Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review
Abstract
:1. Introduction
2. Search Strategy
3. Whole-Body Washdown
4. Regional Sweat Patches
5. Future Directions and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tyler, C.J.; Reeve, T.; Hodges, G.J.; Cheung, S.S. The Effects of Heat Adaptation on Physiology, Perception and Exercise Performance in the Heat: A Meta-Analysis. Sports Med. 2016, 46, 1699–1724. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.H.; Deakin, G.B.; Edwards, A.M.; Miller, C.M.; Pyne, D.B. Adaptation to hot environmental conditions: An exploration of the performance basis, procedures and future directions to optimise opportunities for elite athletes. Sports Med. 2015, 45, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.R.; Barton, C.; Morrissey, D.; Maffulli, N.; Hemmings, S. Pre-cooling for endurance exercise performance in the heat: A systematic review. BMC Med. 2012, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Romanovsky, A.A. Thermoregulation: Some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R37–R46. [Google Scholar] [CrossRef]
- Zhao, Z.-D.; Yang, W.Z.; Gao, C.; Fu, X.; Zhang, W.; Zhou, Q.; Chen, W.; Ni, X.; Lin, J.-K.; Yang, J.; et al. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA 2017, 114, 2042–2047. [Google Scholar] [CrossRef]
- Baker, L.B.; De Chavez, P.J.D.; Ungaro, C.T.; Sopeña, B.C.; Nuccio, R.P.; Reimel, A.J.; Barnes, K.A. Exercise intensity effects on total sweat electrolyte losses and regional vs. whole-body sweat [Na+], [Cl−], and [K+]. Eur. J. Appl. Physiol. 2019, 119, 361–375. [Google Scholar] [CrossRef]
- National Research Council (US). Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. In Recommended Dietary Allowances, 10th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1989. Available online: http://www.ncbi.nlm.nih.gov/books/NBK234932/ (accessed on 6 March 2023).
- Medicine, A.C.O.S.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar]
- Casa, D.J.; Clarkson, P.M.; Roberts, W.O. American College of Sports Medicine Roundtable on Hydration and Physical Activity: Consensus Statements. Curr. Sports Med. Rep. 2005, 4, 115–127. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2004; 617p. [Google Scholar]
- Sawka, M.N.; Montain, S.J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 2000, 72, 564S–572S. [Google Scholar] [CrossRef]
- Senay, L.C.; González-Alonso, J.; Mora-Rodríguez, R.; Below, P.R.; Coyle, E.F. Relationship of evaporative rates to serum [Na+], [K+], and osmolarity in acute heat stress. J. Appl. Physiol. 1968, 25, 149–152. [Google Scholar] [CrossRef]
- Kubica, R.; Nielsen, B.; Bonnesen, A.; Rasmussen, I.B.; Stokłosa, J.; Wilk, B. Relationship between plasma volume reduction and plasma electrolyte changes after prolonged bicycle exercise, passive heating and diuretic dehydration. Acta Physiol. Pol. 1983, 34, 569–579. [Google Scholar] [PubMed]
- Costill, D.L.; Coté, R.; Fink, W.J.; Van Handel, P. Muscle water and electrolyte distribution during prolonged exercise. Int. J. Sports Med. 1981, 2, 130–134. [Google Scholar] [CrossRef]
- Miller, K.C.; McDermott, B.P.; Yeargin, S.W.; Fiol, A.; Schwellnus, M.M.P. An Evidence-Based Review of the Pathophysiology, Treatment, and Prevention of Exercise-Associated Muscle Cramps. J. Athl. Train. 2022, 57, 5–15. [Google Scholar] [CrossRef]
- Lorenzo, I.; Serra-Prat, M.; Yébenes, J.C. The Role of Water Homeostasis in Muscle Function and Frailty: A Review. Nutrients 2019, 11, 1857. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M. Muscle Cramping During Exercise: Causes, Solutions, and Questions Remaining. Sports Med. 2019, 49 (Suppl. S2), 115–124. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: A hierarchy of competing physiological needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK535346/ (accessed on 7 November 2023).
