Serum Concentrations of IGF-1R, ERK2, and EGFR and Their Clinical Significance in Patients with Neuroendocrine Tumors
Abstract
:1. Introduction
2. Materials and Methods
3. Characteristic of the Study Group
4. Statistical Analysis
5. Results
6. Discussion
7. Conclusions
8. Advantages of the Study
9. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basu, B.; Sirohi, B.; Corrie, P. Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin. Endocr. Relat. Cancer 2010, 17, R75–R90. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A.; Bednarczuk, T.; Bolanowski, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Update of the diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–454. [Google Scholar] [CrossRef] [PubMed]
- Adaway, J.E.; Dobson, R.; Walsh, J.; Cuthbertson, D.J.; Monaghan, P.J.; Trainer, P.J.; Valle, J.W.; Keevil, B.G. Serum and plasma 5-hydroxyindoleacetic acid as an alternative to 24-h urine 5-hydroxyindoleacetic acid measurement. Ann. Clin. Biochem. 2016, 53, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Tellez, M.R.; Mamikunian, G.; O’dorisio, T.M.; Vinik, A.I.; Woltering, E.A. A single fasting plasma 5-HIAA value correlates with 24-hour urinary 5-HIAA values and other biomarkers in midgut neuroendocrine tumors (NETs). Pancreas 2013, 42, 405–410. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, S.-J.; Jeon, N.; Lim, B.J. Clinicopathologic characteristics of neuroendocrine tumors with assessment by digital image analysis for Ki-67 index with a focus on the gastroenteropancreatic tract: A single-center study. Int. J. Clin. Exp. Pathol. 2023, 16, 225–234. [Google Scholar] [PubMed]
- Tsoli, M.; Koumarianou, A.; Angelousi, A.; Kaltsas, G. Established and novel circulating neuroendocrine tumor biomarkers for diagnostic, predictive and prognostic use. Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101785. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.; Meisel-Sharon, S.; Bruchim, I. Oncogenic Fusion Proteins Adopt the Insulin-Like Growth Factor Signaling Pathway. Mol. Cancer 2018, 17, 28. [Google Scholar] [CrossRef]
- Cevenini, A.; Orrù, S.; Mancini, A.; Alfieri, A.; Buono, P.; Imperlini, E. Molecular Signatures of the Insulin-Like Growth Factor 1-Mediated Epithelial-Mesenchymal Transition in Breast, Lung and Gastric Cancers. Int. J. Mol. Sci. 2018, 19, 2411. [Google Scholar] [CrossRef]
- Werner, H.; Sarfstein, R.; Bruchim, I. Investigational IGF1R inhibitors in early stage clinical trials for cancer therapy. Expert Opin. Investig. Drugs 2019, 28, 1101–1112. [Google Scholar] [CrossRef]
- Motylewska, E.; Braun, M.; Kazimierczuk, Z.; Ławnicka, H.; Stępień, H. IGF1R and MAPK15 Emerge as Potential Targets of Pentabromobenzylisothioureas in Lung Neuroendocrine Neoplasms. Pharmaceuticals 2020, 13, 354. [Google Scholar] [CrossRef]
- Lamberti, G.; Brighi, N.; Maggio, I.; Manuzzi, L.; Peterle, C.; Ambrosini, V.; Ricci, C.; Casadei, R.; Campana, D. The Role of mTOR in Neuroendocrine Tumors: Future Cornerstone of a Winning Strategy? Int. J. Mol. Sci. 2018, 19, 747. [Google Scholar] [CrossRef]
