Abstract
Insulin resistance is closely associated with metabolic diseases such as type 2 diabetes and cardiovascular disease, and the resting heart rate (RHR) is an important marker of the autonomic nervous system’s status. This study aimed to investigate the association between the RHR and the homeostatic model assessment of insulin resistance (HOMA-IR) in Korean adults. Using data from the 2019 Korea National Health and Nutrition Examination Survey (KNHANES 2019), we analyzed 6118 adults and classified the participants into four groups based on their RHR (≤67, 68–71, 72–79, and ≥80 beats/min [bpm]). Logistic regression analysis considering demographic, lifestyle, and biochemical factors revealed that the odds of having a HOMA-IR of 2.5 or higher were significantly increased in the higher RHR groups. Specifically, compared to the ≤67 bpm group, the 68–71, 72–79, and ≥80 bpm groups had increased odds of 1.277, 1.599, and 1.919 times, respectively. These results are expected to contribute to the development of strategies for the early diagnosis and management of metabolic diseases through the RHR, and further research is needed to deepen the understanding of the physiological mechanisms of this relationship, including the management of the RHR through lifestyle modification and regular exercise and the effect of reducing insulin resistance.
1. Introduction
Insulin resistance is closely associated with important metabolic diseases such as type 2 diabetes mellitus (T2DM) and cardiovascular disease, and the homeostatic model assessment of insulin resistance (HOMA-IR) has been widely used as a marker to assess insulin resistance [1]. The resting heart rate (RHR) is also an important marker of the autonomic nervous system’s status, which represents the balance between sympathetic and parasympathetic activity [2], with a high RHR suggesting an overactive sympathetic nervous system or decreased parasympathetic activity [3]. Epidemiologic studies in the general population have suggested that a high RHR is associated with an increased incidence and mortality of cardiovascular disease, regardless of traditional risk factors [4]. Furthermore, studies in patients with T2DM have identified a high RHR as a risk factor for microvascular complications as well as cardiovascular disease [5,6]. Previous studies have associated the RHR with mortality from multiple causes, including hypertension [7], metabolic syndrome [8], and cardiovascular disease, and studies in general populations in the United States [9] and Japan [10] have also associated the RHR with T2DM. This association is further supported by research suggesting that those with a higher adipose tissue insulin resistance index have a higher RHR, particularly in relation to insulin resistance [11], and by research indicating that insulin resistance plays a mediating role in the association between the RHR and T2DM [12]. Although recent studies have highlighted a link between the RHR and insulin resistance, the specific magnitude and the extent of this relationship are still unclear. Based on the findings of these studies, this study aims to further elucidate the association between the RHR and insulin resistance through quantitative analysis of HOMA-IR in individuals with a high RHR using data from the 2019 Korea National Health and Nutrition Examination Survey [13]. Our intention was to analyze the feasibility of using the RHR as an early marker of insulin resistance and provide practical evidence contributing to the early diagnosis and management of metabolic diseases. Given this background, this study utilized KNHANES 2019 data to investigate whether the HOMA-IR is associated with RHR levels.
2. Materials and Methods
2.1. Study Subjects
This study was based on KNHANES 2019 data and included 8110 participants. After excluding participants with missing data on HOMA-IR and RHR, 6118 participants were included in the final analysis and categorized according to their RHR: ≤67 bpm (n = 2033), 68–71 bpm (n = 1181), 72–79 bpm (n = 1717), and ≥80 bpm (n = 1257) (Figure 1). The survey and materials were reviewed and approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency.
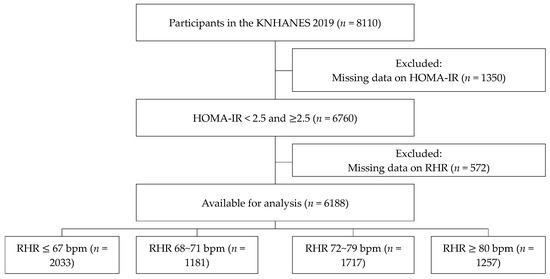
Figure 1.
Study population.
2.2. Variables
2.2.1. General Factors
The participants were categorized according to the following general characteristics: sex, age (20–29, 30–39, 40–49, 50–59, or ≥60 years), marital status (single or married), level of education (high school or less or college or more), and household monthly income (≤2.00 million, 2.01–3.00 million, or ≥3.01 million KRW).
