Abstract
Sweet whey (SW) and yogurt acid whey (YAW) are dairy by-products of the cheese-making process and Greek-style yogurt production, respectively. Both of them are considered pollutants with huge volumes of SW and YAW produced due to the growing demand for dairy products worldwide. Moreover, whey-derived peptides, resulting from fermentation as well as from further hydrolysis during digestion, have been associated with various biological activities. In the present study, the angiotensin-converting enzyme (ACE)-inhibitory activity of 48 SW samples and 33 YAW samples from bovine, ovine, caprine, and ovine/caprine milk obtained were evaluated. Additionally, the SW and YAW digestates and two of their fractions (smaller than 10 kDa, SW-D-P10 and YAW-D-P10, and smaller than 3 kDa, SW-D-P3 and YAW-D-P3), which were obtained after in vitro digestion and subsequent ultrafiltration, were also subjected to evaluation. Our data indicated that the D-P10 and D-P3 fractions exhibited higher ACE-inhibitory activity compared to the corresponding values before digestion. The ACE-inhibitory capacity after in vitro digestion was higher for the ovine SW samples compared to their bovine and caprine counterparts. The effect of the D-P3 fraction on the inhibition of nitric oxide (NO) production and the expression of a selected panel of immune-response-related genes in LPS-stimulated RAW 264.7 macrophages was also evaluated. Fractions from both dairy by-products inhibited NO production in LPS-stimulated RAW 264.7 cells. Especially, ovine SW-D-P3 showed a strong NO inhibitory activity and suppressed inducible nitric oxide synthase (Nos2) mRNA levels. However, YAW-D-P3 could not trigger neither the gene expression of inflammatory macrophage mediators Nos2 and cyclooxygenase-2 (Ptgs2) nor tumor necrosis factor-α (Tnf) and interleukin 6 (Il6) in LPS-stimulated murine macrophages regardless of animal origin. These findings suggest that in vitro digestion could enhance the production of ACE-inhibitory peptides in both dairy by-products, while SW from ovine origin displays higher potential as an anti-inflammatory agent, effectively preventing excessive NO production.
1. Introduction
According to the World Health Organization (WHO), cardiovascular diseases (CVD) are responsible for approximately 18 million deaths annually, accounting for over 30% of global fatalities [1]. Hypertension affects 15–20% of all adults and is often referred to as a “silent killer” because many patients remain asymptomatic for extended periods [2]. Additionally, inflammation is a defense response of the body, it is considered a common pathogenesis factor of chronic diseases [3], and it is closely linked to the early stages and progression of CVD [4]. It is well known that diet is an important tool to effectively lower blood pressure. Consequently, potential candidates for the prevention and/or treatment of a cardiovascular disease could be ingredients capable of decreasing blood pressure after digestion [5].
In recent years, consumption of fermented dairy products has grown worldwide, and market trends indicate that this will further increase. Greek-style yogurt and cheese are two major categories of fermented dairy products [6]. The manufacture of fermented dairy products results in dairy by-products, the composition of which depends mainly on the used raw material [7]. The huge amounts of dairy by-products, sweet whey (SW), and yogurt acid whey (YAW), derived from cheese and Greek-style yogurt, respectively, present both economic and environmental challenges [7,8].
Caseins and whey proteins are the major protein fractions in dairy products, with casein comprising about 80% of milk protein. Protein from dairy products is considered an excellent source of numerous bioactive peptides with multifunctional properties, including antihypertensive, antioxidant, antimicrobial, immunomodulatory, opioid, and antithrombotic activities [9,10,11,12,13]. It is well known that milk proteins are considered a high-quality source of bioactive peptides with antihypertensive and anti-inflammatory functions [14]. However, bioactive peptides are encrypted and inactive in the parental protein and are generated during gastrointestinal digestion by enzymatic hydrolysis or microbial fermentation of food protein [15]. There is an ever-increasing number of studies supporting the idea that whey proteins could be used as potential functional food ingredients, and this idea is attributed to the enhanced bioactivity of milk-derived peptides. Among the several bioactive activities of whey protein peptides, ACE-inhibitory and anti-inflammatory activity have received special attention due to their beneficial effects on hypertension and immunoregulation, respectively.
The exopeptidase angiotensin I-converting enzyme (ACE; EC 3.4.15.1) has been classically associated with the renin–angiotensin system, which plays a central role in the regulation of peripheral blood pressure. ACE controls the levels of endogenous regulatory peptides such as angiotensin II, a potent vasoconstrictor, and bradykinin, a potent vasodilator. Currently, synthetic ACE inhibitors have been extensively used to treat hypertension. Although synthetic drugs are effective, they have several side effects on human health. Therefore, researchers have focused on various ACE inhibitors obtained from natural foods [16] since the inhibition of ACE activity by food-protein-derived peptides can lead to an overall antihypertensive effect [17]. Regarding the ACE activity of dairy proteins, there is one study showing that digested and filtrated dairy proteins have higher values for ACE-inhibitory activity compared to the corresponding plant proteins [18]. Furthermore, the major milk whey proteins (α-lactalbumin and β-lactoglobulin) have been reported to contain peptides which exhibit ACE-inhibitory activity [19].
Inflammation is an important physiological response of the body mediated by immune cells against injury and tissue damage. In more detail, firstly, immune cells (cells of the innate immune system such as monocytes, macrophages, and neutrophils) and inflammatory mediators (such as cytokines and chemokines) are known to be involved in the inflammatory process [20]. Nitric oxide (NO) is one of the inflammatory mediators that regulate a variety of physiological and pathological immune responses, including inflammation [21]. There are two types of nitric oxide synthases (NOSs), with the expression of inducible NOSs (iNOSs) in activated macrophages being responsible for the overproduction of NO, which in turn enhances inflammation. Another important inflammatory macrophage mediator is cyclooxygenase-2 (COX-2), which is related with prostaglandin E2 (PGE2), and the inhibition of its production is considered important for the inflammatory process [22]. Moreover, in response to extracellular stimuli, for instance, the bacterial LPS, tumor necrosis factor-α (TNF-α) is activated, which subsequently stimulates the transcription of inflammation-related genes, resulting in increasing levels of a number of cytokines [23]. Excessive production of interleukin 6 (IL-6), a pleiotropic cytokine, is induced in LPS- activated macrophages, and it has been known to play an important role in the inflammatory response [24].
Despite the high interest for the upcycling of dairy by-products, there are no studies on the antihypertensive and anti-inflammatory capacity of SW and YAW obtained from dairy companies after in vitro digestion. Previous studies from our lab have shown that peptides generated during in vitro gastrointestinal digestion possess antioxidant properties [9,10]. Whether these peptides possess antihypertensive and anti-inflammatory properties is not known. The objective of this study was to determine the aforementioned properties of dairy by-products (SW and YAW) after their in vitro digestion with the harmonized static digestion protocol INFOGEST 2.0 [25]. ACE-inhibitory activity was examined both before and after digestion. Moreover, the effect of D-P3 (for both by-products) on the NO production and gene expression of Nos2, Ptgs2, Il6, and Tnf in LPS-stimulated RAW 264.7 cells were also assessed.
2. Materials and Methods
2.1. Chemicals and Reagents
Chemicals and enzymes used were all high purity or analytical reagent grade. Pepsin from porcine gastric mucosa (≥2.500 units/mg protein), porcine pancreatin (4 × USP, United States Pharmacopeia), porcine bile extract, lipopolysaccharides from Escherichia coli O111:B4 (LPS), captopril, angiotensin-converting enzyme from rabbit lung (ACE), N-[3-(2-Furyl)acryloyl]-Phe-Gly-Gly (FAPGG), sodium nitrite (NaNO2), phosphoric acid (H3PO4), N-(1-Naphthyl)ethylenediamine dihydrochloride (NED), and sulfanilamide were purchased from Sigma-Aldrich (Saint Louis, MO, USA). The 96-well transparent flat-bottom plates were purchased from Sarstedt (Nümbrecht, Germany), while the 96-well cell culture transparent flat-bottom plates were purchased from Kisker Biotech (Steinfurt, Germany). The Amicon Ultra-4 Centrifugal Filter Devices (3 kDa and 10 kDa) and Millex-GP 33 mm PES 0.22 um were from Millipore (Burlington, MA, USA). Cell culture flasks and scrapers were purchased from SPL Life Sciences (Pocheon, Republic of Korea). Fetal bovine serum (FBS) was purchased from Gibco ThermoFisher Scientific (Waltham, MA, USA). DMEM, L-glutamine, sodium pyruvate, non-essential amino acids (NEAAs), and penicillin–streptomycin were obtained from Biosera (Cholet, France), while DMEM without phenol red was purchased from PAN-Biotech (Aidenbach, Germany). 3-(4,5-dimethylthiazol2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Cayman (Michigan, MI, USA). NucleoZOL was obtained from Macherey-Nagel (Düren, Germany). DNase I (RNase-Free) was purchased from New Englands Biolabs (Ipswich, MA, USA). The PrimeScript RT Reagent Kit (Perfect Real Time) kit was obtained from Takara Bio (Shiga, Japan). The FastGene IC Green 2 × IC Green qPCR Universal Mix was obtained from Nippon Genetics (Tokyo, Japan).
2.2. Collection and Preparation of Dairy By-Products
After a systematic search for samples from small-scale cheese plants and dairy companies in Greece, 48 SW and 33 YAW samples were collected. Crude protein of all the samples was determined by the Kjeldahl method (Kjeldahl nitrogen × 6.28) in duplicate. Protein content of SW ranged from 0.3 to 2% w/v, and pH ranged from 4.5 to 6.5. Protein content of YAW ranged from 0.09 to 1.2% w/v, and pH ranged from 3.7 to 4.7.
2.3. In Vitro Digestion Protocol and Digestate Fractionation
SW and YAW samples were concentrated 5 times by freeze-drying followed by rehydration. Freeze-drying was performed under the temperature conditions of −20 °C to 15 °C with a rate of temperature augmentation of 5 °C every 4 h with the maximum difference from shelf to sample at 10 °C. Vacuum pressure was 1 mbar for the duration of the procedure. They were afterwards subjected to the INFOGEST 2.0 method [25,26] separately for two categories of dairy by-products, with minor modifications. Briefly, all digestions were performed on the basis of equal protein amounts. The SW samples had 0.38% w/v in the final digestates, and the YAW samples had 0.11% w/v peptides, as described in [11,26]. Then, in order to obtain the fraction between 0 and 10 kDa (D-P10) and between 0 and 3 kDa (D-P3), membrane filters (Ultracel® low binding regenerated cellulose) with a MWCO of 10 kDa and 3 kDa were used, respectively. All samples were kept at −20 °C until subsequent analyses. In parallel, replicates of blank digestates were also prepared. Blank digestates contained water, instead of SW or YAW, following the same process of the in vitro digestion protocol and fractionation. The resulting blank fractions after digestion are hereby referred to as BL-D for blank digest (digestion without dairy product) and BL-D-P3, corresponding to the fraction of digestate with peptides with a molecular weight below 3 kDa.