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, K.; Ohtsuyama, M.; Samman, G. Eccrine sweat gland disorders. J. Am. Acad. Dermatol. 1991, 24 Pt 1, 1010–1014. [Google Scholar] [CrossRef]
- Sato, K. The mechanism of eccrine sweat secretion. In Exercise, Heat, and Thermoregulation; Brown & Benchmark: Dubuque, IA, USA, 1993; pp. 85–117. [Google Scholar]
- Inoue, R.; Sohara, E.; Rai, T.; Satoh, T.; Yokozeki, H.; Sasaki, S.; Uchida, S. Immunolocalization and translocation of aquaporin-5 water channel in sweat glands. J. Dermatol. Sci. 2013, 70, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Kwon, T.-H.; Jensen, U.B.; Fumagalli, O.; Frøkiaer, J.; Krane, C.M.; Menon, A.G.; King, L.S.; Agre, P.C.; Nielsen, S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc. Natl. Acad. Sci. USA 2002, 99, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jin, L.; Feng, J.; Lv, J. The Expression of AQP5 and UTs in the Sweat Glands of Uremic Patients. BioMed Res. Int. 2017, 2017, 8629783. [Google Scholar] [CrossRef]
- Scott, J.H.; Menouar, M.A.; Dunn, R.J. Physiology, Aldosterone; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470339/ (accessed on 1 May 2023).
- Montain, S.J.; Laird, J.E.; Latzka, W.A.; Sawka, M.N. Aldosterone and vasopressin responses in the heat: Hydration level and exercise intensity effects. Med. Sci. Sports Exerc. 1997, 29, 661–668. [Google Scholar] [CrossRef]
- Patel, P.N.; Horenstein, M.S.; Zwibel, H. Exercise Physiology; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482280/ (accessed on 6 October 2024).
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Stofan, J.R.; Hamilton, A.A.; Horswill, C.A. Comparison of regional patch collection vs. whole body washdown for measuring sweat sodium and potassium loss during exercise. J. Appl. Physiol. 2009, 107, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Casa, D.J. Methods to Evaluate Electrolyte and Water Turnover of Athletes. Athl. Train. Sports Health Care 2009, 1, 169–179. [Google Scholar] [CrossRef]
- Patterson, M.J.; Galloway, S.D.; Nimmo, M.A. Variations in regional sweat composition in normal human males. Exp. Physiol. 2000, 85, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; De Chavez, P.J.D.; Nuccio, R.P.; Brown, S.D.; King, M.A.; Sopeña, B.C.; Barnes, K.A. Explaining variation in sweat sodium concentration: Effect of individual characteristics and exercise, environmental, and dietary factors. J. Appl. Physiol. 2022, 133, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Weschler, L.B. Sweat electrolyte concentrations obtained from within occlusive coverings are falsely high because sweat itself leaches skin electrolytes. J. Appl. Physiol. 2008, 105, 1376–1377. [Google Scholar] [CrossRef]
- Cole, D.E.; Boucher, M.J. Use of a new sample-collection device (Macroduct) in anion analysis of human sweat. Clin. Chem. 1986, 32, 1375–1378. [Google Scholar] [CrossRef]
- Lemon, P.W.; Yarasheski, K.E.; Dolny, D.G.; Brown, M.B.; Haack, K.K.V.; Pollack, B.P.; Millard-Stafford, M.; McCarty, N.A.; Baker, L.B.; Stofan, J.R.; et al. Validity/reliability of sweat analysis by whole-body washdown vs. regional collections. J. Appl. Physiol. 1986, 61, 1967–1971. [Google Scholar] [CrossRef]
- Gibson, L.E.; Cooke, R.E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959, 23, 545–549. [Google Scholar] [CrossRef]
- Barnes, K.A.; Anderson, M.L.; Stofan, J.R.; Dalrymple, K.J.; Reimel, A.J.; Roberts, T.J.; Randell, R.K.; Ungaro, C.T.; Baker, L.B. Normative data for sweating rate, sweat sodium concentration, and sweat sodium loss in athletes: An update and analysis by sport. J. Sports Sci. 2019, 37, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Stofan, J.R.; Lukaski, H.C.; Horswill, C.A. Exercise-induced trace mineral element concentration in regional versus whole-body wash-down sweat. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Kolding, M.; Leslie, E.; Moreno, D.; Norwood, S.; Ordille, A.; Weller, R. Heat acclimation causes a linear decrease in sweat sodium ion concentration. J. Therm. Biol. 2018, 71, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Barnes, K.A.; Anderson, M.L.; Passe, D.H.; Stofan, J.R. Normative data for regional sweat sodium concentration and whole-body sweating rate in athletes. J. Sports Sci. 2016, 34, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.L.; Sekiguchi, Y.; Struder, J.F.; Szymanski, M.R.; Manning, C.N.; Grundstein, A.J.; Lee, E.C.; Huggins, R.A.; Armstrong, L.E.; Casa, D.J. Heat Acclimation Following Heat Acclimatization Elicits Additional Physiological Improvements in Male Endurance Athletes. Int. J. Environ. Res. Public Health 2021, 18, 4366. [Google Scholar] [CrossRef]
