Application of High Hydrostatic Pressures and Refrigerated Storage on the Content of Resistant Starch in Selected Legume Seeds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
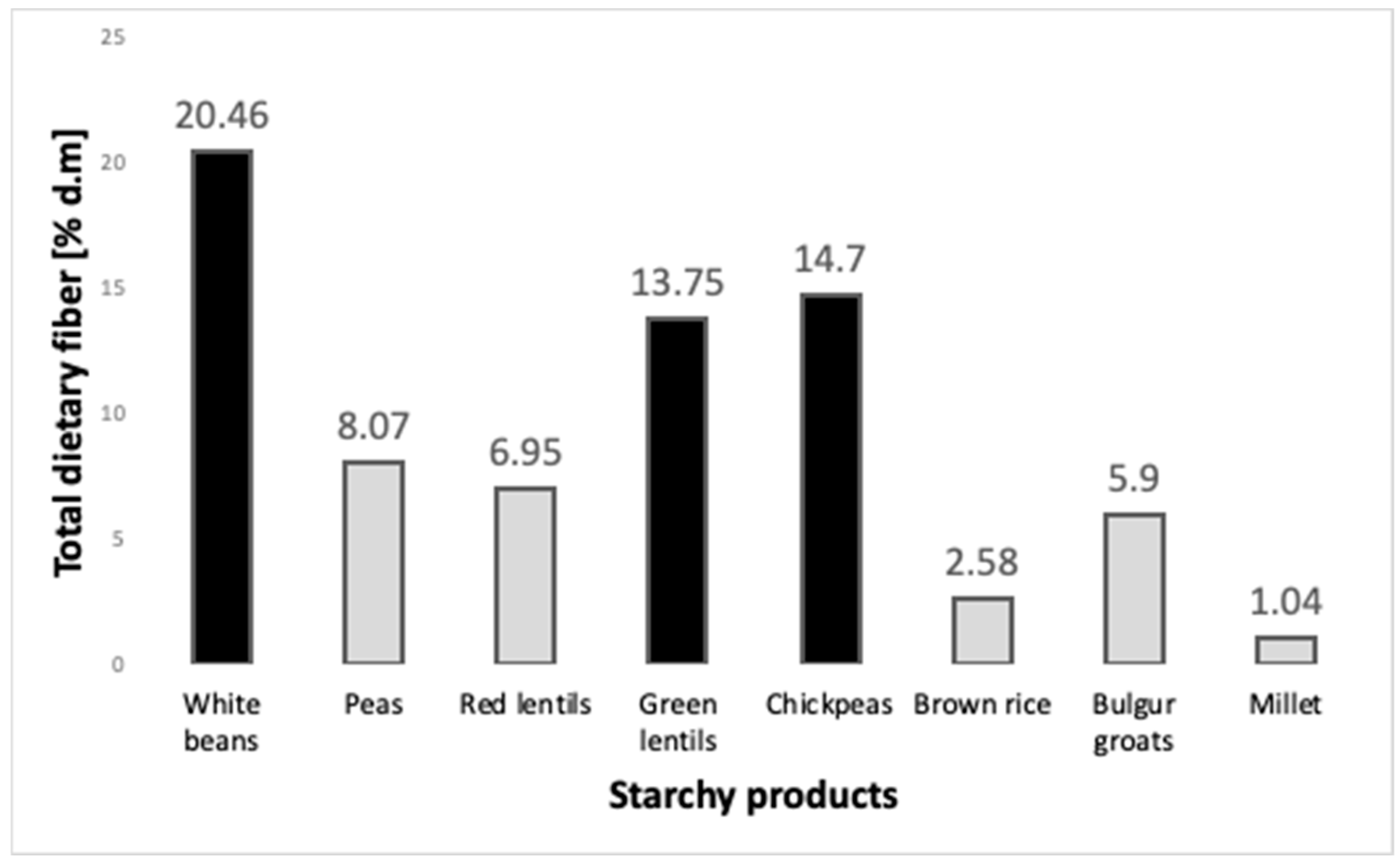
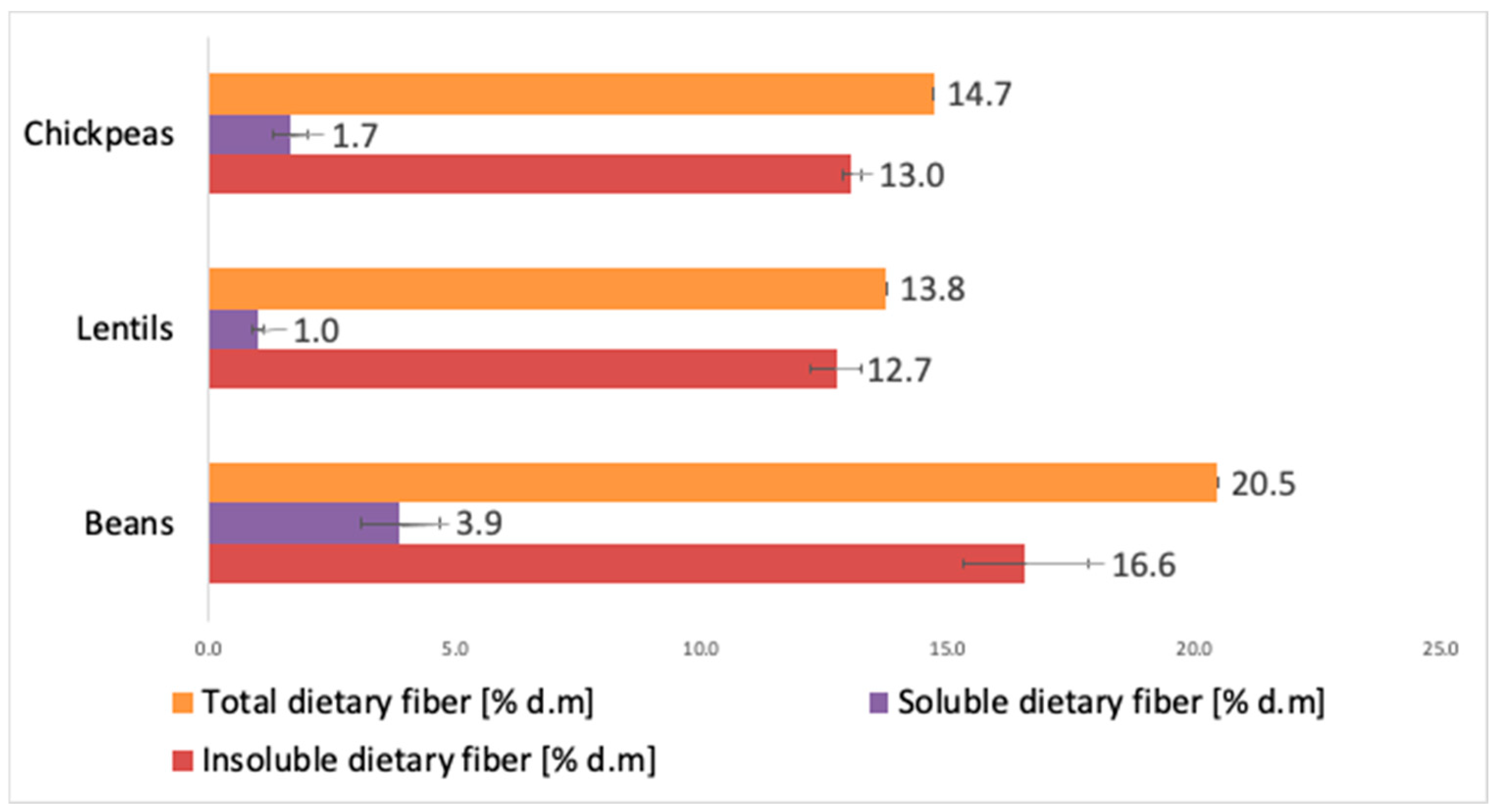
3.1. Legumes as a Source of Dietary Fiber
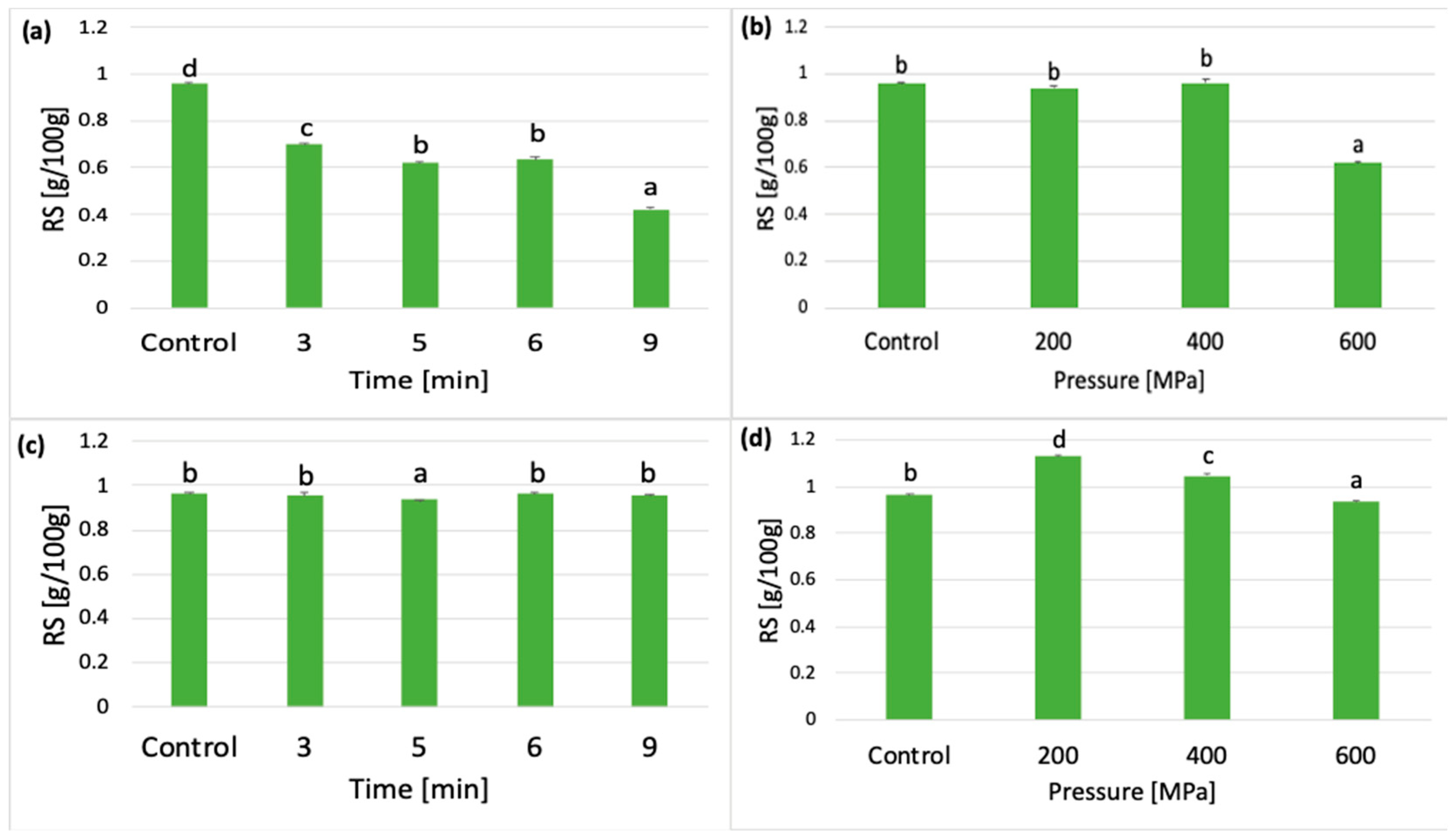
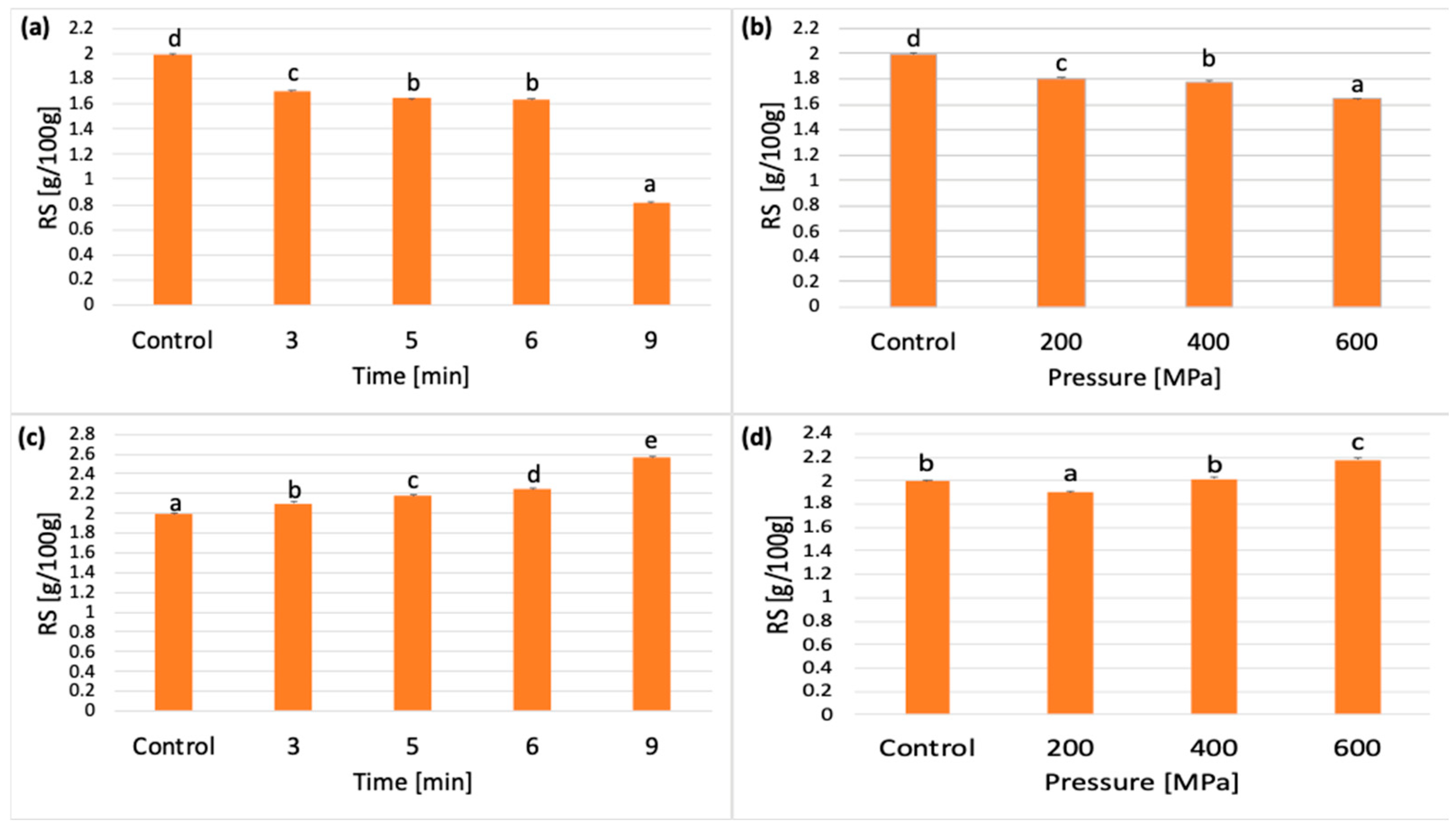
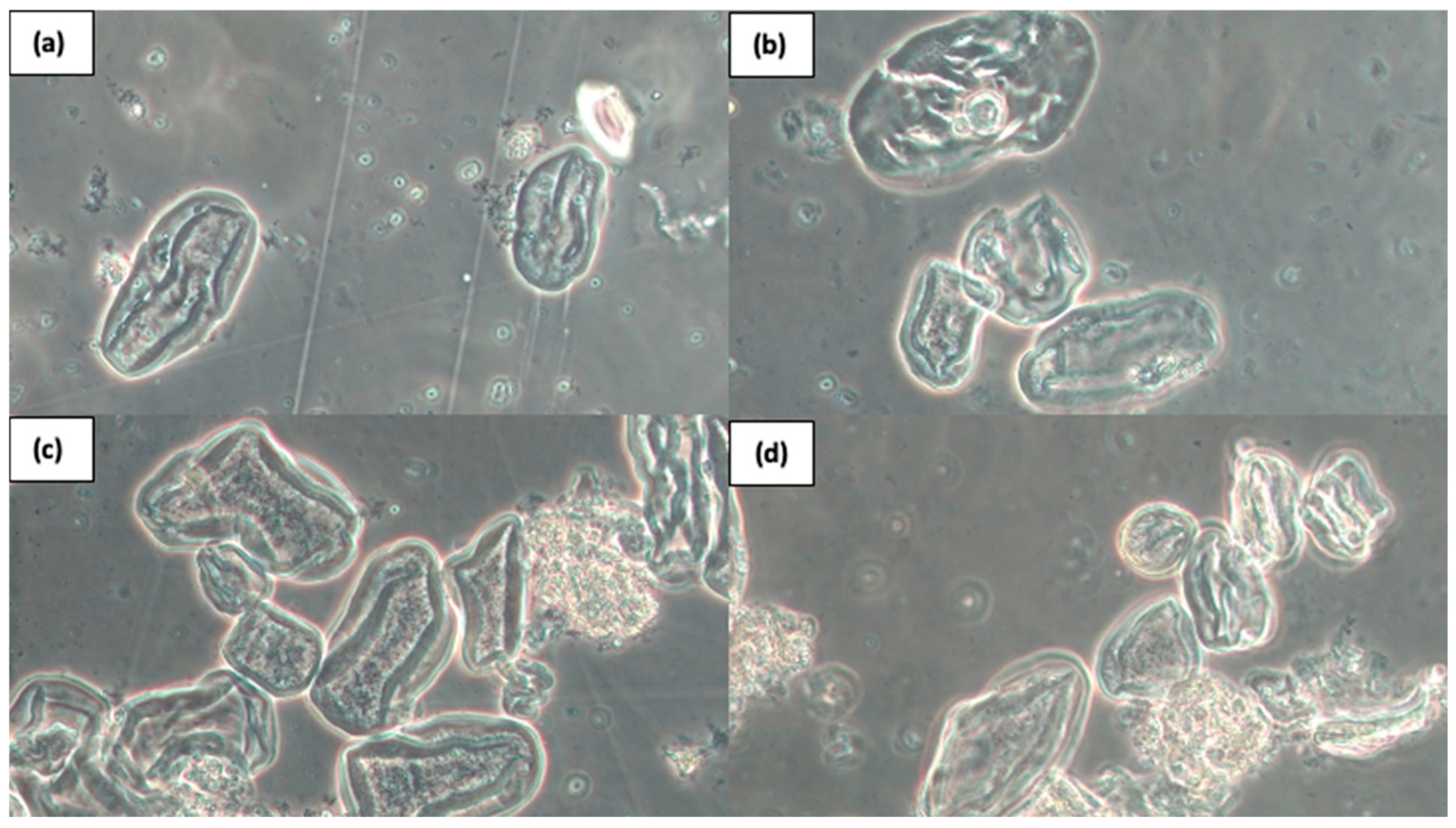
3.2. Effect of HPP on RS Content
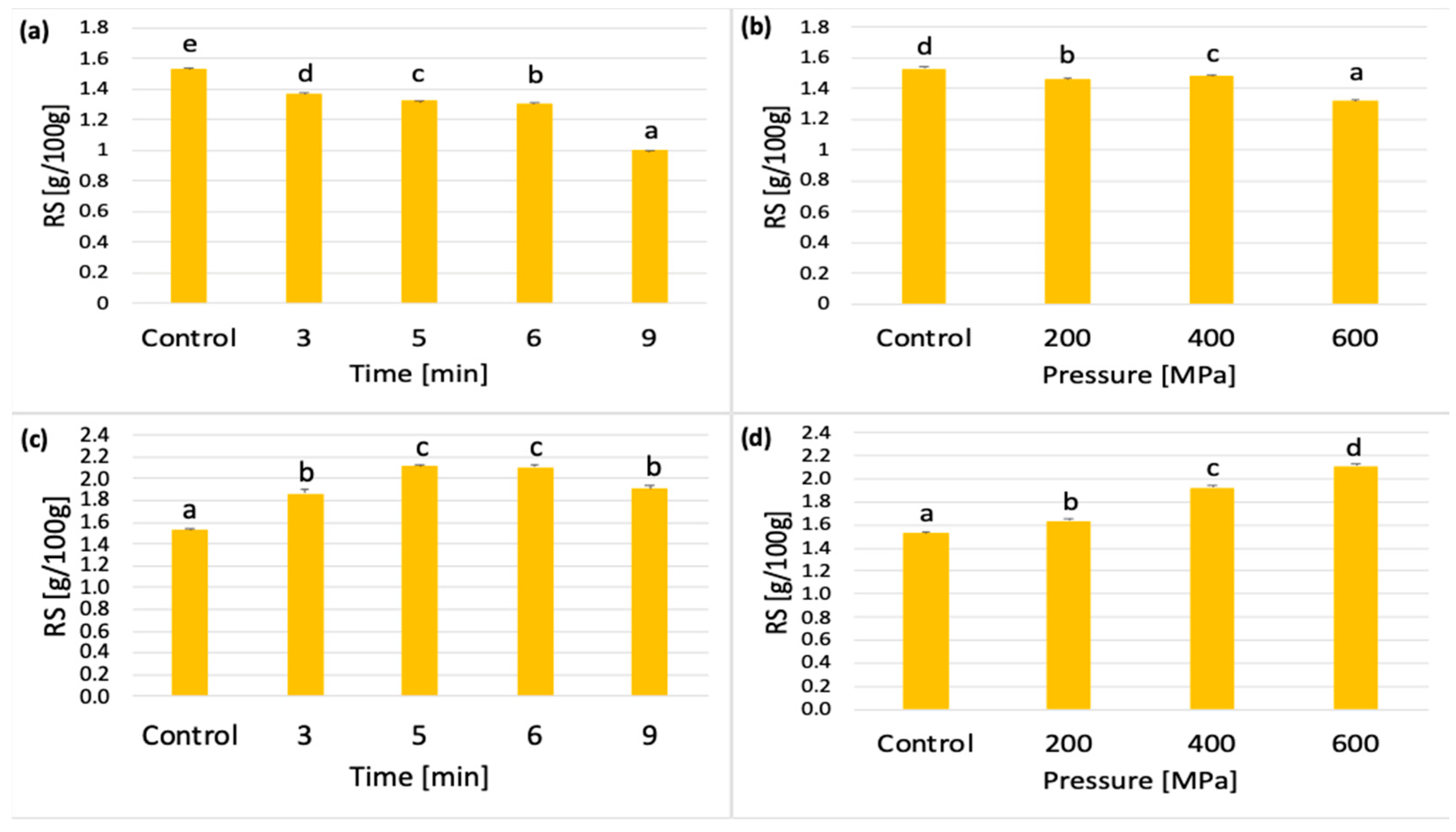
3.2.1. Effect of Pressurization Time and Pressure Value on RS Concentration
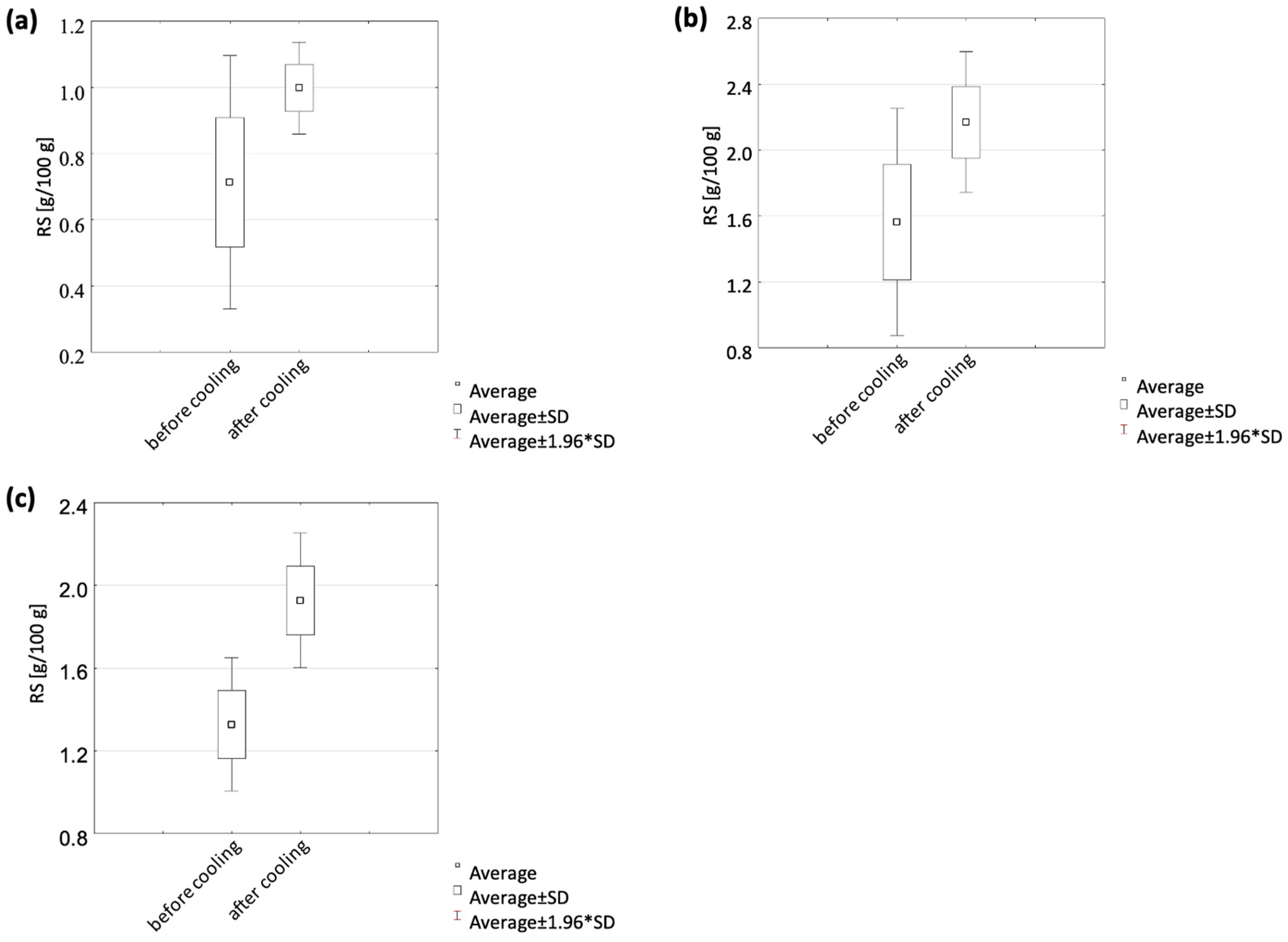
3.2.2. Effect of Cooling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tucker, L.A. Legume Intake, Body Weight, and Abdominal Adiposity: 10-Year Weight Change and Cross-Sectional Results in 15,185 U.S. Adults. Nutrients 2023, 15, 460. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; Harding, S.V.; Zafron, M.; Rideout, T.C. A Systematic Review of the Effect of Dietary Pulses on Microbial Populations Inhabiting the Human Gut. Benef. Microbes 2020, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cui, W.; Hu, X.; Ma, Z. Anti-Hyperlipidemic and Ameliorative Effects of Chickpea Starch and Resistant Starch in Mice with High Fat Diet Induced Obesity Are Associated with Their Multi-Scale Structural Characteristics. Food Funct. 2022, 13, 5135–5152. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Liu, S.; Pang, W.; Lu, B.; Sun, W.; Zhang, N.; Zhou, X.; Zhang, D.; Wang, Y. Beneficial Effect of Kidney Bean Resistant Starch on Hyperlipidemia—Induced Acute Pancreatitis and Related Intestinal Barrier Damage in Rats. Molecules 2022, 27, 2783. [Google Scholar] [CrossRef] [PubMed]
- Kadyan, S.; Sharma, A.; Arjmandi, B.H.; Singh, P.; Nagpal, R. Prebiotic Potential of Dietary Beans and Pulses and Their Resistant Starch for Aging-Associated Gut and Metabolic Health. Nutrients 2022, 14, 1726. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Nugent, A.P. Health Effects of Resistant Starch. Nutr. Bull. 2017, 42, 10–41. [Google Scholar] [CrossRef]