2.4. Measurement of ACE-Inhibitory Activity
The ACE-inhibitory assay was performed in 96-well microtiter plates using FAPGG as substrate, according to the method of Shalaby et al. [27], with slight modifications. Firstly, the antihypertensive drug captopril (as a positive control) was used to evaluate the assay conditions to be used. A standard curve of captopril was performed for a concentration range of 1–27 nM. Briefly, 13.5 μL of dairy by-product and 100 μL of substrate preheated at 37 °C (0.88 mM FAPGG in 50 mM Tris-HCl, pH 7.5, containing 300 mM NaCl) were added into each well. The plate was incubated at 37 °C for 5 min without mixing. Subsequently, the reaction was started by the addition of 10 μL of ACE solution (containing 0.1 units/mL). The plate was immediately transferred to an Epoch 2 microplate spectrophotometer (Biotek, Winooski, VT, USA), which was operated at 37 °C. The absorbance at 340 nm was recorded every 1 min for 40 min, and the slope averaged over a linear interval of 15 min was taken as a measure of the ACE activity. The control samples contained 13.5 μL of buffer (50 mM Tris-HCl, pH 7.5, with 300 mM NaCl) instead of the sample. The slope (ΔA340 min-1), from 5 to 30 min of incubation, was used as a measure of ACE activity. The ACE inhibition (%) was calculated as reported by [28] [1-(ΔAsample-ΔAcontrol)] × 100%, where ΔAsample and ΔAcontrol are the slopes of the sample and of the control sample, respectively. The samples were assayed in duplicate, except for the control samples, which were assayed in four replicates in each plate. The experiment was performed three independent times.
2.5. Anti-Inflammatory Properties of Digestates
2.5.1. Cell Culture, Cell Viability, and Activation of RAW 264.7
Cells of the murine macrophage cell line RAW 264.7 were cultured in Dulbecco’s modified Eagle’s medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 10 U/mL L-glutamine, 100 μΜ NEAA, 1 mM sodium pyruvate, and 10% FBS and incubated at 37 °C in a humidified atmosphere containing 5% CO2. Cells (up to passage 25) were passaged after reaching 80% confluency and detached with cell scraper; a fresh medium was added following the aspiration of old media after centrifugation. RAW 264.7 cells were allowed to attach to 96-well plates at a seeding density of 5 × 104 cells/well overnight before the experiments. After 24 h incubation, the medium was removed, and the cells were washed with PBS. Subsequently, the cells were pretreated with D-P3 (10% of initial protein of dairy by-products) for 1 h and then incubated in the presence of 1 μg/mL LPS (containing DMEM without phenol red) for an additional 24 h.
2.5.2. Determination of Nitric Oxide (NO) Production by LPS-Stimulated Macrophage RAW 264.7 Cells
To quantify NO release by LPS-stimulated macrophage cells, Griess assay, a colorimetric method, was used. The assay is considered an indirect approach and relies on a diazotization reaction that was firstly described by Griess [29]. The Griess assay was performed according to Pinto et al. [30], with some modifications. In brief, 100 μL of each supernatant was transferred to a new 96-well plate, 50 μL of sulfanilamide [1% (w/v) in 5% H3PO4 (v/v)] was added, and reactions were incubated for 10 min at 25 °C in the dark. Subsequently, 50 μL of 10% (w/v) NED [N-(1-naphtlyl) ethylenediamine dihydrochloride] in H2O, then 5% (v/v) in H3PO4, was added and was further incubated for 10–30 min at 25 °C in the dark. The absorbance was measured at 540 nm using an Epoch 2 microplate reader (Biotek, Winooski, VT, USA). The total nitrite concentration was determined using a curve prepared with NaNO2 (concentration range, 1.5–100 μM) and Griess reagent solutions. Each sample was tested in quadruplicate, and the experiment was performed four independent times. The results are expressed as a percentage (%) of NO production to positive control cells (cells exposed only to LPS).
2.5.3. Quantification of Gene Expression in LPS-Stimulated Macrophage RAW 264.7 Cells
Total RNA was extracted from the attached RAW 264.7 cells using the Nucleozol reagent according to the manufacturer’s protocol. To remove DNA traces, samples were treated with DNase I. The RNA quantity and purity were analyzed on a spectrophotometer Q5000 (Quawell Technology Inc., San Jose, CA, USA). Reverse transcription was then performed. In brief, 500 ng of the total RNA was prepared with the PrimeScript RT according to the manufacturer’s instructions. A thermal cycler (SaCycle96, Sacace Biotechnologies, Como, Italy) was used for the qPCR using the FastGene 2 × IC Green qPCR Universal Mix. Primers for target genes (Nos2, Ptgs2, Tnf and Il6) and housekeeping genes (Actb, Cyc1) were designed, with 60 °C for an annealing temperature. Each sample was tested in duplicate. The relative gene expression was calculated using a modified version of the Pfaffl method against the two aforementioned housekeeping genes [31]. Primer details are listed in Table 1.

Table 1.
Sequences, reaction efficiency, and amplicon sizes of oligonucleotide primers used in qPCR.
2.6. Statistical Analysis
The data presented are means ± SEMs of at least two biological replicates. The results were subjected to a Kolmogorov–Smirnov test and transformed in logarithmic or normalized form [32] until the data were normally distributed. Data were compared using one-way ANOVA followed by Duncan’s post hoc test. p-values < 0.05 were considered significant. The statistical analysis was performed with the SPSS version 22.0.0, and graphs were generated using the GraphPad Prism 8 program.
3. Results and Discussion
In the present work, the antihypertensive activity of SW and YAW and their fractions containing peptides with MW below 10 kDa or 3 kDa, resulted after in vitro digestion, was investigated. Both peptidic fractions were selected for the antihypertensive evaluation in order to fully assess the potential of the samples tested. Then, the anti-inflammatory activity of the D-P3 fraction of SW and YAW from the pepsin–pancreatin digestates was tested using a NO radical scavenging activity, in addition to evaluating transcript expression levels of inflammation-related genes, under oxidative stress. Only the 3 kDa fraction was used for the anti-inflammatory evaluation of samples since larger peptides are not absorbed in the digestive tract [33,34].
3.1. Determination of ACE-Inhibitory Activity of Dairy By-Products
There is the notion that ACE-inhibiting potential is associated with low molecular weight peptides [35]. Thus, the ACE-inhibitory activity of SW and YAW was determined both before in vitro digestion and after digestion and fractionation. The results are shown in Figure 1 and Figure 2.
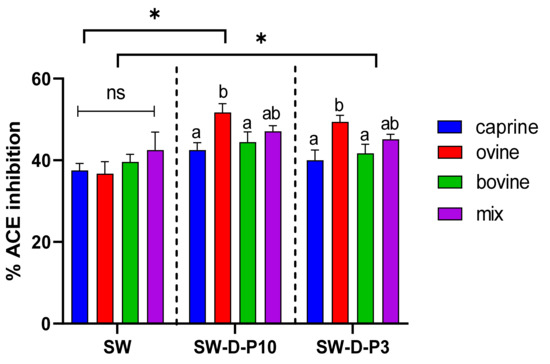
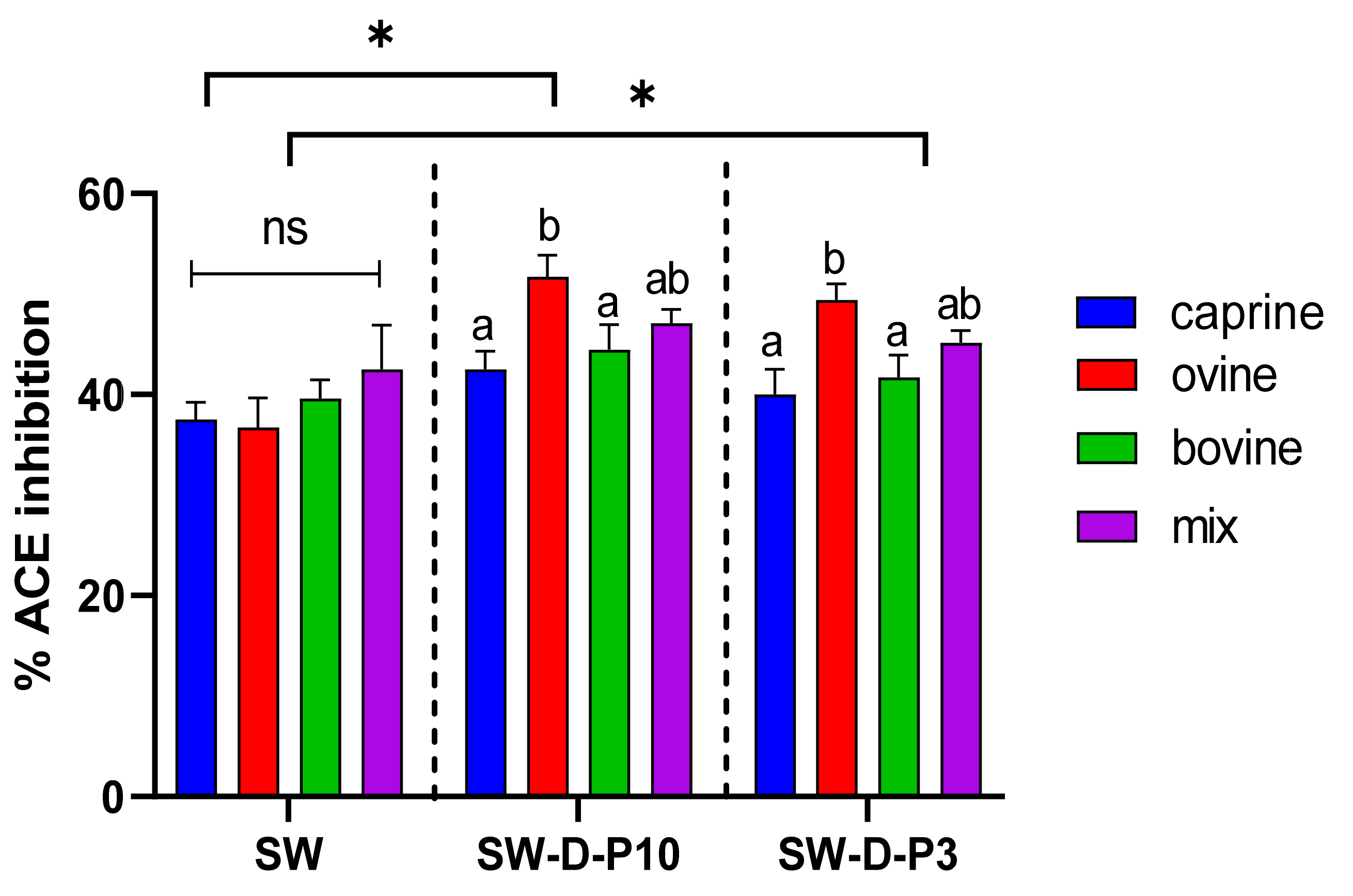
Figure 1.
