Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Methods
2.3. Determination of Moisture Content and Total Soluble Solids (% Brix)
2.4. Determination of pH
2.5. Determination of Free Acidity
2.6. Determination of Electrical Conductivity
2.7. Determination of Diastase Activity (Standard Schade Method)
2.8. Determination of Proline
2.9. Determination of Color Intensity
2.10. Determination of Total Phenolic Content
2.11. Determination of Total Flavonoids Content
2.12. Determination of Phenolic Acids
2.13. Determination of Antioxidant Activity (Assay with DPPH)
2.14. Determination of Carbohydrates
2.15. Determination of Cations
2.16. Determination of Protein
2.17. Determination of Pollen Analysis
2.18. Determination of Grayanotoxin-III Analysis
2.19. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content (%)
3.2. Sugars
3.3. Free Acidity meq/kg
3.4. pH
3.5. Electrical Conductivity (ms/cm)
3.6. Cations
3.7. Color Intensity
3.8. Phenolic Compounds
3.9. Flavonoids
3.10. Phenolic Acids (Aromatic Carbonic Acids)
3.11. Antioxidant Activity by the DPPH Method
3.12. Proline
3.13. The Activities of Enzymes
3.14. Protein
3.15. Melissopalynology, or Pollen Analysis
3.16. The Identification of Grayanotoxin-III
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escuredo, O.; Seijo, M.C. Honey: Chemical Composition, Stability and Authenticity. Foods 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, B.; Abubakar, G.; Aliyu, S. Analysis of Biochemical Composition of Honey Samples from North-East Nigeria. Biochem. Anal. Biochem. 2013, 2, 3. [Google Scholar] [CrossRef]
- Hameed, O.M.; Shaker, O.M.; Ben Slima, A.; Makni, M. Biochemical Profiling and Physicochemical and Biological Valorization of Iraqi Honey:A Comprehensive Analysis. Molecules 2024, 29, 671. [Google Scholar] [CrossRef] [PubMed]
- Ayton, J.; Prenzler, P.; Raman, H.; Warren-Smith, A.; Meyer, R. Review of Chemistry Associated with Honey; Publication No. 19-031; AgriFutures: Wagga Wagga, Australia, 2019; ISBN 978-1-76053-052-5.
- Akalın, H.; Bayram, M.; Anlı, E.R. Determination of some individual phenolic compounds and antioxidant capacity of mead produced from different types of honey. J. Inst. Brew. Distill. 2017, 123, 167–174. [Google Scholar] [CrossRef]
- Kristberg, K.; Otles, S. Functional Properties of Traditional Food; Springer: New York, NY, USA, 2016; ISBN 978-1-4899-7662-8. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Zhuo, Q.; Chen, X.; Liu, T.; Zhang, S.-Q. Quantitative and Discriminative Evaluation of Contents of Phenolic and Flavonoid and Antioxidant Competence for Chinese Honeys from Different Botanical Origins. Molecules 2018, 23, 1110. [Google Scholar] [CrossRef]
- Makowicz, E.; Jasicka-Misiak, I.; Teper, D.; Kafarski, P. HPTLC Fingerprinting—Rapid Method for the Differentiation of Honeys of Different Botanical Origin Based on the Composition of the Lipophilic Fractions. Molecules 2018, 23, 1811. [Google Scholar] [CrossRef] [PubMed]
- Anguebes-Franseschi, F.; Abatal, M.; Pat, L.; Flores, A.; Córdova Quiroz, A.V.; Ramírez-Elias, M.A.; San Pedro, L.; May Tzuc, O.; Bassam, A. Raman Spectroscopy and Chemometric Modeling to Predict Physical-Chemical Honey Properties from Campeche, Mexico. Molecules 2019, 24, 4091. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; Al-Nahari, A.A.M.; Abd El-Ghany, E.S.M.; Barbour, E.; Al Muhayawi, S.M.; Al-Jaouni, S.; Azhar, E.; Qari, M.; Qari, Y.A.; Harakeh, S. Antimicrobial effect of different types of honey on Staphylococcus aureus. Saudi J. Biol. Sci. 2017, 24, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, S.; Koelling-Speer, I.; Speer, K. Confirmation of phenolic acids and flavonoids in honeys by UPLC-MS. Apidologie 2009, 40, 140–150. Available online: https://hal.archives-ouvertes.fr/hal-00892006 (accessed on 30 July 2024). [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Mouhoubi-Tafinine, Z.; Ouchemoukh, S.; Tamendjari, A. Antioxydant activity of some algerian honey and propolis. Ind. Crops Prod. 2016, 88, 85–90. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of flavonoid markers for the botanical origin of Eucalyptus honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Pinto, D.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Portuguese Honeys from Different Geographical and Botanical Origins: A 4-Year Stability Study Regarding Quality Parameters and Antioxidant Activity. Molecules 2017, 22, 1338. [Google Scholar] [CrossRef]
- Kadri, S.M.; Zaluski, R.; Pereira Lima, G.P.; Mazzafera, P.; de Oliveira Orsi, R. Characterization of Coffea arabica monofloral honey from Espírito Santo, Brazil. Food Chem. 2016, 203, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Chin, N.L.; Sowndhararajan, K. A Review on Analytical Methods for Honey Classification, Identification and Authentication. In Honey Analysis—New Advances and Challenges; Intech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Marquele-Oliveira, F.; Carrão, D.B.; de Souza, R.O.; Baptista, N.U.; Nascimento, A.P.; Torres, E.C.; de Padua Moreno, G.; Moreira Buszinski, A.F.; Luis Cuba, G.; dos Reis, T.F.; et al. Fundamentals of Brazilian Honey Analysis: An Overview. In Honey Analysis; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Samsonova, I.; Gryazkin, A.; Smirnov, A.; Mannapov, A.; Beljaev, V. Bioresource potential of forest lands as the source of honey yield in steppe area of the river Don. IOP Conf. Ser. Earth Environ. Sci. 2019, 316, 012057. [Google Scholar] [CrossRef]
- Lowore, J.; Meaton, J.; Wood, A. African Forest Honey: An Overlooked NTFP with Potential to Support Livelihoods and Forests. Environ. Manag. 2018, 62, 15–28. [Google Scholar] [CrossRef]
- Available online: https://www.jarahoney.com/ (accessed on 30 July 2024).