- McCleary, B.V. Measurement of Resistant Starch and Incorporation of Resistant Starch into Dietary Fibre Measurements. In Resistant Starch; Wiley: Hoboken, NJ, USA, 2013; pp. 131–144. [Google Scholar]
- Shen, L.; Keenan, M.J.; Raggio, A.; Williams, C.; Martin, R.J. Dietary-Resistant Starch Improves Maternal Glycemic Control in Goto-Kakizaki Rat. Mol. Nutr. Food Res. 2011, 55, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.H.T.; Louie, J.C.Y. The Relationship between Resistant Starch and Glycemic Control: A Review on Current Evidence and Possible Mechanisms. Starch-Starke 2017, 69, 1600205. [Google Scholar] [CrossRef]
- Robertson, M.D.; Currie, J.M.; Morgan, L.M.; Jewell, D.P.; Frayn, K.N. Prior Short-Term Consumption of Resistant Starch Enhances Postprandial Insulin Sensitivity in Healthy Subjects. Diabetologia 2003, 46, 659–665. [Google Scholar] [CrossRef]
- Johnston, K.L.; Thomas, E.L.; Bell, J.D.; Frost, G.S.; Robertson, M.D. Resistant Starch Improves Insulin Sensitivity in Metabolic Syndrome. Diabet. Med. 2010, 27, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary Fiber Intake Reduces Risk of Inflammatory Bowel Disease: Result from a Meta-Analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Haenen, D.; Zhang, J.; Souza da Silva, C.; Bosch, G.; van der Meer, I.M.; van Arkel, J.; van den Borne, J.J.G.C.; Pérez Gutiérrez, O.; Smidt, H.; Kemp, B.; et al. A Diet High in Resistant Starch Modulates Microbiota Composition, SCFA Concentrations, and Gene Expression in Pig Intestine. J. Nutr. 2013, 143, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Warman, D.J.; Jia, H.; Kato, H. The Potential Roles of Probiotics, Resistant Starch, and Resistant Proteins in Ameliorating Inflammation during Aging (Inflammaging). Nutrients 2022, 14, 747. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of Treatment Methods on the Formation of Resistant Starch in Purple Sweet Potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef] [PubMed]