Effect of animal origin of sweet whey (SW) on angiotensin-converting enzyme (ACE)-inhibitory activity (%) before and after in vitro digestion. The vertical dashed lines separate the three different groups as follows: SW: sweet whey before digestion; SW-D-P10: digested fraction below 10 kDa after digestion; SW-D-P3: digested fraction below 3 kDa. Data are presented as means ± SEM of three independent experiments. Columns with different letters significantly differ within the same group (p < 0.05). * indicates a significant difference between two groups (p < 0.05); ns indicates no significant difference between two groups (p > 0.05).
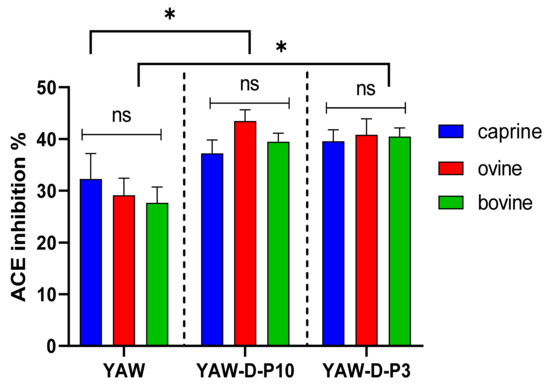
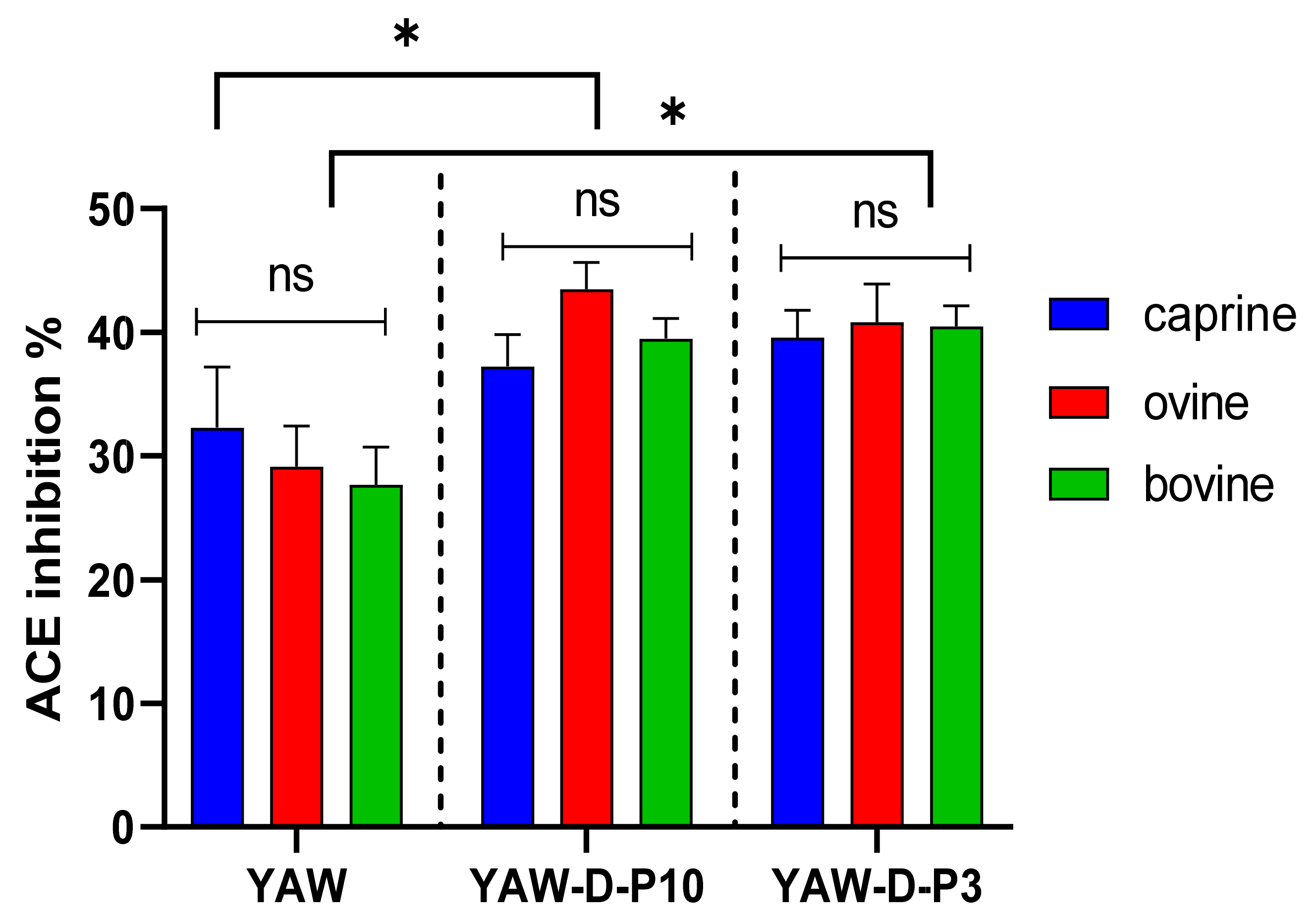
Figure 2.
Effect of animal origin of yogurt acid whey (YAW) on angiotensin-converting enzyme (ACE)-inhibitory activity (%) before and after in vitro digestion. The vertical dashed lines separate the three different groups as follows: YAW: yogurt acid whey before digestion; YAW-D-P10: digested fraction below 10 kDa after digestion; YAW-D-P3: digested fraction below 3 kDa. Data are presented as means ± SEM of three independent experiments. Columns with different letters significantly differ within the same group (p < 0.05). * indicates a significant difference between two groups (p < 0.05); ns indicates no significant difference between two groups (p > 0.05).
Figure 1 and Figure 2 indicate that the ACE-inhibitory activity of SW and YAW, respectively, was significantly increased (p < 0.05) after in vitro digestion, regardless of animal origin. Despite the increase in ACE-inhibitory activity after digestion (D-P10 and D-P3), no difference was observed between these fractions. The digestion process, therefore, had an overall positive effect on the ACE-inhibitory activity of SW and YAW. In Figure 1, the ACE% inhibition values of ovine SW were significantly higher (p < 0.05) after digestion (D-P10 and D-P3) compared to the caprine and bovine counterparts. However, Figure 2 shows no statistically significant differences (p > 0.05) in ACE-inhibition values between the YAW samples from different milk sources after digestion. Between the two by-products tested, SW displayed a higher ACE-inhibitory activity. This difference, which was consistent both before and after digestion, could be attributed to a variety of factors such as different raw material, protein content, structure of peptides, starter cultures used, etc.
There is a strong consensus that peptides with low molecular weight possess stronger inhibitory activity against ACE, which partially explains our results for both by-products. Consistent with our results, Li et al. [36] reported that fractions with low molecular weight (<1 kDa and 1–3 kDa) of whey protein hydrolysate (WPH; 72% of protein was less than 10 kDa) displayed potent ACE-inhibitory activity compared to WPH. Also, a significant decrease in ACE-inhibitory activity for >5 kDa fraction compared to those of <1 kDa and 1–3 kDa fractions was reported [36]. Similarly, Giromini et al. [37] indicated that digested whey protein isolate (WPI) consisting of peptides < 3 kDa induced a greater ACE-inhibitory effect compared with undigested WPI, while no significant difference was observed between undigested WPI and >3 kDa fraction of digested WPI. Likewise, the permeate fraction < 1 kDa of bovine whey proteins (α-lactalbumin and β-lactoglobulin) after hydrolysis, using a variety of different enzymes, had higher ACE-inhibitory potency than the corresponding retentate fractions [13]. Tavares et al. [38] observed a great ACE-inhibitory effect for the hydrolyzed whey protein concentrate (WPC) fraction < 3 kDa. In our study, no significant differences in ACE-inhibitory activity between D-P3 and D-P10 were evident for both SW and YAW.
Although this seems to not be in line with what has been previously reported, it should be taken into consideration that, while in the abovementioned studies, non-overlapping fractions are compared, in our procedure, the D-P10 fraction contains all peptides that are present in the D-P3 fraction. This suggests that peptides with MW below 3 kDa are mainly responsible for the ACE-inhibitory effect also observed in the D-P10 fraction. Furthermore, it is worth noting that the reported differences in ACE-inhibitory activity between studies may be a result of different digestion procedure and enzymes, as well as different food matrices tested and initial protein concentration.
Regarding the animal origin effect, although there are several reports dealing with dairy proteins on several species, there are no available comparative studies between species. Jrad et al. [39] reported that the ACE-inhibitory activity of camel milk and camel colostral whey was enhanced after in vitro digestion, suggesting that ACE-inhibitory peptides corresponding to camel milk and colostrum, respectively, were released. Tagliazucchi reported that the ACE-inhibitory activity of skimmed caprine milk at the end of in vitro gastrointestinal digestion was lower than that observed after gastric digestion. Those results suggested that the ACE-inhibitory activity of caprine milk hydrolysates decreased as the length of the peptides decreased, and further degradation resulted in a decrease in ACE-inhibitory activity [40]. Manso et al. [41] evaluated the ACE-inhibitory activity of bovine, ovine, and caprine κ-casein macropeptides (CMPs) and their tryptic hydrolysates. The results exhibited an increase in ACE-inhibitory activity after in vitro digestion for all three CMPs, without, however, having been compared with each other regarding the different animal origin.