- Wan, X.; Harkavy, B.; Shen, N.; Grohar, P.; Helman, L. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Kim, J.; Jin, H.; Zhao, J.C.; Yang, Y.A.; Li, Y.; Yang, X.; Dong, X.; Yu, J. FOXA1 inhibits prostate cancer neuroendocrine differentiation. Oncogene 2017, 36, 4072–4080. [Google Scholar] [CrossRef]
- Xie, Y.; Ning, S.; Hu, J. Molecular mechanisms of neuroendocrine differentiation in prostate cancer progression. J. Cancer Res. Clin. Oncol. 2022, 148, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Uribe, M.L.; Marrocco, I.; Yarden, Y. EGFR in Cancer: Signaling Mechanisms, Drugs, and Acquired Resistance. Cancers 2021, 13, 2748. [Google Scholar] [CrossRef] [PubMed]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging functions of the EGFR in cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, L.; Chen, C.; Nie, J.; Jiao, B. Can EGFR be a therapeutic target in breast cancer? Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188789. [Google Scholar] [CrossRef]
- Schlessinger, J. Cell signalling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef]
- Zanini, S.; Renzi, S.; Giovinazzo, F.; Bermano, G. mTOR Pathway in Gastroenteropancreatic Neuroendocrine Tumor (GEP-NETs). Front. Endocrinol. 2020, 11, 562505. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Phan, A.; Gupta, S.; Rashid, A.; Yeung, S.-C.J.; Hess, K.; Chen, H.; Tarco, E.; Chen, H.; Wei, C.; et al. Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors. Endocr. Relat. Cancer 2015, 22, 431–441. [Google Scholar] [CrossRef]
- Jin, X.-F.; Spöttl, G.; Maurer, J.; Nölting, S.; Auernhammer, C.J. Inhibition of Wnt/β-Catenin Signaling in Neuroendocrine Tumors in vitro: Antitumoral Effects. Cancers 2020, 12, 345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hochwald, S.; Deng, S.; Zhu, Y.; Tan, C.; Zhong, Q.; Zhou, Y.; Zhao, H.; Huang, H. Evaluation of EGF, EGFR, and E-cadherin as potential biomarkers for gastrointestinal cancers. Front. Lab. Med. 2017, 1, 135–140. [Google Scholar] [CrossRef]
- Xiao, Z.; Xu, H.; Strosberg, J.R.; Lu, R.; Zhu, X.; Deng, S.; Ding, L.; Ni, Q.; Warshaw, A.L.; Yu, X.; et al. EGFR is a potential therapeutic target for highly glycosylated and aggressive pancreatic neuroendocrine neoplasms. Int. J. Cancer 2023, 153, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Liccardi, A.; Cannavale, G.; Minotta, R.; DI Iasi, G.; Colao, A. Recent advances and future challenges in the diagnosis of neuroendocrine neoplasms. Minerva Endocrinol. 2024, 49, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Passhak, M.; McNamara, M.G.; Hubner, R.A.; Ben-Aharon, I.; Valle, J.W. Choosing the best systemic treatment sequence for control of tumour growth in gastro-enteropancreatic neuroendocrine tumours (GEP-NETs): What is the recent evidence? Best. Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101836. [Google Scholar] [CrossRef]
- Dicitore, A.; Cantone, M.C. Targeting receptor tyrosine kinases in neuroendocrine neoplasm: What’s going on with lung carcinoids? Minerva Endocrinol. 2022, 47, 261–263. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Gonzalez-Angulo, A.M. Targeting the mTOR signaling network for cancer therapy. J. Clin. Oncol. 2009, 27, 2278–2287. [Google Scholar] [CrossRef]
