Research on Solidification Methods and Stabilization Mechanisms of Sulfate Saline Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Saline Soils
2.2. Fly Ash, Lime, PAM and Sodium Sulfate
3. Experimental Design and Methods
3.1. Sample Preparation
3.2. Unconfined Compressive Strength and Splitting Strength Test
3.3. Frost Resistance Test
3.4. Material Characterization
4. Results and Analysis
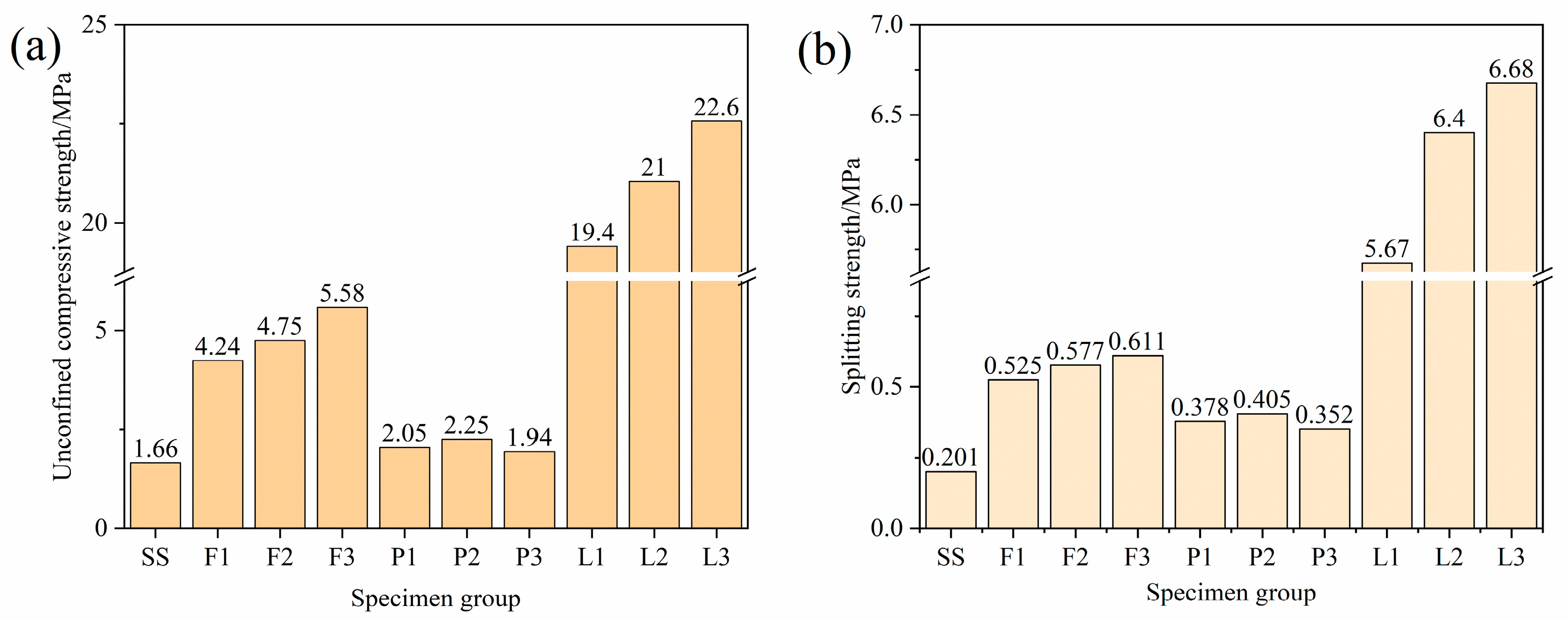
4.1. Mechanical Performance Analysis
4.2. Frost Resistance
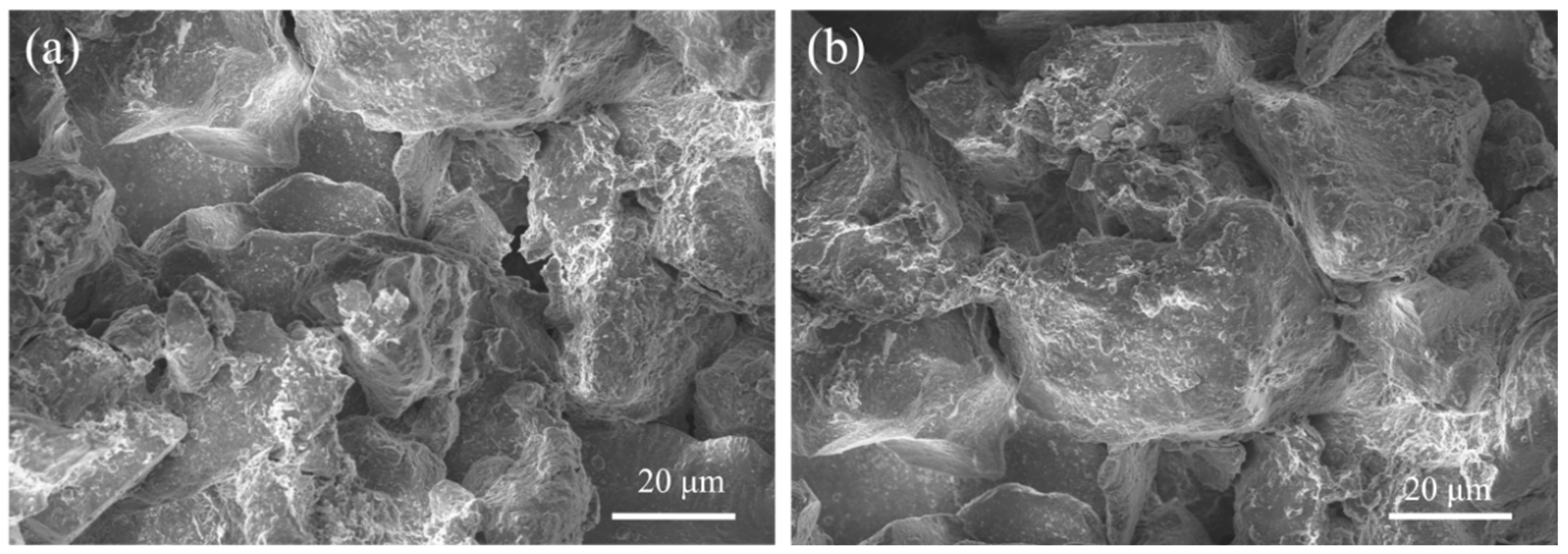