- Benjamin, C.L.; Sekiguchi, Y.; Armstrong, L.E.; Manning, C.N.; Struder, J.F.; Butler, C.R.; Huggins, R.A.; Stearns, R.L.; Lee, E.C.; Casa, D.J. The efficacy of weekly and bi-weekly heat training to maintain the physiological benefits of heat acclimation. J. Sci. Med. Sport 2022, 25, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; Hubbard, R.W.; DeLUCA, J.P.; Christensen, E.L. Heat acclimatization during summer running in the northeastern United States. Med. Sci. Sports Exerc. 1987, 19, 131–136. [Google Scholar] [CrossRef]
- Kaufman, F.; Mills, D.; Hughson, R.; Peake, G. Effects of bromocriptine on sweat gland function during heat acclimatization. Horm. Res. 1988, 29, 31–38. [Google Scholar] [CrossRef]
- Buono, M.J.; Ball, K.D.; Kolkhorst, F.W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 2007, 103, 990–994. [Google Scholar] [CrossRef]
- Inoue, Y.; Havenith, G.; Kenney, W.L.; Loomis, J.L.; Buskirk, E.R. Exercise- and methylcholine-induced sweating responses in older and younger men: Effect of heat acclimation and aerobic fitness. Int. J. Biometeorol. 1999, 42, 210–216. [Google Scholar] [CrossRef]
- Karlsen, A.; Nybo, L.; Nørgaard, S.J.; Jensen, M.V.; Bonne, T.; Racinais, S. Time course of natural heat acclimatization in well-trained cyclists during a 2-week training camp in the heat. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S1), 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.R.; Convertino, V.A.; Tucker, M.A.; Six, A.; Moyen, N.E.; Satterfield, A.Z.; Ganio, M.S.; Keiser, S.; Flück, D.; Hüppin, F.; et al. Plasma aldosterone and sweat sodium concentrations after exercise and heat acclimation. J. Appl. Physiol. 1986, 61, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Klous, L.; de Ruiter, C.; Alkemade, P.; Daanen, H.; Gerrett, N. Sweat rate and sweat composition following active or passive heat re-acclimation: A pilot study. Temperature 2020, 8, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Klous, L.; De Ruiter, C.; Alkemade, P.; Daanen, H.; Gerrett, N. Sweat rate and sweat composition during heat acclimation. J. Therm. Biol. 2020, 93, 102697. [Google Scholar] [CrossRef]
- Magalhães, F.C.; Passos, R.L.F.; Fonseca, M.A.; Oliveira, K.P.M.; Ferreira-Júnior, J.B.; Martini, A.R.P.; Lima, M.R.M.; Guimarães, J.B.; Baraúna, V.G.; Silami-Garcia, E.; et al. Thermoregulatory efficiency is increased after heat acclimation in tropical natives. J. Physiol. Anthr. 2010, 29, 1–12. [Google Scholar] [CrossRef]
- Marshall, H.C.; Campbell, S.A.; Roberts, C.W.; Nimmo, M.A. Human physiological and heat shock protein 72 adaptations during the initial phase of humid-heat acclimation. J. Therm. Biol. 2007, 32, 341–348. [Google Scholar] [CrossRef]
- McCleave, E.L.; Slattery, K.M.; Duffield, R.; Saunders, P.U.; Sharma, A.P.; Crowcroft, S.; Coutts, A.J. Impaired Heat Adaptation From Combined Heat Training and “Live High, Train Low” Hypoxia. Int. J. Sports Physiol. Perform. 2019, 14, 635–643. [Google Scholar] [CrossRef]
- Mikkelsen, C.J.; Junge, N.; Piil, J.F.; Morris, N.B.; Oberholzer, L.; Siebenmann, C.; Lundby, C.; Nybo, L. Prolonged Heat Acclimation and Aerobic Performance in Endurance Trained Athletes. Front. Physiol. 2019, 10, 1372. [Google Scholar] [CrossRef]
- Petersen, C.J.; Portus, M.R.; Pyne, D.B.; Dawson, B.T.; Cramer, M.N.; Kellett, A.D. Partial heat acclimation in cricketers using a 4-day high intensity cycling protocol. Int. J. Sports Physiol. Perform. 2010, 5, 535–545. [Google Scholar] [CrossRef]