- Chorfa, N.; Nlandu, H.; Belkacemi, K.; Hamoudi, S. Physical and Enzymatic Hydrolysis Modifications of Potato Starch Granules. Polymers 2022, 14, 2027. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Dhital, S.; Junejo, S.A.; Ding, L.; Huang, Q.; Fu, X.; He, X.; Zhang, B. Starch Retrogradation in Potato Cells: Structure and in Vitro Digestion Paradigm. Carbohydr. Polym. 2022, 286, 119261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Brennan, M.; Brennan, C.; Zheng, H. Thermal, Pasting and Structural Studies of Oat Starch-Caseinate Interactions. Food Chem. 2022, 373, 131433. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Starch Fine Molecular Structures: The Basis for Designer Rice with Slower Digestibility and Desirable Texture Properties. Carbohydr. Polym. 2023, 299, 120217. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Y.; Li, H.; Chen, Z.; Xu, B. Improving the Cold Water Swelling Properties of Oat Starch by Subcritical Ethanol-Water Treatment. Int. J. Biol. Macromol. 2022, 194, 594–601. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, D.; Zhou, H.; Wu, F.; Xu, X. Rheological, Microstructure and Mixing Behaviors of Frozen Dough Reconstituted by Wheat Starch and Gluten. Int. J. Biol. Macromol. 2022, 212, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Donmez, D.; Pinho, L.; Patel, B.; Desam, P.; Campanella, O.H. Characterization of Starch–Water Interactions and Their Effects on Two Key Functional Properties: Starch Gelatinization and Retrogradation. Curr. Opin. Food Sci. 2021, 39, 103–109. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Akintayo, O.A.; Adebo, O.A.; Kayitesi, E.; Njobeh, P.B. A Review on the Physicochemical Properties of Starches Modified by Microwave Alone and in Combination with Other Methods. Int. J. Biol. Macromol. 2021, 176, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Górecki, A.R.; Błaszczak, W.