The beneficial effects of dairy fermented foods on health can be more correctly assessed when tested following in vitro digestion, as bioactive peptides that are contained in larger peptides are thus released. In a study comparing ACE-inhibitory activity after fermentation combined with in vitro digestion of milk whey protein to pea protein, Vermeirssen et al. [42] suggested that in vitro digestion is the dominant factor controlling ACE inhibition, closely resembling the in vivo condition. However, the hydrolysis of bioactive peptides into inactive fragments during digestion is sometimes likely to occur [43]. Lignitto et al. [44] observed a significant interaction regarding ACE-inhibitory activity between the molecular mass of Asiago d’allevo semi-hard cheese and gastrointestinal digestion. This interaction results from a decreased ACE-inhibitory activity in <3 kDa fraction and an increased activity in <10 kDa fraction after digestion. It should be noted however that in this study, fractions were produced from water-soluble extracts of cheese samples before in vitro digestion and are therefore not representative of an actual food component. In our study, in vitro digestion was performed on raw materials (SW and YAW) that contained a variety of peptides regarding their molecular mass and the observed increased ACE-inhibitory following in vitro digestion is in accordance with the observed results of Lignitto et al. for the <10 kDa fraction. Similarly, Abedin et al. reported that simulated gastrointestinal digestion had a positive effect (5-fold increase) on the ACE-inhibitory activity of a yak and cow hard, fermented dairy product, chhurpi [43]. This increase is notably higher than the one reported by us. This can be attributed to the higher percentage of caseins in cheese, which are almost absent in YAW [45] and very low in SW [46,47], mainly in the form of caseinomacropeptide [48], as well as the possible higher initial activity of our SW and YAW samples compared to the cheese samples.
Concerning in vivo reports on the antihypertensive effect of whey as feed or a food component, Xia et al. [49] reported a positive effect of whey, with a blood pressure drop of 2.33 kPa when fed to spontaneously hypertensive rats, when compared with those in a pre-gavage state; with a long-term gavage treatment resulting in a greater decrease of 7.46 kPa. Clinical trial studies suggest that dairy intake could have a beneficial effect on blood pressure [50]. Similarly, meta-analysis studies show an inverse association between the consumption of fermented dairy products and hypertension risks [51].
The high activity observed after digestion suggests that SW and YAW could be suitable for use as functional feed/food supplements for reducing or preventing hypertension. However, there is not always a stable relation between ACE inhibition in vitro and the antihypertensive effect of the fermented food in vivo [52]. Thus, human or animal interventions trials are necessary to demonstrate the efficacy of food-derived peptides in blood pressure control, as well as validate their therapeutic potential.
3.2. Effect of D-P3 on Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells
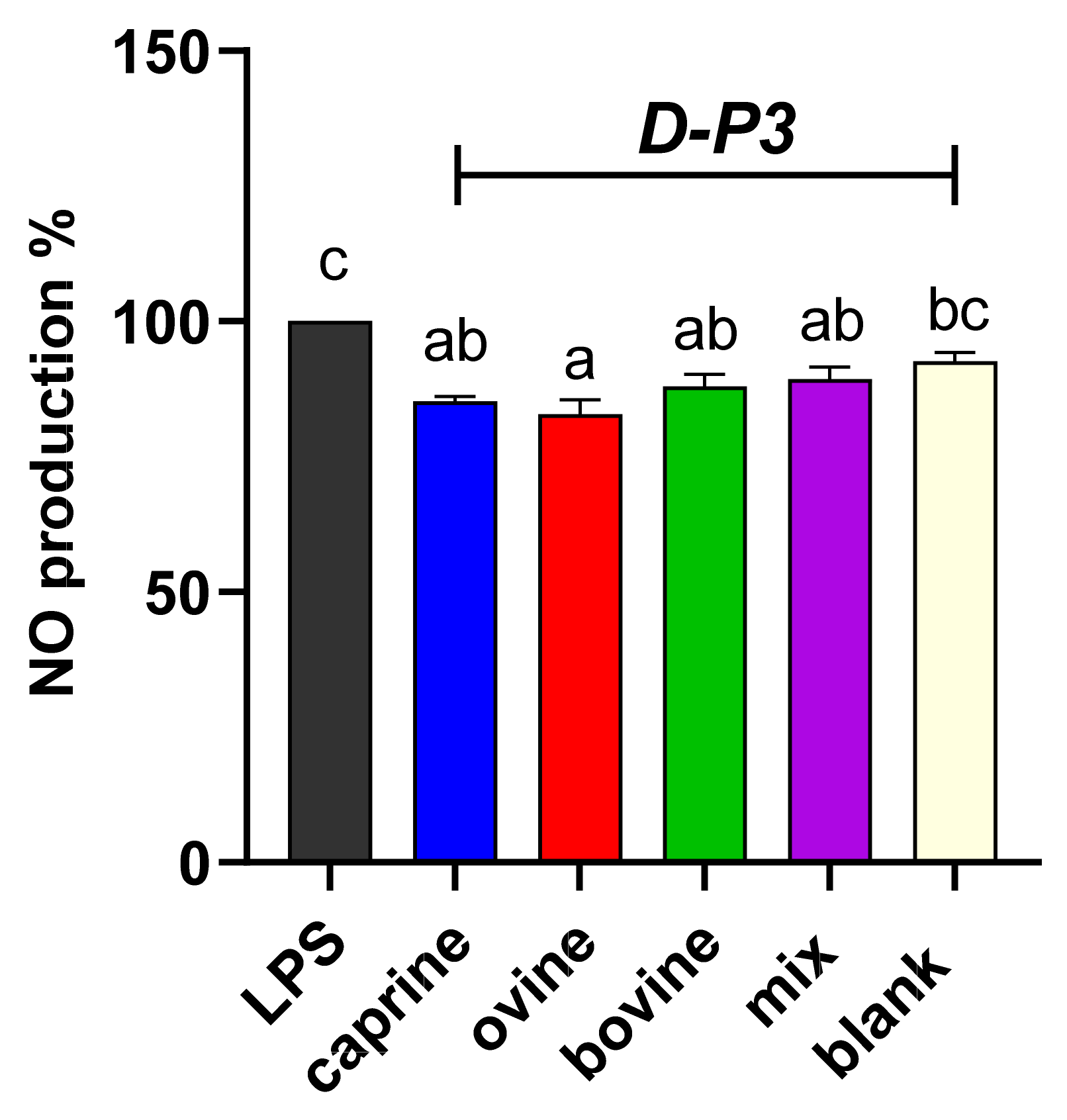
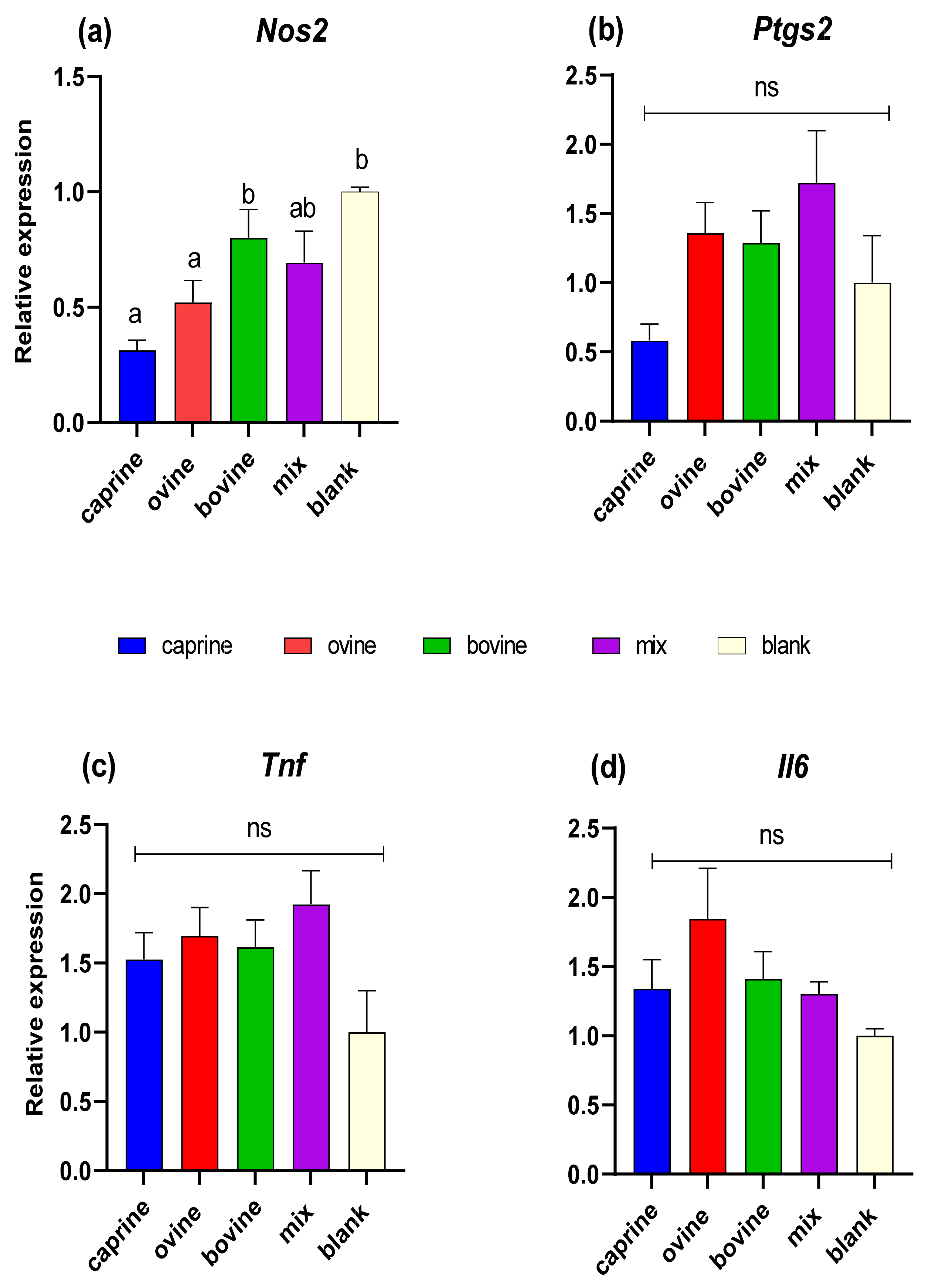
To evaluate the effect of digested whey in preventing induced NO formation, LPS-stimulated RAW 264.7 macrophage cells were used due to the potential interaction of bioactive components with inducible NO, since the iNOS molecule is expressed in these cells [53]. NO production was evaluated for the resultant fraction after digestion, referred to as SW-D-P3 and YAW-D-P3, and the results are shown in Figure 3 and Figure 4, respectively. Figure 3 and Figure 4 illustrate that both aforesaid dairy by-products fractions reduced the NO levels compared to the LPS group in the activated macrophage cell line, RAW 264.7. As shown in Figure 3, the production of NO was lowered significantly (p < 0.05) only in the cells incubated with ovine SW-D-P3 compared to the cells incubated with BL-D-P3. However, no statistically significant differences were observed between SW-D-P3 fractions of different animal origins (p > 0.05; Figure 3).