- Alliances Caucasus Programme (ALCP). Prospects for the Export of Georgian Honey; ALCP: Seaside, CA, USA, 2017. [Google Scholar]
- Abashidze, N.; Vanidze, M.; Kharadze, M.; Djaparidze, I.; Kalandia, A. West Georgian honey cations. Open J. Syst. 2018, 6, 990–994. [Google Scholar] [CrossRef][Green Version]
- Abashidze, N.; Japaridze, I.; Chikovani, D.; Kalandia, A.; Vanidze, M. Antibiotics and heavy metals in Georgian honey. Ann. Agrar. Sci. 2020, 18, 404–408. [Google Scholar]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, pesticide, and microbial contaminants of honey: Human health hazards. Sci. World J. 2012, 2012, 930849. [Google Scholar] [CrossRef] [PubMed]
- Fulya, G.D.; Goc, R.P.; Meral, K.; Kaya, S.T. Rhododendron honey and active substance Grayanotoxin III induced chromosome abnormalities in mice bone marrow cells. Toxicol. Lett. 2016, 258, S62–S324. [Google Scholar] [CrossRef]
- Shen, C.; Guo, S.; Ding, T.; Liu, Y.; Chen, L.; Fei, X.; Zhang, R.; Wu, B.; Shen, W.; Chen, L.; et al. Determination of characteristic compound in manuka honey by automatic on-line solid phase extraction-liquid chromatography-high resolution mass spectrometry. Se Pu 2017, 35, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Turumtay, E.A.; Yildiz, O.; Kolayli, S. Grayanotoxin-III Detection and Antioxidant Activity of Mad Honey. Int. J. Food Prop. 2015, 18, 2665–2674. [Google Scholar] [CrossRef]
- Harmonized Methods of the International Honey Commission. Available online: https://www.bee-hexagon.net/english/network/ (accessed on 30 July 2024).
- Sarmento Silva, T.M.; Pereira dos Santos, F.; Evangelista-Rodriges, A.; Sarmento da Silva, E.M.; Sarmento da Silva, G.; de Novais, J.S.; de Assis Ribeiro dos Santos, F.; Amorim Camara, C. Phenolic Compounds, Melissopalynological, Physicochemical Analysis and Antioxidant Activity of Jandaira (Melipona subnitida) Honey. J. Food Compos. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef]
- Bouddine, T.; Laaroussi, H.; Bakour, M.; Guirrou, I.; Khallouki, F.; Mazouz, H.; Hajjaj, H.; Hajji, L. Organic Honey from the Middle Atlas of Morocco: Physicochemical Parameters, Antioxidant Properties, Pollen Spectra, and Sugar Profiles. Foods 2022, 11, 3362. [Google Scholar] [CrossRef] [PubMed]
- Pauliuc, D.; Dranca, F.; Ropciuc, S.; Oroian, M. Advanced Characterization of Monofloral Honeys from Romania. Agriculture 2022, 12, 526. [Google Scholar] [CrossRef]
- Ciucure, G.E.-I.; Costinel, C.T.; Ionete, D.; Elena, R. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. J. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Živkov Baloš, M.; Popov, N.; Jakšić, S.; Mihaljev, Ž.; Pelić, M.; Ratajac, R.; Ljubojević Pelić, D. Sunflower Honey—Evaluation of Quality and Stability during Storage. Foods 2023, 12, 2585. [Google Scholar] [CrossRef] [PubMed]
- Sakač, N.; Sak-Bosnar, M. A rapid method for the determination of honey diastase activity. Talanta 2012, 93, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Al-Amri, A.; Al-Hadhrami, A.; Al-Belushi, S. Color, flavonoids, phenolics and antioxidants of Omani honey. Heliyon 2018, 4, e00874. [Google Scholar] [CrossRef]
- Marshall, S.M. Identipication and Concentration of Phenolic and Carbonyl Compounds in Florida Honey. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2013. [Google Scholar]
- Albu, A.; Radu-Rusu, C.-G.; Pop, I.M.; Frunza, G.; Nacu, G. Quality Assessment of Raw Honey Issued from Eastern Romania. Agriculture 2021, 11, 247. [Google Scholar] [CrossRef]
- A-Rahaman, N.L.; Chua, L.S.; Sarmidi, M.R.; Aziz, R. Physicochemical and radical scavenging activities of honey samples from Malaysia. Agric. Sci. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsı, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- Mehdi, R.; Zrira, S.; Vadalà, R.; Nava, V.; Condurso, C.; Cicero, N.; Costa, R. A Preliminary Investigation of Special Types of Honey Marketed in Morocco. J. Exp. Theor. Anal. 2023, 1, 1–20. [Google Scholar] [CrossRef]
- Pătruică, S.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Radulov, I.; Berbecea, A.; Lazăr, R.N.; Simiz, E.; Vicar, N.M.; Hulea, A.; et al. Chemical Composition, Antioxidant and Antimicrobial Activity of Some Types of Honey from Banat Region, Romania. Molecules 2022, 27, 4179. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-Q.; Zhang, J.; Li, Y.; Chen, L.; Zhao, W.; Zhou, J.; Jin, Y. Characterization of Chinese Unifloral Honeys Based on Proline and Phenolic Content as Markers of Botanical Origin, Using Multivariate Analysis. Molecules 2017, 22, 735. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.A.; Kleerekooper, I.; Hofman, Z.L.; Kappen, I.F.; Stary-Weinzinger, A.; van der Heyden, M.A. Grayanotoxin Poisoning: ‘Mad Honey Disease’ and Beyond. Cardiovasc. Toxicol. 2012, 12, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, K.; Christou, A.; Goulas, V. Optimization of Green Sample Preparation for the Determination of Hydroxycinnamic Acids in Multi-Floral Honey Using Response Surface Methodology. Appl. Sci. 2024, 14, 5781. [Google Scholar] [CrossRef]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Yung An, C.; Viswerwara Rao, P.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of Phenolic Acids and Flavonoids in Monofloral Honey from Bangladesh by High Performance Liquid Chromatography: Determination of Antioxidant Capacity. Corp. Bio Med Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Fechner, D.C.; Hidalgo, M.J.; Ruiz, D.; Juan, D.; Gil, R.A.; Pellerano, R.G. Geographical origin authentication of honey produced in Argentina. J. Food Biosci. 2019, 33, 100483. [Google Scholar] [CrossRef]
- Meoa, S.A.; Al-Asirib, S.A.; Mahesarc, A.L.; Ansarid, M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017, 24, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.I.; Mahaneem, M.; Jamalullail, S.M.S.; Alam, N.; Sulaiman, S.A. Evaluation of Radical Scavenging Activity and Colour Intensity of Nine Malaysian Honeys of Different Origin. J. ApiProduct ApiMedical Sci. 2011, 3, 4–11. [Google Scholar] [CrossRef]
- Schievano, E.; Sbrizza, M.; Zuccato, V.; Piana, L.; Tessari, M. NMR carbohydrate profile in tracing acacia honey authenticity. Food Chem. 2020, 309, 125788. [Google Scholar] [CrossRef] [PubMed]
- Ioannis, P.; Charalampos, P. HMF and diastase activity in honeys: A fully validated approach and a Chemometric analvsis for identification of honey freshness and adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef]
- Bogdanov, S. Chapter 5: Honey Composition. The Honey Book. Bee Product Science. 2016. Available online: www.bee-hexagon.net (accessed on 30 July 2024).