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Penkacik, K. Influence of High Pressure or Autoclaving-Cooling Cycles and Pullulanase Treatment on Buckwheat Starch Properties and Resistant Starch Formation. Pol. J. Food Nutr. Sci. 2018, 68, 235–242. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Comparative Effects of High Pressure Processing and Heat Treatment on in Vitro Digestibility of Pea Protein and Starch. NPJ Sci. Food 2022, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Zhang, W.; Li, C.; Yu, J.; Wang, S. Molecular Order and Functional Properties of Starches from Three Waxy Wheat Varieties Grown in China. Food Chem. 2015, 181, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Pottier, L.; Villamonte, G.; de Lamballerie, M. Applications of High Pressure for Healthier Foods. Curr. Opin. Food Sci. 2017, 16, 21–27. [Google Scholar] [CrossRef]
- Andrés, V.; Mateo-Vivaracho, L.; Guillamón, E.; Villanueva, M.J.; Tenorio, M.D. High Hydrostatic Pressure Treatment and Storage of Soy-Smoothies: Colour, Bioactive Compounds and Antioxidant Capacity. LWT-Food Sci. Technol. 2016, 69, 123–130. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of Thermal and High Pressure Processing on Quality Parameters of Beetroot (Beta vulgaris L.). LWT-Food Sci. Technol. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Bauer, B.A.; Knorr, D. The Impact of Pressure, Temperature and Treatment Time on Starches: Pressure-Induced Starch Gelatinisation as Pressure Time Temperature Indicator for High Hydrostatic Pressure Processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Liu, P.; Bai, Y.; Gao, L.; Shen, Q. Effect of High Hydrostatic Pressure on Physicochemical, Thermal and Morphological Properties of Mung Bean (Vigna radiata L.) Starch. J. Food Eng. 2011, 103, 388–393. [Google Scholar] [CrossRef]
- Ruiz, R.P. Gravimetric Determination of Water by Drying and Weighing. Curr. Protoc. Food Anal. Chem. 2001, A1.1.1. [Google Scholar] [CrossRef]
- Thompson, H.J.; Brick, M.A. Perspective: Closing the Dietary Fiber Gap: An Ancient Solution for a 21st Century Problem. Adv. Nutr. 2016, 7, 623–626. [Google Scholar] [CrossRef]