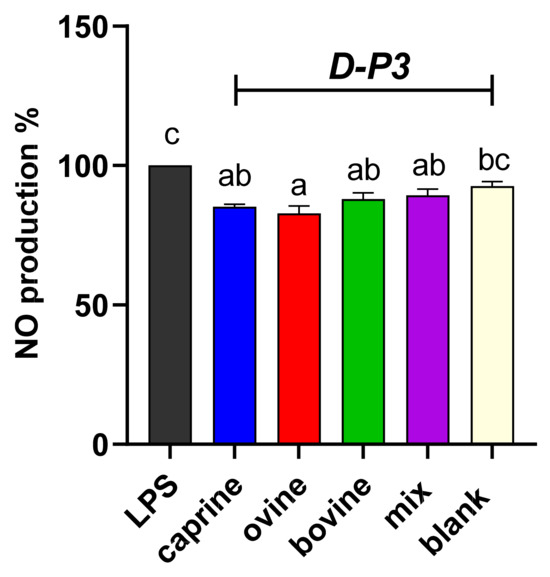
Figure 3.
Effect of SW-D-P3 on LPS-induced NO production in RAW 264.7 cells. RAW 264.7 cells were treated as follows: LPS, cells stimulated only with 1 μg/mL LPS; D-P3, cells stimulated with LPS and subsequently incubated in the presence of SW-D-P3 (caprine, ovine, bovine, and mix; 0.038% w/v refers to starting SW-D protein) or BL-D-P3 (blank) for 24 h. Data are presented as means ± SEM of four independent experiments. Columns with different letters are significantly different (p < 0.05).
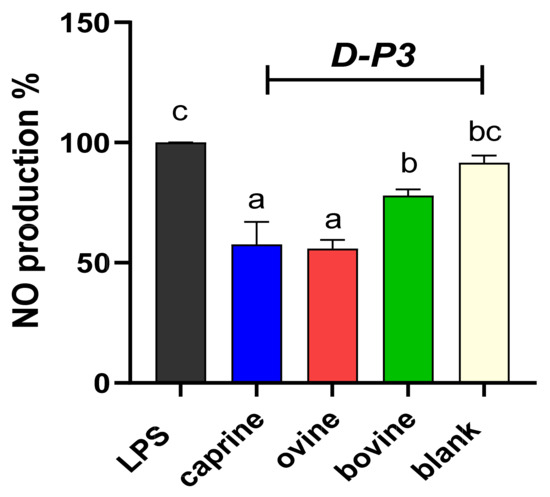
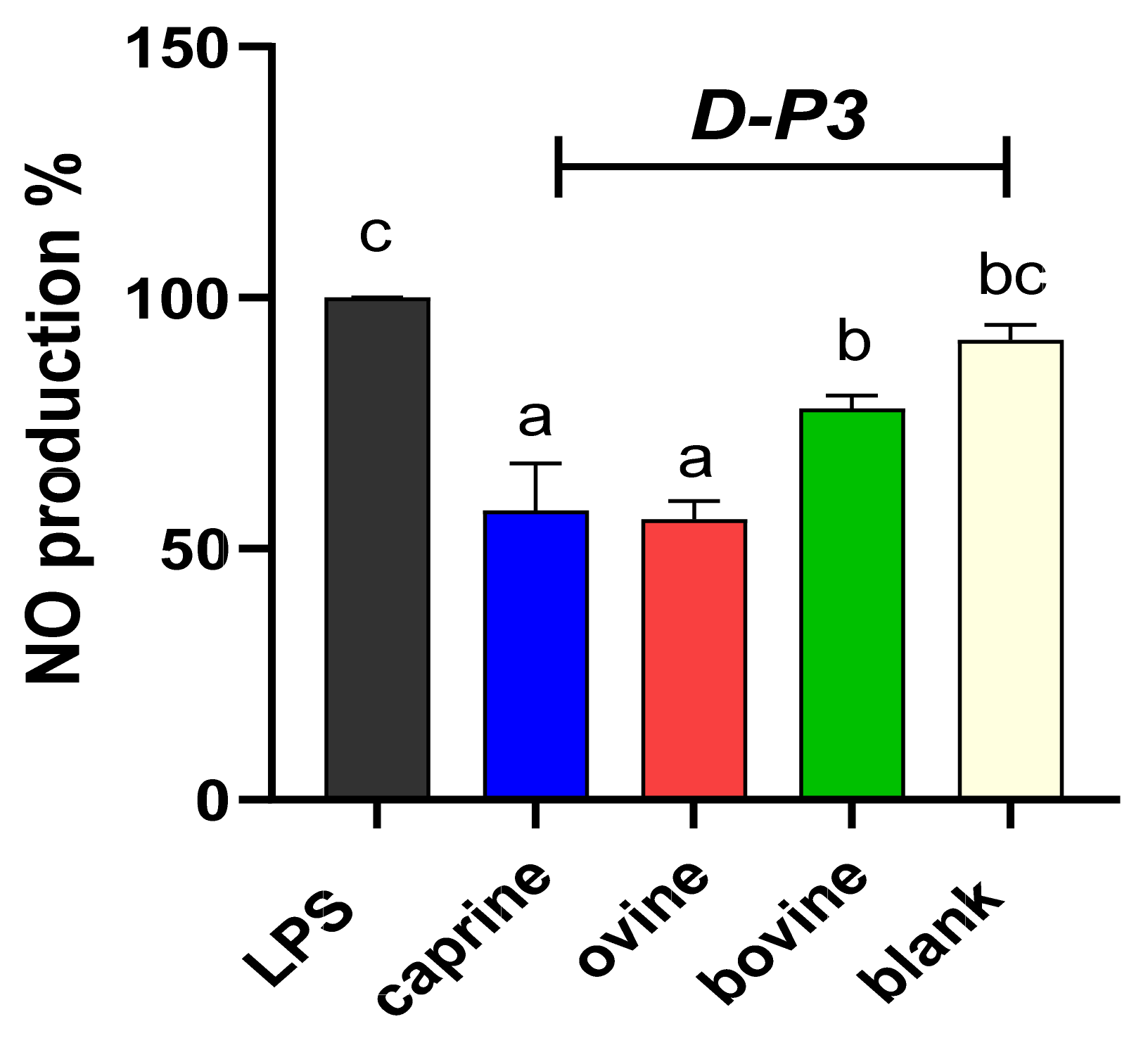
Figure 4.
Effect of YAW-D-P3 on LPS-induced NO production in RAW 264.7 cells. RAW 264.7 cells were treated as follows: LPS, cells stimulated only with 1 μg/mL LPS; D-P3, cells stimulated with LPS and subsequently incubated in the presence of YAW-D-P3 (caprine, ovine, and bovine; 0.011% w/v refers to starting YAW-D protein) or BL-D-P3 (blank) for 24 h. Data are presented as means ± SEM of four independent experiments. Columns with different letters are significantly different (p < 0.05).
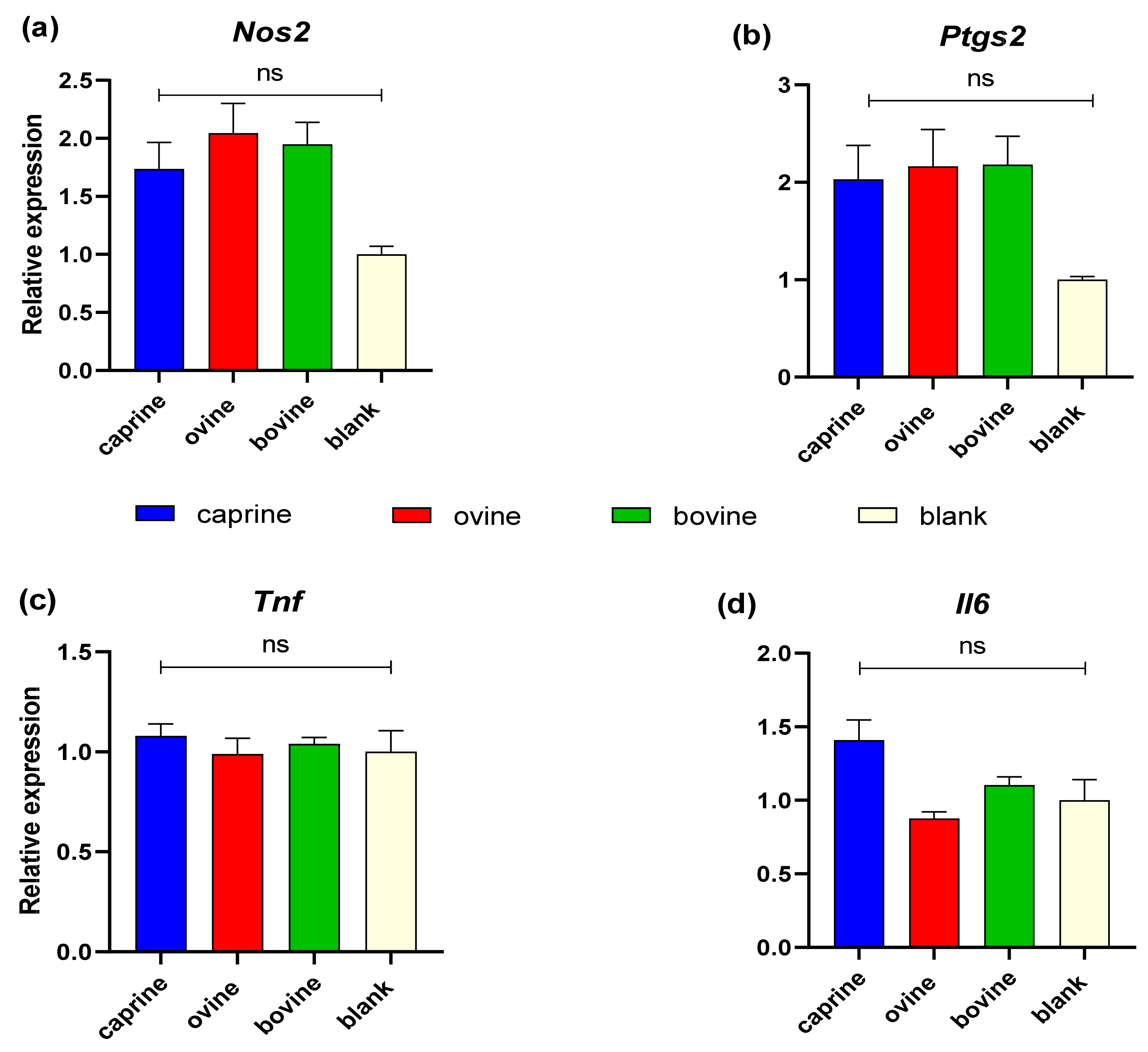
The NO production of cells incubated in the presence of caprine, ovine, and bovine YAW-D-P3 are displayed in Figure 4. NO values were significantly reduced for all treatment groups compared to the LPS-stimulated cells (p < 0.05), while ovine and caprine YAW-D-P3-treated cells exhibited lower NO production levels compared to both cells treated with bovine YAW-D-P3 and BL-D-P3 (p < 0.05).
