Inhibitory Effect of Dipeptides Containing Acidic Amino Acid Residue on Degranulation of RBL-2H3 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Antigen-Induced Degranulation Assay
2.4. Calcium Ionophore-Induced Degranulation Assay
2.5. Cell Viability Assay
2.6. Monitoring of Cytosolic Calcium Ion Concentration
2.7. Morphological Analysis of RBL-2H3 Cells
2.8. Immunoblot Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Inhibitory Effect of Acidic Amino Acid Residue-Containing Dipeptides on IgE-Mediated Degranulation of RBL-2H3 Cells
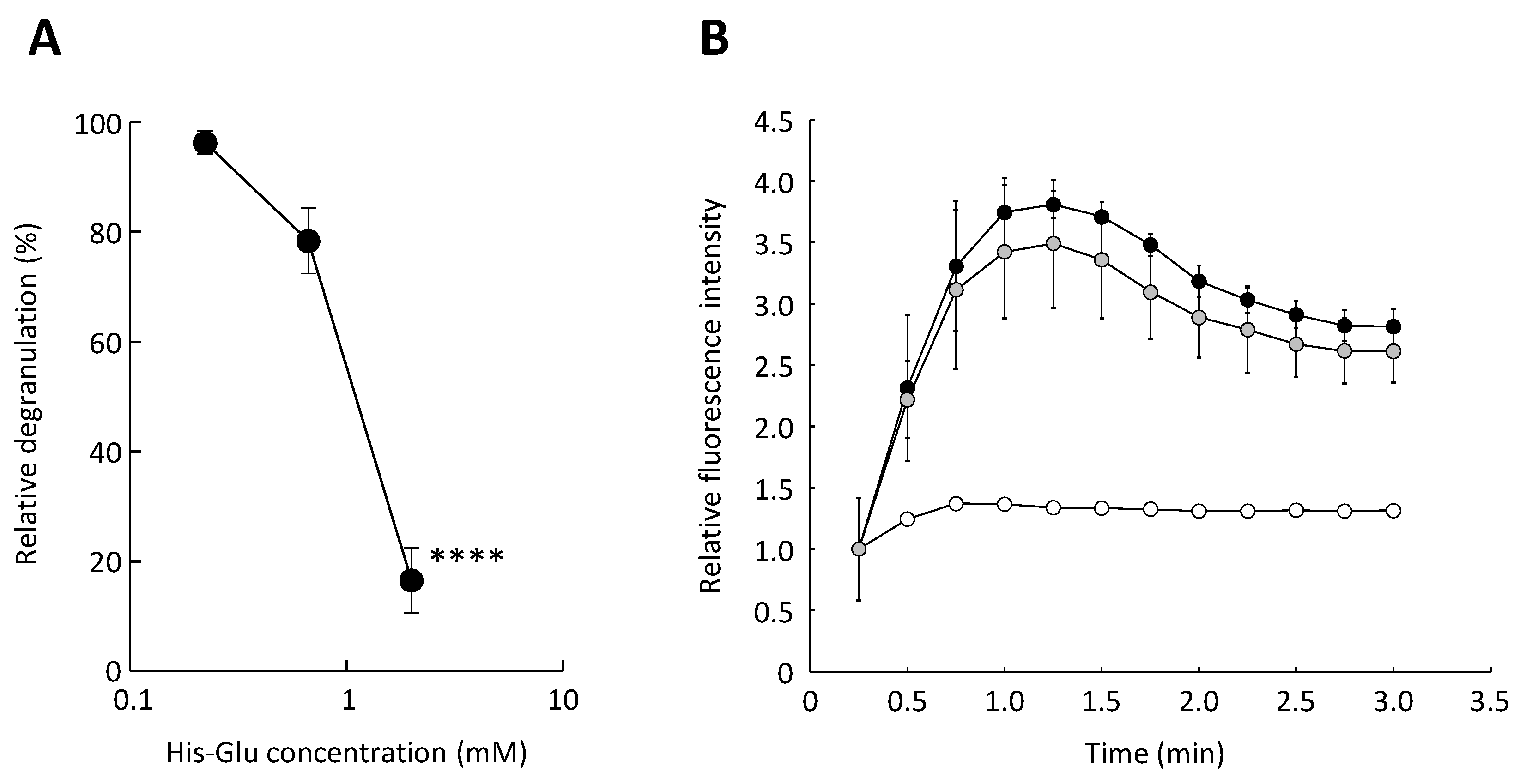
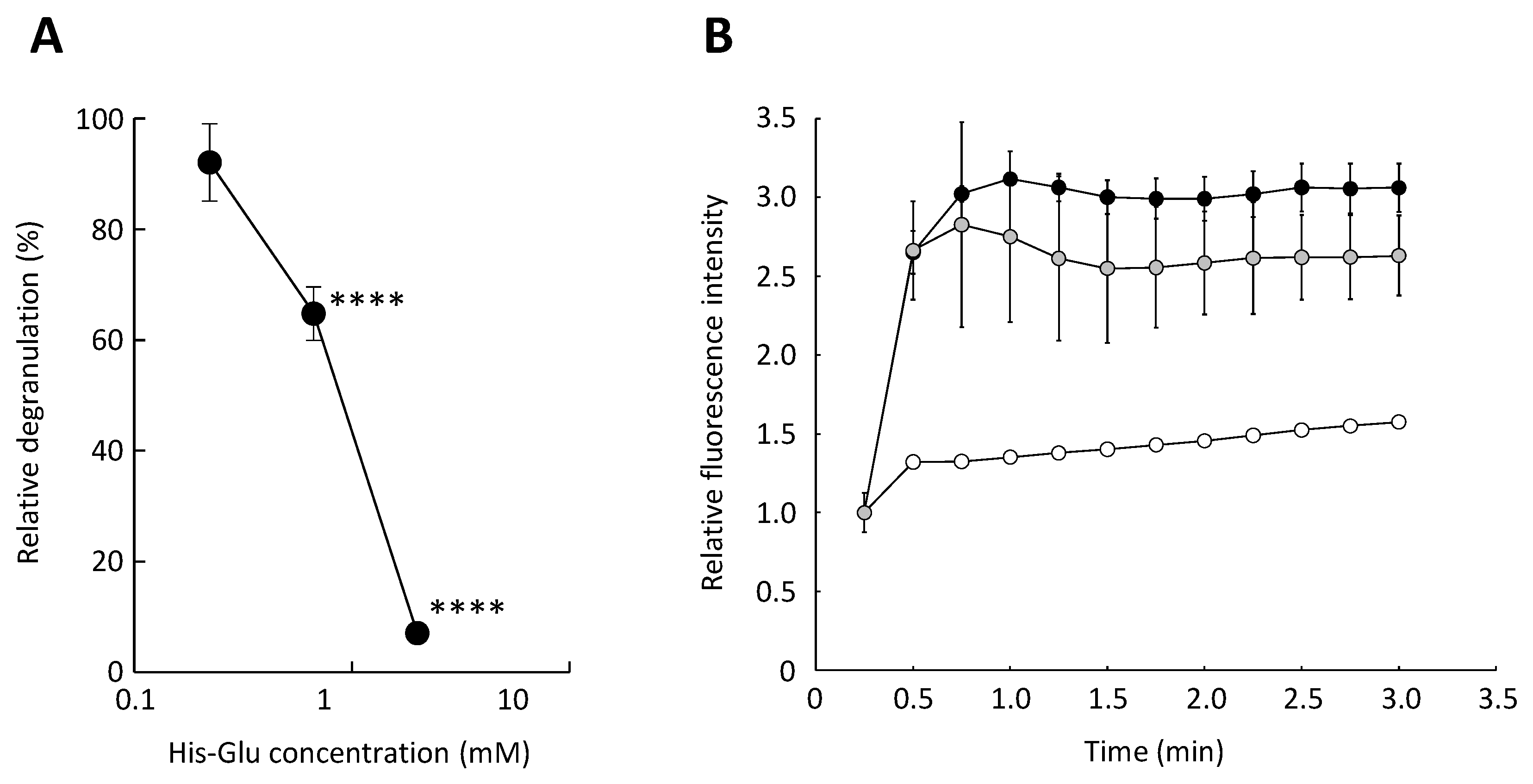
3.2. Effect of an L-Glutamic Acid-Containing Dipeptide His-Glu on the Antigen-Induced Elevation of Cytosolic Calcium Ion Concentration in RBL-2H3 Cells
3.3. Inhibitory Effect of His-Glu on Calcium Ionophore-Mediated Degranulation of RBL-2H3 Cells
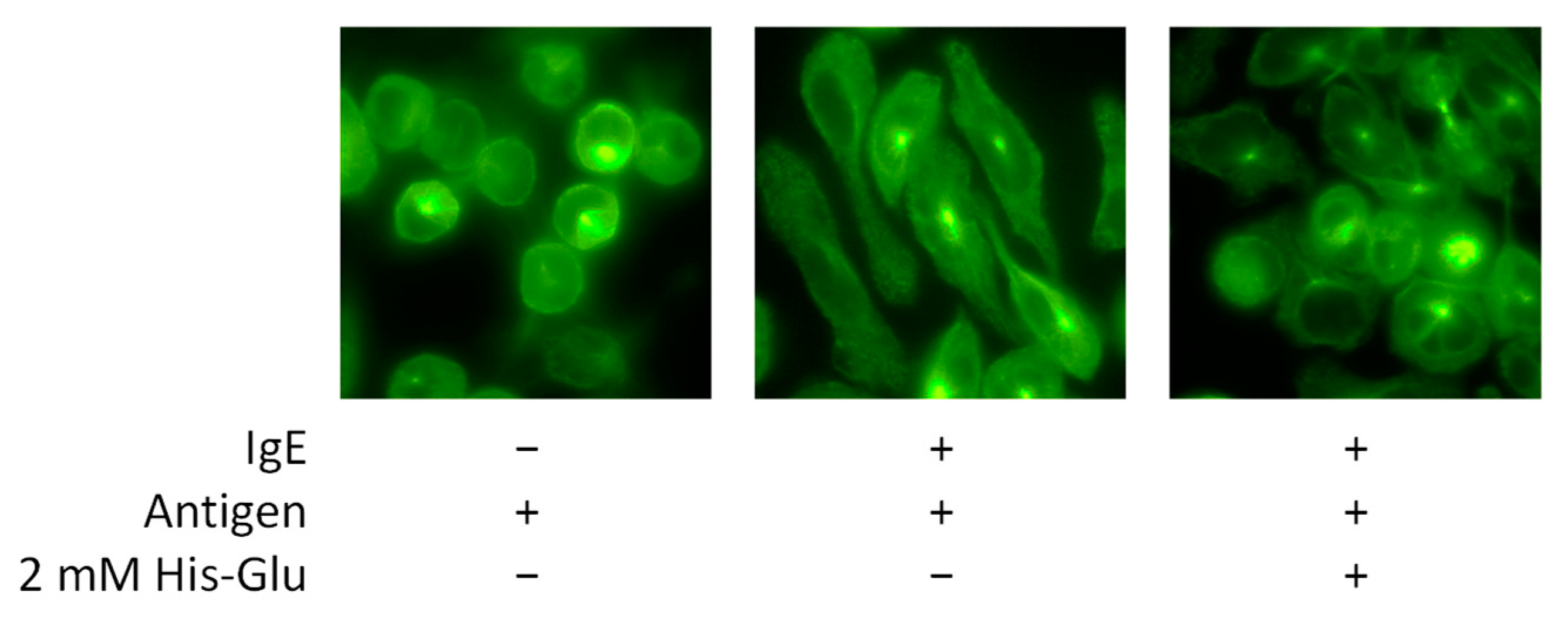
3.4. Effect of His-Glu on the Antigen-Induced Morphological Change of RBL-2H3 Cells
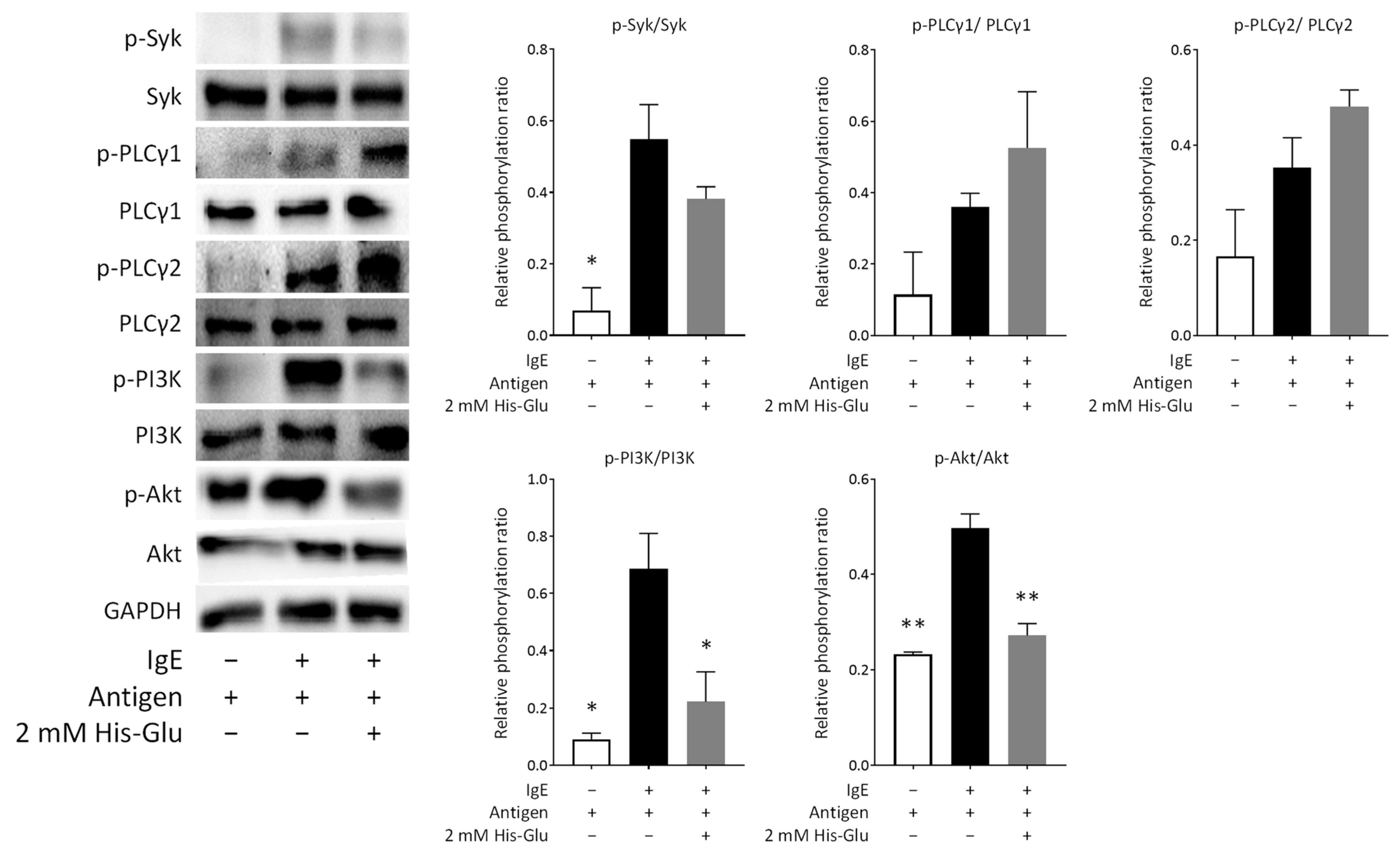
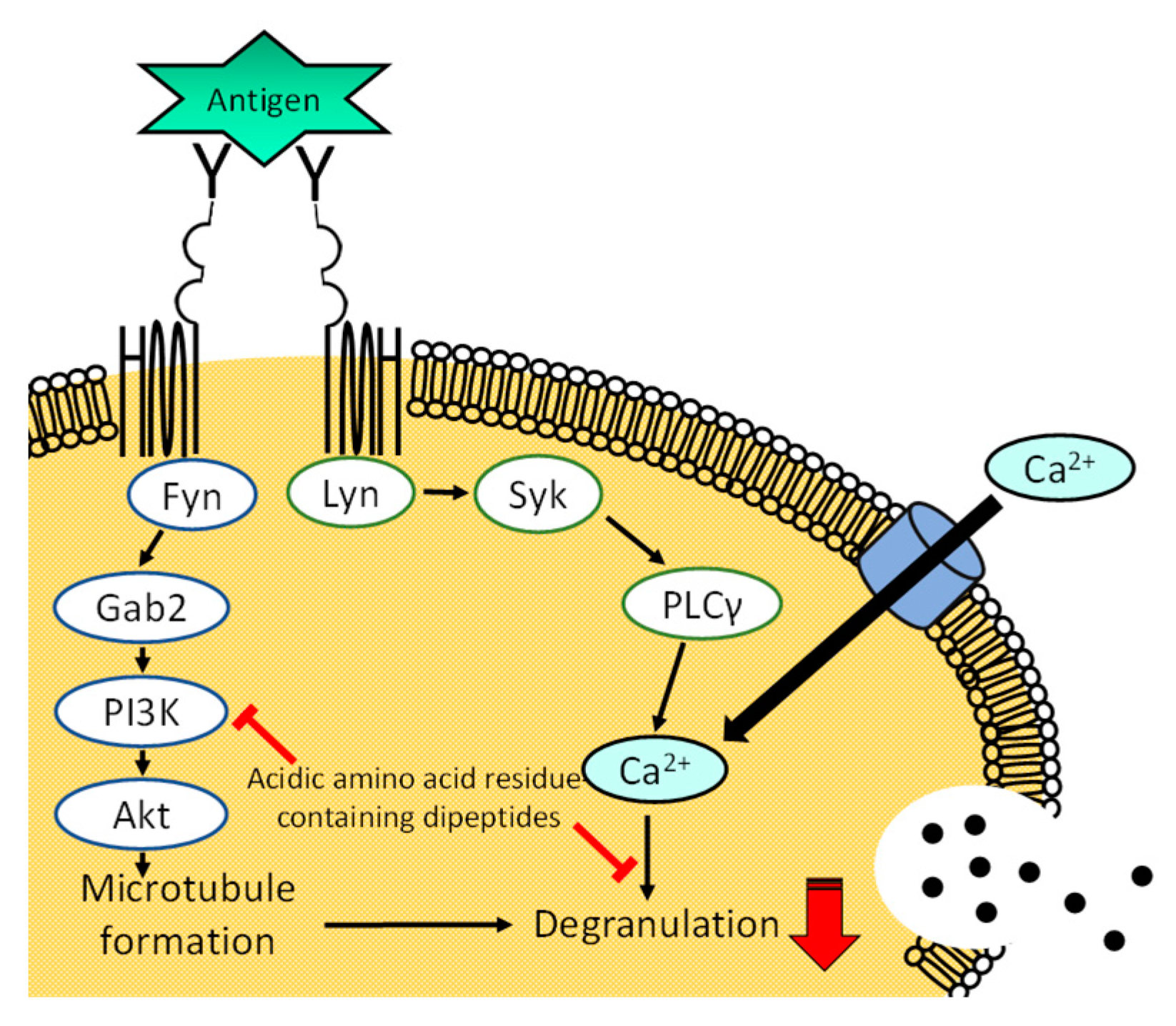