- McGinley, J.; Fitzgerald, V.; Neil, E.; Omerigic, H.; Heuberger, A.; Weir, T.; McGee, R.; Vandemark, G.; Thompson, H. Pulse Crop Effects on Gut Microbial Populations, Intestinal Function, and Adiposity in a Mouse Model of Diet-Induced Obesity. Nutrients 2020, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Vasconcelos, M.; Gil, A.M.; Pinto, E. Benefits of Pulse Consumption on Metabolism and Health: A Systematic Review of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 85–96. [Google Scholar] [CrossRef]
- Gowd, V.; Xie, L.; Zheng, X.; Chen, W. Dietary Fibers as Emerging Nutritional Factors against Diabetes: Focus on the Involvement of Gut Microbiota. Crit. Rev. Biotechnol. 2019, 39, 524–540. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary Fiber Sources and Human Benefits: The Case Study of Cereal and Pseudocereals. In Advances in Food and Nutrition Research; Elsiever: Amsterdam, The Netherlands, 2019; pp. 83–134. [Google Scholar]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy Bean Supplemented High-Fat Diet Improves Intestinal Health, Epithelial Barrier Integrity and Critical Aspects of the Obese Inflammatory Phenotype. J. Nutr. Biochem. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Lin, D.; Peters, B.A.; Friedlander, C.; Freiman, H.J.; Goedert, J.J.; Sinha, R.; Miller, G.; Bernstein, M.A.; Hayes, R.B.; Ahn, J. Association of Dietary Fibre Intake and Gut Microbiota in Adults. Br. J. Nutr. 2018, 120, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, W.; Chen, Y.; Lei, L.; Li, F.; Zhao, J.; Zeng, K.; Ming, J. Dietary Fiber of Tartary Buckwheat Bran Modified by Steam Explosion Alleviates Hyperglycemia and Modulates Gut Microbiota in Db/Db Mice. Food Res. Int. 2022, 157, 111386. [Google Scholar] [CrossRef]