Our results are in accordance with those previously reported by other groups using undigested and digested milk proteins from different animal sources. Pinho et al. [54] reported that the incubation of in vitro-digested skimmed milk in LPS-stimulated RAW 264.7 cells is able to reduce NO production. Similarly, the NO production was decreased in cells treated with digested β-lactoglobulin-lactose conjugate after a 1-day co-incubation with LPS [55]. Zhao et al. [56] reported that undigested caprine milk whey reduced NO production significantly in a dose-dependent manner in LPS-stimulated RAW 264.7 cells. Similarly, Prakash et al. reported reduced NO production in the same murine macrophage cell line in WPH-treated cells compared to the LPS-stimulated group [57]. Another study reported that a combination of whey protein isolate and galactose did not inhibit NO production in LPS-stimulated RAW 264.7 cells, while the corresponding glycated and fermented sample was able to reduce NO levels [58], which serves as proof of the major role of the fermentation process in releasing anti-inflammatory peptides from whey proteins. In line with this notion, Dharmisthaben et al. demonstrated a positive effect of camel milk fermentation on NO inhibition in RAW 264.7 [59]. In contrast to our results and those of the aforementioned studies, Espindola et al. reported that NO secretion levels were not affected by WPH treatment. It should be noted though that in this study, a different cell model and stimuli (Caco-2 and H2O2) were used, leading the authors to conclude that this intestinal epithelial cell line is not responsive to NO production [60].
In general, a combined model of human-simulated digestion with immunocompetent cell lines provides a good representation of in vivo conditions with fewer ethical restrictions, although in vitro efficacy may differ from in vivo results. In the case of NO, which, as a key messenger molecule for activated macrophages, has a vital role in immune regulation, the fact that it not only protects against exogenous pathogens but is also involved in the regulation of cellular activities associated with the immune system makes more complex the translation of in vitro results to in vivo [61]. Previously, Park et al. [62] observed no difference between spontaneously hypertensive rats fed WPC and its hydrolysate in the nitrate-to-nitrite ratio. Gandhi et al. [63] reported that WPC–iron complex supplementation in weaning and anemic rats reduced NO production, and this complex may fight against anemia and iron-deficiency-related disorders.
3.3. Effect of D-P3 on Expression of Inflammation-Related Genes
Monocytes differentiate into macrophages, following polarization depending on a variety of stimuli, and diverse inflammatory pathways are activated. In response to different microenvironment, various functional phenotypes are detected [64]. Macrophages secrete inflammatory cytokines, including IL-1β and IL-6, as well as TNF-α. In addition, certain enzymes, such as iNOS and COX-2, play an important role in the development of inflammation-related diseases.
In this study, the relative expression of Nos2, Ptgs2, Il6, and Tnf inflammation-related genes was quantified in response to treatment with D-P3 in LPS-stimulated RAW 264.7 macrophages. As shown in Figure 5a, Nos2 expression was found to be higher (p < 0.05) in BL-D-P3 when compared with caprine and ovine SW-D-P3 but not with bovine and mixed ones. Also, higher Nos2 mRNA levels were observed for bovine SW-D-P3 compared to caprine and ovine SW-D-P3. On the other hand, no statistically significant differences were observed between SW treatments and BL-D-P3 regarding Ptgs2, Tnf, and Il6 expression (p > 0.05; Figure 5b, c, and d, respectively).
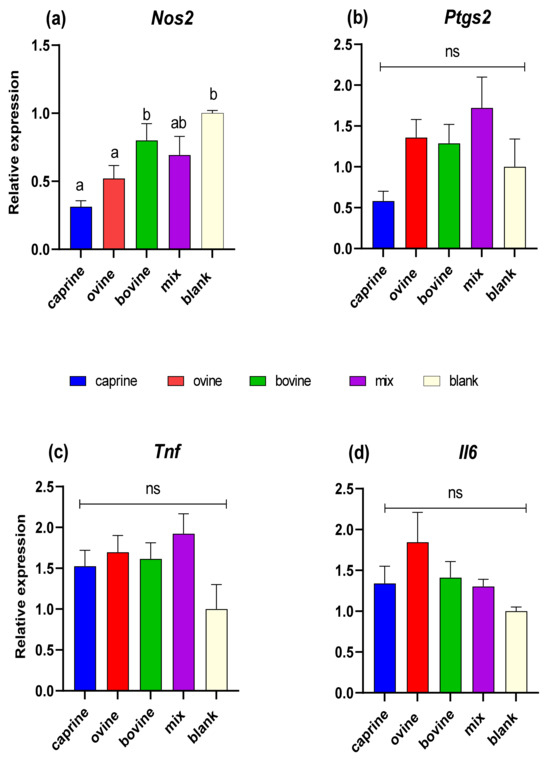
Figure 5.
Effect of SW-D-P3 on LPS-induced mRNA expression in RAW 264.7 cells. RAW 264.7 cells were treated as follows: LPS, cells stimulated only with 1 μg/mL LPS; D-P3, cells stimulated with LPS and subsequently incubated in the presence of SW-D-P3 (caprine, ovine, bovine, and mix; 0.038% w/v refers to starting SW-D protein) or BL-D-P3 (blank) for 24 h. The expression levels of (a) Nos2, (b) Ptgs2, (c) Tnf, and (d) Il6 were measured using qPCR and were normalized to housekeeping genes (Actb and Cyc1). Data are presented as mean ± SEM of two independent experiments. Columns with different letters within the same panel are significantly different (p < 0.05); ns = not significant (p > 0.05).
Figure 6 shows the quantification of transcription levels of genes relevant to the immune response for YAW fractions. No statistically significant differences were observed between the YAW-D-P3 samples either between different animal origins or with BL-D-P3-treated cells regarding Nos2, Ptgs2, Tnf, and Il6 expression (p > 0.05).
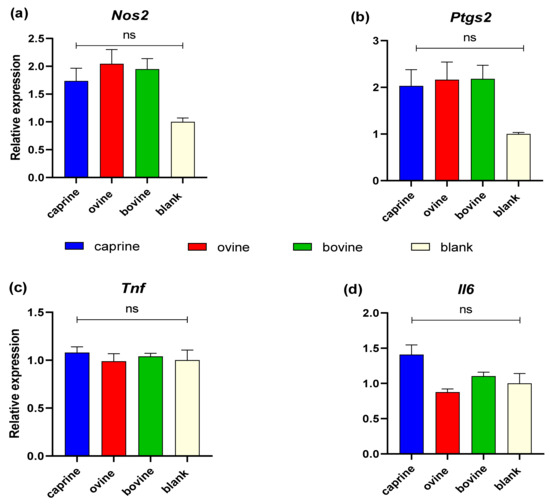
Figure 6.
Effect of YAW-D-P3 on LPS-induced mRNA expression in RAW 264.7 cells. RAW 264.7 cells were treated as follows: LPS, cells stimulated only with 1 μg/mL LPS; D-P3, cells stimulated with LPS and subsequently incubated in the presence of YAW-D-P3 (caprine, ovine, and bovine; 0.011% w/v refers to starting YAW-D protein) or BL-D-P3 (blank) for 24 h. The expression levels of (a) Nos2, (b) Ptgs2, (c) Tnf, and (d) Il6 were measured using qPCR and were normalized to housekeeping genes (Actb and Cyc1). Data are presented as mean ± SEM of two independent experiments. ns = not significant (p > 0.05).
Previous studies reported that natural non-dairy compounds, including protein and peptides, have anti-inflammatory activity by suppressing Nos2 expression, resulting in the inhibition of NO production, as well as by down-regulating pro-inflammatory gene expression, most of the time, in a dose-dependent manner [65,66,67,68,69]. The nuclear factor-kappa light-chain-enhancer of activated B cells (NF-κB) pathway is a crucial signaling pathway responsible for maintaining the defense system against inflammatory stimuli, either endogenous or exogenous (like LPS). The production of cytokines (IL-1β, IL-6, and TNF-α), the induction of iNOS, and the expression of COX-2 are regulated by the participants in the NF-κB canonical pathway [66]. Zhao et al. reported that [70] two buffalo whey peptides significantly inhibited the expression of inflammatory mediators, iNOS, TNF-α, and IL-6, in stimulated with 1 μg/mL LPS murine RAW 264.7 macrophages, in a dose-dependent manner (50–200 μg/mL), indicating the protective mechanism of these peptides against cell inflammation damage. Another study indicated that the <1 kDa fraction from WPH, as well as two subsequent sub-fractions, showed inhibitory activity on IL-1β, COX-2, and TNF-α mRNA expression in LPS-induced RAW 264.7 cells [71]. According to a previous study, the addition of fermented camel milk (at concentration of 0.25 mg/mL) resulted in the inhibition of pro-inflammatory cytokine production of TNF-α, IL-6, and IL-1β in LPS-stimulated murine macrophages [72]. Another study reported the anti-inflammatory properties of hydrolysate of β-LG under high hydrostatic pressure. In more detail, the suppression of the synthesis of pro-inflammatory cytokines (TNF-α and Il-1β) was observed in LPS-stimulated macrophage cells [73].