3.5. Effect of His-Glu on Intracellular Signaling Pathways in RBL-2H3 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, R.; Peng, C.; Chen, X.; Li, J. Pharmacogenomics for the efficacy and side effects of antihistamines. Exp. Dermatol. 2022, 31, 993–1004. [Google Scholar] [CrossRef]
- Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023, 15, 3683. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Wu, J. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, P.J.; Devika, V.; Aiswarya, L.S.; Jeevan, S.R.; Ramanunni, K.; Nair, P.B.; Sadanandan, S. Recent Advances in Bioactive Peptides as Functional Food for Health Promotions and Medicinal Applications. Protein Pept. Lett. 2023, 30, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Soy bioactive peptides and the gut microbiota modulation. Appl. Microbiol. Biotechnol. 2020, 104, 9009–9017. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food protein-derived bioactive peptides in management of type 2 diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Antony, P.; Vijayan, R. Bioactive Peptides as Potential Nutraceuticals for Diabetes Therapy: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 9059. [Google Scholar] [CrossRef]
- Mojica, L.; Luna-Vital, D.A.; González de Mejía, E. Characterization of peptides from common bean protein isolates and their potential to inhibit markers of type-2 diabetes, hypertension and oxidative stress. J. Sci. Food Agric. 2017, 97, 2401–2410. [Google Scholar] [CrossRef]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Awane, S.; Nishi, K.; Nakamoto, M.; Osajima, K.; Suemitsu, T.; Sugahara, T. Inhibitory effects of enzyme-treated dried sardine extract on IgE-mediated degranulation of RBL-2H3 cells and a murine model of Japanese cedar pollinosis. RSC Adv. 2016, 6, 85718–85726. [Google Scholar] [CrossRef]
- Nishi, K.; Kanayama, Y.; Kim, I.H.; Nakata, A.; Nishiwaki, H.; Sugahara, T. Docosahexaenoyl ethanolamide mitigates IgE-mediated allergic reactions by inhibiting mast cell degranulation and regulating allergy-related immune cells. Sci. Rep. 2019, 9, 16213. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Nishi, K.; Kadota, A.; Nishimoto, S.; Liu, M.C.; Sugahara, T. Nobiletin suppresses adipocyte differentiation of 3T3-L1 cells by an insulin and IBMX mixture induction. Biochim. Biophys. Acta 2012, 1820, 461–468. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, Z.; Ban, Z.; Reiter, R.J.; Ma, Q.; Liang, Z.; Yang, M.; Li, X.; Li, L. Bioactive peptides of plant origin: Distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022, 13, 3133–3158. [Google Scholar] [CrossRef] [PubMed]
- Kussmann, M. Prediction, Discovery, and Characterization of Plant- and Food-Derived Health-Beneficial Bioactive Peptides. Nutrients 2022, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Beaven, M.A. Regulators of Ca2+ signaling in mast cells: Potential targets for treatment of mast cell-related diseases? Adv. Exp. Med. Biol. 2011, 716, 62–90. [Google Scholar]
- Zhao, L.; Huang, S.; Cai, X.; Hong, J.; Wang, S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Tang, N.; Skibsted, L.H. Calcium Binding to Amino Acids and Small Glycine Peptides in Aqueous Solution: Toward Peptide Design for Better Calcium Bioavailability. J. Agric. Food Chem. 2016, 64, 4376–4389. [Google Scholar] [CrossRef]