- Ruoff, K.; Luginbühl, W.; Kilchenmann, V.; Bosset, J.O.; Von Der Ohe, K.; Von Der Ohe, W.; Amadò, R. Authentication of the botanical origin of honey using profiles of classical measurands and discriminant analysis. Apidologie 2007, 38, 438–452. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Li, H.; Li, Y.; Hu, J.; Li, J.; Wang, H.; Deng, Z. Chemical and molecular dynamics analysis of crystallization properties of honey. Int. J. Food Prop. 2016, 20, 725–733. [Google Scholar] [CrossRef]
- Karabagiasa, I.K.; Karabourniotib, S.; Karabagiasa, V.K.; Badekaa, A.V. Palynological, physico-chemical and bioactivity parameters determination, of a less common Greek honeydew honey: “dryomelo”. J. Food Control 2020, 109, 106940. [Google Scholar] [CrossRef]
- Hagr, T.E.; Mirghani, M.E.S.; Elnour, A.A.H.M.; Bkharsa, B.E. Antioxidant capacity and sugar content of honey from Blue Nile State, Sudan. Int. Food Res. J. 2017, 24, S452–S456. [Google Scholar]
- Azonwade, F.E.; Paraso, A.; Dossa, C.P.A.G.; Dougnon, V.T.; Ntcha, C.; Mousse, W.; Moussa, B.L. Physicochemical Characteristics and Microbiological Quality of Honey Produced in Benin. J. Food Qual. 2018, 2018, 1896057. [Google Scholar] [CrossRef]
- Abashidze, N.; Djaparidze, I.; Vanidze, M.; Kalandia, A. Antioxidant activity of chestnut honey produced in Western Georgia. Bull. Georg. Natl. Acad. Sci. 2018, 12, 145–151. [Google Scholar]
- Khalil, M.I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, M.A.; Islam, M.N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Algerian Honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.; Feás, X.; Rodrigues, S.; Seijas, J.A.; Vázquez-Tato, M.P.; Dias, L.G.; Estevinho, L.M. Comprehensive Study of Honey with Protected Denomination of Origin and Contribution to the Enhancement of Legal Specifications. Molecules 2012, 17, 8561–8577. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Douglas, A.B. Physicochemical Characterization of Maltese Honey. In Honey Analysis; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Sniderman, J.K.; Matley, K.A.; Haberle, S.G.; Cantrill, D.J. Pollen analysis of Australian honey. PLoS ONE 2018, 13, e0197545. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Sulaiman, S.A.; Khalil, M.I.; Gan, S.H. Evaluation of physicochemical and antioxidant properties of sourwood and other Malaysian honeys: A comparison with manuka honey. Chem. Cent. J. 2013, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, T.; Stamatovska, V.; Kalevska, T. Quality Characteriteristy of Honey: A Review. Proc. Univ. Ruse 2018, 57, 10.2. [Google Scholar]
- Živkov Baloš, M.; Popov, N.; Vidaković, S.; Ljubojević Pelić, D.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical conductivity and acidity of honey. Arh. Vet. Med. 2018, 11, 91–101. Available online: https://niv.ns.ac.rs/e-avm/index.php/e-avm/article/view/20 (accessed on 30 July 2024). [CrossRef]
- Wieczorek, J.; Pietrzak, M.; Pomianowski, J.; Wieczorek, Z. Honey as a source of bioactive compounds. Pol. J. Nat. Sci. 2014, 29, 275–285. [Google Scholar]
- Dhawan, A.S.; Pawar, K.W.; Lokhande, A.A. Analysis of pollen grains in different honey samples from the region of Newasa tehsil in Maharashtra. J. Pharmacogn. Phytochem. 2018, 7, 3438–3442. [Google Scholar]
- Kıvrak, Ş.; Kıvrak, İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2016, 20, 864–876. [Google Scholar] [CrossRef]
- Sabo, M.; Potocnjak, M.; Banjari, I.; Petrovic, D. Pollen analysis of honeys from Varaždin County, Croatia. Turk. J. Bot. 2011, 35, 9. [Google Scholar] [CrossRef]
- Salvador, L.; Guijarro, M.; Rubio, D.; Aucatoma, B.; Guillén, T.; Vargas Jentzsch, P.; Ciobotă, V.; Stolker, L.; Ulic, S.; Vásquez, L.; et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Keka, S.P.; China, N.L.; Yusofa, Y.A.; Tanb, S.W.; Chua, L.S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Lachman, J.; Hejtmánková, A.; Sýkora, J.; Karban, J.; Orsák, M.; Rygerová, B. Contents of major phenolic and flavonoid antioxidants in selected Czech honey. Czech J. Food Sci. 2010, 28, 412–426. [Google Scholar] [CrossRef]