2.2.2. Lifestyle
Alcohol consumption was determined by a survey item asking whether the participants were lifelong abstainers or had had at least one drink per month in the past year. Smoking status was categorized as non-smoker (never smoked), past smoker (smoked in the past but do not currently smoke), and current smoker. The cutoff for glycated hemoglobin (HbA1c) was set at ≥6.5%. Participants with a systolic blood pressure of ≤120 mmHg and a diastolic blood pressure of ≤80 mmHg were classified as nonhypertensive; those with a systolic blood pressure of >120 or ≤140 mmHg and a diastolic blood pressure of >80 or ≤90 mmHg as prehypertensive; and those with a systolic blood pressure of >140 mmHg or a diastolic blood pressure of >90 mmHg and who were diagnosed as hypertensive by a doctor or taking medication for hypertension were classified as hypertensive [14]. The body mass index (BMI) was calculated as weight (kg)/height (m2), and a BMI of ≤18.5 kg/m2 was classified as underweight, 18.5–22.9 kg/m2 as normal weight, 23–24.9 kg/m2 as overweight, and ≥25 kg/m2 as obese [15].
2.2.3. Blood Chemistry
Blood chemistry test markers, including HbA1c, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were analyzed. Diabetes was defined as HbA1c ≥ 6.5%, and dyslipidemia was defined as a TC level ≥ 260 mg/dL, TG level ≥ 200 mg/dL, HDL-C level < 40 mg/dL, and LDL-C level ≥ 160 mg/dL [16].
2.2.4. HOMA-IR
The HOMA-IR was calculated using the Matthews equation: HOMA-IR [fasting blood glucose (mg/dL) × insulin (µU/mL)/405] [17]. A HOMA-IR of 2.5 was classified as insulin resistance.
2.3. Statistical Analysis
The general characteristics, lifestyle, and biochemical factors of the participants are presented as frequencies and percentages, and the independence of the RHR (≤67, 68–71, 72–79, and ≥80 bpm) was analyzed using the chi-square test. Logistic regression was performed to analyze the HOMA-IR, and the results are presented with 95% confidence intervals (CIs). All the analyses were performed using SPSS (version 25.0), with statistical significance set at 0.05.
3. Results
3.1. Demographic Status According to RHR
Table 1 shows a comparison of the general and socioeconomic characteristics according to the RHR (≤64, 68–71, 72–79, and ≥80 bpm). In the group with the highest RHR of ≥80 bpm, there was a higher proportion of women (56.7%) than of men (43.3%), and the age group with the highest proportion (31.2%) was ≥60 years. There was a higher proportion of participants who were not married (60%) than were married (40%) in the highest RHR group, and those with an education level of high school or less accounted for the most at 69.4%. Finally, those with a household income of ≥3.01 million KRW accounted for the most at 58.1%, but there was no significant association. All factors except household income showed statistically significant differences depending on the RHR.

Table 1.
General characteristics according to resting heart rate.
3.2. Lifestyle Factors According to RHR
Table 2 shows the lifestyle factors according to the RHR. In the ≥80 bpm group, the drinking group accounted for the most at 79.8%, and the smoking group accounted for the most at 68%. Those with HbA1c ≥ 6.5% accounted for 9.6%, and those with normal blood pressure accounted for the most at 53.7%. Finally, in terms of BMI, the normal weight group accounted for the most at 41%. All lifestyle factors showed statistically significant differences depending on the RHR.

Table 2.
Lifestyle factors according to resting heart rate.
3.3. Blood Chemistry According to RHR
Table 3 shows the blood chemistry test results according to the RHR. In the ≥80 bpm group, those with TC ≥ 240 mg/dL accounted for only 9.5%, those with HDL-C < 40 mg/dL accounted for 12.6%, and those with LDL-C ≥ 160 mg/dL accounted for 7.3%, none of which were statistically significant. Those with TG ≥ 200 mg/dL accounted for 15.5%, and those with HOMA-IR ≥ 2.5 accounted for 42.6%, showing a statistically significant difference.

Table 3.
Blood chemistry test results according to resting heart rate.