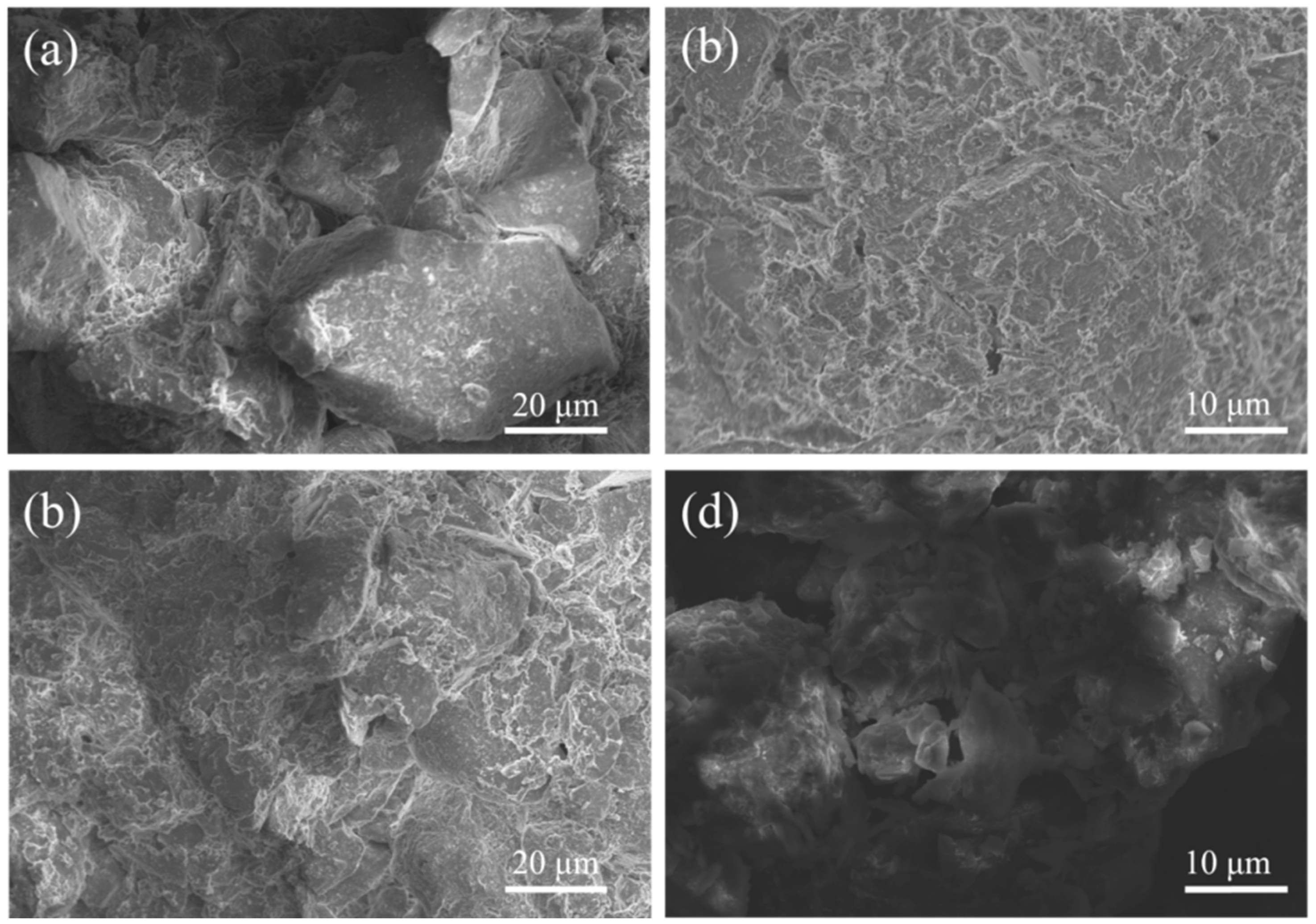
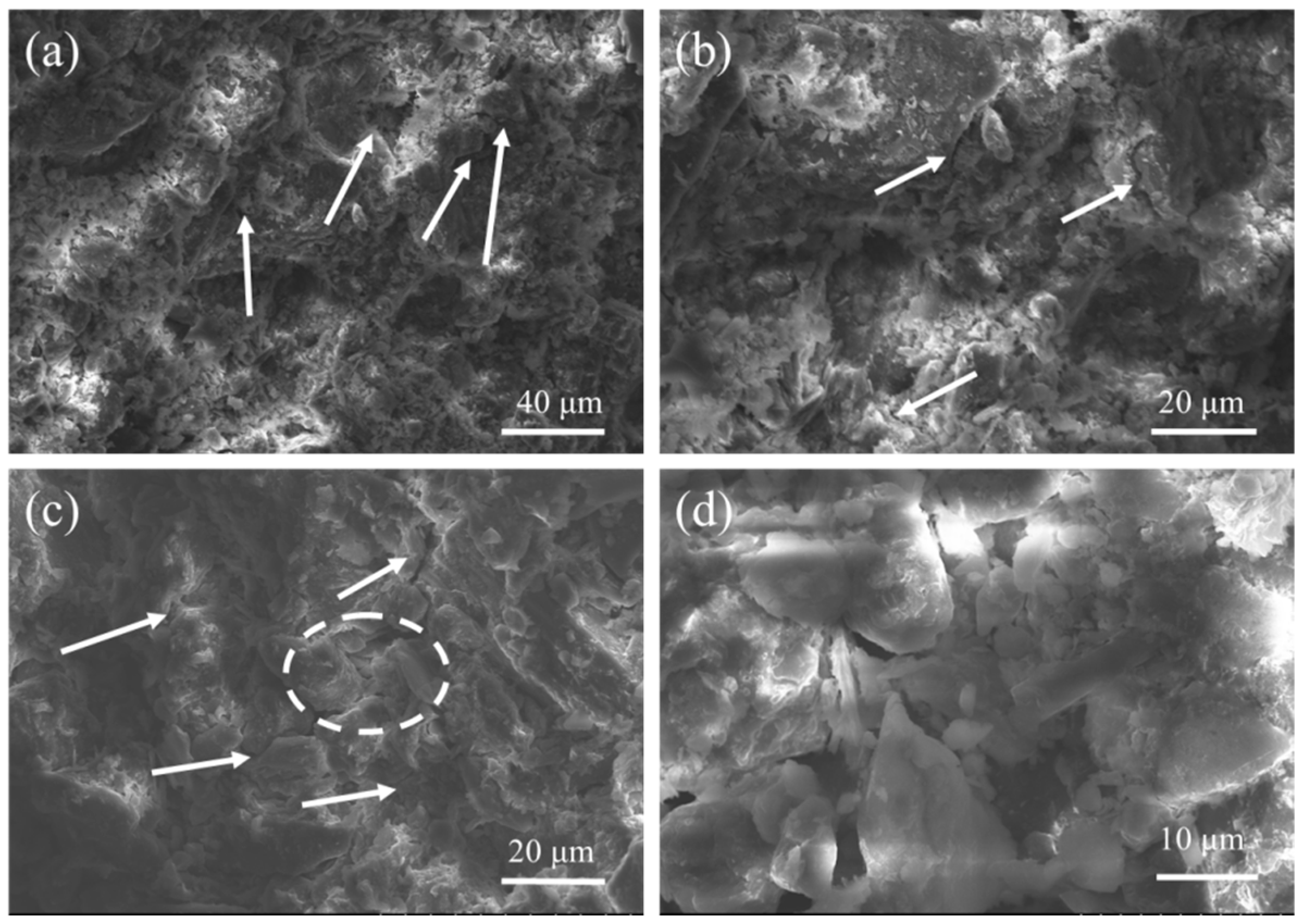
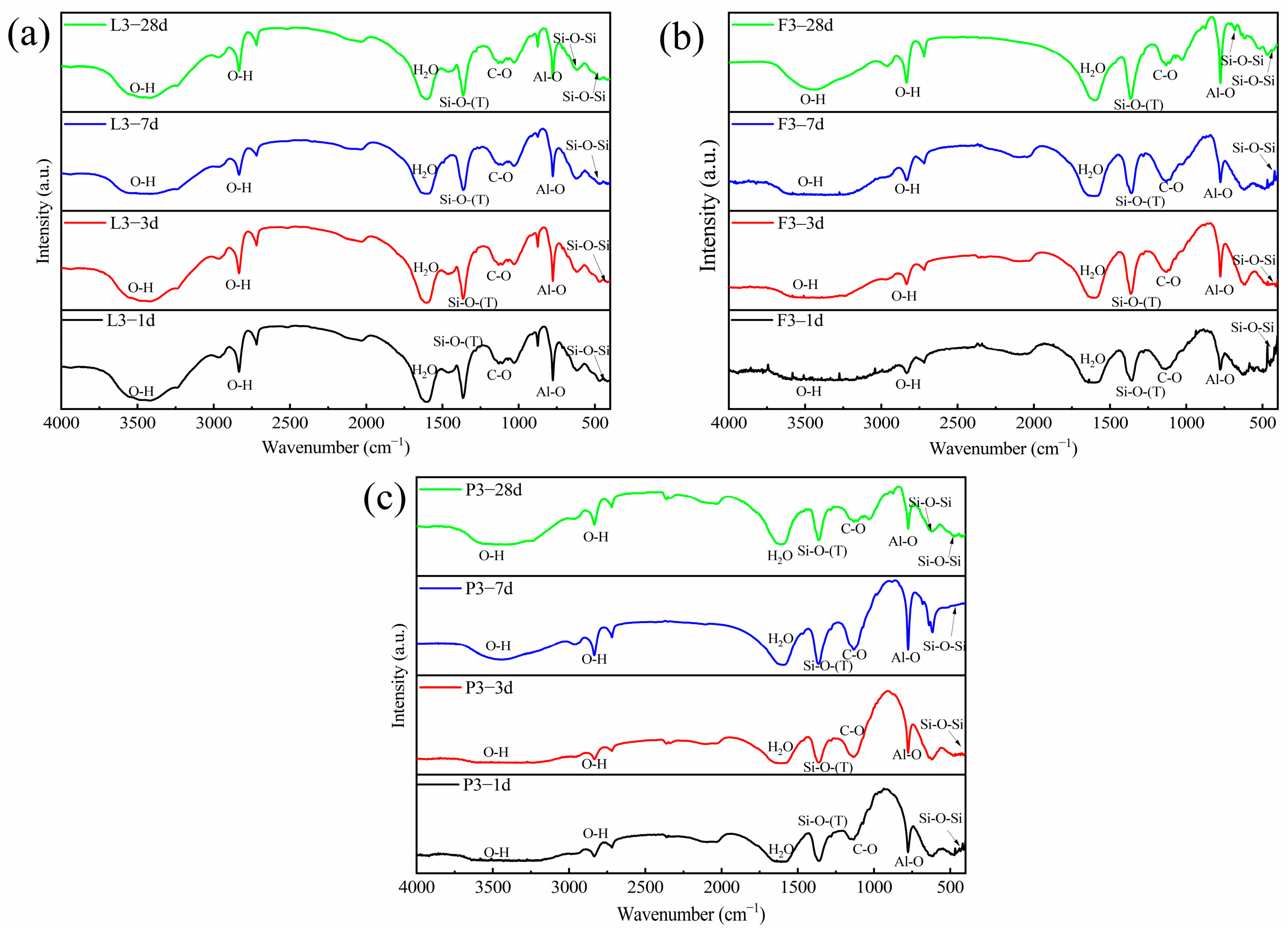
4.3. Microstructure Analysis of Stabilized Soils
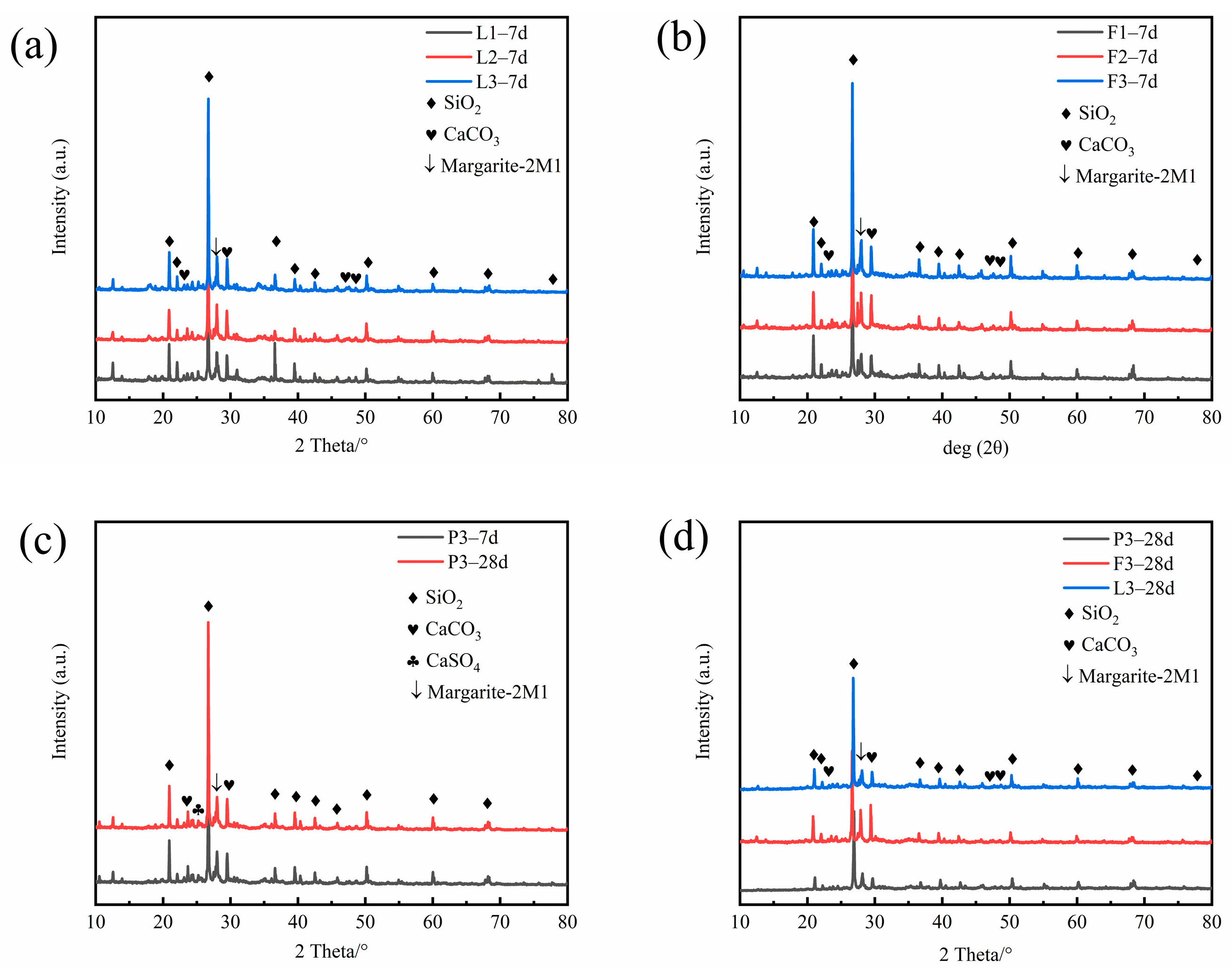
4.3.1. XRD Analysis
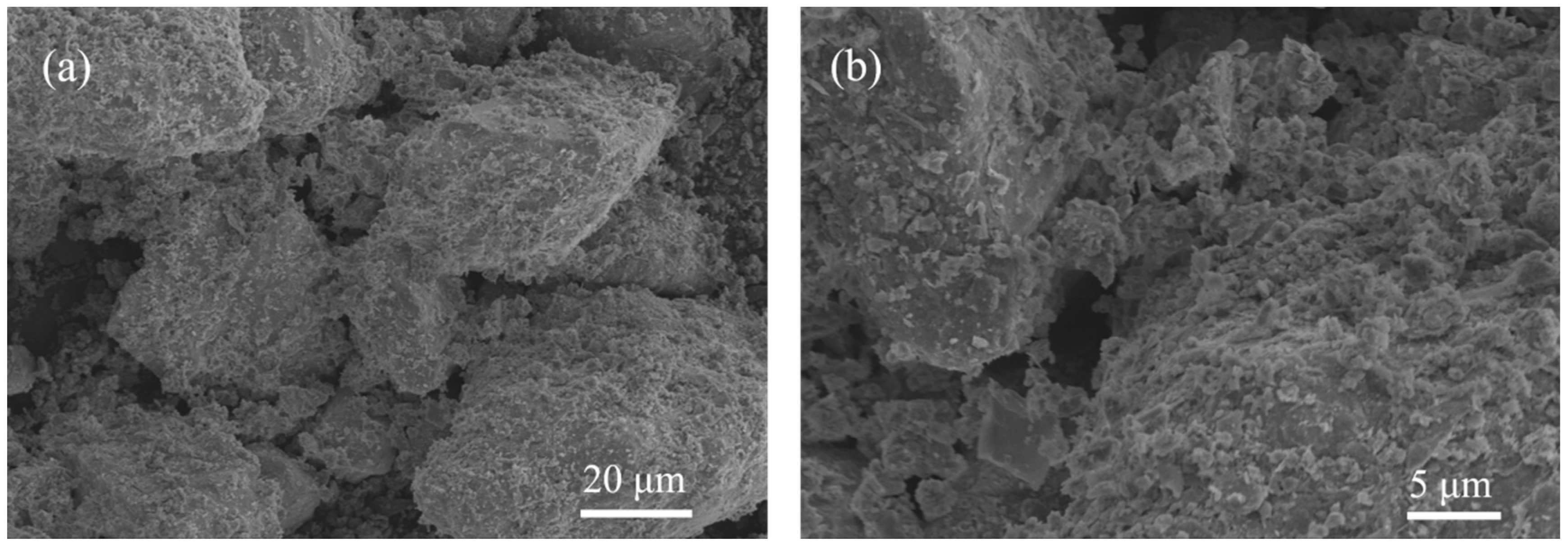
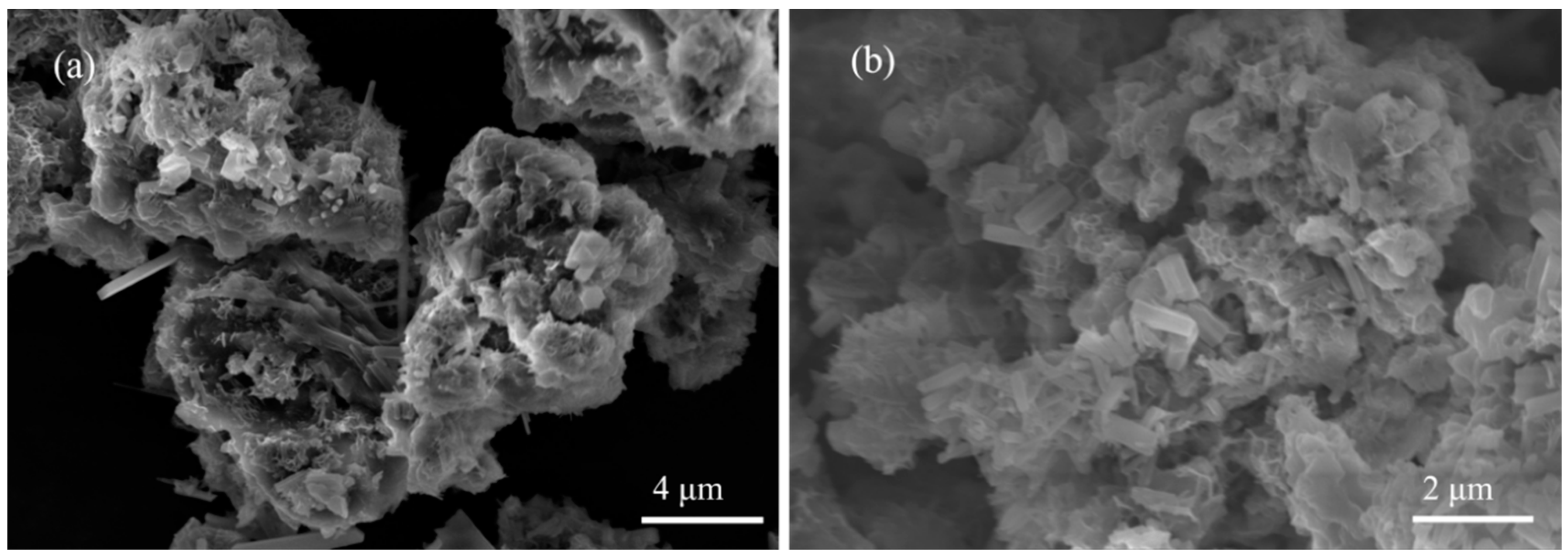
4.3.2. SEM Analysis
4.3.3. FTIR Analysis
4.3.4. TG Analysis
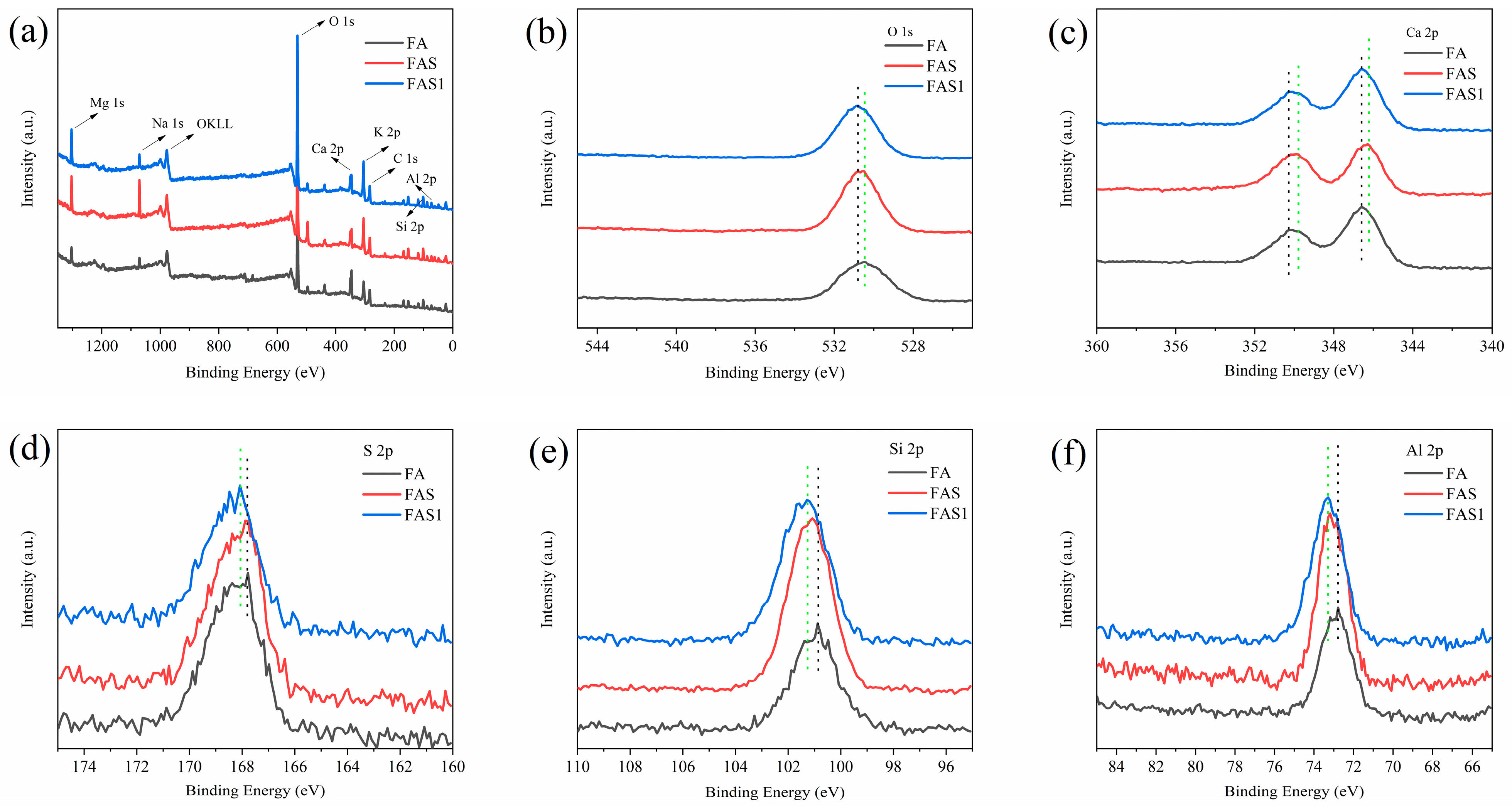
4.3.5. XPS Analysis
5. Discussions and Conclusions
5.1. Discussions
5.2. Conclusions
- (1)