- Makawi, S.Z.A.; Gadkriem, E.A.; Ayoub, S.M.H. Determination of Antioxidant Flavonoids in Sudanese Honey Samples by Solid Phase Extraction and High Performance Liquid Chromatography. J. Chem. 2009, 6, S429–S437. [Google Scholar] [CrossRef]
- Istasse, T.; Jacquet, N.; Berchem, T.; Haubruge, E.; Nguyen, B.K.; Richel, A. Extraction of Honey Polyphenols: Method Development and Evidence of Cis Isomerization ubertas Academica. Anal. Chem. Insights 2016, 11, ACI–S39739. [Google Scholar] [CrossRef] [PubMed]
- Predescu, C.; Papuc, C.; Nicorscu, V. Antioxidant Activity of Sanflower and Meadow Honey. Sci. Work. Ser. C Vet. Med. 2015, 61, 46–50. [Google Scholar]
- Tu, J.-Q.; Zhang, Z.-Y.; Cui, C.-X.; Ang, M.; Li, Y.Y.; Zhang, Y.-P. Fast Separation and Determination of Flavonoids in Honey Samples by Capillary Zone Electrophoresis. Kem. Ind. 2017, 66, 129–134. [Google Scholar] [CrossRef]
- Jibril, F.I.; Hilmi, A.B.M.; Manivannan, L. Isolation and characterization of polyphenols in natural honey for the treatment of human diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Ozkoki, A.; Ozenirler, C.; Canli, D.; Mayda, N.; Sorkun, K. Monofloral Features of Turkish Honeys According to Melissopalynologic, Total Phenolic and Total Flavonoid Content. Gazi Univ. J. Sci. 2018, 31, 713–723. [Google Scholar]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as Possible Markers of Botanical Origin of Honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef]
- Gomes de Dliveira, R.; Jain, S.; Cândido, L.A.; Freitas, L.d.S. Edilson Divino de Araujd Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae). Food Sci. Technol. 2017, 37, 552–557. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Tan, T.T.E. Antioxidant Activity of Three Honey Samples in relation with Their Biochemical Components. J. Anal. Methods Chem. 2013, 2013, 313798. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, L.; Li, G.; Li, H.; Ye, D.; Li, X. Nondestructive Determination of Diastase Activity of Honey Based on Visible and Near-Infrared Spectroscopy. Molecules 2019, 24, 1244. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Oddo, L.P.; Sabatini, A.-G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standards: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Tosi, E.; Martinet, R.; Ortega, M.; Lucero, H.; Re, E. Honey diastase activity modified by heating. Food Chem. 2008, 106, 883–887. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S. A Preliminary Cost Effective Qualitative Assay for Diastase Analysis in Honey. Insights Enzym. Res. 2017, 1, 1. [Google Scholar] [CrossRef]
- Linkon, K.M.M.R.; Prodhan, U.K.; Hakim, A.; Alim, A. Study on the Physicochemical and Antioxidant Properties of Nigella Honey. Int. J. Nutr. Food Sci. 2015, 4, 137–140. [Google Scholar] [CrossRef]
- Czipa, N.; BorBély, M.; Győri, Z. Proteine content of different Honey types. Acta Aliment. 2012, 41, 26–32. [Google Scholar] [CrossRef]
- Uršulin-Trstenjak, N.; Puntaric, D.; Levanic, D.; Gvozdic, V.; Pavlek, C.; Puntaric, A.; Puntaric, E.; Puntaric, I.; Vidosavljevic, D.; Lasic, D.; et al. Pollen, Physicochemical, and Mineral Analysis of Croatian Acacia Honey Samples: Applicability for Identification of Botanical and Geographical Origin. J. Food Qual. 2017, 2017, 8538693. [Google Scholar] [CrossRef]