Sullivan et al. [70] evaluated the anti-inflammatory activity of the 5 kDa permeates of casein hydrolysates using different proteolytic sources (mammalian, plant, fungal) in two cell lines (RAW 264.7 and Jurkat T cells) with two concentrations of LPS. Each of the hydrolysate fractions caused a significant decrease in IL-6 production at 0.05% w/v, although no difference was noted at 0.005% w/v. Consistent with our study, TNF-a production was unaffected after treatment with the 5 kDa permeates at any concentration for both cell lines used. Notably, the 5 kDa permeate fraction of casein hydrolysates was used in the study of Sullivan et al. [74], while in this study, the 3 kDa permeate fractions of SW and YAW were used. Τhis may imply that the enhanced cell-protective effect is associated not only with the protein itself (casein vs. whey) but also with the peptides with a higher molecular mass.
4. Conclusions
To the best of our knowledge, this is the first work evaluating the antihypertensive capacity of SW and YAW before and after in vitro digestion, as well as the anti-inflammatory potential of the fraction between 0 and 3 kDa (D-P3) for both by-products. From the obtained results concerning in vitro digestion, it is obvious that the ACE-inhibitory values of SW and YAW were significantly lower than those of the digested counterparts (D-P10 and D-P3), regardless of animal origin. Also, after in vitro digestion, ovine SW-D-P10 and SW-D-P3 showed greater ACE-inhibitory activity compared to their caprine and bovine counterparts. Furthermore, ovine SW-D-P3 exhibited suppressed NO production on LPS-stimulated macrophage cells compared to BL-D-P3. Of special interest was the gene expression quantification of pro-inflammatory mediators in response to treatment with D-P3 in LPS-stimulated RAW 264.7 cells. Ovine and caprine SW-D-P3 could significantly down-regulate the Nos2 expression in LPS-stimulated RAW 264.7 cells. Similarly, caprine and ovine YAW-D-P3 inhibited NO production, although this was not accompanied by changes in the expression of pro-inflammatory cytokines and enzymes involved in inflammation-related diseases. Due to the prevalence of hypertension and the rise of inflammatory diseases in the global population, leading to a growing demand for nutraceuticals—in conjunction with environmental concern regarding the disposal of dairy by-products—the positive results presented here regarding the antihypertensive and anti-inflammatory properties of dairy by-products could pave the way for their targeted use in human nutrition. However, further extensive in vivo studies are required to establish the health claim for each by-product and its constituents individually.
Author Contributions
Conceptualization, G.T. and E.D.; methodology and investigation, E.D.; writing—original draft preparation, E.D.; writing—review and editing, G.T., G.C.S. and I.P.; supervision, G.T.; project administration, G.T.; funding acquisition, I.P., G.T. and E.D. All authors have read and agreed to the published version of the manuscript.
Funding
E.D. received a scholarship co-financed by Greece and the European Union (European Social Fund—ESF) through the Operational Program “Human Resources Development, Education and Lifelong Learning” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (MIS-5000432), implemented by the State Scholarships Foundation (ΙΚΥ). This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: Τ2EDK-00783; MIS-5074577). The results of this study are part of a research project (MIS 5033108) funded by the Operational Program of the Region of Epirus, co-financed by Greece and the European Union—European Regional Development Fund (ERDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The RAW 264.7 cell line was kindly provided by Tsatsanis Christos from the Department of Laboratory Medicine, School of Medicine (University of Crete, Greece). We would like to thank Maria Kapsokefalou for giving us access to the instrument Epoch 2 microplate spectrophotometer (Laboratory of Food Chemistry and Analysis, Agricultural University of Athens). Finally, we would like to thank all the dairy companies for providing us with sweet and yogurt acid whey samples.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 December 2023).
- Lin, L.; Lv, S.; Li, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory and Antihypertensive Properties of Squid Skin Gelatin Hydrolysates. Food Chem. 2012, 131, 225–230. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Iaccarino, G. Inflammation and Cardiovascular Diseases: The Most Recent Findings. Int. J. Mol. Sci. 2019, 20, 3879. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Hayes, M.; Carney, B. Angiotensin-I-Converting Enzyme and Prolyl Endopeptidase Inhibitory Peptides from Natural Sources with a Focus on Marine Processing by-Products. Food Chem. 2011, 129, 235–244. [Google Scholar] [CrossRef] [PubMed]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New Perspectives in Fermented Dairy Products and Their Health Relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Bong, D.D.; Moraru, C.I. Use of Micellar Casein Concentrate for Greek-Style Yogurt Manufacturing: Effects on Processing and Product Properties. J. Dairy Sci. 2014, 97, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Dalaka, E.; Stefos, G.C.; Politis, I.; Theodorou, G. Effect of Milk Origin and Seasonality of Yogurt Acid Whey on Antioxidant Activity before and after In Vitro Gastrointestinal Digestion. Antioxidants 2023, 12, 2130. [Google Scholar] [CrossRef]
- Dalaka, E.; Politis, I.; Theodorou, G. Antioxidant Activity of Sweet Whey Derived from Bovine, Ovine and Caprine Milk Obtained from Various Small-Scale Cheese Plants in Greece before and after In Vitro Simulated Gastrointestinal Digestion. Antioxidants 2023, 12, 1676. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory Peptides Obtained by the Enzymatic Hydrolysis of Whey Proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Phelan, M.; Khaldi, N.; Shields, D.C.; Kerins, D.M. Angiotensin Converting Enzyme and Nitric Oxide Inhibitory Activities Ofnovel Milk Derived Peptides. Int. Dairy J. 2014, 35, 38–42. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Koskinen, P.; Phlola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-Converting Enzyme Inhibitory Properties of Whey Protein Digests: Concentration and Characterization of Active Peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, P.; Chen, X. Bioactive Peptides Derived from Fermented Foods: Preparation and Biological Activities. J. Funct. Foods 2023, 101, 105422. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 2666–5662. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhuang, Y.; Lin, L.; Li, L.; Fan, X.; Sun, L. In Vitro Simulated Gastrointestinal Digestion Stability and in Vivo Antihypertensive Effect of the Peptide KYPHVF and Its Network Pharmacology. J. Funct. Foods 2023, 107, 105672. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive Peptides Derived from Bovine Whey Proteins: Opioid and Ace-Inhibitory Peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Giromini, C.; Fekete, Á.A.; Ian Givens, D.I.; Baldi, A.; Lovegrove, J.A. Short-Communication: A Comparison of the in Vitro Angiotensin-1-Converting Enzyme Inhibitory Capacity of Dairy and Plant Protein Supplements. Nutrients 2017, 9, 1352. [Google Scholar] [CrossRef]
- Pihlanto-Leppaïa, A.; Rokka, T.; Korhonen, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Bovine Milk Proteins. Int. Dairy J. 1998, 8, 325–331. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of Chitosan Molecular Weight on Anti-Inflammatory Activity in the RAW 264.7 Macrophage Model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Ahn, G.N.; Kang, S.M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.K.; Jeon, Y.J. Evaluation of Anti-Inflammatory Effect of Fucoxanthin Isolated from Brown Algae in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Wang, S.Y. Lucidone Inhibits INOS and COX-2 Expression in LPS-Induced RAW 264.7 Murine Macrophage Cells via NF-KappaB and MAPKs Signaling Pathways. Planta Med. 2009, 75, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Kim, S.A.; Lee, J.S.; Kim, H.J.; Choi, I.W.; Jung, W.K. The Antioxidant and Anti-Inflammatory Effects of Abalone Intestine Digest, Haliotis Discus Hannai in RAW 264.7 Macrophages. Biotechnol. Bioprocess Eng. 2012, 17, 475–484. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.M.; Zakora, M.; Otte, J. Performance of Two Commonly Used Angiotensin-Converting Enzyme Inhibition Assays Using FA-PGG and HHL as Substrates. J. Dairy Res. 2006, 73, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.; Shalaby, S.M.; Zakora, M.; Pripp, A.H.; El-Shabrawy, S.A. Angiotensin-Converting Enzyme Inhibitory Activity of Milk Protein Hydrolysates: Effect of Substrate, Enzyme and Time of Hydrolysis. Int. Dairy J. 2007, 17, 488–503. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen Zu Der Abhandlung Der HH. Weselsky Und Benedikt „Ueber Einige Azoverbindungen”. Berichte Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Pinto, R.V.; Antunes, F.; Pires, J.; Silva-Herdade, A.; Pinto, M.L. A Comparison of Different Approaches to Quantify Nitric Oxide Release from NO-Releasing Materials in Relevant Biological Media. Molecules 2020, 25, 2580. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 2008, 8, R19. [Google Scholar] [CrossRef]
- Templeton, G.F.; Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 41–58. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of Chain Length on Absorption of Biologically Active Peptides from the Gastrointestinal Tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Barac, M.; Vucic, T.; Zilic, S.; Pesic, M.; Sokovic, M.; Petrovic, J.; Kostic, A.; Ignjatovic, I.S.; Milincic, D. The Effect of in Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, C.; Hong, H.; Zhang, Y.; Luo, Z.; Wang, Q.; Luo, Y.; Tan, Y. Novel ACE Inhibitory Peptides Derived from Whey Protein Hydrolysates: Identification and Molecular Docking Analysis. Food Biosci. 2022, 48, 101737. [Google Scholar] [CrossRef]
