 ± 7.76 | 0.615 | 0.29 | (−12.31 to 7.62) |
| VM_average actEMG (%) | 54.13 ± 54.13 | 53.94 ± 17.31 | 0.979 | 0.01 | (−15.79 to 16.18) | 47.07 ± 15.42 | 51.83 ± 10.47 | 0.518 | 0.36 | (−6.88 to 11.61) |
| GMax_average actEMG (%) | 48.86 ± 48.86 | 55.83 ± 10.88 | 0.178 | 0.71 | (−17.53 to 3.58) | 59.82 ± 5.54 | 52.25 ± 5.66 | 0.038 * | 1.35 | (0.52 to 14.62) |
| Gm_average actEMG (%) | 56.79 ± 56.79 | 58.57 ± 18.71 | 0.801 | 0.13 | (−16.68 to 13.12) | 60.09 ± 5.37 | 60.02 ± 8.34 | 0.986 | 0.01 | (−9.20 to 9.36) |
| DOMINANT LIMB (DL) | NO DOMINANT LIMB (NDL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | ACL | NI | p-Value | Cohen’s d | Confidence Interval (95%) | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| H_peak_Torque (Nm/kg) | 1.55 ± 1.55 | 1.50 ± 0.50 | 0.831 | 0.11 | (−0.47 to 0.58) | 1.25 ± 0.91 | 1.47 ± 0.57 | 0.596 | 0.29 | (−1.11 to 0.67) |
| Q_peak_Torque (Nm/kg) | 2.86 ± 2.86 | 2.69 ± 0.98 | 0.640 | 0.23 | (−0.59 to 0.94) | 2.54 ± 0.97 | 2.50 ± 1.08 | 0.958 | 0.03 | (−1.27 to 1.34) |
| GMax_peak_Torque (Nm/kg) | 3.15 ± 3.15 | 2.93 ± 1.12 | 0.175 | 0.22 | (−0.85 to 1.29) | 2.71 ± 0.93 | 2.99 ± 1.10 | 0.649 | 0.27 | (−1.58 to 1.03) |
| Gm_peak_Torque (Nm/kg) | 2.22 ± 2.22 | 1.61 ± 0.65 | 0.092 | 0.94 | (−0.11 to 1.32) | 1.78 ± 0.78 | 1.52 ± 0.68 | 0.540 | 0.35 | (−0.64 to 1.16) |
| ACL INJURED DOMINANT LIMB (ACL_DL) | ACL INJURED NO DOMINANT LIMB (ACL_NDL) | NO INJURED (NI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | |
| (Injured) | (No Injured) | (No Injured) | (Injured) | ||||||||||||
| Mean | Mean | Mean | Mean | Mean | Mean | ||||||||||
| ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | ||||||||||
| BF_peak | 85.81 | 91.29 | 0.041 * | 0.91 | (−10.67 to −0.30) | 80.45 | 91.22 | 0.151 | 1.14 | (−27.65 to 6.10) | 90.06 | 93.61 | 0.227 | 0.67 | (−9.88 to 2.78) |
| actEMG (%) | ±6.50 | ±5.53 | ±12.14 | ±5.59 | ±5.05 | ±5.45 | |||||||||
| ST_peak | 83.79 | 90.39 | 0.290 | 0.60 | (−20.04 to 6.83) | 84.86 | 91.34 | 0.402 | 0.53 | (−25.66 to 12.71) | 89.38 | 86.44 | 0.356 | 0.42 | (−4.09 to 9.96) |
| actEMG (%) | ±83.79 | ±6.80 | ±84.86 | ±6.98 | ±89.38 | ±5.96 | |||||||||
| VL_peak | 87.04 | 88.21 | 0.827 | 0.12 | (−13.11 to 10.77) | 83.14 | 86.14 | 0.409 | 0.37 | (−12.04 to 6.05) | 89.18 | 89.39 | 0.949 | 0.03 | (−7.84 to 7.42) |
| actEMG (%) | ±87.04 | ±8.75 | ±83.14 | ±8.59 | ±89.18 | ±5.16 | |||||||||
| VM_peak | 82.68 | 89.68 | 0.201 | 0.74 | (−18.59 to 4.59) | 86.08 | 78.84 | 0.298 | 0.85 | (−9.59 to 24.05) | 87.44 | 86.78 | 0.855 | 0.07 | (−7.52 to 8.83) |
| actEMG (%) | ±82.68 | ±8.77 | ±86.08 | ±7.34 | ±87.44 | ±7.43 | |||||||||
| GMax_peak | 85.83 | 89.27 | 0.642 | 0.36 | (−22.50 to 15.61) | 86.02 | 94.37 | 0.043 * | 1.21 | (−18.12 to 1.42) | 90.08 | 88.09 | 0.502 | 0.31 | (−4.67 to 8.67) |