3.4. HOMA-IR According to RHR
Table 4 and Figure 2 shows HOMA-IR values according to the RHR. A logistic regression analysis was performed after adjusting for sex, age, marital status, education level, family income, alcohol drinking, smoking status, blood pressure, BMI, and HbA1c TC, TG, HDL-C, and LDL-C levels. The HOMA-IR was found to have a statistically significant effect on the RHR (p < 0.05). Compared with ≤67 bpm, the odds ratio (OR) was 1.277 (95% confidence interval [CI], 1.045–1.560) for 68–71 bpm, 1.599 (95% CI, 1.336–1.913) for 72–79 bpm, and 1.919 times (95% CI, 1.567–2.350) for ≥80 bpm. In Figure 2, the dashed line represents the represents the reference value(≤67 bpm), and the gray lines represent the 95% confidence intervals for each odds ratio.

Table 4.
Logistic regression for the effect of HOMA-IR on resting heart rate.
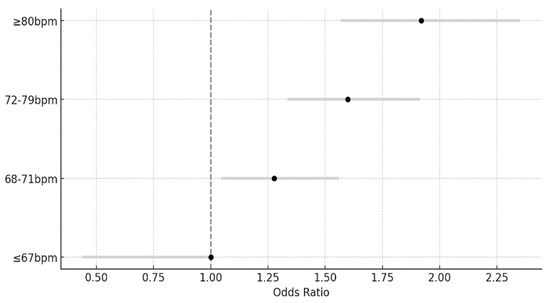
Figure 2.
Odds ratios for homeostatic model assessment of insulin resistance by resting heart rate categories.
4. Discussion
This study investigated the association between the RHR and the HOMA-IR in 6188 participants in the KNHANES who had HOMA-IR and RHR data. The key outcomes indicated a significant association between RHR and HOMA-IR, with a higher RHR being associated with a higher probability of having a HOMA-IR ≥ 2.5 after adjusting for all variables, including the general characteristics, lifestyle habits, and biochemical factors of the participants. After adjusting for the relevant variables, the ORs were 1.277 for 68–71 bpm, 1.599 for 72–79 bpm, and 1.919 for ≥80 bpm, indicating that an increase in the RHR tends to rise in proportion to an increase in HOMA-IR and, thus, that RHR levels may have a linear relationship with the level of insulin resistance.
The RHR has potential for clinical use due to its simplicity, safety, affordability, and high efficiency [18]. Previous studies have explained the relationship between the RHR and T2DM in terms of biological mechanisms, suggesting that an elevated RHR is driven by the activation of the sympathetic nervous system and represents an imbalance in the autonomic nervous system [19,20]. Overactivation of the sympathetic nervous system can lead to decreased insulin sensitivity, increasing the risk of T2DM. There are several studies on such an influence of the RHR on diabetes [21,22,23,24]. Previous studies have reported that insulin resistance tends to increase with increasing RHR, with a significantly higher risk of developing T2DM in individuals with higher RHR [25] and that insulin resistance mediates the association between the RHR and T2DM, emphasizing the association of the RHR with T2DM [9].
This is in line with the findings of the present study suggesting that the RHR may be a useful predictor of insulin resistance and related metabolic diseases. We hypothesized that the RHR may contribute to insulin resistance, and given that the RHR affects T2DM [26] and insulin resistance is a major contributor to the development of T2DM, this analysis clarified the extent to which different levels of the RHR affected the relationship with the HOMA-IR. The clinical implications of a linear relationship between the RHR and HOMA-IR suggest that the early detection and management of insulin resistance through the RHR may be crucial in the prevention of and treatment strategies for metabolic diseases. The linear relationship between the RHR and insulin resistance identified in this study is quite interesting. Most importantly, the finding that HOMA-IR levels tended to show a linear increase with increasing RHR suggested that the heart rate could play an important role in the development and progression of metabolic disease beyond just a physical marker, further emphasizing the possibility of the RHR having a direct association with insulin resistance. Although the mechanism has not been fully elucidated, a possible biological explanation is that the RHR is a marker of autonomic nerve activity [27].