- The addition of lime, FA, and PAM significantly increased the strength of saline soils. The UCS and splitting strength of stabilized saline soils gradually increased with an increase in binding material content. The best performance was obtained for lime, followed by FA, and the worst performance was obtained for PAM. The results of XRD, FTIR, SEM, and TG−DSC analyses showed that lime chemically solidified the soil by generating CaSO4 and CaCO3 as binding substances through ion exchange and carbonation.
- (2)
- The number of freeze–thaw cycles has a greater effect on the strength of stabilized soil; with an increase in the number of freeze–thaw cycles, the UCS and splitting residual value of stabilized soil decrease. SEM test results show that the internal structure of stabilized soil is more compact before freeze–thaw cycles. With an increase in the number of freeze–thaw cycles, the porosity of the stabilized soil increased, the contact between soil particles began to change, and the structure became loose; after 20 freeze–thaw cycles, a small amount of voids appeared in the lime-stabilized soil, a large crack appeared in the FA-stabilized soil, and the structure of the PAM-stabilized soil was destroyed.
- (3)
- Saline soils have a granular structure, and the contact mode is point to point. Lime-stabilized soil changes the cementing substances from Ca(OH)2 and Na2SO4 to CaSO4 and CaCO3 through ion exchange and carbonation, which fill the pores between soil particles and change the contact between particles to face-to-face contact, thus enhancing the mechanical properties of saline soils.