- Zhu, F. Dietary Fiber Polysaccharides of Amaranth, Buckwheat and Quinoa Grains: A Review of Chemical Structure, Biological Functions and Food Uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Kabisch, S.; Weickert, M.O.; Pfeiffer, A.F.H. The Role of Cereal Soluble Fiber in the Beneficial Modulation of Glycometabolic Gastrointestinal Hormones. Crit. Rev. Food Sci. Nutr. 2022, 64, 4331–4347. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Nevola, G.; Mazzeo, R.; Cucciniello, L.; Totaro, F.; Bertuzzi, C.A.; Caccialanza, R.; Pedrazzoli, P.; Puglisi, F. The Impact of Cereal Grain Composition on the Health and Disease Outcomes. Front. Nutr. 2022, 9, 888974. [Google Scholar] [CrossRef]
- Iversen, K.N.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.A.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McGee, R.; Vandemark, G.; Brick, M.; Thompson, H. Dietary Fiber Analysis of Four Pulses Using AOAC 2011.25: Implications for Human Health. Nutrients 2016, 8, 829. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-Supplemented Diet Alters the Gut Microbiome and Enhances Gut Barrier Integrity in C57Bl/6 Male Mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary Fibres in Pulse Seeds and Fractions: Characterization, Functional Attributes, and Applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Rodríguez, R.; Jiménez, A.; Fernández-Bolaños, J.; Guillén, R.; Heredia, A. Dietary Fibre from Vegetable Products as Source of Functional Ingredients. Trends Food Sci. Technol. 2006, 17, 3–15. [Google Scholar] [CrossRef]
- Kutoš, T.; Golob, T.; Kač, M.; Plestenjak, A. Dietary Fibre Content of Dry and Processed Beans. Food Chem. 2003, 80, 231–235. [Google Scholar] [CrossRef]
- Granito, M.; Frias, J.; Doblado, R.; Guerra, M.; Champ, M.; Vidal-Valverde, C. Nutritional Improvement of Beans (Phaseolus vulgaris) by Natural Fermentation. Eur. Food Res. Technol. 2002, 214, 226–231. [Google Scholar] [CrossRef]
- Dalgetty, D.D.; Baik, B.-K. Isolation and Characterization of Cotyledon Fibers from Peas, Lentils, and Chickpeas. Cereal Chem. J. 2003, 80, 310–315. [Google Scholar] [CrossRef]