Whereas activation of the parasympathetic nerves stimulates insulin secretion from the beta cells of the pancreas, lowering blood sugar levels, activation of the sympathetic nerves suppresses insulin production in the pancreas, resulting in a high fasting blood sugar level. Furthermore, overactivation of the sympathetic nervous system can induce vasoconstriction, which reduces blood flow to the skeletal muscle, thereby impairing glucose uptake [28]. Activation of the sympathetic nervous system also increases lipolytic activity within the white adipose tissue. This process is mediated by the activation of hormone-sensitive lipase (HSL), which catalyzes the breakdown of triglycerides into glycerol and free fatty acids. Glycerol, utilized in the liver for gluconeogenesis, elevates circulating glucose levels [29]. The resultant excess free fatty acids migrate to the peripheral tissues such as the skeletal muscle, where they accumulate as ectopic lipids, thereby impeding insulin signaling pathways and inducing insulin resistance [30]. These physiological mechanisms provide crucial insights into why individuals with a higher RHR exhibit increased HOMA-IR levels. In particular, the negative correlation between cholesterol levels and the RHR is intriguing and may significantly contribute to the current literature. According to the research findings, total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels did not show a significant relationship with the RHR. However, in the group with an RHR of 80 bpm or higher, 15.5% had triglyceride (TG) levels of 200 mg/dL or higher, which was statistically significant. This suggests that individuals with lower cholesterol levels tend to have a lower RHR. Particularly, higher TG levels may increase sympathetic nervous system activation, leading to a higher RHR. This indicates a complex interaction between lipid metabolism and autonomic nervous system activity, necessitating further research into these interactions. Other previous studies have also mentioned that demographic characteristics (e.g., age, gender) and lifestyle factors (e.g., smoking status, alcohol consumption) are associated with the RHR, consistent with our findings. For example, older adults generally have a higher RHR, and smokers and drinkers have a higher RHR compared to non-smokers and non-drinkers [25]. Our study also found that these factors support the association with the RHR. Another study contends that overactivation of the sympathetic nervous system is associated with stimulation of the renin–angiotensin–aldosterone system (RAAS), which can lead to insulin resistance and eventually diabetes [31]. In this study, activation of the sympathetic nervous system in relation to an elevated RHR is hypothesized to inhibit insulin in pancreatic beta cells and activate the RAAS to increase and cause insulin resistance [19], but further investigations into the physiological mechanisms are needed.
This also raises other questions about the mechanisms driving the association between the RHR and HOMA-IR. The RHR is commonly used as a marker of cardiovascular fitness in epidemiologic studies. A low RHR is often observed in athletes and people engaging in endurance exercise and is attributed to an increased one-time cardiac output. The independent role of cardiovascular fitness has been noted in other studies, and the findings of the present study imply that this effect may be attributed in part to the effect of cardiovascular fitness on insulin sensitivity [32]. Such a diversity of opinion needs to be considered, and further studies are needed to explore how the relationship between cardiovascular fitness and insulin sensitivity may influence the correlation between the RHR and HOMA-IR. Although this study suggests that the RHR may be an important predictor of insulin resistance, a more comprehensive approach that considers the role of cardiovascular fitness is required. Therefore, further studies including variables related to cardiovascular fitness may contribute to the accurate prediction and management of insulin resistance. There are some limitations to interpreting the results of this study. First, as this is a cross-sectional study using the KNHANES data, no causal inferences can be made. There is also the possibility of under- or over-measurement as the survey progresses. Second, not all influences on HOMA-IR could be considered outside of the variables included in the analysis. Third, the cardiovascular fitness levels were directly measured in assessing the impact of the RHR on insulin resistance. Nevertheless, this study is significant in that it analyzed the association between the RHR and HOMA-IR using the KNHANES data, which are representative and reliable. In conclusion, the findings of this study showed that an increase in the RHR significantly increased the odds of having a HOMA-IR ≥ 2.5. To improve fitness in relation to the RHR, lifestyle modifications should be made with encouragement to engage in regular exercise. However, further studies will be required to measure the effectiveness of these interventions in improving the RHR.