- Ponnuchamy, R.; Bonhomme, V.P.S.; Das, L.; Patel, P.; Gaucherel, C.; Pragasam, A.; Anupama, K. Honey Pollen: Using Melissopalynology to Understand Foraging Preferences of Bees in Tropical South India. PLoS ONE 2014, 9, e101618. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, K.; Vikram, P.; Soon, J.M.; Krishnan, K.T.; Mohammed, A. Melissopalynologica, phisicochemica and antioxidant properties of honey from West Coast of Malaysia. J. Food Sci. Technol. 2019, 56, 2508–2521. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, K.; Luginbühl, W.; Künzli, R.; Iglesias, M.T.; Bogdanov, S.; Bosset, J.O.; von der Ohe, K.; von der Ohe, W.; Amado, R. Authentication of the botanical and geographical origin of honey by mid-infrared spectroscopy. J. Agric. Food Chem. 2006, 54, 6873–6880. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.C.; Dos Santos, F.d.A.R. Pollen diversity in honey from Sergipe, Brazil. Grana 2014, 53, 159–170. [Google Scholar] [CrossRef]
- da Silva, C.I.; Radaeski, J.N.; Arena MV, N.; Bauermann, S.G. Soraia Atlas of Pollen and Plants Used by Bees; Consultoria Inteligente en Servicios Ecosistemicos: Rio Claro, Brazil, 2020; ISBN 978-65-86372-01-4. [Google Scholar]
- Saric, G.; Markovic, K.; Major, N.; Krpan, M.; Ur{ulin-Trstenjak, N.; Hruckar, M.; Vahcic, N. Changes of Antioxidant Activity and Phenolic Content in Acacia and Multifloral Honey During Storage. Food Technol. Biotechnol 2012, 50, 434–441. [Google Scholar]
- Özkök, D.; Silici, S. Effects of crystallization on antioxidant property of honey. J. Apitherapy 2018, 3, 24–30. [Google Scholar] [CrossRef]


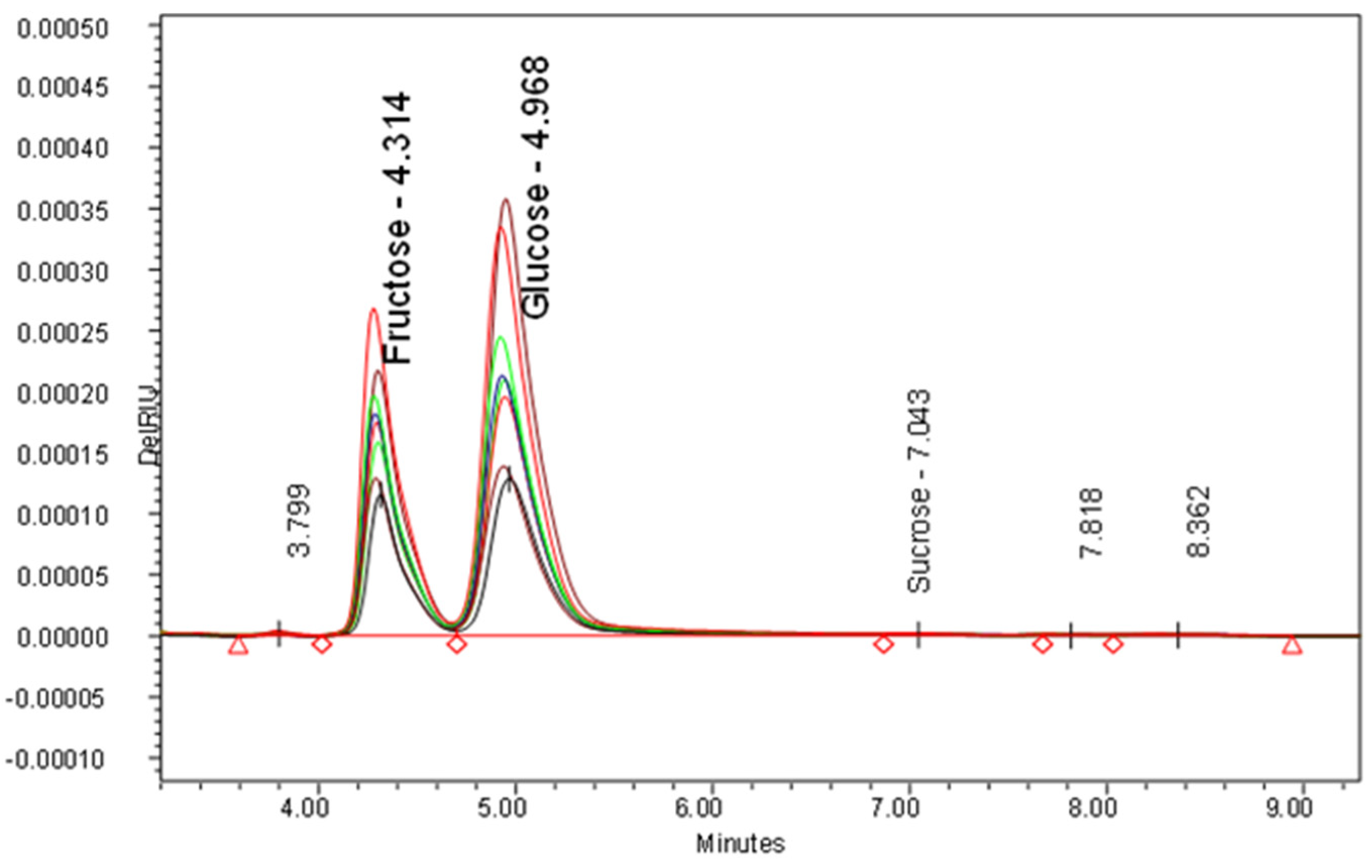

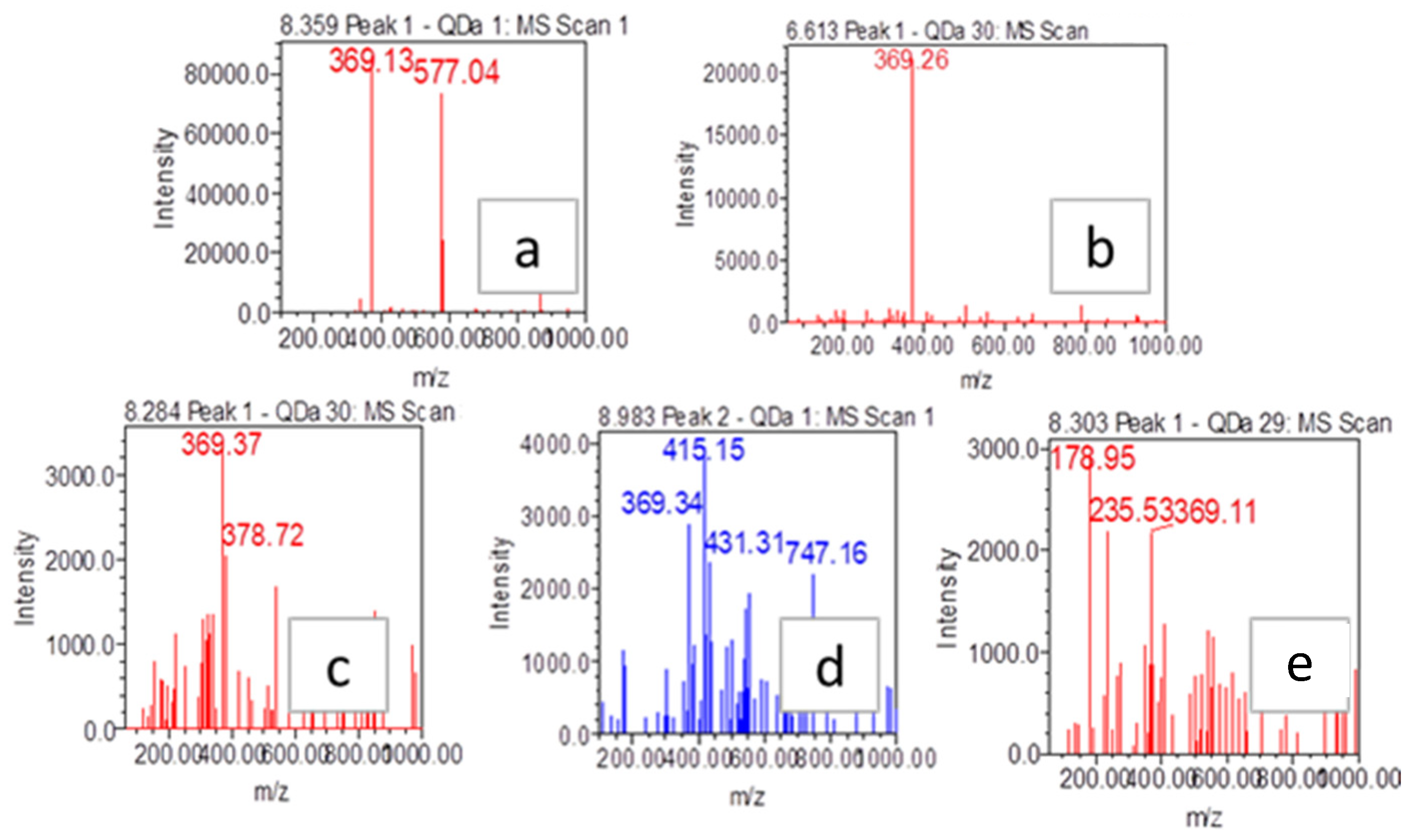
| Samples | Samplers Code | Sampling Place (Municipality) | Height above Mean Sea Level, m | Coordinates |
|---|---|---|---|---|
| Jara Honey 1 | JH-1 | Keda–Gobroneti | 607 | 41°39′8.5″ N, 42°2′18.6″ E |
| Jara Honey 2 | JH-2 | Keda–Zesopeli | 536 | 41°37′14.2″ N, 41°57′44.6″ E |
| Jara Honey 3 | JH-3 | Keda–Tskhmorisi | 523 | 41°38′22″ N, 42°2′50″ E |
| Jara Honey 4 | JH-4 | Keda–Zendidi | 336 | 41°36′10″ N, 41°55′55″ E |
| Jara Honey 5 | JH-5 | Keda–Zundaga | 400 | 41°34′42.4″ N, 41°49′32.9″ E |
| Jara Honey 6 | JH-6 | Keda–Namonastrevi | 820 | 41°34′13″ N, 42°3′10″ E |
| Jara Honey 7 | JH-7 | Keda–Silibauri | 490 | 41°34′46.3″ N, 42°0′47.1″ E |
| Jara Honey 8 | JH-8 | Keda–Medzibna | 640 | 41°33′51.9″ N, 41°58′29″ E |
| Jara Honey 9 | JH-9 | Keda–Merisi | 700 | 41°34′54″ N, 42°0′18″ E |
| Jara Honey 10 | JH-10 | Shuakhevi–Instkirveli | 1385 | 41°42′57.4″ N, 42°14′9.3″ E |
| Jara Honey 11 | JH-11 | Shuakhevi–Khabelashvilebi | 766 | 41°42′28″ N, 42°10′45.7″ E |
| Jara Honey 12 | JH-12 | Shuakhevi–Kidzinidzeebi | 1040 | 41°35′6.1″ N, 42°11′37.4″ E |
| Jara Honey 13 | JH-13 | Shuakhevi–Karapeti | 1334 | 41°33′8.1″ N, 42°15′24.5″ E |
| Jara Honey 14 | JH-14 | Khulo–Skhalta | 800 | 41°35′4.84″ N, 42°19′46.21″ E |
| Jara Honey 15 | JH-15 | Khulo–Kvatia | 1090 | 41°34′40″ N, 42°24′17″ E |
| Jara Honey 16 | JH-16 | Khulo–Pushrukauli | 1180 | 41°33′41″ N, 42°27′0″ E |
| Jara Honey 17 | JH-17 | Khulo–Rakvta | 1350 | 41°34′11″ N, 42°28′58″ E |
| Jara Honey 18 | JH-18 | Khulo–Bardnali | 570 | 42°36′22″ N, 42°42′50″ E |
| Name | Moisture (%) | Dry Substances, g/100 g | Free Acidity, mEq/kg | pH | Electrical Conductivity (µs/sm) |
|---|---|---|---|---|---|
| JH-1 | 17.0 ± 0.26 | 83.0 ± 1.25 | 22.48 ± 34 | 5.18 ± 0.08 | 1.185 ± 0.018 |
| JH-2 | 17.8 ± 0.26 | 82.2 ± 1.23 | 26.4 ± 0.40 | 5.3 ± 0.08 | 1.159 ± 0.017 |
| JH-3 | 17.0 ± 0.26 | 83.0 ± 1.25 | 27.5 ± 0.41 | 4.23 ± 0.06 | 1.071 ± 0.016 |
| JH-4 | 17.36 ± 0.26 | 82.6 ± 1.24 | 23.32 ± 0.35 | 5.14 ± 0.08 | 1.099 ± 0.016 |
| JH-5 | 18.4 ± 0.27 | 81.6 ± 1.22 | 25.04 ± 0.38 | 4.86 ± 0.07 | 1.706 ± 0.026 |
| JH-6 | 18.2 ± 0.27 | 81.8 ± 1.23 | 23.08 ± 0.35 | 5.06 ± 0.08 | 1.552 ± 0.023 |
| JH-7 | 17.6 ± 0.26 | 82.4 ± 1.24 | 27.00 ± 0.41 | 5.2 ± 0.08 | 1.606 ± 0.024 |
| JH-8 | 18.1 ± 0.27 | 81.9 ± 1.23 | 20.96 ± 0.31 | 5.62 ± 0.08 | 1.181 ± 0.018 |
| JH-9 | 18.6 ± 0.27 | 81.4 ± 1.22 | 23.24 ± 0.41 | 5.63 ± 0.08 | 1.447 ± 0.022 |