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Akcakanat, A.; Chen, H.; Do, K.-A.; Sangai, T.; Adkins, F.; Gonzalez-Angulo, A.M.; Rashid, A.; Crosby, K.; Dong, M.; et al. PIK3CA/PTEN mutations and Akt activation as markers of sensitivity to allosteric mTOR inhibitors. Clin. Cancer Res. 2012, 18, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- von Wichert, G.; Jehle, P.M.; Hoeflich, A.; Koschnick, S.; Dralle, H.; Wolf, E.; Wiedenmann, B.; Boehm, B.O.; Adler, G.; Seufferlein, T. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000, 60, 4573–4581. [Google Scholar] [PubMed]
- Münzberg, C.; Höhn, K.; Krndija, D.; Maaß, U.; Bartsch, D.K.; Slater, E.P.; Oswald, F.; Walther, P.; Seufferlein, T.; von Wichert, G. IGF-1 drives chromogranin A secretion via activation of Arf1 in human neuroendocrine tumour cells. J. Cell. Mol. Med. 2015, 19, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Höpfner, M.; Baradari, V.; Huether, A.; Schöfl, C.; Scherübl, H. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr. Relat. Cancer 2006, 13, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Thimmaiah, K.N.; Easton, J.; Huang, S.; Veverka, K.A.; Germain, G.S.; Harwood, F.C.; Houghton, P.J. Insulin-like growth factor I-mediated protection from rapamycin-induced apoptosis is independent of Ras-Erk1-Erk2 and phosphatidylinositol 3’-kinase-Akt signaling pathways. Cancer Res. 2003, 63, 364–374. [Google Scholar]
- O’Reilly, K.E.; Rojo, F.; She, Q.-B.; Solit, D.; Mills, G.B.; Smith, D.; Lane, H.; Hofmann, F.; Hicklin, D.J.; Ludwig, D.L.; et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006, 6, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Lawnicka, H.; Motylewska, E.; Borkowska, M.; Kuzdak, K.; Siejka, A.; Swietoslawski, J.; Stepien, H.; Stepien, T. Elevated serum concentrations of IGF-1 and IGF-1R in patients with thyroid cancers. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2020, 164, 77–83. [Google Scholar] [CrossRef]
- Manzella, L.; Massimino, M.; Stella, S.; Tirrò, E.; Pennisi, M.S.; Martorana, F.; Motta, G.; Vitale, S.R.; Puma, A.; Romano, C.; et al. Activation of the IGF Axis in Thyroid Cancer: Implications for Tumorigenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 3258. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, X.; Wang, Y.; Sun, G.; Zhang, J.; Li, Z. Obesity and endocrine-related cancer: The important role of IGF-1. Front. Endocrinol. 2023, 14, 1093257. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, M.M.; Lodhia, K.A.; Aleksic, T.; Gao, S.; Protheroe, A.S.; Macaulay, V.M. IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene 2014, 33, 5262–5273. [Google Scholar] [CrossRef] [PubMed]
- Ramcharan, R.; Aleksic, T.; Kamdoum, W.P.; Gao, S.; Pfister, S.X.; Tanner, J.; Bridges, E.; Asher, R.; Watson, A.J.; Margison, G.P.; et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget 2015, 6, 39877–39890. [Google Scholar] [CrossRef] [PubMed]