- Giromini, C.; Lovegrove, J.A.; Givens, D.I.; Rebucci, R.; Pinotti, L.; Maffioli, E.; Tedeschi, G.; Sundaram, T.S.; Baldi, A. In Vitro-Digested Milk Proteins: Evaluation of Angiotensin-1-Converting Enzyme Inhibitory and Antioxidant Activities, Peptidomic Profile, and Mucin Gene Expression in HT29-MTX Cells. J. Dairy Sci. 2019, 102, 10760–10771. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.; Contreras, M.D.M.; Amorim, M.; Pintado, M.; Recio, I.; Malcata, F.X. Novel Whey-Derived Peptides with Inhibitory Effect against Angiotensin-Converting Enzyme: In Vitro Effect and Stability to Gastrointestinal Enzymes. Peptides 2011, 32, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Jrad, Z.; El Hatmi, H.; Adt, I.; Girardet, J.-M.; Cakir-Kiefer, C.; Jardin, J.; Degraeve, P.; Khorchani, T.; Oulahal, N.; Jrad, Z.; et al. Effect of Digestive Enzymes on Antimicrobial, Radical Scavenging and Angiotensin I-Converting Enzyme Inhibitory Activities of Camel Colostrum and Milk Proteins. Dairy Sci. Technol. 2014, 94, 205–224. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Shamsia, S.; Helal, A.; Conte, A. Angiotensin-Converting Enzyme Inhibitory Peptides from Goats’ Milk Released by in Vitro Gastro-Intestinal Digestion. Int. Dairy J. 2017, 71, 6–16. [Google Scholar] [CrossRef]
- Manso, M.A.; López, R.; López-Fandiño, L.; Fandiño, F. Angiotensin I Converting Enzyme-Inhibitory Activity of Bovine, Ovine, and Caprine k-Casein Macropeptides and Their Tryptic Hydrolysates. J. Food Prot. 2003, 66, 1686–1692. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The Impact of Fermentation and in Vitro Digestion on the Formation of Angiotensin-I-Converting Enzyme Inhibitory Activity from Pea and Whey Protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Chiring Phukon, L.; Singh, S.P.; Kumar Rai, A. Characterization of ACE Inhibitory and Antioxidant Peptides in Yak and Cow Milk Hard Chhurpi Cheese of the Sikkim Himalayan Region. Food Chem. X 2022, 13, 100231. [Google Scholar] [CrossRef]
- Lignitto, L.; Cavatorta, V.; Balzan, S.; Gabai, G.; Galaverna, G.; Novelli, E.; Sforza, S.; Segato, S. Angiotensin-Converting Enzyme Inhibitory Activity of Water-Soluble Extracts of Asiago d’allevo Cheese. Int. Dairy J. 2010, 20, 11–17. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited Review: Acid Whey Trends and Health Benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- Baig, D.; Sabikhi, L.; Khetra, Y.; Kumar, D. Effect of Casein to Fat Ratio of Camel Milk on Solids Losses in Cheese Whey and Their Recovery in Camel Milk Cheese. Int. Dairy J. 2022, 124, 105185. [Google Scholar] [CrossRef]
- Outinen, M.; Heino, A.; Uusi-Rauva, J. Pre-Treatment Methods of Edam Cheese Milk. Effect on the Whey Composition. LWT-Food Sci. Technol. 2010, 43, 647–654. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Fraga, M.J.; Fontecha, J. Isolation and Characterisation of Caseinmacropeptide from Bovine, Ovine, and Caprine Cheese Whey. Eur. Food Res. Technol. 2002, 214, 282–286. [Google Scholar] [CrossRef]
- Xia, Y.; Yu, J.; Xu, W.; Shuang, Q. Purification and Characterization of Angiotensin-I-Converting Enzyme Inhibitory Peptides Isolated from Whey Proteins of Milk Fermented with Lactobacillus Plantarum QS670. J. Dairy Sci. 2020, 103, 4919–4928. [Google Scholar] [CrossRef]
- McGrane, M.M.; Essery, E.; Obbagy, J.; Lyon, J.; MacNeil, P.; Spahn, J.; van Horn, L. Dairy Consumption, Blood Pressure, and Risk of Hypertension: An Evidence-Based Review of Recent Literature. Curr. Cardiovasc. Risk Rep. 2011, 5, 287–298. [Google Scholar] [CrossRef]
- Vajdi, M.; Musazadeh, V.; Zareei, M.; Adeli, S.; Karimi, A.; Hojjati, A.; Darzi, M.; Shoorei, H.; Abbasalizad Farhangi, M. The Effects of Whey Protein on Blood Pressure: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Yin, R.; Howell, K.; Zhang, P. Activity and Bioavailability of Food Protein-Derived Angiotensin-I-Converting Enzyme–Inhibitory Peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1150–1187. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Kim, Y.-S.; Kim, Y.; Yoo, S.-H. Regulation of Inflammation by Sucrose Isomer, Turanose, in Raw 264.7 Cells. J. Cancer Prev. 2017, 22, 195. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.C.M.; Faria, M.A.; Melo, A.; Pinto, E.; Almeida, A.; Alves, R.; Cabrita, A.R.J.; Fonseca, A.J.M.; Ferreira, I.M.P.L.V.O. Effect of Skimmed Milk on Intestinal Tract: Prevention of Increased Reactive Oxygen Species and Nitric Oxide Formation. Int. Dairy J. 2021, 118, 105046. [Google Scholar] [CrossRef]
- Chun, S.H.; Lee, K.W. Immune-Enhancing Effects of β-Lactoglobulin Glycated with Lactose Following in Vitro Digestion on Cyclophosphamide-Induced Immunosuppressed Mice. J. Dairy Sci. 2022, 105, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, K.; Jiang, K.; Yuan, Z.; Xiao, M.; Wei, G.; Zheng, W.; Wang, X.; Huang, A. Proteomic Approach-Based Comparison of Metabolic Pathways and Functional Activities of Whey Proteins Derived from Guishan and Saanen Goat Milk. J. Dairy Sci. 2023, 106, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.K.; Eligar, S.M.; Prakruthi, M.; Jyothi Lakshmi, A. setty Comparative Assessment of Antioxidant and Immunomodulatory Properties of Skimmed Milk Protein Hydrolysates and Their Incorporation in Beverage Mix. J. Sci. Food Agric. 2022, 102, 6414–6422. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chun, S.H.; Oh, N.S.; Lee, J.Y.; Lee, K.W. Anti-Inflammatory Activities of Maillard Reaction Products from Whey Protein Isolate Fermented by Lactobacillus Gasseri 4M13 in Lipopolysaccharide-Stimulated RAW264.7 Cells. J. Dairy Sci. 2019, 102, 7707–7716. [Google Scholar] [CrossRef] [PubMed]
- Dharmisthaben, P.; Sakure, A.; Liu, Z.; Maurya, R.; Das, S.; Basaiawmoit, B.; Kumari, R.; Bishnoi, M.; Kondepudi, K.K.; Gawai, K.M.; et al. Identification and Molecular Mechanisms of Novel Antioxidative Peptides from Fermented Camel Milk (Kachchi Breed, India) with Anti-Inflammatory Activity in Raw Macrophages Cell Lines. Int. J. Dairy Technol. 2023, 76, 111–125. [Google Scholar] [CrossRef]
- De Espindola, J.S.; Ferreira Taccóla, M.; da Silva, V.S.N.; dos Santos, L.D.; Rossini, B.C.; Mendonça, B.C.; Pacheco, M.T.B.; Galland, F. Digestion-Resistant Whey Peptides Promote Antioxidant Effect on Caco-2 Cells. Food Res. Int. 2023, 173, 113291. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Park, E.; Seo, B.-Y.; Yoon, Y.-C.; Lee, S.-M. Beneficial Effects of Hydrolysates of Whey Proteins in Spontaneously Hypertensive Rats. J. Food Nutr. Res. 2017, 5, 794–800. [Google Scholar] [CrossRef]
- Gandhi, K.; Devi, S.; Gautam, P.B.; Sharma, R.; Mann, B.; Ranvir, S.; Sao, K.; Pandey, V. Enhanced Bioavailability of Iron from Spray Dried Whey Protein Concentrate-Iron (WPC-Fe) Complex in Anaemic and Weaning Conditions. J. Funct. Foods 2019, 58, 275–281. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte Differentiation and Macrophage Polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, J.H.; Moon, S.H.; Ahn, D.U.; Paik, H.D. Ovalbumin Hydrolysates Inhibit Nitric Oxide Production in LPS-Induced 264.7 Macrophages. Food Sci. Anim. Resour. 2020, 40, 274. [Google Scholar] [CrossRef]
- Olejnik, A.; Kowalska, K.; Olkowicz, M.; Rychlik, J.; Juzwa, W.; Myszka, K.; Dembczyński, R.; Białas, W. Anti-Inflammatory Effects of Gastrointestinal Digested Sambucus Nigra L. Fruit Extract Analysed in Co-Cultured Intestinal Epithelial Cells and Lipopolysaccharide-Stimulated Macrophages. J. Funct. Foods 2015, 19, 649–660. [Google Scholar] [CrossRef]
- Montoya-Rodríguez, A.; de Mejía, E.G.; Dia, V.P.; Reyes-Moreno, C.; Milán-Carrillo, J. Extrusion Improved the Anti-Inflammatory Effect of Amaranth (Amaranthus hypochondriacus) Hydrolysates in LPS-Induced Human THP-1 Macrophage-like and Mouse RAW 264.7 Macrophages by Preventing Activation of NF-ΚB Signaling. Mol. Nutr. Food Res. 2014, 58, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, X.; Xiong, H.; Qiu, T.; Zhang, H.; Guo, F.; Jiang, L.; Sun, Y. Anti-Inflammatory Effects of Three Selenium-Enriched Brown Rice Protein Hydrolysates in LPS-Induced RAW264.7 Macrophages via NF-ΚB/MAPKs Signaling Pathways. J. Funct. Foods 2021, 76, 104320. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.; Yao, Y. Peptides from Extruded Lupin (Lupinus albus L.) Regulate Inflammatory Activity via the P38 MAPK Signal Transduction Pathway in RAW 264.7 Cells. J. Agric. Food Chem. 2020, 68, 11702–11709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zheng, W.; Yuan, Z.; Wang, X.; Huang, A. Anti-Inflammatory Effect of Two Novel Peptides Derived from Binglangjiang Buffalo Whey Protein in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Food Chem. 2023, 429, 136804. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, J.; Shi, H.; Yu, L.L. Isolation and Characterization of Anti-Inflammatory Peptides Derived from Whey Protein. J. Dairy Sci. 2016, 99, 6902–6912. [Google Scholar] [CrossRef]
- Shukla, P.; Sakure, A.; Basaiawmoit, B.; Khakhariya, R.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Liu, Z.; Padhi, S.; Rai, A.K.; et al. Molecular Binding Mechanism and Novel Antidiabetic and Anti-Hypertensive Bioactive Peptides from Fermented Camel Milk with Anti-Inflammatory Activity in Raw Macrophages Cell Lines. Amino Acids 2023, 55, 1621–1640. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Shin, S.H.; Suh, J.W.; Nimalaratne, C.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Casein Hydrolysate Produced Using High Hydrostatic Pressure Combined with Proteolytic Enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.M.; O’Callaghan, Y.C.; O’Keeffe, M.B.; FitzGerald, R.J.; O’Brien, N.M. Immunomodulatory Activity of 5 KDa Permeate Fractions of Casein Hydrolysates Generated Using a Range of Enzymes in Jurkat T Cells and RAW264.7 Macrophages. Int. Dairy J. 2019, 91, 9–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).