| JH-10 | 18.18 ± 0.27 | 81.8 ± 1.23 | 28.5 ± 0.43 | 5.07 ± 0.08 | 1.111 ± 0.017 |
| JH-11 | 16.1 ± 0.24 | 83.9 ± 1.26 | 23.28 ± 0.35 | 5.11 ± 0.08 | 1.1742 ± 0.018 |
| JH-12 | 15.0 ± 0.23 | 85.0 ± 1.28 | 27.5 ± 0.41 | 4.71 ± 0.07 | 1.1008 ± 0.017 |
| JH-13 | 16.6 ± 0.24 | 83.4 ± 1.25 | 24.96 ± 0.37 | 4.96 ± 0.07 | 1.190 ± 0.020 |
| JH-14 | 14.3 ± 0.23 | 85.7 ± 1.29 | 25.3 ± 0.38 | 5.09 ± 0.08 | 1.122 ± 0.017 |
| JH-15 | 15.4 ± 0.23 | 84.6 ± 1.27 | 28.41 ± 0.43 | 5.31 ± 0.08 | 1.166 ± 0.017 |
| JH-16 | 16.4 ± 0.24 | 83.6 ± 1.25 | 25.64 ± 0.38 | 5.17 ± 0.08 | 1.150 ± 0.017 |
| JH-17 | 18.4 ± 0.27 | 84.6 ± 1.27 | 24.4 ± 0.37 | 5.14 ± 0.08 | 1.137 ± 0.017 |
| JH-18 | 18.0 ± 0.27 | 82.0 ± 1.23 | 26.64 ± 0.40 | 4.4 ± 0.07 | 1.123 ± 0.017 |
| Samples | Fructose % | Glucose % | Sucrose % | Maltose % | The Total Amount of Carbohydrates % |
|---|---|---|---|---|---|
| JH-1 | 45.175 ± 0.68 | 17.8 ± 0.27 | - | - | 82.246 ± 1.23 |
| JH-2 | 46.523 ± 0.70 | 34.974 ± 0.52 | 0.326 ± 0.0049 | - | 81.823 ± 1.23 |
| JH-3 | 48.576 ± 0.73 | 33.605 ± 0.44 | 0.065 ± 0.0010 | - | 82.246 ± 1.23 |
| JH-4 | 53.266 ± 0.80 | 29.045 ± 0.55 | - | -- | 82.312 ± 1.23 |
| JH-5 | 44.543 ± 0.67 | 36.472 ± 0.44 | - | - | 81.015 ± 1.22 |
| JH-6 | 50.998 ± 0.76 | 29.607 ± 0.43 | - | - | 80.605 ± 1.21 |
| JH-7 | 52.580 ± 0.79 | 28.590 ± 0.52 | - | - | 81.170 ± 1.22 |
| JH-8 | 46.144 ± 0.69 | 34.619 ± 0.44 | - | - | 80.763 ± 1.21 |
| JH-9 | 51.368 ± 0.77 | 29.356 ± 0.48 | - | - | 80.724 ± 1.21 |
| JH-10 | 49.541 ± 0.74 | 31.786 ± 0.43 | 0.123 ± 0.0018 | - | 81.450 ± 1.22 |
| JH-10 | 54.438 ± 0.82 | 28.651 ± 0.41 | - | - | 83.089 ± 1.25 |
| JH-12 | 54.402 ± 0.82 | 27.656 ± 0.56 | - | 2.006 ± 0.0301 | 84.064 ± 1.26 |
| JH-13 | 45.740 ± 0.69 | 37.226 ± 0.46 | 0.217 ± 0.0033 | - | 83.183 ± 1.25 |
| JH-14 | 54.457 ± 0.82 | 30.986 ± 0.51 | - | - | 85.443 ± 1.28 |
| JH-15 | 48.355 ± 0.73 | 34.036 ± 0.50 | - | 1.800 ± 0.0270 | 84.191 ± 1.26 |
| JH-16 | 49.688 ± 0.75 | 33.125 ± 0.41 | 0.403 ± 0.0060 | 83.216 ± 1.25 | |
| JH-17 | 56.400 ± 0.85 | 27.180 ± 0.54 | 0.754 ± 0.0113 | 84.334 ± 1.27 | |
| JH-18 | 46.043 ± 0.69 | 35.739 ± 0.27 | - | - | 81.782 ± 1.23 |
| Samples | Fructose/Glucose (F/G) | Glucose/Water (G/W) |
|---|---|---|
| JH-1 | 1.22 ± 0.018 | 0.458 ± 0.0069 |
| JH-2 | 1.33 ± 0.020 | 1.96 ± 0.0294 |
| JH-3 | 1.45 ± 0.022 | 2.0 ± 0.0300 |
| JH-4 | 1.83 ± 0.027 | 2.25 ± 0.0338 |
| JH-5 | 1.22 ± 0.018 | 0.504 ± 0.0076 |
| JH-6 | 1.72 ± 0.026 | 1.63 ± 0.0245 |
| JH-7 | 1.84 ± 0.028 | 1.62 ± 0.0243 |
| JH-8 | 1.33 ± 0.020 | 1.91 ± 0.0287 |
| JH-9 | 1.75 ± 0.026 | 1.58 ± 0.0237 |
| JH-10 | 1.56 ± 0.023 | 1.75 ± 0.0263 |
| JH-10 | 1.90 ± 0.029 | 1.78 ± 0.0267 |
| JH-12 | 1.97 ± 0.030 | 1.84 ± 0.0276 |
| JH-13 | 1.23 ± 0.018 | 0.445 ± 0.0067 |
| JH-14 | 1.76 ± 0.026 | 2.16 ± 0.0324 |
| JH-15 | 1.42 ± 0.021 | 2.21 ± 0.0332 |
| JH-16 | 1.50 ± 0.023 | 2.02 ± 0.0303 |
| JH-17 | 2.08 ± 0.031 | 1.47 ± 0.0221 |
| JH-18 | 1.09 ± 0.016 | 0.5 ± 0.0075 |
| Samples | Lithium ppm | Sodium ppm | Potassium ppm | Magnesium ppm | Calcium ppm | Total Ions ppm |
|---|---|---|---|---|---|---|
| JH-1 | 10.54 ± 0.26 | 17.58 ± 0.44 | 2998.04 ± 89.9 | 80.22 ± 2.4 | 270.44 ± 8.1 | 3376.8 ± 101.3 |
| JH-2 | 11.22 ± 0.28 | 17.82 ± 0.45 | 2252.34 ± 67.6 | 56.58 ± 1.7 | 292.64 ± 8.8 | 2630.6 ± 78.9 |
| JH-3 | 12.7 ± 0.32 | 18.44 ± 0.46 | 2850.78 ± 85.5 | 72.54 ± 2.2 | 289.68 ± 8.7 | 3244.1 ± 97.3 |
| JH-4 | 15.06 ± 0.38 | 10.06 ± 0.25 | 4810.24 ± 144.3 | 78.52 ± 2.4 | 376.72 ± 11.3 | 5290.6 ± 158.7 |
| JH-5 | 14.76 ± 0.37 | 2.4 ± 0.06 | 4860.36 ± 145.8 | 171.04 ± 5.1 | 245.74 ± 7.4 | 5294.3 ± 158.8 |
| JH-6 | 10.78 ± 0.27 | 29.72 ± 0.74 | 2239.88 ± 67.2 | 88.32 ± 2.6 | 373.12 ± 11.2 | 2741.8 ± 82.3 |
| JH-7 | 14.94 ± 0.37 | 2.66 ± 0.07 | 3262.64 ± 97.9 | 262.34 ± 7.9 | 795.06 ± 23.9 | 4337.6 ± 130.1 |
| JH-8 | 15.94 ± 0.40 | 4.74 ± 0.12 | 4702.58 ± 141.1 | 55.48 ± 1.7 | 181.74 ± 5.5 | 4960.4 ± 148.8 |