- Rendell, R.A.; Prout, J.; Costello, J.T.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effects of 10 days of separate heat and hypoxic exposure on heat acclimation and temperate exercise performance. Am. J. Physiol. Integr. Comp. Physiol. 2017, 313, R191–R201. [Google Scholar] [CrossRef] [PubMed]
- Saat, M.; Sirisinghe, R.G.; Singh, R.; Tochihara, Y. Effects of short-term exercise in the heat on thermoregulation, blood parameters, sweat secretion and sweat composition of tropic-dwelling subjects. J. Physiol. Anthr. Appl. Hum. Sci. 2005, 24, 541–549. [Google Scholar] [CrossRef]
- Willmott, A.G.B.; Hayes, M.; James, C.A.; Gibson, O.R.; Maxwell, N.S. Heat acclimation attenuates the increased sensations of fatigue reported during acute exercise-heat stress. Temperature 2019, 7, 178–190. [Google Scholar] [CrossRef]
- Roussey, G.; Bernard, T.; Fontanari, P.; Louis, J. Heat acclimation training with intermittent and self-regulated intensity may be used as an alternative to traditional steady state and power-regulated intensity in endurance cyclists. J. Therm. Biol. 2021, 98, 102935. [Google Scholar] [CrossRef]
- Racinais, S.; Mohr, M.; Buchheit, M.; Voss, S.C.; Gaoua, N.; Grantham, J.; Nybo, L. Individual responses to short-term heat acclimatisation as predictors of football per-formance in a hot, dry environment. Br. J. Sports Med. 2012, 46, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Racinais, S.; Buchheit, M.; Bilsborough, J.; Bourdon, P.C.; Cordy, J.; Coutts, A.J. Physiological and performance responses to a training camp in the heat in professional Australian football players. Int. J. Sports Physiol. Perform. 2014, 9, 598–603. [Google Scholar] [CrossRef]
- Tan, S.C.C.; Ang, W.H.; Lim, L.S.X.; Low, I.C.C.; Lee, J.K.W. Efficacy of Isothermic Conditioning over Military-Based Heat Acclimatization and Interval Training in Tropical Native Males. Med. Sci. Sports Exerc. 2022, 54, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Zurawlew, M.J.; Mee, J.A.; Walsh, N.P. Post-exercise Hot Water Immersion Elicits Heat Acclimation Adaptations That Are Retained for at Least Two Weeks. Front. Physiol. 2019, 10, 1080. [Google Scholar] [CrossRef]
- Taylor, N.A.; A Machado-Moreira, C. Regional variations in transepidermal water loss, eccrine sweat gland density, sweat secretion rates and electrolyte composition in resting and exercising humans. Extrem. Physiol. Med. 2013, 2, 4. [Google Scholar] [CrossRef]
- Bijker, K.; De Groot, G.; Hollander, A. Differences in leg muscle activity during running and cycling in humans. Eur. J. Appl. Physiol. 2002, 87, 556–561. [Google Scholar]
- Hori, S. Adaptation to heat. Jpn. J. Physiol. 1995, 45, 921–946. [Google Scholar] [CrossRef] [PubMed]
- Saat, M.; Tochihara, Y.; Hashiguchi, N.; Sirisinghe, R.G.; Fujita, M.; Chou, C.M. Effects of exercise in the heat on thermoregulation of Japanese and Malaysian males. J. Physiol. Anthr. Appl. Hum. Sci. 2005, 24, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Brown, A.M.; Quiñones-González, J.R. Normative Data for Sweat Rate and Whole-Body Sodium Concentration in Athletes Indigenous to Tropical Climate. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Tebeck, S.T.; Buckley, J.D.; Bellenger, C.R.; Stanley, J. Differing Physiological Adaptations Induced by Dry and Humid Short-Term Heat Acclimation. Int. J. Sports Physiol. Perform. 2020, 15, 133–140. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, K.; Raj, M.S.; Lee, B.; Reeder, J.T.; Koo, J.; Hourlier-Fargette, A.; Bandodkar, A.J.; Won, S.M.; Sekine, Y.; et al. Soft, Skin-Interfaced Microfluidic Systems with Wireless, Battery-Free Electronics for Digital, Real-Time Tracking of Sweat Loss and Electrolyte Composition. Small 2018, 14, e1802876. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P.; et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Rodin, D.; Shapiro, Y.; Pinhasov, A.; Kreinin, A.; Kirby, M. An accurate wearable hydration sensor: Real-world evaluation of practical use. PLoS ONE 2022, 17, e0272646. [Google Scholar] [CrossRef]
- Choi, J.; Ghaffari, R.; Baker, L.B.; Rogers, J.A. Skin-interfaced systems for sweat collection and analytics. Sci. Adv. 2018, 4, eaar3921. [Google Scholar] [CrossRef]