- Perez-Hidalgo, M.A.; Guerra-Hernández, E.; García-Villanova, B. Dietary Fiber in Three Raw Legumes and Processing Effect on Chick Peas by an Enzymatic-Gravimetric Method. J. Food Compos. Anal. 1997, 10, 66–72. [Google Scholar] [CrossRef]
- Thevelein, J.M.; Van Assche, J.A.; Heremans, K.; Gerlsma, S.Y. Gelatinisation Temperature of Starch, as Influenced by High Pressure. Carbohydr. Res. 1981, 93, 304–307. [Google Scholar] [CrossRef]
- Liu, H.; Guo, X.; Li, Y.; Li, H.; Fan, H.; Wang, M. In Vitro Digestibility and Changes in Physicochemical and Textural Properties of Tartary Buckwheat Starch under High Hydrostatic Pressure. J. Food Eng. 2016, 189, 64–71. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Cao, R.; Blanchard, C.; Wang, M. Physicochemical Properties and in Vitro Digestibility of Sorghum Starch Altered by High Hydrostatic Pressure. Int. J. Biol. Macromol. 2016, 92, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, J.; Saleh, A.S.M.; Tian, X.; Wang, P.; Jiang, H.; Zhang, G. The Modifications in Physicochemical and Functional Properties of Proso Millet Starch after Ultra-High Pressure (UHP) Process. Starch-Stärke 2018, 70, 1700235. [Google Scholar] [CrossRef]
- D’Aniello, A.; Koshenaj, K.; Ferrari, G. A Preliminary Study on the Release of Bioactive Compounds from Rice Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 521. [Google Scholar] [CrossRef]
- Pulgarín, O.; Larrea-Wachtendorff, D.; Ferrari, G. Effects of the Amylose/Amylopectin Content and Storage Conditions on Corn Starch Hydrogels Produced by High-Pressure Processing (HPP). Gels 2023, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Al-Attar, H. Structural Properties of High-Pressure-Treated Chestnut Flour Dispersions. Int. J. Food Prop. 2017, 20, S766–S778. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Taher, A.; Joseph, A. Impact of High Pressure Treatment on Functional, Rheological, Pasting, and Structural Properties of Lentil Starch Dispersions. Carbohydr. Polym. 2016, 152, 639–647. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, Y.; Luo, Y.; Zhong, Y.; Yue, J.; Song, X.; Zhao, Y. Impact of Continuous or Cycle High Hydrostatic Pressure on the Ultrastructure and Digestibility of Rice Starch Granules. J. Cereal Sci. 2014, 60, 302–310. [Google Scholar] [CrossRef]