| JH-9 | 15.38 ± 0.38 | 6.7 ± 0.17 | 3943.58 ± 118.3 | 59.22 ± 1.8 | 141.28 ± 4.2 | 4166.1 ± 125. 0 |
| JH-10 | 15.76 ± 0.39 | 6.82 ± 0.17 | 3856.74 ± 115.7 | 79.14 ± 2.4 | 258.3 ± 7.7 | 4216.7 ± 126.5 |
| JH-10 | 10.76 ± 0.27 | 10.72 ± 0.27 | 3594.96 ± 107.8 | 41.6 ± 1.2 | 390.38 ± 11.7 | 4048.4 ± 121.5 |
| JH-12 | 17.82 ± 0.45 | 2.94 ± 0.07 | 3259.64 ± 97.8 | 85.72 ± 2.6 | 402.72 ± 12.1 | 3768.8 ± 113.1 |
| JH-13 | 12.4 ± 0.31 | 2.82 ± 0.07 | 3296.6 ± 98.9 | 83.32 ± 2.5 | 201.86 ± 6.1 | 3597 ± 107.9 |
| JH-14 | 16.48 ± 0.41 | 23.06 ± 0.58 | 5074.36 ± 152.2 | 84.12 ± 2.5 | 370.42 ± 11.1 | 5568.4 ± 167.1 |
| JH-15 | 8.22 ± 0.21 | 13.64 ± 0.34 | 3803.18 ± 114.1 | 43.2 ± 1.3 | 272.3 ± 8.2 | 4140.5 ± 124.2 |
| JH-16 | 9.84 ± 0.25 | 15.8 ± 0.40 | 2356.68 ± 70.7 | 58.5 ± 1.8 | 288.66 ± 8.7 | 2729.4 ± 81.9 |
| JH-17 | 14.74 ± 0.37 | 33.58 ± 0.84 | 2174.86 ± 65.2 | 84.02 ± 2.5 | 306.32 ± 9.2 | 2613.5 ± 78.4 |
| JH-18 | 19.44 ± 0.49 | 7.18 ± 0.18 | 2395.72 ± 71.9 | 67.42 ± 2.0 | 183.62 ± 5.5 | 2673.3 ± 80.2 |
| Samples | Total Phenols mg/kg | Total Phenolic Acids mg/kg | Total Flavonoids mg/kg | Antioxidant Activity DPPH-50% Inhibition mg of Samples | Color (mmPfund) |
|---|---|---|---|---|---|
| JH-1 | 801.61 ± 20.04 | 453.3 ± 11.33 | 231.8 ± 5.80 | 90.01 ± 2.25 | 181.61 ± 4.54 |
| JH-2 | 1105.56 ± 27.64 | 682.4 ± 17.06 | 321.4 ± 8.04 | 75.1 ± 1.88 | 294.81 ± 7.37 |
| JH-3 | 987.19 ± 24.68 | 543.8 ± 13.60 | 307.9 ± 7.70 | 88.29 ± 2.21 | 249.31 ± 6.23 |
| JH-4 | 872.48 ± 21.81 | 418.5 ± 10.46 | 265.8 ± 6.65 | 79.59 ± 1.99 | 189.98 ± 4.75 |
| JH-5 | 752.99 ± 18.82 | 452.1 ± 11.30 | 229.4 ± 5.74 | 94.75 ± 2.37 | 180.76 ± 4.52 |
| JH-6 | 670.61 ± 16.77 | 379.9 ± 9.50 | 203.7 ± 5.09 | 107.15 ± 2.68 | 146.68 ± 3.67 |
| JH-7 | 622.34 ± 15.56 | 388.7 ± 9.72 | 154 ± 3.85 | 128.08 ± 3.20 | 148.26 ± 3.71 |
| JH-8 | 634.22 ± 15.86 | 359.8 ± 9.00 | 185.7 ± 4.64 | 125.77 ± 3.14 | 131.11 ± 3.28 |
| JH-9 | 647.77 ± 16.19 | 419 ± 10.48 | 143.6 ± 3.59 | 111.58 ± 2.79 | 130.44 ± 3.26 |
| JH-10 | 900.55 ± 22.51 | 533.8 ± 13.35 | 291.4 ± 7.29 | 82.38 ± 2.06 | 219.16 ± 5.48 |
| JH-11 | 988.79 ± 24.72 | 580.2 ± 14. 51 | 299.7 ± 7.49 | 89.83 ± 2.25 | 271.29 ± 6.78 |
| JH-12 | 902.03 ± 22.55 | 567.2 ± 14.18 | 232 ± 5.80 | 85.88 ± 2.15 | 232.87 ± 5.82 |
| JH-13 | 931.49 ± 23.29 | 612.4 ± 15.31 | 221 ± 5.53 | 86.43 ± 2.16 | 274.29 ± 6.86 |
| Samples | Proline mg/kg | Diastase Activity (Shade) | Protein % |
|---|---|---|---|
| JH-1 | 913.98 ± 27.41 | 14.01 ± 0.21 | 0.31 ± 0.0047 |
| JH-2 | 761.28 ± 22.83 | 9.23 ± 0.14 | 0.86 ± 0.0129 |
| JH-3 | 790.62 ± 23.71 | 20.0 ± 0.30 | 0.91 ± 0.0137 |
| JH-4 | 1109.17 ± 33.27 | 16.5 ± 0.25 | 0.91 ± 0.0137 |
| JH-5 | 1248.39 ± 37.45 | 18.75 ± 0.28 | 0.43 ± 0.0065 |
| JH-6 | 1054.08 ± 31.62 | 17 ± 0.26 | 0.46 ± 0.0069 |
| JH-7 | 1311.06 ± 39.33 | 13.33 ± 0.20 | 0.46 ± 0.0069 |
| JH-8 | 1090.02 ± 32.70 | 11.56 ± 0.17 | 0.46 ± 0.0069 |
| JH-9 | 896.04 ± 26.88 | 9.68 ± 0.15 | 0.40 ± 0.0060 |
| JH-10 | 985.25 ± 29.55 | 21 ± 0.32 | 0.89 ± 0.0134 |
| JH-10 | 927.39 ± 27.82 | 13.63 ± 0.20 | 0.41 ± 0.0062 |
| JH-12 | 1234.90 ± 37.04 | 21.11 ± 0.32 | 0.43 ± 0.0065 |
| JH-13 | 1198.16 ± 35.94 | 27 ± 0.41 | 0.42 ± 0.0063 |
| JH-14 | 920.53 ± 27.61 | 21.6 ± 0.32 | 0.91 ± 0.0137 |
| JH-15 | 1097.91 ± 32.93 | 16.2 ± 0.24 | 0.42 ± 0.0063 |
| JH-16 | 1148.98 ± 34.46 | 12.9 ± 0.19 | 0.43 ± 0.0065 |
| JH-17 | 1077.14 ± 32.31 | 14.2 ± 0.21 | 0.47 ± 0.0071 |
| JH-18 | 1372.29 ± 41.16 | 18.3 ± 0.27 | 0.41 ± 0.0062 |
| Pollen Types in the Honey Samples (in Percentages) | ||||
|---|---|---|---|---|
| Samples | Dominant Pollen (>45%) | Secondary Pollen (16–45%) | Important Minor Pollen (3–15%) | Minor Pollen (<3%) |
| JH-1 | - | Chestnut—37.6, Tilia platyphyllos—35.7 | Prunus domestica—11.2, Rubus idaeus—12.6 | Juglans regia, Prunus laurocerasus, Rhododenron, and eq.t. |