- Basheer, A. The Art and Science of Writing Narrative Reviews. Int. J. Adv. Med. Health Res. 2022, 9, 124–126. [Google Scholar] [CrossRef]


| Study | Participant Characteristics | HA Classification | Sweat Electrolyte Assessment | Na+ Sweat Conc. | Cl− Sweat Conc. | K+ Sweat Conc. |
|---|---|---|---|---|---|---|
| Buono et al. (2018) [40] | 4 healthy individuals (f = 1) | 7-day HA | Forearm regional sweat collector |  | -- | -- |
| Benjamin et al. (2021) [42] | 24 male endurance athletes | 4-month HAz + 5-days HA | WBW | 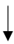 | 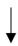 |  |
| Benjamin et al. (2022) [43] | 24 male endurance athletes | 4- and 8-wk IHT after 5-day HA | WBW |  |  |  |
| Armstrong (1987) [44] | 5 highly trained distance runners (f = 1) | 14.5-wk HAz | WBW |  | -- |  |
| Kaufman et al. (1988) [45] | 8 healthy males | 10-day HA | WBW | 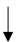 | -- |  |
| Buono et al. (2007) [46] | 8 healthy males | 10-day HA | Chest regional sweat collector |  | -- | -- |
| Inoue et al. (1999) [47] | 5 young, 4 highly fit old, and 5 normally fit old males | 8-day HA | Chest, back, forearm, &and thigh sweat patch |  | -- | -- |
| Karlsen et al. (2015) [48] | 9 trained cyclists | 2-wk HAz | Back sweat patch |  | -- | -- |
| Kirby and Convertino (1986) [49] | 10 healthy males | 10-day HA | Chest regional sweat patch |  | -- | -- |
| Klous et al. (2020b) [50] | 15 healthy participants (f = 5) | 10-day HA | Back and arm sweat patch |  |  |  |
| Klous et al. (2020a) [51] | 8 healthy participants (f = 2) | 10-day HA | Back and arm sweat patch |  |  |  |
| Magalhães et al. (2010) [52] | 9 male tropical natives | 11-day HA | Forehead, chest, arm, forearm, and thigh sweat patch |  | -- | -- |
| Marshall et al. (2007) [53] | 7 healthy males | 3-day HA | Forearm sweat patch |  |  | -- |
| McCleave et al. (2019) [54] | 9 trained male and female runners (f = 3) | 3-wk heat training | Back sweat patch | 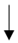 | -- | -- |
| Mikkelsen et al. (2019) [55] | 12 male sub-elite cyclists | 5.5-wk HA | Back sweat patch | 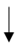 | -- | -- |
| Petersen et al. (2010) [56] | 6 male cricket players | 4-day HA | Upper forearm sweat patch |  | 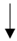 | 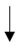 |
| Rendell et al. (2017) [57] | 8 healthy males | 11-day HA | Back sweat patch |  | -- | -- |
| Saat et al. (2005) [58] | 16 Malaysian-Malay males | 2-wk HA | Back sweat patch |  | -- |  |
| Willmott et al. (2019) [59] | 20 healthy males | 5- and 10-day HA | Unspecified regional sweat patch | 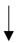 | -- | -- |
| Roussey et al. (2021) [60] | 17 male competitive-level athletes | 5-day HA | Back sweat patch |  | -- | -- |
| Racinais et al. (2012) [61] | 19 male semiprofessional soccer players | 6-day HAz | Back sweat patch |  | -- | -- |
| Racinais et al. (2014) [62] | 18 male Australian football players | 2-wk HAz | Back sweat patch |  | -- | -- |
| Tan et al. (2022) [63] | 51 untrained tropical native males | 10-day HA | Thigh sweat patch |  | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keefe, M.S.; Benjamin, C.L.; Casa, D.J.; Sekiguchi, Y. Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review. Appl. Sci. 2024, 14, 10103. https://doi.org/10.3390/app142210103
Keefe MS, Benjamin CL, Casa DJ, Sekiguchi Y. Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review. Applied Sciences. 2024; 14(22):10103. https://doi.org/10.3390/app142210103
Chicago/Turabian StyleKeefe, Marcos S., Courteney L. Benjamin, Douglas J. Casa, and Yasuki Sekiguchi. 2024. "Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review" Applied Sciences 14, no. 22: 10103. https://doi.org/10.3390/app142210103
APA StyleKeefe, M. S., Benjamin, C. L., Casa, D. J., & Sekiguchi, Y. (2024). Importance of Electrolytes in Exercise Performance and Assessment Methodology After Heat Training: A Narrative Review. Applied Sciences, 14(22), 10103. https://doi.org/10.3390/app142210103