- (4)
- XRD, SEM, FTIR, and TG analyses of FA-stabilized soil were consistent with the observed mechanical properties. The hydration of FA to form a C−(A)−S−H gel altered the microstructure of saline soils.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, Y.; Yang, F.; Wang, Z.; Shao, X.; Gneg, Y. Progresaline soil of research on the improvement of saline-sodic soil by acidic substances. Chin. J. Eco-Agric. 2022, 31, 373–384. [Google Scholar] [CrossRef]
- Qadir, M.; Tubeileh, A.; Akhtar, J.; Larbi, A.; Minhas, P.S.; Khan, M.A. Productivity enhancement of salt-affected environments through crop diversification. Land Degrad. Dev. 2008, 19, 429–453. [Google Scholar] [CrossRef]
- Yang, J.; Yao, R.; Wang, X.; Xie, W.; Zhang, X.; Zhu, W.; Zhang, L.; Sun, R. Salt-affected Soils in China: History, Status Quo and Prospect. Acta Pedol. Sin. 2022, 59, 10–27. [Google Scholar] [CrossRef]
- Pan, X.P.; Li, G.L. Experimental Research on Strength Characteristics of Highway Cured Saline soil Subgrade. Highw. Traffic Sci. Technol. Appl. Technol. Ed. 2009, 5, 83–85. [Google Scholar]
- Xing, G.; Zhang, L.; Xuan, W.; Pan, Y.; Zhao, Y.; Zhang, B. Influence of Alkaline Activators on Unconfined Compresaline soilive Strength of Saline Soils Stabilised with Ground Granulated Blast Furnace Slags. Adv. Civ. Eng. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Lai, Y.; Wu, D.; Zhang, M. Crystallization deformation of a saline soil during freeze-thaw procesaline soiles. Appl. Therm. Eng. 2017, 120, 463–473. [Google Scholar] [CrossRef]
- Wan, X.; Hu, Q.; Liao, M. Salt crystallization in cold sulfate saline soil. Cold Reg. Sci. Technol. 2017, 137, 36–47. [Google Scholar] [CrossRef]
- Zhang, J.; Pei, X.; Wei, L. Research on Salt Swelling Inhibitors for Cement Reinforcement of Sulphate Saline soil. J. Geotech. Eng. 2018, 40, 155–161. [Google Scholar]
- Zhang, H.O. Distribution and evolution of saline soils in China. Agric. Technol. 2022, 42, 104–107. [Google Scholar] [CrossRef]
- Song, J.T. Research on Improved Utilisation of Fill Material for Saline Soil Roadbase. Master’s Thesis, Chang’an University, Xi’an, China, 2009. [Google Scholar]
- Zhou, G. Research on Microscopic Characteristics of Solidified Sulphate Saline Soil. Master’s Thesis, Lanzhou University, Lanzhou, China, 2018. [Google Scholar]
- Xian, S.; Lu, Z.; Yao, H.; Fang, R.; She, J. Comparative Study on Mechanical Properties of Compacted Clay under Freeze–Thaw Cycles with Closed and Open Systems. Adv. Mater. Sci. Eng. 2019, 2019, 9206372. [Google Scholar] [CrossRef]
- Aksakal, E.L.; Angin, I.; Sari, S. Effects of freeze–thaw cycles on consistency limits of soils amended with diatomite. Soil Tillage Res. 2021, 213, 105144. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, S.; ShangGuan, Y.; Fu, H.; Ma, B.; Chen, H.; Yuan, X. Experimental investigation of the geotechnical properties and microstructure of lime-stabilized saline soils under freeze-thaw cycling. Cold Reg. Sci. Technol. 2019, 161, 32–42. [Google Scholar] [CrossRef]
- Zimar, Z.; Robert, D.; Zhou, A.; Giustozzi, F.; Setunge, S.; Kodikara, J. Application of coal fly ash in pavement subgrade stabilisation: A review. J. Environ. Manag. 2022, 312, 114926. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, C.S.; Liu, F.; Fan, F. Feasibility Study of Loesaline soil Stabilization with Fly Ash–Based Geopolymer. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Shi, X. Reactivity of coal fly ash used in cementitious binder systems: A state-of-the-art overview. Fuel 2021, 301. [Google Scholar] [CrossRef]
- Yang, S.; Liu, W. The Effect of Changing Fly Ash Content on the Modulus of Compresaline soilion of Stabilized Soil. Materials 2019, 12, 2925. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Rout, P.K. A Review of Coal Fly Ash Utilization to Save the Environment. Water Air Soil Pollut. 2023, 234, 128. [Google Scholar] [CrossRef]
- Awn, S.H.A.; Abbas, H.O. Study the posaline soilibility of treating saline soils using cement-activated fly ash. Arab. J. Geosci. 2023, 16, 647. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Li, S.; Shan, L.; Zhang, Z.; Wang, Z. Characterization and mechanism study of sulfate saline soil solidification in seasonal frozen regions using ternary solid waste-cement synergy. Constr. Build. Mater. 2024, 427. [Google Scholar] [CrossRef]
- Ying, Z.; Cui, Y.-J.; Benahmed, N.; Duc, M. Changes in microstructure and water retention property of a lime-treated saline soil during curing. Acta Geotech. 2021, 17, 319–326. [Google Scholar] [CrossRef]
- Chen, K.; Huang, S.; Liu, Y.; Ding, L. Improving Carbonate Saline Soil in a Seasonally Frozen Region Using Lime and Fly Ash. Geofluids 2022, 2022, 7472284. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, A.; Deng, Y.; Cui, Y.; Yu, Z.; Yu, C. Strength performance of cement/slag-based stabilized soft clays. Constr. Build. Mater. 2019, 211, 909–918. [Google Scholar] [CrossRef]
- Xu, T.; Zhou, Z.; Wang, M.; Zhu, L.; Tian, Y.; Han, D. Damage mechanism of pier concrete subjected to combined compressive stress, freeze-thaw, and salt attacks in saline soil. Constr. Build. Mater. 2022, 324, 126567. [Google Scholar] [CrossRef]
- Zhi, F.; Jiang, Y.; Guo, M.-Z.; Jin, W.; Yan, X.; Zhu, P.; Jiang, L. Effect of polyacrylamide on the carbonation behavior of cement paste. Cem. Concr. Res. 2022, 156, 106756. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Di, H.; Liu, D.; Wang, X.; Zhao, Y. Influence of anionic polyacrylamide on the freeze–thaw resistance of silty clay. Cold Reg. Sci. Technol. 2024, 219, 104111. [Google Scholar] [CrossRef]
- Lv, J.; Sun, B.; Jin, J.; Jiang, W. Mechanical and slow-released property of poly(acrylamide) hydrogel reinforced by diatomite. Mater. Sci. Eng. C 2019, 99, 315–321. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, Z.; Liang, X. Aggregate-Breaking Mechanism Response to Polyacrylamide Application of Purple Soils in Southwestern China Using Le Bisaline soilonnais Method. Agronomy 2023, 13, 2222. [Google Scholar] [CrossRef]
- Georgees, R.N.; Hassan, R.A.; Evans, R.P. A potential use of a hydrophilic polymeric material to enhance durability properties of pavement materials. Constr. Build. Mater. 2017, 148, 686–695. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, Y.; Lan, H.; Zhang, F.; Zhang, H.; Wang, C.; Tan, Y.; Yu, R. Experimental Investigation of the Compactability and Cracking Behavior of Polyacrylamide-Treated Saline Soil in Gansu Province, China. Polymers 2019, 11, 90. [Google Scholar] [CrossRef]
- Soltani-Jigheh, H.; Bagheri, M.; Amani-Ghadim, A.R. Use of hydrophilic polymeric stabilizer to improve strength and durability of fine-grained soils. Cold Reg. Sci. Technol. 2019, 157, 187–195. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2019, 31, 1317–1324. [Google Scholar] [CrossRef]
- Sujatha, E.R.; Saisree, S. Geotechnical behaviour of guar gum-treated soil. Soils Found. 2019, 59, 2155–2166. [Google Scholar] [CrossRef]
- Highway Engineering Inorganic Binding Material Stabilized Material Test Specification (JTG 3441–2024). Available online: https://xxgk.mot.gov.cn/2020/jigou/glj/202403/t20240301_4032012.html (accessed on 13 August 2024).