| JH-2 | Chestnut—50.0 | Acacia—24.2, Trifolium pratense—20.0 | Prunus laurocerasus—5.8 | - |
| JH-3 | Chestnut—65.5 | Trifolium pratense—14.03 | Acacia—11.74, Tilia platyphyllos—6.0 | Prunus laurocerasus, Juglans regia, and eq.t. |
| JH-4 | Chestnut—47.5 | Umbelliferae—36.2 | Tilia platyphyllos—9.6, Prunus laurocerasus—6.7 | - |
| JH-5 | Chestnut—62.4 | Acacia—17.74, Trifolium pratense—17.03 | - | Rhododenron and eq.t. |
| JH-6 | - | Chestnut—36.2, Tilia platyphyllos—19.0, Acacia—17.8 | Prunus laurocerasus—14.0, Prunus domestica L.—7.2, Rubus idaeus—5.8 | - |
| JH-7 | Chestnut—73.36 | - | Rubus idaeus—10.98, Trifolium pratense—9.13, Acacia—4.5 | Tilia platyphyllos, Taraxacum officinale, Solidago virgaurea, and eq.t. |
| JH-8 | Chestnut—96.89 | - | - | Juglans regia, Acacia, Solidago virgaurea, and eq.t. |
| JH-9 | Chestnut—93.3 | - | Rhododenron—4.45 | Acacia, Tilia platyphyllos, Trifolium pratense, Umbelliferae, and eq.t. |
| JH-10 | Chestnut —96.6 | - | - | Acacia, Tilia platyphyllos, Trifolium pratense, Rhododenron |
| JH-11 | Chestnut—53.28 | Acacia—33.46 | Umbelliferae—8.1 | Tilia, Trifolium pratense, Taraxacum, Rhododenron, and eq.t. |
| JH-12 | Chestnut—90.0 | - | Acacia—7.11 | Umbelliferae, Taraxacum officinale, and eq.t. |
| JH-13 | Chestnut—63.47 | Umbelliferae—21.88 | Acacia—6.03, Rhododenron—4.88 | Tilia, Taraxacum, and eq.t. |
| JH-14 | Chestnut—50.38 | Acacia—25.0 | Trifolium pratense—10.2, Tilia—11.7 | Alnus incana, Rubus idaeus, Rhododenron, and eq.t. |
| JH-15 | - | Chestnut—38.28, Trifolium pratense—25.52 | Malus domestica—12.87, Pyrus communis L.—15.9 | Umbelliferae, Rhododenron, and eq.t. |
| JH-16 | Chestnut—80.47 | - | Acacia—5.65, Umbelliferae—4.62 Rhododenron—4.26 | Tilia platyphyllos, Trifolium pratense, Taraxacum, Solidago virgaurea, and eq.t. |
| JH-17 | Chestnut—46.19 | Acacia—21.8 | Trifolium pratense—10.3, Rubus idaeus—8.13, Tilia platyphyllos—5.57, Juglans regia—5.02 | Rhododenron and eq.t. |
| JH-18 | Chestnut—52.3 | Tilia—37.8 | Umbelliferae—7.6 | Taraxacum, Solidago virgaurea, and eq.t. |
| Correlation between the Color Intensity of Honey Samples and Biochemical Parameters | Total Phenols mg/kg | Phenolic Acids mg/kg | Total Flavonoids mg/kg | Antioxidant Activity DPPH-50% Inhibition mg of Samples | Color (mmPfund) |
|---|---|---|---|---|---|
| Total phenols mg/kg | 1.00000 | 0.92120 | 0.88781 | −0.86301 | 0.95475 |
| Phenolic acids mg/kg | 0.92120 | 1.00000 | 0.67985 | −0.71814 | 0.96380 |
| Total flavonoids mg/kg | 0.88781 | 0.67985 | 1.00000 | −0.82727 | 0.76718 |
| Antioxidant activity DPPH-50% inhibition mg of samples | −0.86301 | −0.71814 | −0.82727 | 1.00000 | −0.74267 |
| Color (mmPfund) | 0.95475 | 0.96380 | 0.76718 | −0.74267 | 1.00000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abashidze, N.; Djafaridze, I.; Vanidze, M.; Khakhutaishvili, M.; Kharadze, M.; Kartsivadze, I.; Davitadze, R.; Kalandia, A. Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia. Appl. Sci. 2024, 14, 6874. https://doi.org/10.3390/app14166874
Abashidze N, Djafaridze I, Vanidze M, Khakhutaishvili M, Kharadze M, Kartsivadze I, Davitadze R, Kalandia A. Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia. Applied Sciences. 2024; 14(16):6874. https://doi.org/10.3390/app14166874
Chicago/Turabian StyleAbashidze, Nona, Indira Djafaridze, Maia Vanidze, Meri Khakhutaishvili, Maia Kharadze, Inga Kartsivadze, Ruslan Davitadze, and Aleko Kalandia. 2024. "Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia" Applied Sciences 14, no. 16: 6874. https://doi.org/10.3390/app14166874
APA StyleAbashidze, N., Djafaridze, I., Vanidze, M., Khakhutaishvili, M., Kharadze, M., Kartsivadze, I., Davitadze, R., & Kalandia, A. (2024). Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia. Applied Sciences, 14(16), 6874. https://doi.org/10.3390/app14166874






