Research on the Corrosion Inhibition Effect of Xanthium sibiricum on Reinforced Steel and the Prediction of Reinforced Concrete Performance under a Stray Current and Chloride Environment
Abstract
:Featured Application
Abstract
1. Introduction
2. Experiments and Methods
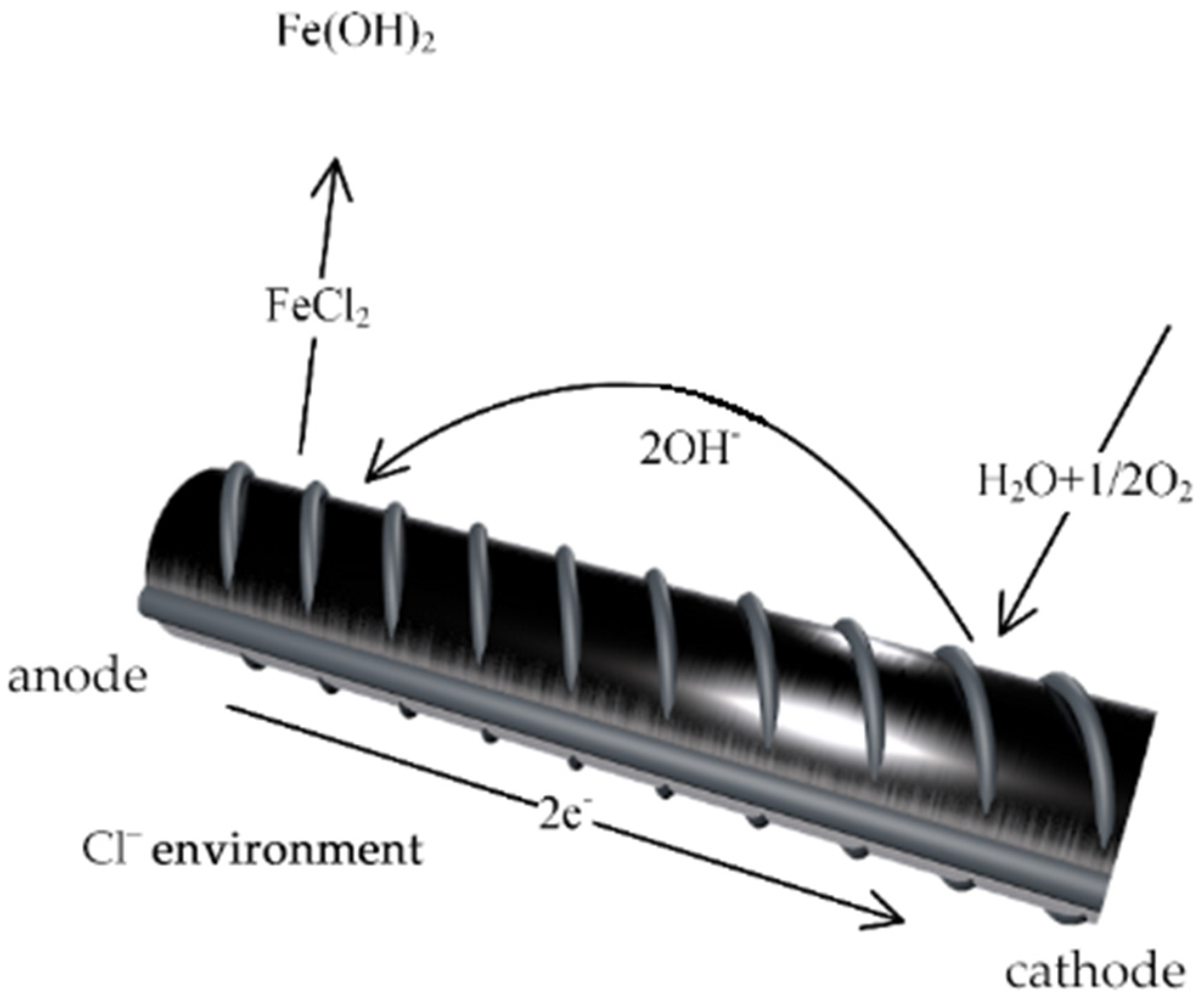
2.1. Verification Test of Xanthium sibiricum’s Corrosion Inhibition Effect on Reinforcing Steel
2.1.1. Experimental Materials
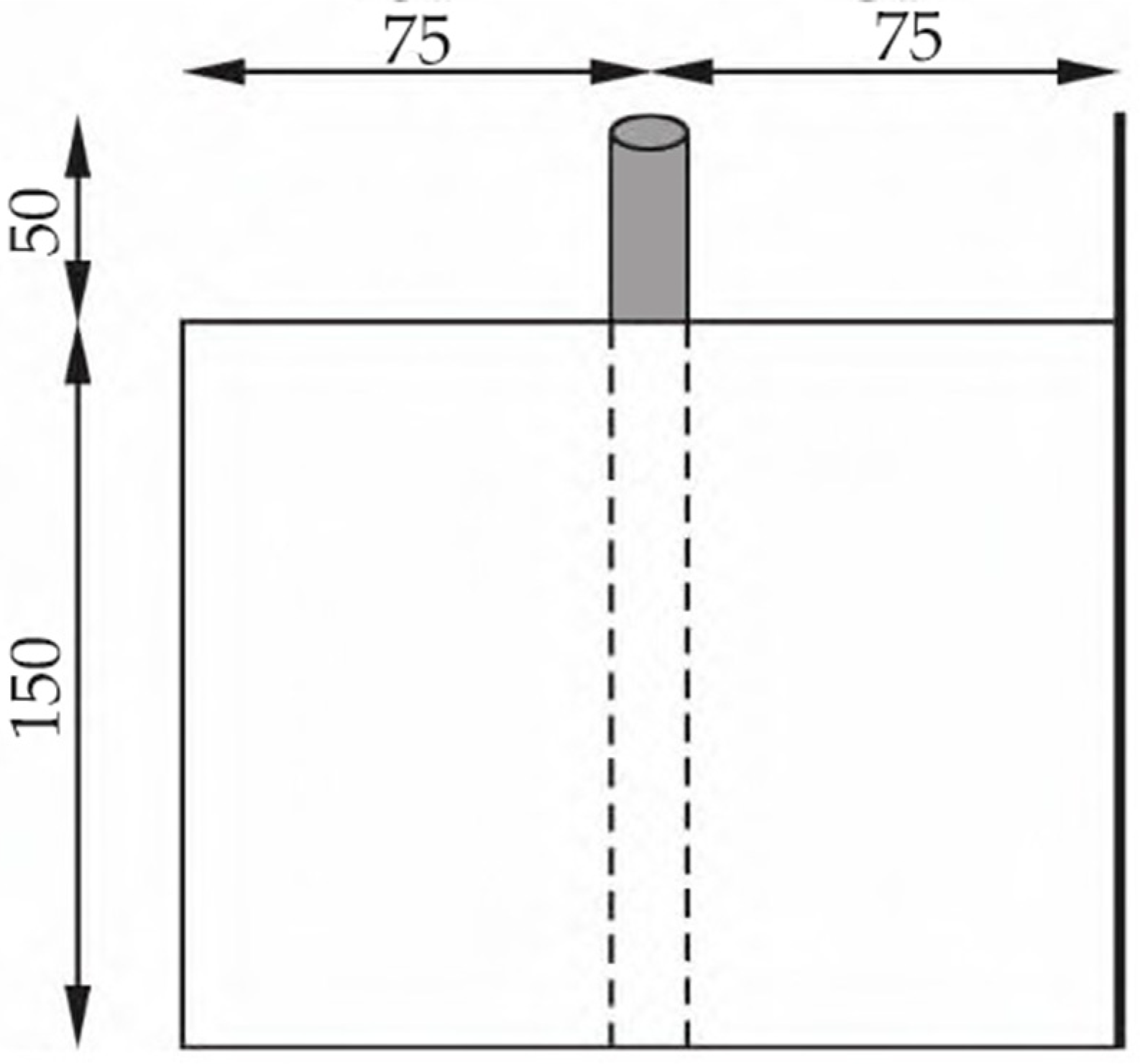
2.1.2. Preparation of Concrete Specimens
2.1.3. Natural Potential Method Testing
2.1.4. Reinforcing Steel Mass Loss Test and Corrosion Area Test
- Reinforcing Steel Mass Loss Method: After cutting the concrete specimens, the reinforcing steel samples were obtained and initially weighed, with their mass