5. Conclusions
This study analyzed the association between the RHR and insulin resistance (HOMA-IR) in Korean adults using data from the KNHANES 2019 data. Participants with a higher RHR tended to have significantly higher insulin resistance. This suggests that the RHR could be a useful predictor of insulin resistance and related metabolic diseases. These findings indicate that monitoring and managing the RHR may be crucial for the prevention and management of metabolic diseases and may contribute to the improvement of early diagnosis and treatment strategies for metabolic diseases. Based on these findings, further research on the relationship between the RHR and insulin resistance will be needed to develop metabolic disease management strategies.
Author Contributions
Conceptualization, A.-S.H. and J.-C.L.; Formal analysis, A.-S.H. and J.-C.L.; Investigation, A.-S.H. and J.-C.L.; Methodology, A.-S.H. and J.-C.L.; Writing—original draft, A.-S.H. and J.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The survey and materials were reviewed and approved by the Institutional Review Board of the Korea Disease Control and Prevention Agency, 2018-01-03-C-A, approval date: 3 January 2018.
Informed Consent Statement
Patient consent was not applicable for an observational study in which we analyzed pre-existing data. Given the nature of the study, there was no direct interaction with patients, and informed consent was deemed not required.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kasuga, M. Insulin resistance and pancreatic β-cell failure. J. Clin. Investig. 2007, 30, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, M.K.; Kannankeril, P.J.; Goldberger, J.J. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J. Am. Coll. Cardiol. 2008, 51, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Nanchen, D.; Stott, D.J.; Gussekloo, J.; Mooijaart, S.P.; Westendorp, R.G.; Jukema, J.W.; Macfarlane, P.W.; Cornuz, J.; Rodondi, N.; Buckley, B.M.; et al. Resting heart rate and incident heart failure and cardiovascular mortality in older adults: Role of inflammation and endothelial dysfunction: The PROSPER study. Eur. J. Heart Fail. 2013, 15, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Johansen, C.D.; Olsen, R.H.; Pedersen, L.R.; Kumarathurai, P.; Mouridsen, M.R.; Binici, Z.; Intzilakis, T.; Køber, L.; Sajadieh, A. Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur. Heart J. 2013, 34, 1732–1739. [Google Scholar] [CrossRef]
- Hillis, G.S.; Woodward, M.; Rodgers, A.; Chow, C.K.; Li, Q.; Zoungas, S.; Patel, A.; Webster, R.; Batty, G.D.; Ninomiya, T.; et al. Resting heart rate and the risk of death and cardiovascular complications in patients with type 2 diabetes mellitus. Diabetologia 2012, 55, 1283–1290. [Google Scholar] [CrossRef]
- Hillis, G.S.; Hata, J.; Woodward, M.; Perkovic, V.; Arima, H.; Chow, C.K.; Zoungas, S.; Patel, A.; Poulter, N.R.; Mancia, G.; et al. Resting heart rate and the risk of microvascular complications in patients with type 2 diabetes mellitus. J. Am. Heart Assoc. 2012, 1, e002832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, P.; Sun, H.; Yin, Z.; Li, H.; Sun, X.; Liu, F.; Ren, Y.; Liu, D.; Chen, X.; et al. Resting heart rate and its dynamic change and the risk of hypertension: The Rural Chinese Cohort Study. J. Hum. Hypertens. 2020, 34, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, X.; Liu, Y.; Sun, X.; Han, C.; Zhang, L.; Wang, B.; Ren, Y.; Zhao, Y.; Zhang, D.; et al. Resting heart rate and risk of metabolic syndrome in adults: A dose-response meta-analysis of observational studies. Acta Diabetol. 2017, 54, 223–235. [Google Scholar] [CrossRef]
- Lee, D.H.; de Rezende, L.F.M.; Hu, F.B.; Jeon, J.Y.; Giovannucci, E.L. Resting heart rate and risk of type 2 diabetes: A prospective cohort study and meta-analysis. Diabetes Metab. Res. Rev. 2019, 35, e3095. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Yoshida, H.; Takahashi, H.; Kawai, M. Resting heart rate and blood pressure, independent of each other, proportionally raise the risk for type-2 diabetes mellitus. Int. J. Epidemiol. 2010, 39, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Nojima, T.; Matsubayashi, Y.; Yoshida, A.; Suganami, H.; Abe, T.; Ishizawa, M.; Fujihara, K.; Tanaka, S.; Kaku, K.; Sone, H. Influence of an SGLT2 inhibitor, tofogliflozin, on the resting heart rate in relation to adipose tissue insulin resistance. Diabet. Med. 2020, 37, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Qin, P.; Liu, Y.; Sun, X.; Li, H.; Wu, X.; Lu, J. Sex-specific association of resting heart rate with type 2 diabetes mellitus. J. Diabetes Complicat. 2020, 34, 107754. [Google Scholar] [CrossRef] [PubMed]