- Lloret, M.; Lara, P.C.; Bordón, E.; Pinar, B.; Rey, A.; Falcón, O.; Molano, F.; Hernández, M.A. IGF-1R expression in localized cervical carcinoma patients treated by radiochemotherapy. Gynecol. Oncol. 2007, 106, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Dale, O.T.; Aleksic, T.; Shah, K.A.; Han, C.; Mehanna, H.; Rapozo, D.C.; Sheard, J.D.H.; Goodyear, P.; Upile, N.S.; Robinson, M.; et al. IGF-1R expression is associated with HPV-negative status and adverse survival in head and neck squamous cell cancer. Carcinogenesis 2015, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, H.; Xu, X.; Ye, M.; Cao, H.; Xu, L.; Hou, Y.; Tang, J.; Zhou, D.; Bai, Y.; et al. Insulin-like growth factor-1 receptor knockdown enhances radiosensitivity via the HIF-1α pathway and attenuates ATM/H2AX/53BP1 DNA repair activation in human lung squamous carcinoma cells. Oncol. Lett. 2018, 16, 1332–1340. [Google Scholar] [CrossRef]
- Ferté, C.; Loriot, Y.; Clémenson, C.; Commo, F.; Gombos, A.; Bibault, J.-E.; Fumagalli, I.; Hamama, S.; Auger, N.; Lahon, B.; et al. IGF-1R targeting increases the antitumor effects of DNA-damaging agents in SCLC model: An opportunity to increase the efficacy of standard therapy. Mol. Cancer Ther. 2013, 12, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Acosta, P.; Gamboa, O.; Sanchez-Gomez, M.; Cendales, R.; Diaz, G.D.; Romero, A.; Serra, J.B.; Conrado, Z.; Levy, A.; Chargari, C.; et al. IGF1R gene expression as a predictive marker of response to ionizing radiation for patients with locally advanced HPV16-positive cervical cancer. Anticancer Res. 2012, 32, 4319–4325. [Google Scholar]
- Valsecchi, M.E.; McDonald, M.; Brody, J.R.; Hyslop, T.; Freydin, B.; Yeo, C.J.; Solomides, C.; Peiper, S.C.; Witkiewicz, A.K. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer 2012, 118, 3484–3493. [Google Scholar] [CrossRef]
- Obr, A.E.; Kumar, S.; Chang, Y.-J.; Bulatowicz, J.J.; Barnes, B.J.; Birge, R.B.; Lazzarino, D.A.; Gallagher, E.; LeRoith, D.; Wood, T.L. Insulin-like growth factor receptor signaling in breast tumor epithelium protects cells from endoplasmic reticulum stress and regulates the tumor microenvironment. Breast Cancer Res. 2018, 20, 138. [Google Scholar] [CrossRef]
- Ekyalongo, R.C.; Yee, D. Revisiting the IGF-1R as a breast cancer target. npj Precis. Oncol. 2017, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Tocheny, C.E.; Shaw, L.M. The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target. Life 2022, 12, 1992. [Google Scholar] [CrossRef] [PubMed]
- Soni, U.K.; Jenny, L.; Hegde, R.S. IGF-1R targeting in cancer–does sub-cellular localization matter? J. Exp. Clin. Cancer Res. 2023, 42, 273. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.T.; Alexi, X.; Opdam, M.; Schuurman, K.; Voorwerk, L.; Sanders, J.; van der Noort, V.; Boven, E.; Zwart, W.; Linn, S.C. IGF-1R pathway activation as putative biomarker for linsitinib therapy to revert tamoxifen resistance in ER-positive breast cancer. Int. J. Cancer 2020, 146, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-T.; Lin, R.-J.; Wang, Y.-H.; Hung, T.-H.; Huang, Y.; Yu, J.; Yu, J.-C.; Yu, A.L. The interplay between IGF-1R signaling and Hippo-YAP in breast cancer stem cells. Cell Commun. Signal. 