recorded as m1. The samples were then immersed in a 10% ammonium citrate solution to remove corrosion products. After this treatment, they were weighed again, and their mass was recorded as m2. The following formula was used to calculate the mass loss ratio of the reinforcing steel:where R represents the rebar weight loss rate (%), m1 represents the initial quality of steel bars (g), m2 represents the quality of steel bars after rust removal (g).
- 2.
- Corrosion Area Method: After cutting the concrete specimens, the reinforcing steel was extracted. Transparent sulfuric acid test paper was used to trace the corroded areas on the surface, as shown in Figure 3. Relevant analytical tools were employed to calculate the corroded area. The following formula was used to determine the corrosion area ratio of the reinforcing steel:where P represents the corrosion area rate of steel bars (%), s represents the corrosion area of steel bars (mm2), and s0 represents the total surface area of steel bars (mm2).
2.1.5. Linear Polarization Method Testing
2.2. Simulation of Chloride Ion Transport in Concrete
2.2.1. Finite Element Model and Calculation Parameters
2.2.2. Calculation Conditions
2.3. Field Tests in Actual Engineering Projects
2.3.1. Experimental Materials and Equipment
2.3.2. Specimen Preparation
3. Results and Discussions
3.1. Evaluation of the Corrosion Inhibition Effect of the Inhibitor on Chloride-Containing Reinforced Concrete
3.1.1. Natural Potential of Reinforcing Steel
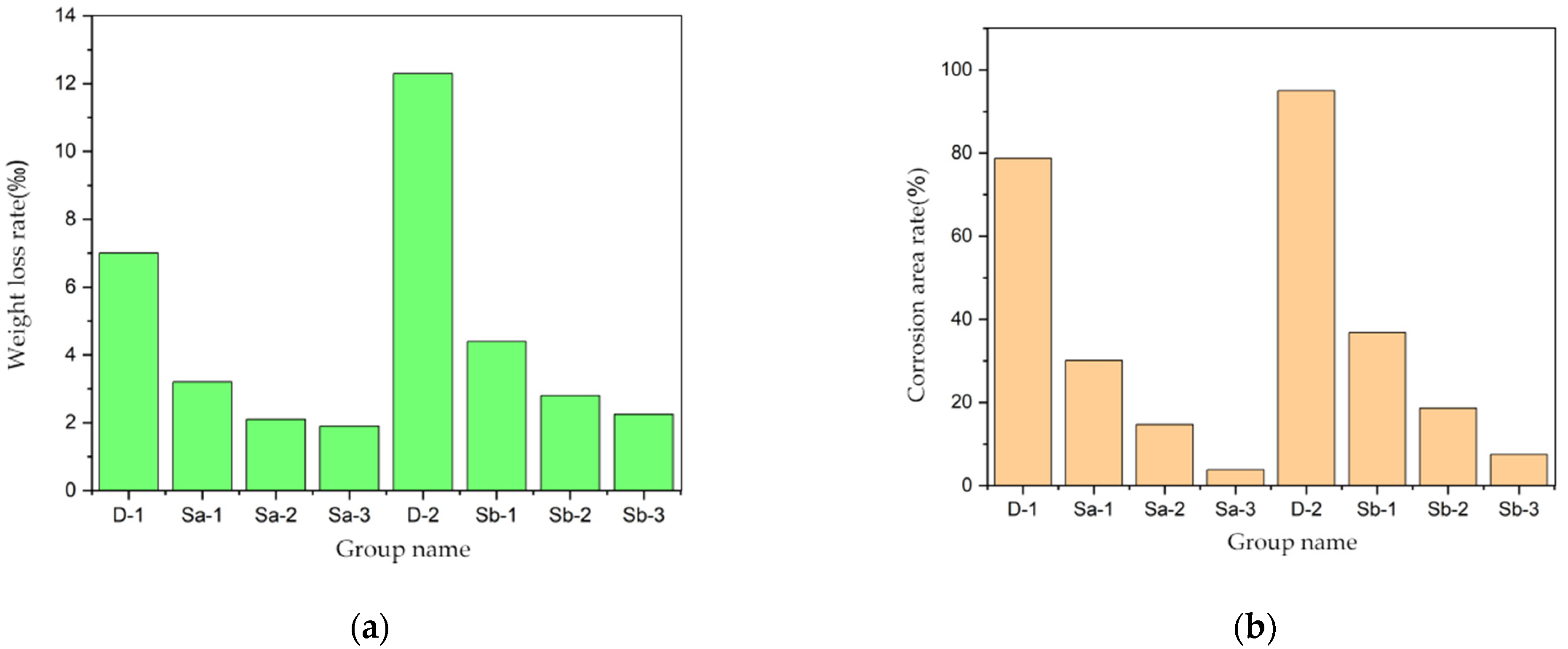