- Xiao, Z.; Hou, Z.; Zhu, L.; Dong, X. Experimental investigation of the influence of salt on the phase transition temperature in saline soil. Cold Reg. Sci. Technol. 2021, 183, 103229. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Z.; Ning, J.; Li, T.; Zhou, Z. Viscoelastic–plastic constitutive model with damage of frozen soil under impact loading and freeze–thaw loading. Int. J. Mech. Sci. 2022, 214, 106890. [Google Scholar] [CrossRef]
- Duan, Z.; Song, K.; Zhang, N.; Zheng, L.-C.; Yan, X.-S.; Zhang, M.-M. Characteristics and mechanisms of soil structure damage under salt weathering. Soil Tillage Res. 2024, 238. [Google Scholar] [CrossRef]
- Wan, Q.; Yang, X.; Wang, R.; Zhu, Z. Dynamic deformation and meso-structure of coarse-grained saline soil under cyclic loading with freeze–thaw cycles. Front. Earth Sci. 2024, 12, 1361620. [Google Scholar] [CrossRef]
- Hewage, S.A.; Tang, C.-S.; Mehta, Y.; Zhu, C. Investigating cracking behavior of saline clayey soil under cyclic freezing-thawing effects. Eng. Geol. 2023, 326, 107319. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Liu, J.; Renqingcairang, J.; Fang, J. Macro-micro characteristics of geopolymer-stabilized saline soil in seasonal frozen soil region. Case Stud. Constr. Mater. 2023, 19, e02496. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; He, W.; Zhang, L. Durability analysis of seashore saline soil bound with a slag compound binder. Soils Found. 2019, 59, 1456–1467. [Google Scholar] [CrossRef]
- Qiu, K.; Zeng, G.; Shu, B.; Luo, D. Study on the Performance and Solidification Mechanism of Multi-Source Solid-Waste-Based Soft Soil Solidification Materials. Materials 2023, 16, 4517. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Q.; Chen, Y.; Han, Y.; Zhang, X.; Liu, Y. Evolution procesaline soil of the microstructure of saline soil with different compaction degrees during freeze–thaw cycles. Eng. Geol. 2022, 304, 106699. [Google Scholar] [CrossRef]
- Elbarbary, A.M.; El-Rehim, H.A.A.; El-Sawy, N.M.; Hegazy, E.-S.A.; Soliman, E.-S.A. Radiation induced crosaline soillinking of polyacrylamide incorporated low molecular weights natural polymers for posaline soilible use in the agricultural applications. Carbohydr. Polym. 2017, 176, 19–28. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; He, B.; Jing, X.; Cang, D.; Zhang, L. Study on evolution of pores channel in carbonation steel slag samples with fly ash. Constr. Build. Mater. 2024, 411, 134471. [Google Scholar] [CrossRef]
- Lv, Q.; Jiang, L.; Ma, B.; Zhao, B.; Huo, Z. A study on the effect of the salt content on the solidification of sulfate saline soil solidified with an alkali-activated geopolymer. Constr. Build. Mater. 2018, 176, 68–74. [Google Scholar] [CrossRef]
- Mollah, M.Y.; Kesmez, M.; Cocke, D.L. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total Environ. 2004, 325, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Eyley, S.; Thielemans, W.; Yuan, Q.; Li, J. Valorization of deep soil mixing residue in cement-based materials. Resour. Conserv. Recycl. 2022, 187, 106597. [Google Scholar] [CrossRef]
- Wu, J.; Deng, Y.; Zhang, G.; Zhou, A.; Tan, Y.; Xiao, H.; Zheng, Q. A Generic Framework of Unifying Industrial By-products for Soil Stabilization. J. Clean. Prod. 2021, 321, 128920. [Google Scholar] [CrossRef]