- Korea Centers for Disease Control and Prevention. Korea National Health and Nutrition Examination Survey (KNHANES 2019); Korea Centers for Disease Control and Prevention: Seoul, Republic of Korea, 2019. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on prevention, Detection, Evaluation, and Treatment of high blood pressure, the 7 report. JAMA 2003, 239, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Appropriate body mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program(NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2011, 285, 2486–2497. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Shigetoh, Y.; Adachi, H.; Yamagishi, S.I.; Enomoto, M.; Fukami, A.; Otsuka, M.; Kumagae, S.I.; Furuki, K.; Nanjo, Y.; Imaizumi, T. Higher heart rate amy predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am. J. Hypertens. 2009, 22, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Bousquet, P.; Elghozi, J.L.; Esler, M.; Grassi, G.; Julius, S.; Reid, J.; Van Zwieten, P.A. The sympathetic nervous system and the metabolic syndrome. J. Hypertens. 2007, 25, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D.E.; Vaile, J.C.; Petley, G.W.; Moore, V.M.; Godsland, I.F.; Cockington, R.A.; Robinson, J.S.; Phillips, D.I. The autonomic control of heart rate and insulin resistance in young adults. J. Clin. Endocrinol. Metab. 1999, 84, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Ó Hartaigh, B.; Vatten, L.J. Resting heart rate and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of cohort studies. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 526–534. [Google Scholar] [CrossRef]
- Yang, W.; Lu, J.; Weng, J.; Jia, W.; Ji, L.; Xiao, J.; Shan, Z.; Liu, J.; Tian, H.; Ji, Q.; et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010, 362, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Yang, H.I.; Park, J.H.; Lee, M.K.; Kang, D.W.; Chae, J.S.; Lee, J.H.; Jeon, J.Y. The association between resting heart rate and type 2 diabetes and hypertension in Korean adults. Heart 2016, 102, 1757–1781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, T.; Zhang, W.; Zhang, M.; Zhang, Y.; Zhang, S. Higher heart rates increase risk of diabetes and cardiovascular events: A prospective cohort study among Inner Mongolians. Diabetes Metab. 2020, 46, 20–26. [Google Scholar] [CrossRef]
- Saito, I.; Maruyama, K.; Kato, T.; Takata, Y.; Tomooka, K.; Kawamura, R.; Osawa, H.; Tanigawa, T. Role of insulin resistance in the association between resting heart rate and type 2 diabetes: A prospective study. J. Diabetes Complicat. 2022, 36, 108319. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Schumacher, H.; Teo, K.K.; Lonn, E.M.; Mahfoud, F.; Ukena, C.; Mann, J.F.E.; Mancia, G.; Redon, J.; Schmieder, R.E.; et al. Resting heart rate and cardiovascular outcomes in diabetic and non-diabetic individuals at high cardiovascular risk: Analysis from the ONTARGET/TRANSCEND trials. Eur. Heart J. 2020, 41, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Vailati, S.; Bertinieri, G.; Seravalle, G.; Stella, M.L.; Dell’Oro, R.; Mancia, G. Heart rate as marker of sympathetic activity. J. Hypertens. 1998, 16, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, A.; Liu, D.M.K.; Kaholokula, J.K.A. Relationship of resting heart rate and physical activity with insulin sensitivity in a population-based survey. J. Diabetes Metab. Disord. 2015, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Human fat cell lipolysis: Biochemistry, regulation and clinical role. Best. Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Julius, S.; Gudbrandsson, T.; Jamerson, K.; Andersson, O. The interconnection between sympathetics, microcirculation, and insulin resistance in hypertension. Blood Press. 1992, 1, 9–19. [Google Scholar] [CrossRef]
- Perin, P.C.; Maule, S.; Quadri, R. Sympathetic nervous system, diabetes, and hypertension. Clin. Exp. Hypertens. 2001, 23, 45–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).