3.1.2. Mass Loss Rate and Corrosion Area Rate of Reinforcing Steel
3.1.3. Linear Polarization Method
3.2. Analysis of Finite Element Calculation Results
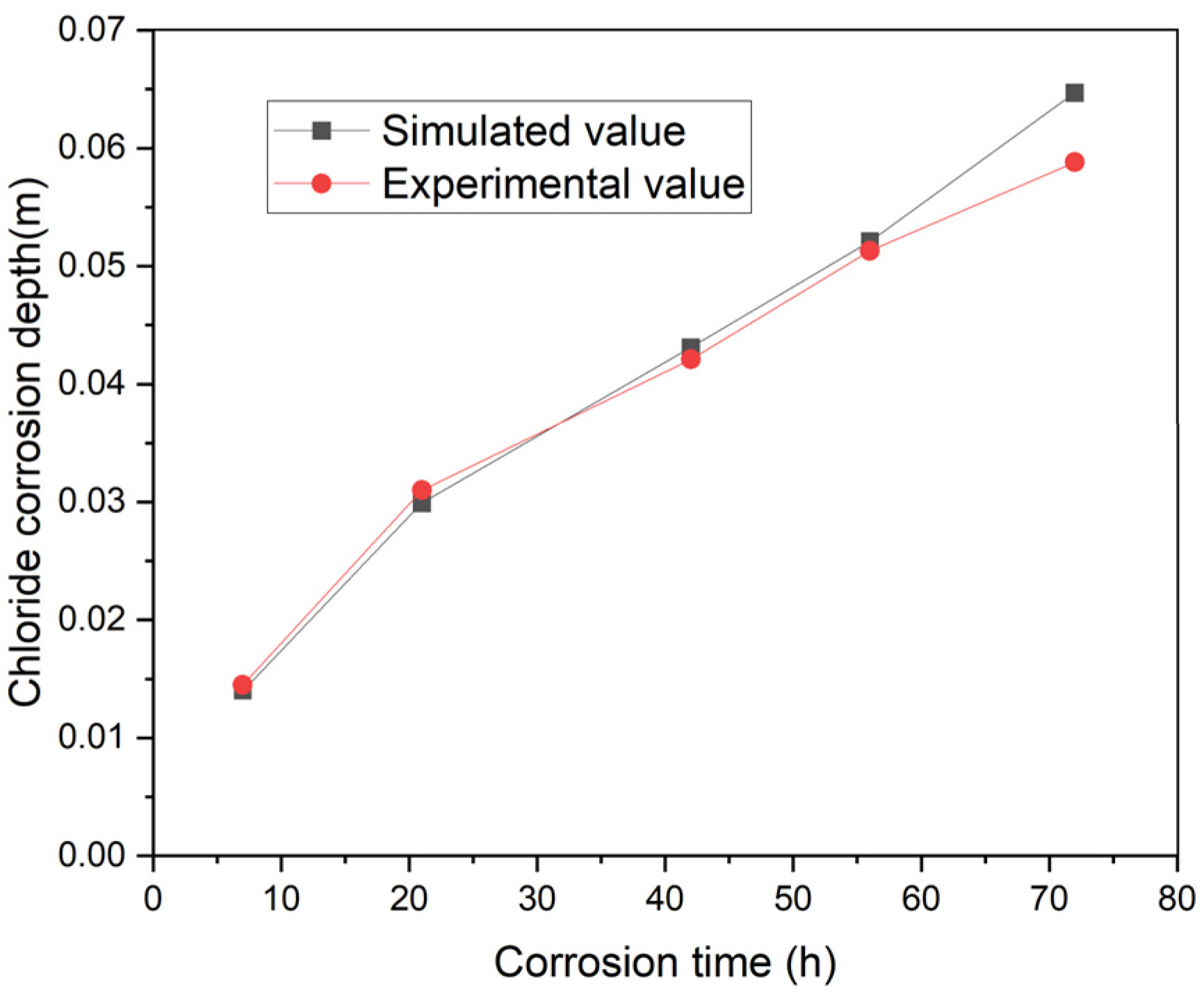
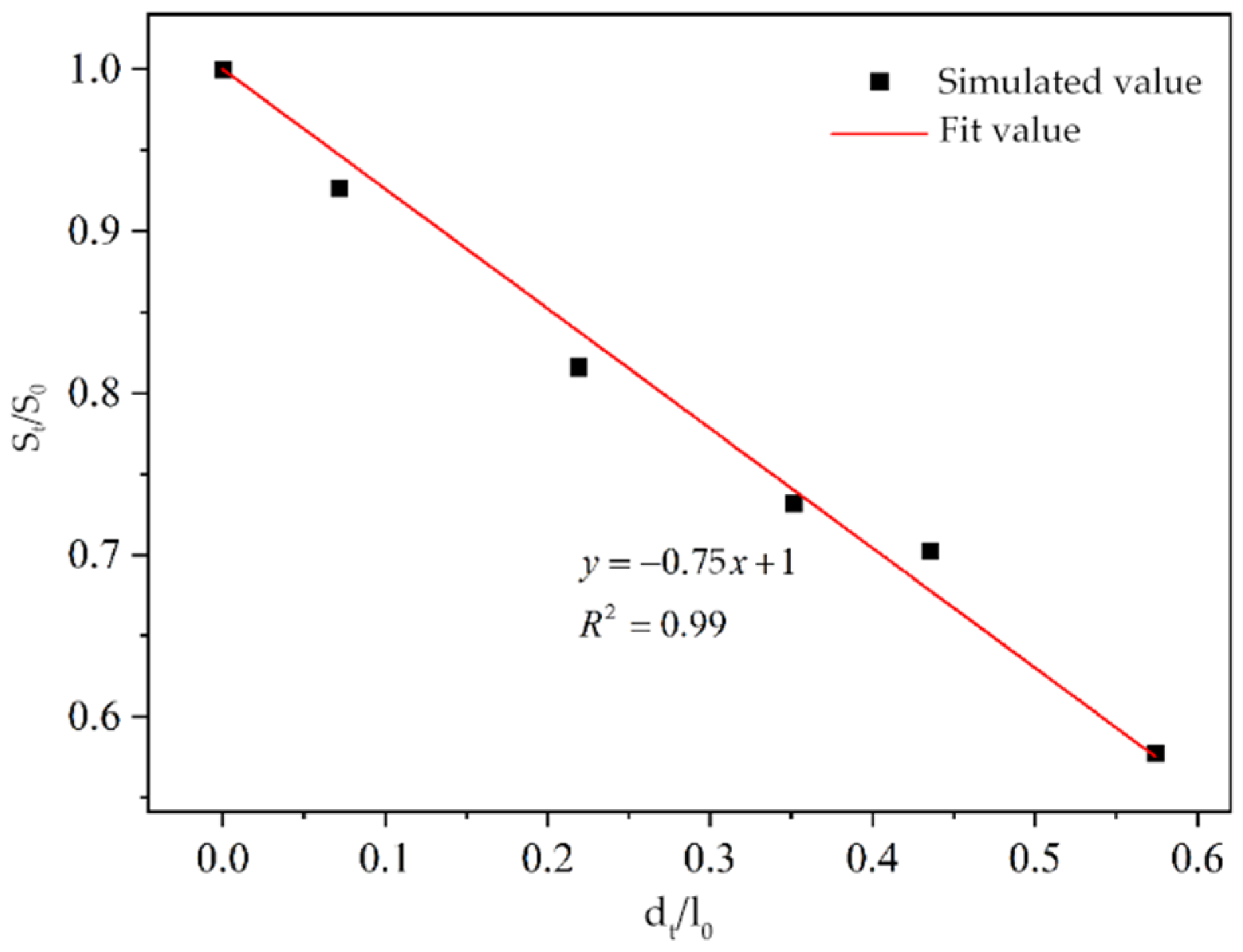
3.2.1. Model Validation
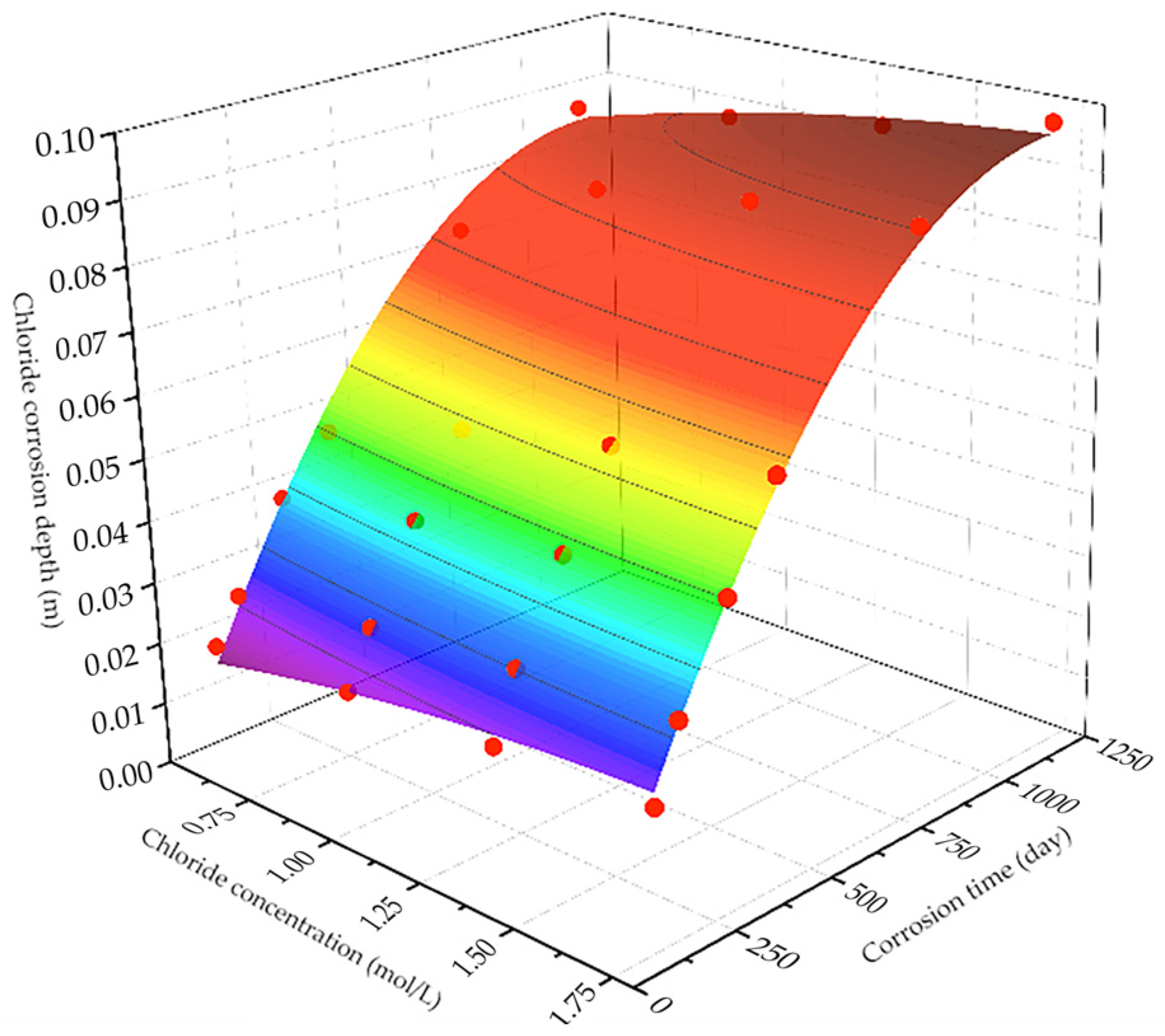
3.2.2. Prediction of Chloride Ion Penetration Depth in Concrete
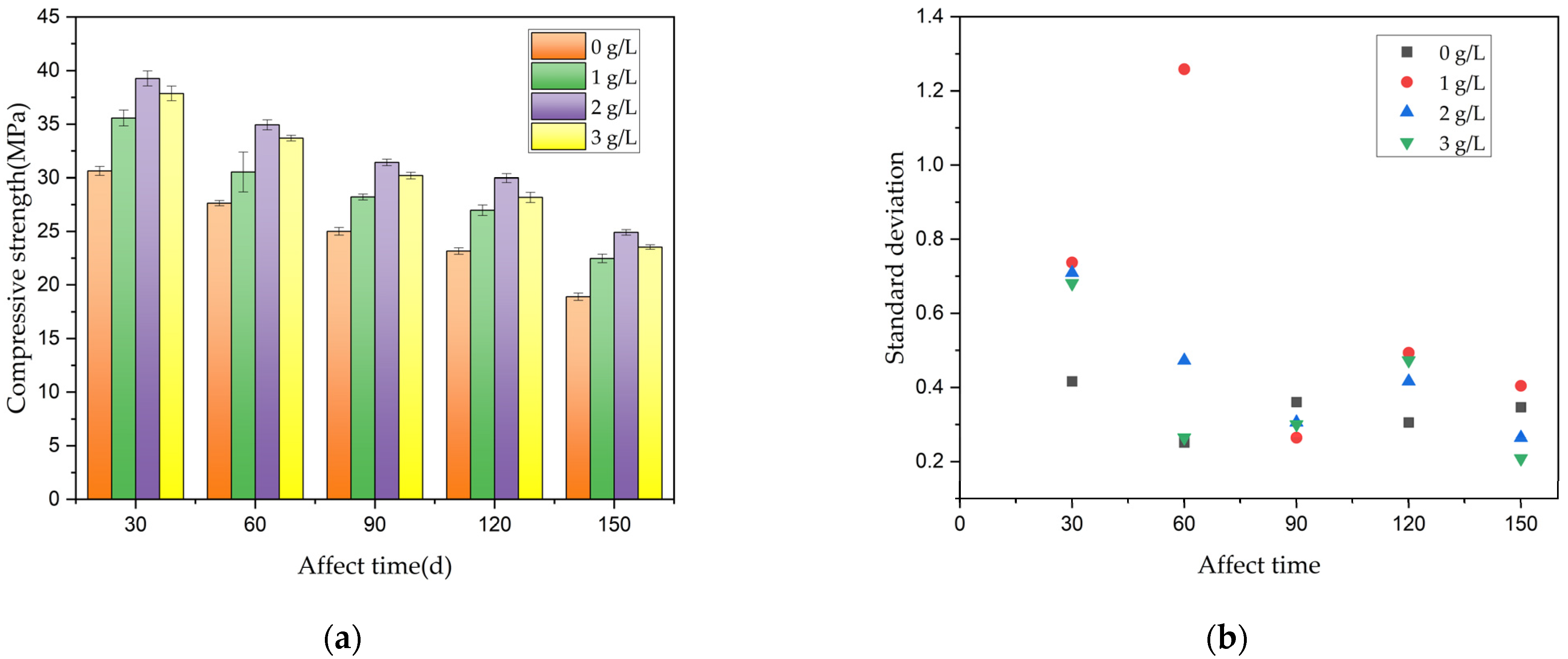
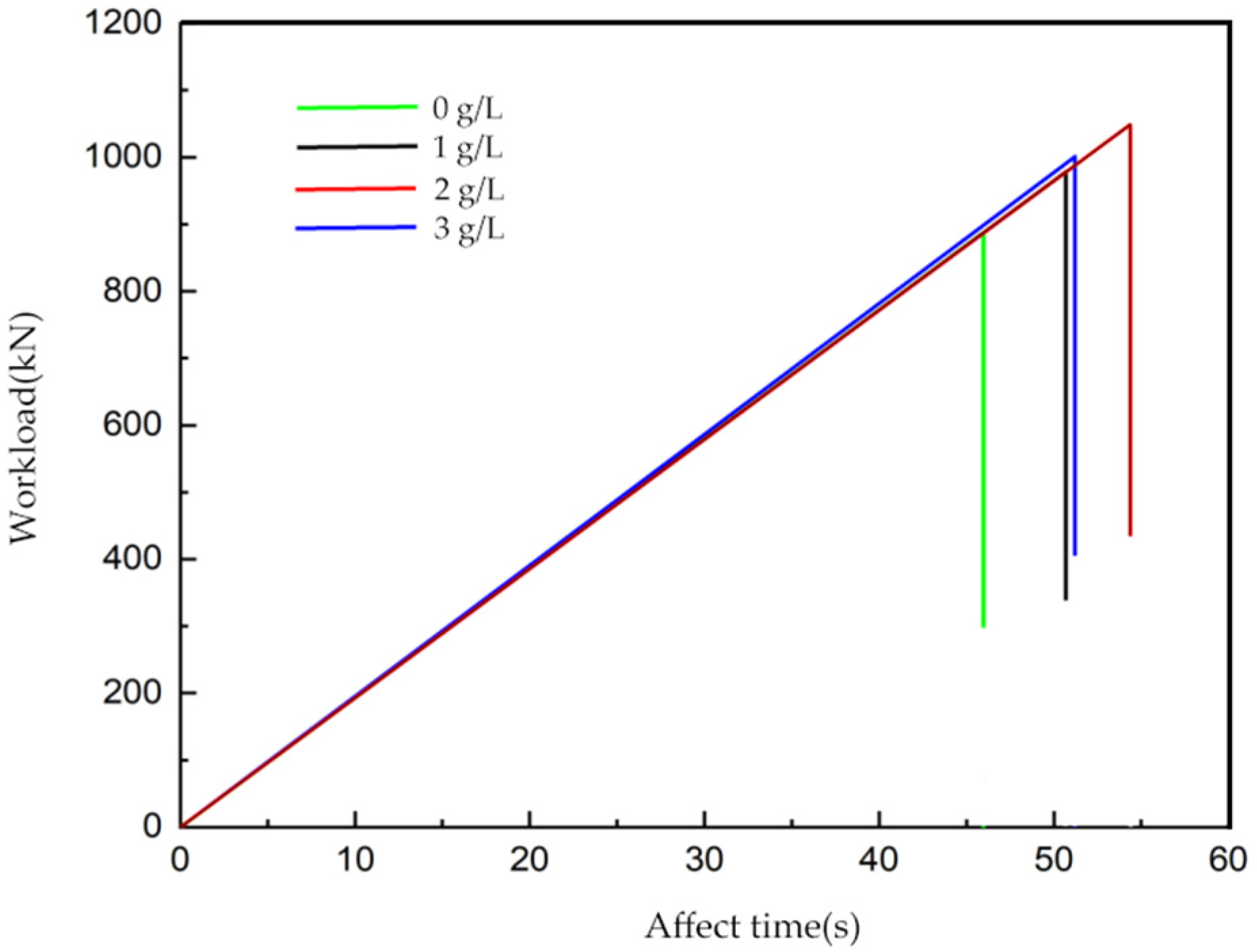
3.2.3. Prediction of Remaining Compressive Strength of Concrete
3.3. Analysis of Field Test Results
3.3.1. Optimal Dosage of Xanthium sibiricum Corrosion Inhibitor in Engineering Applications
3.3.2. Prediction of Remaining Compressive Strength of Reinforced Concrete Containing Corrosion Inhibitor
4. Conclusions
- (a)
- As a corrosion inhibitor, Xanthium sibiricum can increase the natural potential of reinforcing steel within concrete. It raises the potential from −350 mV (indicating inevitable corrosion) to below −200 mV (indicating resistance to corrosion). It effectively increases the self-corrosion potential of reinforcing steel and reduces the self-corrosion current. The best corrosion inhibition effect is achieved when the mass ratio of Xanthium sibiricum to chloride ions is 3:1. After three months of curing, the corrosion rate of reinforcing steel without the inhibitor is approximately 47.5% faster than the experimental group, with the steel loss rate being about 40% more severe. Additionally, an increased chloride content in concrete affects the efficacy of the corrosion inhibitor.
- (b)
- As the corrosion time extends, the corrosion depth of reinforced concrete increases linearly while the compressive strength decreases linearly, indicating a negative correlation between corrosion depth and compressive strength. The two-dimensional multiphase ion transport model based on the finite element method can accurately simulate the chloride ion transport process under the coupled effects of stray currents and a brine environment. Based on this, a quantitative relationship between corrosion depth and corrosion time under different voltages and chloride ion concentrations was obtained, leading to the derivation of prediction formula (5) for the remaining compressive strength of reinforced concrete materials after a certain corrosion time under different voltages and chloride ion concentrations.