2023, 21, 81. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, C.; Formentini, I.; Bondanza, A.; Bonini, C.; Naldini, L.; Brambilla, R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J. Biol. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Sig. Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Lefloch, R.; Pouysségur, J.; Lenormand, P. Single and combined silencing of ERK1 and ERK2 reveals their positive contribution to growth signaling depending on their expression levels. Mol. Cell. Biol. 2008, 28, 511–527. [Google Scholar] [CrossRef]
- Karhoff, D.; Sauer, S.; Schrader, J.; Arnold, R.; Fendrich, V.; Bartsch, D.K.; Hörsch, D. Rap1/B-Raf signaling is activated in neuroendocrine tumors of the digestive tract and Raf kinase inhibition constitutes a putative therapeutic target. Neuroendocrinology 2007, 85, 45–53. [Google Scholar] [CrossRef]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Li, Y.; Dillon, T.J.; Stork, P.J. Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP. J. Biol. Chem. 2017, 292, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Buzzelli, J.N.; Chalinor, H.V.; Pavlic, D.I.; Sutton, P.; Menheniott, T.R.; Giraud, A.S.; Judd, L.M. Decoding the Molecular and Mutational Ambiguities of Gastroenteropancreatic Neuroendocrine Neoplasm Pathobiology. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 131–153. [Google Scholar] [CrossRef]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Freudlsperger, C.; Burnett, J.R.; Friedman, J.A.; Kannabiran, V.R.; Chen, Z.; Van Waes, C. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas: Attractive targets for molecular-oriented therapy. Expert. Opin. Ther. Targets 2011, 15, 63–74. [Google Scholar] [CrossRef]
- Gan, Y.; Shi, C.; Inge, L.; Hibner, M.; Balducci, J.; Huang, Y. Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 2010, 29, 4947–4958. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, S.; Zhao, W.; Qin, S.; Chu, Q.; Wu, K. EGFR-TKIs resistance via EGFR-independent signaling pathways. Mol. Cancer 2018, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Agelaki, S.; Kalykaki, A.; Stournaras, C.; Mavroudis, D.; Georgoulias, V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008, 10, R80. [Google Scholar] [CrossRef]
- Novoplansky, O.; Shnerb, A.B.; Marripati, D.; Jagadeeshan, S.; Abu Shareb, R.; Conde-López, C.; Zorea, J.; Prasad, M.; Ben Lulu, T.; Yegodayev, K.M.; et al. Activation of the EGFR/PI3K/AKT pathway limits the efficacy of trametinib treatment in head and neck cancer. Mol. Oncol. 2023, 17, 2618–2636. [Google Scholar] [CrossRef]
- Toffoli, L.; Ditsiou, A.; Gagliano, T. Exploring Emerging Therapeutic Targets and Opportunities in Neuroendocrine Tumors: Updates on Receptor Tyrosine Kinases. Receptors 2024, 3, 145–154. [Google Scholar] [CrossRef]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. Epidermal growth factor receptor expression and activation in neuroendocrine tumours. J. Neuroendocrinol. 2006, 18, 355–360. [Google Scholar] [CrossRef]