- Johansen, T. Mechanism of histamine release from rat mast cells induced by the ionophore A23187: Effects of calcium and temperature. Br. J. Pharmacol. 1978, 63, 643–649. [Google Scholar] [CrossRef]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kalesnikoff, J.; Galli, S. New developments in mast cell biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Lazki-Hagenbach, P.; Klein, O.; Sagi-Eisenberg, R. The actin cytoskeleton and mast cell function. Curr. Opin. Immunol. 2021, 72, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Siraganian, R.P.; de Castro, R.O.; Barbu, E.A.; Zhang, J. Mast cell signaling: The role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010, 584, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
| Sample | Relative Degranulation-Inhibitory Effect (%) at 1 mM | Relative Cell Viability (%) at 1 mM | Sample | Relative Degranulation-Inhibitory Effect (%) at 1 mM | Relative Cell Viability (%) at 1 mM |
|---|---|---|---|---|---|
| Glutamic acid | −1.3 ± 2.0 | 95.0 ± 4.7 | Aspartic acid | 4.5 ± 1.6 | 97.9 ± 2.9 |
| Glu-Glu | 16.7 ± 1.0 | 106.2 ± 12.2 | Asp-Asp | 37.3 ± 1.0 | 99.4 ± 3.4 |
| Glu-Asp | 51.9 ± 3.9 | 93.7 ± 3.5 | Asp-Glu | 39.8 ± 2.0 | 93.1 ± 1.1 |
| Glu-Ala | 38.9 ± 3.3 | 106.6 ± 9.3 | Asp-Ala | 27.9 ± 1.5 | 100.1 ± 8.6 |
| Ala-Glu | 45.8 ± 2.5 | 97.4 ± 8.8 | Ala-Asp | 21.4 ± 1.5 | 105.8 ± 4.9 |
| Glu-Gly | 47.2 ± 4.3 | 86.8 ± 9.8 | Asp-Gly | 18.6 ± 0.9 | 101.1 ± 7.8 |
| Gly-Glu | 31.3 ± 3.1 | 92.2 ± 5.7 | Gly-Asp | 28.2 ± 0.8 | 109.4 ± 11.1 |
| Glu-His | 17.1 ± 1.1 | 93.7 ± 9.0 | Asp-His | 39.4 ± 5.1 | 98.3 ± 6.4 |
| His-Glu | 41.4 ± 0.5 | 106.1 ± 12.7 | His-Asp | 39.4 ± 4.4 | 93.2 ± 4.4 |
| Glu-Lys | 31.5 ± 4.3 | 105.8 ± 9.6 | Asp-Lys | 31.7 ± 2.3 | 116.5 ± 12.8 |
| Lys-Glu | 33.4 ± 2.3 | 90.8 ± 4.0 | Lys-Asp | 28.0 ± 0.8 | 115.1 ± 8.5 |
| Glu-Arg | 39.3 ± 2.4 | 96.8 ± 3.9 | Asp-Arg | 22.6 ± 0.2 | 104.2 ± 3.0 |
| Arg-Glu | 25.7 ± 1.5 | 93.6 ± 0.6 | Arg-Asp | 41.5 ± 1.7 | 111.5 ± 4.2 |
| Glu-Ser | 52.3 ± 2.4 | 94.0 ± 7.5 | Asp-Ser | 23.3 ± 1.5 | 95.6 ± 8.2 |
| Ser-Glu | 47.1 ± 5.1 | 97.0 ± 2.6 | Ser-Asp | 27.8 ± 1.5 | 96.4 ± 8.1 |