| actEMG (%) | ±85.83 | ±4.97 | ±86.02 | ±4.67 | ±90.08 | ±6.38 | |||||||||
| Gm_peak | 92.27 | 85.17 | 0.126 | 1.03 | (−3.11 to 17.31) | 92.92 | 91.59 | 0.663 | 0.30 | (−6.52 to 9.18) | 88.04 | 91.08 | 0.411 | 0.49 | (−11.25 to 5.17) |
| actEMG (%) | ±92.27 | ±9.05 | ±92.92 | ±4.86 | ±88.04 | ±6.16 | |||||||||
| ACL INJURED DOMINANT LIMB (ACL_DL) | ACL INJURED NO DOMINANT LIMB (ACL_NDL) | NO INJURED (NI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p Value | Cohen’s d | Confidence Interval 95% | |
| (Injured) | (No Injured) | (No Injured) | (Injured) | ||||||||||||
| Mean | Mean | Mean | Mean | Mean | Mean | ||||||||||
| ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | ||||||||||
| BF_average | 57.64 | 57.68 | 0.984 | 0.01 | (−4.32 to 4.24) | 51.17 | 49.49 | 0.851 | 0.15 | (−21.64 to 25.00) | 63.60 | 60.56 | 0.525 | 0.29 | (−7.72 to 13.80) |
| actEMG (%) | ±57.64 | ±6.27 | ±51.17 | ±10.01 | ±63.60 | ±5.28 | |||||||||
| ST_average | 48.57 | 54.23 | 0.311 | 0.43 | (−17.73 to 6.41) | 52.30 | 54.77 | 0.73 | 0.17 | (−20.99 to 16.05) | 60.21 | 53.55 | 0.215 | 0.57 | (−4.89 to 18.20) |
| actEMG (%) | ±48.57 | ±9.75 | ±52.30 | ±10.99 | ±60.21 | ±6.38 | |||||||||
| VL_average | 58.95 | 54.63 | 0.184 | 0.63 | (−2.53 to 11.18) | 51.12 | 55.57 | 0.175 | 0.43 | (−11.95 to 3.06) | 55.97 | 57.92 | 0.749 | 0.19 | (−15.81 to 11.91) |
| actEMG (%) | ±58.95 | ±6.50 | ±51.12 | ±8.25 | ±55.97 | ±7.76 | |||||||||
| VM_average | 54.13 | 54.20 | 0.991 | 0.01 | (−11.86 to 11.73) | 52.80 | 47.07 | 0.472 | 0.49 | (−14.31 to 25.76) | 53.94 | 51.83 | 0.760 | 0.15 | (−13.53 to 17.73) |
| actEMG (%) | ±54.13 | ±5.89 | ±52.80 | ±15.42 | ±53.94 | ±10.47 | |||||||||
| GMax_average | 47.33 | 54.44 | 0.347 | 0.81 | (−25.65 to 11.43) | 53.40 | 59.82 | 0.090 | 0.91 | (−14.41 to 1.57) | 55.83 | 52.25 | 0.501 | 0.41 | (−8.35 to 15.51) |
| actEMG (%) | ±47.33 | ±6.36 | ±53.40 | ±5.54 | ±55.83 | ±5.66 | |||||||||
| Gm_average | 59.51 | 52.30 | 0.202 | 0.93 | (−5.93 to 20.36) | 57.25 | 60.09 | 0.404 | 0.51 | (−11.30 to 5.62) | 58.57 | 60.02 | 0.829 | 0.10 | (−16.69 to 13.80) |
| actEMG (%) | ±59.51 | ±10.86 | ±57.25 | ±5.37 | ±58.57 | ±8.34 | |||||||||
| ACL INJURED DOMINANT LIMB (ACL_DL) | ACL INJURED NO DOMINANT LIMB (ACL_NDL) | NO INJURED (NI) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL | NDL | p-Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p-Value | Cohen’s d | Confidence Interval 95% | DL | NDL | p-Value | Cohen’s d | Confidence Interval 95% | |
| (Injured) | (No Injured) | (No Injured) | (Injured) | ||||||||||||
| Mean | Mean | Mean | Mean | Mean | Mean | ||||||||||
| ± SD | ± SD | ± SD | ± SD | ± SD | ± SD | ||||||||||
| H_peak_ | 1.55 | 1.50 | 0.720 | 0.12 | (−0.29 to 0.40) | 1.72 | 1.25 | 0.011 * | 0.56 | (0.18 to 0.77) | 1.50 | 1.47 | 0.770 | 0.05 | (−0.19 to 0.25) |