- McClellan, K.; Chen, E.Y.; Kardosh, A.; Lopez, C.D.; Del Rivero, J.; Mallak, N.; Rocha, F.G.; Koethe, Y.; Pommier, R.; Mittra, E.; et al. Therapy Resistant Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2022, 14, 4769. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Bilgin, E.; Karabulut, S.; Duranyildiz, D. Clinical significance of serum epidermal growth factor receptor (EGFR) concentrations in patients with breast cancer. Cytokine 2015, 71, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsson, K.S.; Agrawal, A.; Allen, C.; Hitch, A.; Ellis, I.O.; Chapman, C.; Cheung, K.L.; Robertson, J.F. Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients. Breast Cancer Res. 2007, 9, R75. [Google Scholar] [CrossRef] [PubMed]
- Kar, T.; Dugam, P.; Shivhare, S.; Shetty, S.R.; Choudhury, S.; Sen, D.; Deb, B.; Majumdar, S.; Debnath, S.; Das, A. Epidermal growth factor receptor inhibition potentiates chemotherapeutics-mediated sensitization of metastatic breast cancer stem cells. Cancer Rep. 2024, 7, e2049. [Google Scholar] [CrossRef] [PubMed]
- Kjær, I.M.; Olsen, D.A.; Brandslund, I.; Bechmann, T.; Jakobsen, E.H.; Bogh, S.B.; Madsen, J.S. Prognostic impact of serum levels of EGFR and EGFR ligands in early-stage breast cancer. Sci. Rep. 2020, 10, 16558. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Witzel, I.; Riethdorf, S.; Rack, B.; Janni, W.; Fasching, P.A.; Solomayer, E.F.; Aktas, B.; Kasimir-Bauer, S.; Pantel, K.; et al. Evaluation of serum epidermal growth factor receptor (EGFR) in correlation to circulating tumor cells in patients with metastatic breast cancer. Sci. Rep. 2017, 7, 17307. [Google Scholar] [CrossRef] [PubMed]
- Angelescu, R.; Burada, F.; Angelescu, C.; Gheonea, D.I.; Mixich, F.; Ioana, M.; Săftoiu, A. Expression of vascular endothelial growth factor and epidermal growth factor receptor in pancreatic ductal adenocarcinomas, neuroendocrine tumours and chronic pancreatitis. Endosc. Ultrasound. 2013, 2, 86–91. [Google Scholar] [CrossRef]
- Marcoux, N.; Gettinger, S.N.; O’kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Evangelou, G.; Vamvakaris, I.; Papafili, A.; Anagnostakis, M.; Peppa, M. Lung NETs and GEPNETs: One Cancer with Different Origins or Two Distinct Cancers? Cancers 2024, 16, 1177. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, Z.; Bai, C.; Yan, X.; Qin, R.; Meng, C.; Ying, H. Serum chromogranin A levels for the diagnosis and follow-up of well-differentiated non-functioning neuroendocrine tumors. Tumor Biol. 2016, 37, 2863–2869. [Google Scholar] [CrossRef]
- Chevallier, M.; Borgeaud, M.; Addeo, A.; Friedlaender, A. Oncogenic driver mutations in non-small cell lung cancer: Past, present and future. World J. Clin. Oncol. 2021, 12, 217–237. [Google Scholar] [CrossRef] [PubMed]