- Yadav, B.S.; Sharma, A.; Yadav, R.B. Studies on Effect of Multiple Heating/Cooling Cycles on the Resistant Starch Formation in Cereals, Legumes and Tubers. Int. J. Food Sci. Nutr. 2009, 60, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, E.; Cabrera-Ramírez, A.H.; Gaytán-Martínez, M.; Mendoza-Zuvillaga, A.L.; Velázquez, G.; Méndez-Montealvo, M.G.; Rodríguez-García, M.E. Heating-Cooling Extrusion Cycles as a Method to Improve the Physicochemical Properties of Extruded Corn Starch. Int. J. Biol. Macromol. 2021, 188, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Zhou, X.; Lim, S.-T. Pasting Viscosity and in Vitro Digestibility of Retrograded Waxy and Normal Corn Starch Powders. Carbohydr. Polym. 2012, 87, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Baik, B.-K.; Wang, R.; Lim, S.-T. Retrogradation of Waxy and Normal Corn Starch Gels by Temperature Cycling. J. Cereal Sci. 2010, 51, 57–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarczuk, A.; Le-Thanh-Blicharz, J.; Michałowska, D.; Kotyrba, D.; Marszałek, K. Application of High Hydrostatic Pressures and Refrigerated Storage on the Content of Resistant Starch in Selected Legume Seeds. Appl. Sci. 2024, 14, 7049. https://doi.org/10.3390/app14167049
Bojarczuk A, Le-Thanh-Blicharz J, Michałowska D, Kotyrba D, Marszałek K. Application of High Hydrostatic Pressures and Refrigerated Storage on the Content of Resistant Starch in Selected Legume Seeds. Applied Sciences. 2024; 14(16):7049. https://doi.org/10.3390/app14167049
Chicago/Turabian StyleBojarczuk, Adrianna, Joanna Le-Thanh-Blicharz, Dorota Michałowska, Danuta Kotyrba, and Krystian Marszałek. 2024. "Application of High Hydrostatic Pressures and Refrigerated Storage on the Content of Resistant Starch in Selected Legume Seeds" Applied Sciences 14, no. 16: 7049. https://doi.org/10.3390/app14167049