| Torque (Nm/kg) | ±1.55 | ±0.39 | ±1.72 | ±0.91 | ±1.50 | ±0.57 | |||||||||
| Q_peak_ | 2.86 | 2.55 | 0.138 | 0.63 | (−0.12 to 0.73) | 2.92 | 2.54 | 0.270 | 0.32 | (−0.45 to 1.21) | 2.69 | 2.50 | 0.017 * | 0.18 | (0.04 to 0.32) |
| Torque (Nm/kg) | ±2.86 | ±0.53 | ±2.92 | ±0.97 | ±2.69 | ±1.08 | |||||||||
| GMax_peak_ | 3.15 | 2.34 | 0.066 | 0.83 | (−0.07 to 1.70) | 2.67 | 2.71 | 0.877 | 0.04 | (−0.60 to 0.54) | 2.93 | 2.99 | 0.865 | 0.05 | (−0.78 to 0.67) |
| Torque (Nm/kg) | ±3.15 | ±1.09 | ±2.67 | ±0.93 | ±2.93 | ±1.10 | |||||||||
| Gm_peak_ | 2.22 | 1.80 | 0.245 | 0.47 | (−0.37 to 1.20) | 1.77 | 1.78 | 0.984 | 0.01 | (−0.80 to 0.79) | 1.61 | 1.52 | 0.305 | 0.14 | (−0.11 to 0.30) |
| Torque (Nm/kg) | ±2.22 | ±1.07 | ±1.77 | ±0.78 | ±1.61 | ±0.68 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrández-Laliena, L.; Sánchez-Rodríguez, R.; Vicente-Pina, L.; Lucha-López, M.O.; Ambrus, M.; Hidalgo-García, C.; Monti-Ballano, S.; Tricás-Moreno, J.M. Comparative Analysis of Neuromuscular Activation Patterns Associated with Force between Semi-Professional Female Soccer Players with Previous Anterior Cruciate Ligament Surgery and Healthy Players in Thigh Musculature Related to Valgus Collapse. Appl. Sci. 2024, 14, 6869. https://doi.org/10.3390/app14166869
Ferrández-Laliena L, Sánchez-Rodríguez R, Vicente-Pina L, Lucha-López MO, Ambrus M, Hidalgo-García C, Monti-Ballano S, Tricás-Moreno JM. Comparative Analysis of Neuromuscular Activation Patterns Associated with Force between Semi-Professional Female Soccer Players with Previous Anterior Cruciate Ligament Surgery and Healthy Players in Thigh Musculature Related to Valgus Collapse. Applied Sciences. 2024; 14(16):6869. https://doi.org/10.3390/app14166869
Chicago/Turabian StyleFerrández-Laliena, Loreto, Rocío Sánchez-Rodríguez, Lucía Vicente-Pina, María Orosia Lucha-López, Mira Ambrus, César Hidalgo-García, Sofía Monti-Ballano, and José Miguel Tricás-Moreno. 2024. "Comparative Analysis of Neuromuscular Activation Patterns Associated with Force between Semi-Professional Female Soccer Players with Previous Anterior Cruciate Ligament Surgery and Healthy Players in Thigh Musculature Related to Valgus Collapse" Applied Sciences 14, no. 16: 6869. https://doi.org/10.3390/app14166869
APA StyleFerrández-Laliena, L., Sánchez-Rodríguez, R., Vicente-Pina, L., Lucha-López, M. O., Ambrus, M., Hidalgo-García, C., Monti-Ballano, S., & Tricás-Moreno, J. M. (2024). Comparative Analysis of Neuromuscular Activation Patterns Associated with Force between Semi-Professional Female Soccer Players with Previous Anterior Cruciate Ligament Surgery and Healthy Players in Thigh Musculature Related to Valgus Collapse. Applied Sciences, 14(16), 6869. https://doi.org/10.3390/app14166869