- O’leary, C.; Gasper, H.; Sahin, K.B.; Tang, M.; Kulasinghe, A.; Adams, M.N.; Richard, D.J.; O’byrne, K.J. Epidermal Growth Factor Receptor (EGFR)-Mutated Non-Small-Cell Lung Cancer (NSCLC). Pharmaceuticals 2020, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Gou, Q.; Gan, X.; Xie, Y. Novel therapeutic strategies for rare mutations in non-small cell lung cancer. Sci. Rep. 2024, 14, 10317. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Jang, T.W.; Choi, C.M.; Kim, M.-H.; Lee, S.Y.; Chang, Y.S.; Lee, K.Y.; Kim, S.J.; Yang, S.H.; Ryu, J.S.; et al. Final Report on Real-World Effectiveness of Sequential Afatinib and Osimertinib in EGFR-Positive Advanced Non-Small Cell Lung Cancer: Updated Analysis of the RESET Study. Cancer Res. Treat. 2023, 55, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.S.; Needham, S.R.; Galdadas, I.; Davis, B.M.; Roberts, S.K.; Man, R.C.H.; Zanetti-Domingues, L.C.; Clarke, D.T.; Fruhwirth, G.O.; Parker, P.J.; et al. Drug-resistant EGFR mutations promote lung cancer by stabilizing interfaces in ligand-free kinase-active EGFR oligomers. Nat. Commun. 2024, 15, 2130. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Basu, S.; Lall, S.P.; Ganti, A.K.; Batra, S.K.; Seshacharyulu, P. Targeting the EGFR signaling pathway in cancer therapy: What’s new in 2023? Expert Opin. Ther. Targets 2023, 27, 305–324. [Google Scholar] [CrossRef]
- Friedlaender, A.; Perol, M.; Banna, G.L.; Parikh, K.; Addeo, A. Oncogenic alterations in advanced NSCLC: A molecular super-highway. Biomark. Res. 2024, 12, 24. [Google Scholar] [CrossRef]
- Hernandez-Rienda, L.; Del Olmo-García, M.I.; Merino-Torres, J.F. Impact of Diabetes Mellitus in Patients with Pancreatic Neuro-Endocrine Tumors: Causes, Consequences, and Future Perspectives. Metabolites 2022, 12, 1103. [Google Scholar] [CrossRef]
- Gallo, M.; Ruggeri, R.M.; Muscogiuri, G.; Pizza, G.; Faggiano, A.; Colao, A. NIKE Group. Diabetes and pancreatic neuroendocrine tumours: Which interplays, if any? Cancer Treat. Rev. 2018, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Study Group Me (Q1–Q3) |
|---|---|
| Chromogranin A [µg/L] | 48.1 (29.7–82.2) |
| Serotonin [ng/mL] | 139.1 (91.2–259.4) |
| 5-hydroxyindole acetic acid (5-HIAA) [mg/24 h] | 3.1 (2.0–4.7) |
| Glucose [mg/dL] | 96.0 (88.6–106.5) |
| Total cholesterol (TCH) [mg/dL] | 209.9 (169.5–235.0) |
| Triglycerides (TG) [mg/dL] | 96.5 (75.5–124.5) |
| Classification | n (% of Patients) | ||
|---|---|---|---|
| Location of the primary tumor | BP-NET cases | Lung | 16 (20%) |
| GEP-NET cases | Pancreas | 24 (30%) | |
| Small intestine | 21 (26%) | ||
| Stomach | 8 (10%) | ||
| Rectum | 7 (9%) | ||
| Duodenum | 4 (5%) | ||
| Histological grade for GEP-NET cases | NET G1 | 40 (50%) | |
| NET G2 | 21 (26%) | ||
| NET G3 | 3 (4%) | ||
| Histological grade for BP-NET cases | Typical carcinoid (TC) | 10 (11%) | |
| Atypical (ATC) | 6 (8%) | ||
| Metastasis | Yes | 35 (44%) | |
| No | 45 (56%) | ||
| Liver metastasis | Yes | 20 (25%) | |
| No | 60 (75%) | ||
| Lymph node metastasis | Yes | 27 (34%) | |
| No | 53 (66%) | ||
| Bone metastasis | Yes | 7 (9%) | |
| No | 73 (91%) | ||
| Clinical stage | I | 36 (45%) | |
| II | 8 (10%) | ||
| III | 11 (14%) | ||
| IV | 25 (31%) | ||
| Ki-67 | <3% | 45 (56%) | |
| 3% to 20% | 31 (39%) | ||
| >21% | 4 (5%) | ||
| Control Group M ± SD | Study Group M ± SD | t | p | |
|---|---|---|---|---|
| Age [years] | 52.7 ± 11.5 | 56.2 ± 12.5 | 1.75 | 0.083 |
| BMI [kg/m2] | 26.3 ± 4.1 | 26.2 ± 5.3 | 0.87 | 0.385 |