- Acarturk, B.C.; Sandalci, I.; Hull, N.M.; Bundur, Z.B.; Burris, L.E. Calcium sulfoaluminate cement and supplementary cementitious materials-containing binders in self-healing systems. Cem. Concr. Compos. 2023, 141, 105115. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, T.; Han, Q.; Zhu, Y.; Hou, D.; Dong, B. Effects of fly ash on MgO-based shrinkage-compensating cement: Microstructure and properties. Constr. Build. Mater. 2022, 339, 127648. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, Y.; Liu, S.; Liu, Q.; Chen, Y.; Zha, F. Strength and micro-structure evolution of compacted soils modified by admixtures of cement and metakaolin. Appl. Clay Sci. 2016, 127–128, 44–51. [Google Scholar] [CrossRef]
- Xu, F.; Wei, H.; Qian, W.; Cai, Y. Composite alkaline activator on cemented soil: Multiple tests and mechanism analyses. Constr. Build. Mater. 2018, 188, 433–443. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, H.; Zhao, J. Removal of Pb(II) from aqueous solution using hydroxyapatite/calcium silicate hydrate (HAP/C-S-H) composite adsorbent prepared by a phosphate recovery procesaline soil. Chem. Eng. J. 2018, 344, 53–61. [Google Scholar] [CrossRef]
- Kurumisawa, K.; Nawa, T.; Owada, H.; Shibata, M. Deteriorated hardened cement paste structure analyzed by XPS and 29Si NMR techniques. Cem. Concr. Res. 2013, 52, 190–195. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Zhang, Z.; Ma, X. The impacts of sodium nitrate on hydration and microstructure of Portland cement and the leaching behavior of Sr2+. J. Hazard. Mater. 2020, 388, 121805. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Wang, H.; Liu, S.; Liu, M. Solidification/stabilization of lead-contaminated soils by phosphogypsum slag-based cementitious materials. Sci. Total Environ. 2023, 857, 159552. [Google Scholar] [CrossRef]
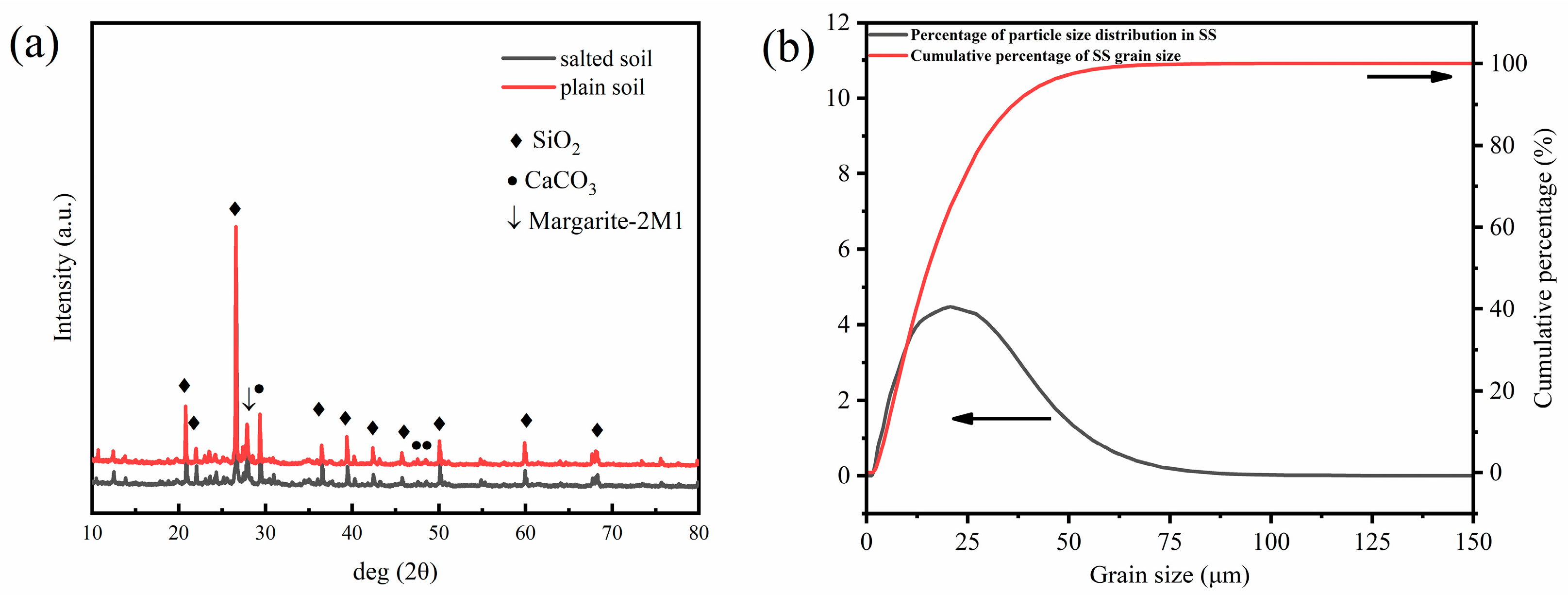
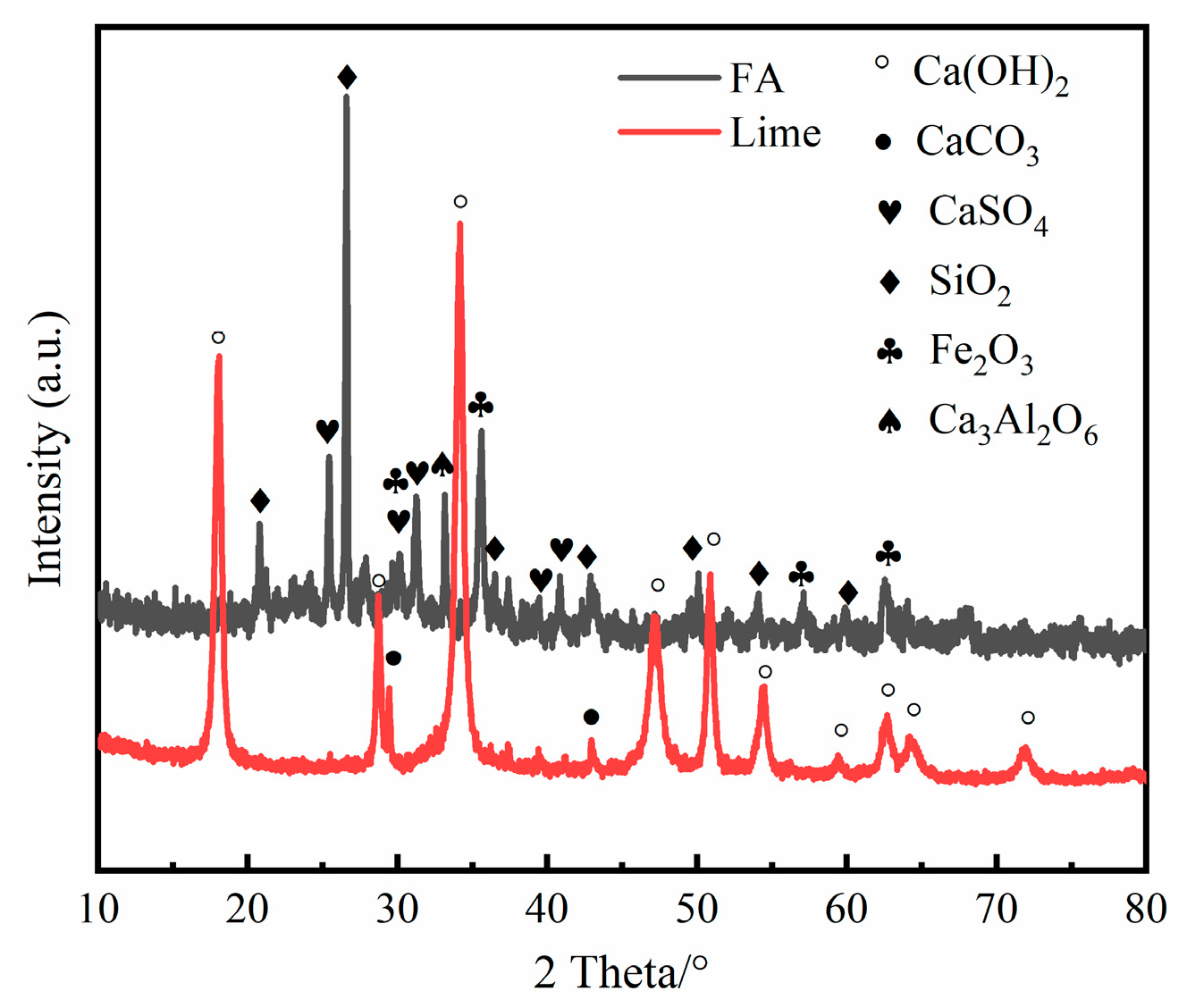




| Saline Soils | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | Na2O | Cl | SO3 |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 61.78 | 15.63 | 8.57 | 4.97 | 3.39 | 2.79 | 1.66 | 0.17 | 0.09 |
| Grain Size Distribution/% | Maximum Dry Density (g.cm−3) | Optimum Water Content (%) | Salt Content (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| >50 μm | 20~50 μm | 10~20 μm | <10 μm | Na2SO4 | NaCl | Na2CO3 | |||
| saline soils | 2.41 | 26.64 | 33.36 | 31.59 | 1.894 | 12.5 | 0.86 | 0.11 | 0 |
| FA | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | K2O | Na2O | Cl | SO3 |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 37.31 | 17.38 | 13.14 | 15.07 | 5.95 | 1.28 | 2.60 | 0.27 | 5.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Huang, Y.; Sun, J.; Yu, R.; Liu, Y.; Cui, Q. Research on Solidification Methods and Stabilization Mechanisms of Sulfate Saline Soils. Appl. Sci. 2024, 14, 7246. https://doi.org/10.3390/app14167246
Li S, Huang Y, Sun J, Yu R, Liu Y, Cui Q. Research on Solidification Methods and Stabilization Mechanisms of Sulfate Saline Soils. Applied Sciences. 2024; 14(16):7246. https://doi.org/10.3390/app14167246
Chicago/Turabian StyleLi, Sining, Yong Huang, Jian Sun, Rui Yu, Yubin Liu, and Qiushuang Cui. 2024. "Research on Solidification Methods and Stabilization Mechanisms of Sulfate Saline Soils" Applied Sciences 14, no. 16: 7246. https://doi.org/10.3390/app14167246
APA StyleLi, S., Huang, Y., Sun, J., Yu, R., Liu, Y., & Cui, Q. (2024). Research on Solidification Methods and Stabilization Mechanisms of Sulfate Saline Soils. Applied Sciences, 14(16), 7246. https://doi.org/10.3390/app14167246