- (c)
- Combined with a pumping station project serving in a similar environment, the optimal dosage of the Xanthium sibiricum corrosion inhibitor in practical engineering was determined to be 2 g/L. Under this dosage, the strength of reinforced concrete specimens increased by about 31.1%. Prediction formula (6) was derived for the remaining compressive strength of reinforced concrete after a certain corrosion time under different voltages and chloride ion concentrations, following the addition of the corrosion inhibitor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.F.; Hu, C.; Chen, Z. Stochastic and time-dependent diffusion of chloride ion in concrete and its concentration distribution. J. Build. Mater. 2013, 16, 210–216. [Google Scholar]
- Liu, Q. Study on Green Corrosion Inhibition of Reinforced Concrete in Pump Stations under Salt Brine Environments. Master’s Thesis, Hohai University, Nanjing, China, 2024. [Google Scholar]
- Haiyan, Y.; Cuina, Q.; Lintong, H.; Qing, X. Research on expansion and stability of Friedel’s salt. Ferroelectrics 2022, 596, 39–55. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, L.; Zhu, Y.; Ai, F.; Li, H.; Li, Y.; Jiang, Z. The effect of immersion corrosion time on electrochemical corrosion behavior and the corrosion mechanism of EH47 ship steel in seawater. Metals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, W.; Liu, S.; Yuan, M.; Qin, X. Multi-ion erosion test and molecular dynamics simulation of carbon nanotube concrete under stray current and salt brine environment. Dev. Built Environ. 2024, 17, 100335. [Google Scholar] [CrossRef]
- Marichev, V.A. First experimental evaluation of partial charge transfer during anion adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 28–34. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Seman, A.A.; Masri, M.N.; Ter Teo, P. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Yatmaz, H.A.; Gokoglu, N. Effects of plant extract-sulphide combinations on melanosis inhibition and quality in shrimp (Aristeus antennatus). Int. J. Food Prop. 2016, 19, 359–370. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Lai, J.; Qiang, S. Inhibition effect and mechanism explanation of perilla seed extract as a green corrosion inhibitor on Q235 carbon steel. Materials 2022, 15, 5394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Yang, H.R.; Zhou, A.P. Experimental analysis of the rust inhibition performance of Spartina alterniflora extracts. Shandong Water Resour. 2019, 3, 20–21. (In Chinese) [Google Scholar]
- Bui, H.T.; Dang, T.D.; Le, H.T.; Hoang, T.T. Comparative study on corrosion inhibition of vietnam orange peel essential oil with urotropine and insight of corrosion inhibition mechanism for mild steel in hydrochloric solution. J. Electrochem. Sci. Technol. 2019, 10, 69–81. [Google Scholar]
- Pradipta, I.; Kong, D.; Tan, J.B.L. Natural organic antioxidants from green tea inhibit corrosion of steel reinforcing bars embedded in mortar. Constr. Build. Mater. 2019, 227, 117058. [Google Scholar] [CrossRef]
- Shen, D. Electrochemical impedance spectroscopy study on corrosion inhibitor for reinforced concrete. Int. J. Electrochem. Sci. 2017, 12, 4183–4192. [Google Scholar] [CrossRef]
- Bo, X.; Yan, Y.; Xu, T.; Zhong, A.J.; Wu, Z.J. A Comparative Study on the Chemical Composition Changes and Antioxidant Activity of Xanthium sibiricum after Stir-frying to a Yellow Color. Ginseng Res. 2024, 3, 46–48. [Google Scholar]
- Tsukahara, E. Estimation Method of Corroded Portion of Reinforcing Steel Bar by Natural Potential Measurement. Non-Destr. Test. Civ. Eng. 2000, 261, 671–678. [Google Scholar]
- Tada, Y.; Miura, T.; Nakamura, H. Detection method of corrosion area of rebar and corrosion induced internal crack by using electromagnetic wave radar. In Bridge Maintenance, Safety, Management, Life-Cycle Sustainability and Innovations; CRC Press: Boca Raton, FL, USA, 2021; pp. 1672–1679. [Google Scholar]
- Law, D.W.; Cairns, J.; Millard, S.G.; Bungey, J.H. Measurement of loss of steel from reinforcing bars in concrete using linear polarisation resistance measurements. Ndt E Int. 2004, 37, 381–388. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Zhu, Y.-L. Evaluation of inhibitors in an artificially synthesized oilfield production water. Corros. Sci. Protetion Technol. 2009, 20, 298–300. [Google Scholar]
- JGJ/T 192-2009; Technical Specifications for the Application of Reinforcement Corrosion Inhibitors. China Architecture and Building Press: Beijing, China, 2009. (In Chinese)