| Variant | Control Group n (%) | Study Group n (%) | χ2 | p | |
|---|---|---|---|---|---|
| Sex | Male | 23 (37%) | 36 (45%) | 0.90 | 0.343 |
| Female | 39 (63%) | 44 (55%) | |||
| Diabetes | Yes | 2 (3%) | 13 (16%) | 6.27 | <0.05 |
| No | 60 (97%) | 67 (84%) | |||
| Hypertension | Yes | 17 (27%) | 30 (38%) | 1.60 | 0.205 |
| No | 45 (73%) | 50 (62%) | |||
| Smoking | Yes | 9 (14%) | 12 (15%) | 0.01 | 0.911 |
| No | 53 (86%) | 68 (85%) | |||
| Parameters | Control Group Me (Q1–Q3) | Study Group Me (Q1–Q3) | z | p |
|---|---|---|---|---|
| IGF-1R [pg/mL] | 22.9 (18.6–22.9) | 27.2 (22.9–27.2) | 1.05 | 0.289 |
| ERK2 [pg/mL] | 432.9 (312.0–632.9) | 430.4 (359.2–568.9) | 0.12 | 0.901 |
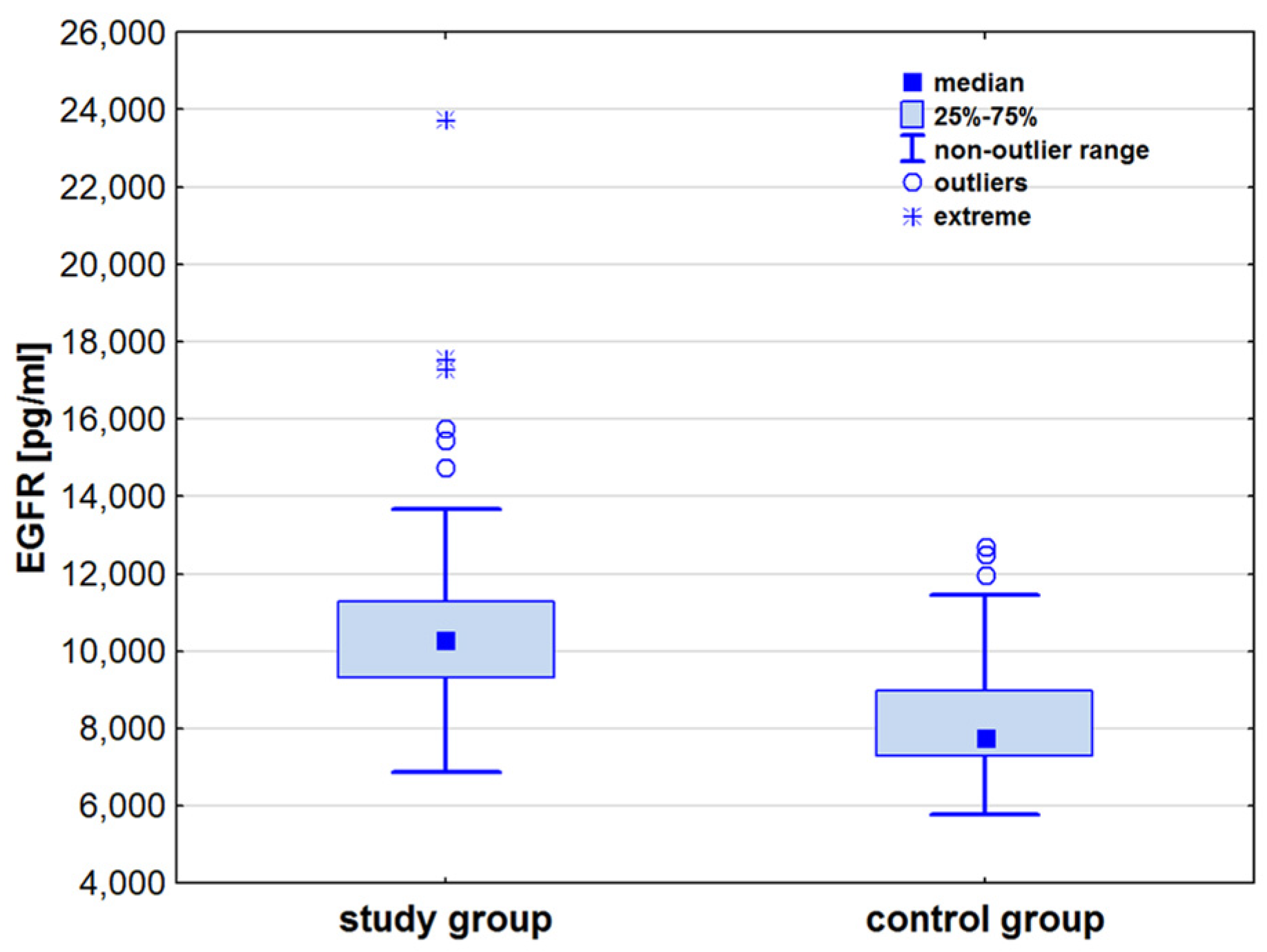
| EGFR [pg/mL] | 7771.1 (7296.5–8966.5) | 10,257.7 (9314.7–11,290.9) | 7.25 | <0.001 |
| Parameters | Control Group | Study Group | |||
|---|---|---|---|---|---|
| ρ | p | ρ | p | ||
| Age | IGF-1R [pg/mL] | 0.120 | 0.359 | 0.073 | 0.523 |
| ERK2 [pg/mL] | 0.178 | 0.165 | 0.028 | 0.807 | |
| EGFR [pg/mL] | 0.135 | 0.294 | 0.130 | 0.267 | |
| BMI | IGF-1R [pg/mL] | 0.247 | 0.057 | 0.143 | 0.207 |
| ERK2 [pg/mL] | −0.042 | 0.743 | 0.119 | 0.298 | |
| EGFR [pg/mL] | −0.289 | <0.05 | −0.673 | 0.503 | |
| Ki-67 | IGF-1R [pg/mL] | - | - | 0.055 | 0.633 |
| ERK2 [pg/mL] | - | - | 0.177 | 0.141 | |
| EGFR [pg/mL] | - | - | −0.013 | 0.910 | |
| Clinical stage | IGF-1R [pg/mL] | - | - | −0.012 | 0.921 |
| ERK2 [pg/mL] | - | - | −0.059 | 0.606 | |
| EGFR [pg/mL] | - | - | 0.204 | 0.079 | |
| Parameters | BP-NET Me (Q1–Q3) | GEP-NET Me (Q1–Q3) | z | p |
|---|---|---|---|---|
| IGF-1R [pg/mL] | 27.2 (22.9–27.2) | 27.2 (19.7–27.2) | 0.04 | 0.968 |
| ERK2 [pg/mL] | 461.8 (378.0–560.5) | 430.4 (356.5–595.1) | 0.03 | 0.980 |
| EGFR [pg/mL] | 10,087 (9413–10,852) | 10,408 (9314–11,291) | 0.27 | <0.001 |
| Parameters | Study Group | ||
|---|---|---|---|
| ρ | p | ||
| Chromogranin A [µg/L] | IGF-1R | −0.041 | 0.722 |
| ERK2 | 0.025 | 0.830 | |
| EGFR | 0.296 | <0.05 | |
| Serotonin [ng/mL] | IGF-1R | 0.067 | 0.568 |
| ERK2 | −0.145 | 0.214 | |
| EGFR | 0.222 | 0.061 | |
| 5-hydroxyindole acetic acid (5-HIAA) [mg/24 h] | IGF-1R | 0.092 | 0.422 |
| ERK2 | 0.121 | 0.293 | |
| EGFR | 0.150 | 0.200 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duszkiewicz, R.; Strzelczyk, J.; Chełmecka, E.; Strzelczyk, J.K. Serum Concentrations of IGF-1R, ERK2, and EGFR and Their Clinical Significance in Patients with Neuroendocrine Tumors. Appl. Sci. 2024, 14, 6998. https://doi.org/10.3390/app14166998
Duszkiewicz R, Strzelczyk J, Chełmecka E, Strzelczyk JK. Serum Concentrations of IGF-1R, ERK2, and EGFR and Their Clinical Significance in Patients with Neuroendocrine Tumors. Applied Sciences. 2024; 14(16):6998. https://doi.org/10.3390/app14166998
Chicago/Turabian StyleDuszkiewicz, Roksana, Janusz Strzelczyk, Elżbieta Chełmecka, and Joanna Katarzyna Strzelczyk. 2024. "Serum Concentrations of IGF-1R, ERK2, and EGFR and Their Clinical Significance in Patients with Neuroendocrine Tumors" Applied Sciences 14, no. 16: 6998. https://doi.org/10.3390/app14166998