| Glu-Thr | 49.9 ± 1.1 | 97.8 ± 11.4 | Asp-Thr | 21.7 ± 1.5 | 94.0 ± 6.4 |
| Thr-Glu | 29.2 ± 0.9 | 102.5 ± 8.9 | Thr-Asp | 19.9 ± 0.9 | 107.9 ± 6.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, K.; Hirakawa, T.; Izumi, M.; Kageyama, N.; Yurue, S.; Ozaki, A.; Toga, Y.; Ishida, M.; Sugahara, T. Inhibitory Effect of Dipeptides Containing Acidic Amino Acid Residue on Degranulation of RBL-2H3 Cells. Appl. Sci. 2024, 14, 7048. https://doi.org/10.3390/app14167048
Nishi K, Hirakawa T, Izumi M, Kageyama N, Yurue S, Ozaki A, Toga Y, Ishida M, Sugahara T. Inhibitory Effect of Dipeptides Containing Acidic Amino Acid Residue on Degranulation of RBL-2H3 Cells. Applied Sciences. 2024; 14(16):7048. https://doi.org/10.3390/app14167048
Chicago/Turabian StyleNishi, Kosuke, Taiki Hirakawa, Mitsumasa Izumi, Naoki Kageyama, Senri Yurue, Akari Ozaki, Yuki Toga, Momoko Ishida, and Takuya Sugahara. 2024. "Inhibitory Effect of Dipeptides Containing Acidic Amino Acid Residue on Degranulation of RBL-2H3 Cells" Applied Sciences 14, no. 16: 7048. https://doi.org/10.3390/app14167048