- Yang, P.; Sant, G.; Neithalath, N. A refined, self-consistent Poisson-Nernst-Planck (PNP) model for electrically induced transport of multiple ionic species through concrete. Cem. Concr. Compos. 2017, 82, 80–94. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Li, H.; Lai, J.; Qiang, S.; Luo, T. Multi-ion erosion experiment and corrosion mechanism verification of steel fiber–reinforced concrete under stray current. J. Mater. Civ. Eng. 2022, 34, 04022355. [Google Scholar] [CrossRef]
- Li, X. Study on Bending Performance of Reinforced Concrete Beams Damaged by HDC Reinforcement under Sulfate Environment. Master’s Thesis, Xi’an University of Technology, Xi’an, China, 2023. [Google Scholar]
- Huang, J.Y.; Hu, X.D.; Hong, D.H.; Li, Z.Q.; Liu, N. Effect of Electrochemical Injection of Corrosion Inhibitor into Rebar Deteriorated by Chlorides in Concrete. J. Build. Mater. 2011, 4, 546–549. [Google Scholar]
- Yang, J.Y.; Xia, B.H.; Li, R.J.; Yang, W.J.; Jiang, W.L. Experimental Study on Degradation of Compressive Strength of Concrete under Steel Corrosion Expansion. Chongqing Archit. 2022, 10, 38–41. [Google Scholar]
- Farahmand, R.; Sohrabi, B.; Ghaffarinejad, A.; Meymian, M.R.Z. Synergistic effect of molybdenum coating and SDS surfactant on corrosion inhibition of mild steel in presence of 3.5% NaCl. Corros. Sci. 2018, 136, 393–401. [Google Scholar] [CrossRef]
- Fei, F.L.; Hu, J.; Yu, Q.J.; Wei, J.X.; Nong, Y.B. The effect of a tailored electro-migrating corrosion inhibitor on the corrosion performance of chloride-contaminated reinforced concrete. Mater. Corros. 2015, 66, 1039–1050. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, L.; Wei, Q.; Huang, D.; Luo, M.; Tang, X. Drying Kinetics of a Single Biomass Particle Using Fick’s Second Law of Diffusion. Processes 2023, 11, 984. [Google Scholar] [CrossRef]










| Cement | Sand | Stone | Water | Admixtures | Sand Ratio (%) | Slump (cm) | Gas Content (%) |
|---|---|---|---|---|---|---|---|
| 300 | 767 | 1190 | 129 | 91 | 33 | 16 | 4 |
| Control Group | Experimental Group | ||||
|---|---|---|---|---|---|
| Group Name | Cl− (%) | Inhibitor (%) | Group Name | Cl− (%) | Inhibitor (%) |
| D-1 | 0.5 | 0 | Sa-1 | 0.5 | 0.5 |
| D-2 | 1.0 | 0 | Sa-2 | 0.5 | 1.0 |
| Sa-3 | 0.5 | 1.5 | |||
| Sb-1 | 1.0 | 1.0 | |||
| Sb-2 | 1.0 | 2.0 | |||
| Sb-3 | 1.0 | 3.0 | |||
| Potential Measurement Value (mV) | Corrosion Possibility |
|---|---|
| E > −200 | corrosion possibility is lower than 10% |
| −200 > E > −350 | uncertain |
| E < −350 | corrosion possibility is higher than 90% |
| Polarization Resistance (Ω·cm2) | Corrosion Current Density (μA/cm2) | Steel Bar Loss Rate (mm/year) | Corrosion Rate |
|---|---|---|---|
| 0.25~2.5 | 100~10 | 0.1~1 | very high |
| 2.5~25 | 10~1 | 0.01~0.1 | high |
| 25~250 | 1~0.1 | 0.001~0.01 | medium |
| >250 | <0.1 | <0.001 | hard to erode |
| Material | Diffusion Coefficient (DClα·m2/s) | Chloride Ion Boundary Concentration (C0,Cl·mol/m3) | |||
|---|---|---|---|---|---|
| cement mortar | 0.09 | 8.60 | 1.33 | ||
| aggregate | 0.01 | 16.10 | 1.33 |
| Group Name | Corrosion Current Density (μA/cm2) | Corrosion Potential (V) | βa (mV) | βc (mV) | Corrosion Rate (g/(m2·h)) | Steel Bar Loss Rate (mm/Year) |
|---|---|---|---|---|---|---|
| D-1 | 1.49 | −0.782 | 284.76 | −34.15 | 1.18 × 10−2 | 1.33 × 10−2 |
| Sa-1 | 0.91 | −0.749 | 200.88 | −38.51 | 0.95 × 10−2 | 0.97 × 10−2 |
| Sa-2 | 0.80 | −0.725 | 239.57 | −35.82 | 0.84 × 10−2 | 0.96 × 10−2 |
| Sa-3 | 0.56 | −0.721 | 214.76 | −40.70 | 0.80 × 10−2 | 0.95 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Yuan, M.; Zhang, J.; Qiang, S. Research on the Corrosion Inhibition Effect of Xanthium sibiricum on Reinforced Steel and the Prediction of Reinforced Concrete Performance under a Stray Current and Chloride Environment. Appl. Sci. 2024, 14, 6986. https://doi.org/10.3390/app14166986
Liu Q, Yuan M, Zhang J, Qiang S. Research on the Corrosion Inhibition Effect of Xanthium sibiricum on Reinforced Steel and the Prediction of Reinforced Concrete Performance under a Stray Current and Chloride Environment. Applied Sciences. 2024; 14(16):6986. https://doi.org/10.3390/app14166986
Chicago/Turabian StyleLiu, Qi, Min Yuan, Jiaming Zhang, and Sheng Qiang. 2024. "Research on the Corrosion Inhibition Effect of Xanthium sibiricum on Reinforced Steel and the Prediction of Reinforced Concrete Performance under a Stray Current and Chloride Environment" Applied Sciences 14, no. 16: 6986. https://doi.org/10.3390/app14166986
APA StyleLiu, Q., Yuan, M., Zhang, J., & Qiang, S. (2024). Research on the Corrosion Inhibition Effect of Xanthium sibiricum on Reinforced Steel and the Prediction of Reinforced Concrete Performance under a Stray Current and Chloride Environment. Applied Sciences, 14(16), 6986. https://doi.org/10.3390/app14166986








