Comparison of Four Rapid N-Glycan Analytical Methods and Great Application Potential in Cell Line Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
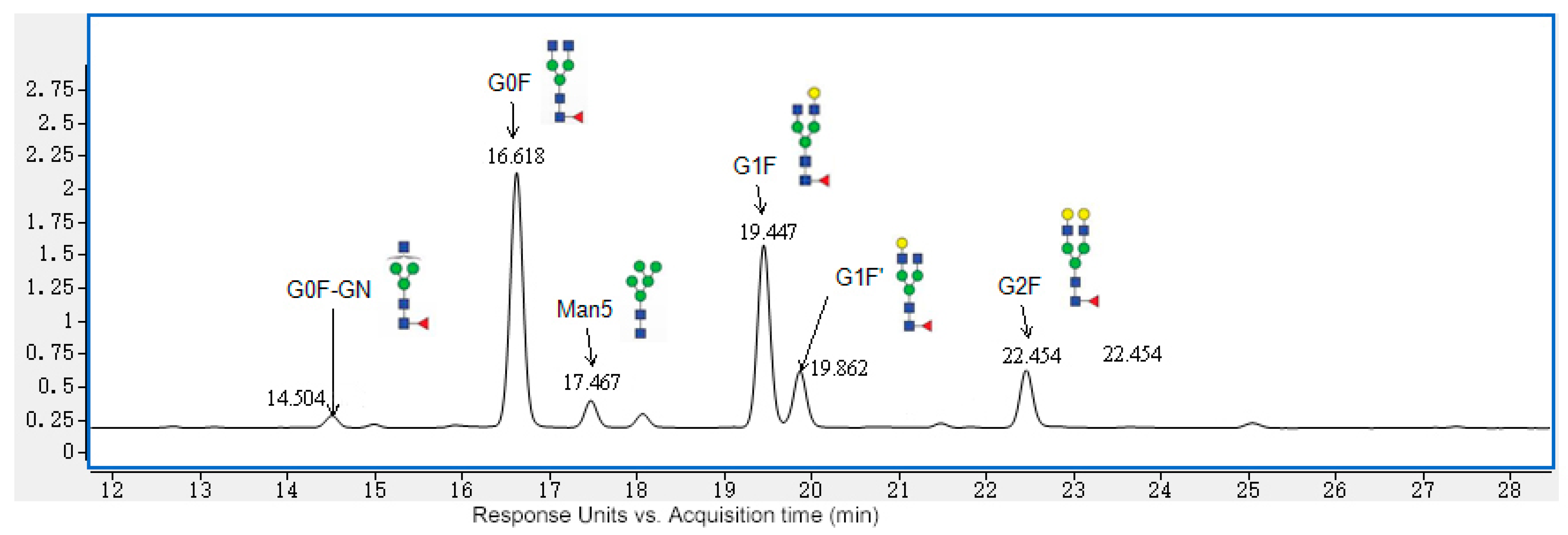
2.3. Rapid 2-AB Method
2.4. Reduction Method
2.5. Off-Line IdeS Digestion Method
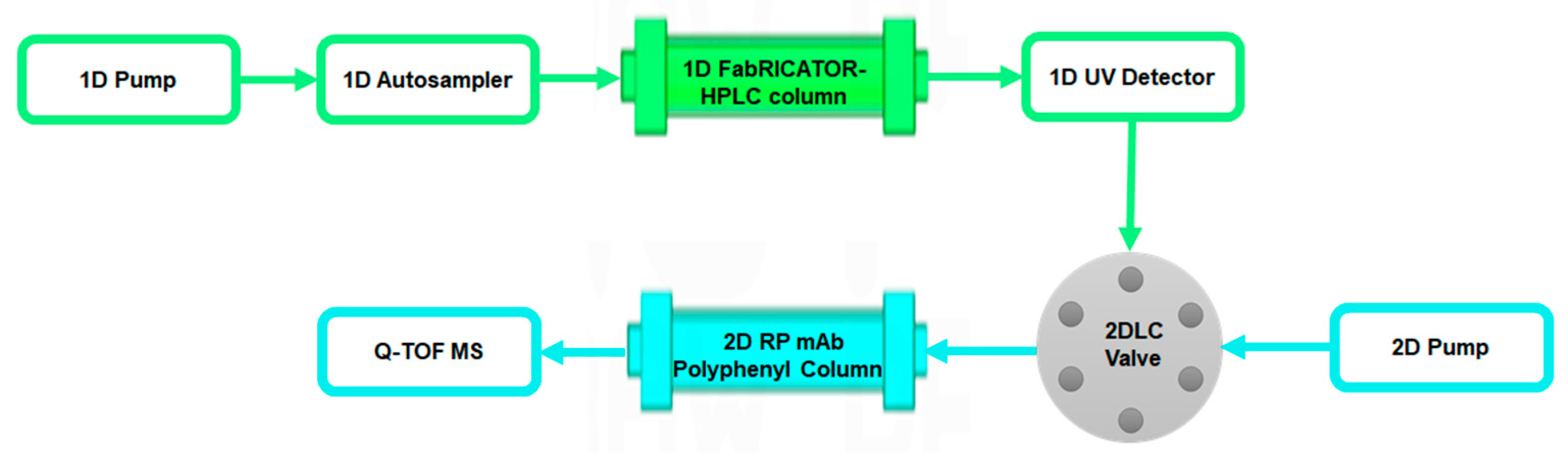
2.6. 2D-LC-MS Method
3. Results and Discussion
3.1. N-Glycan Data from the Rapid 2-AB Method
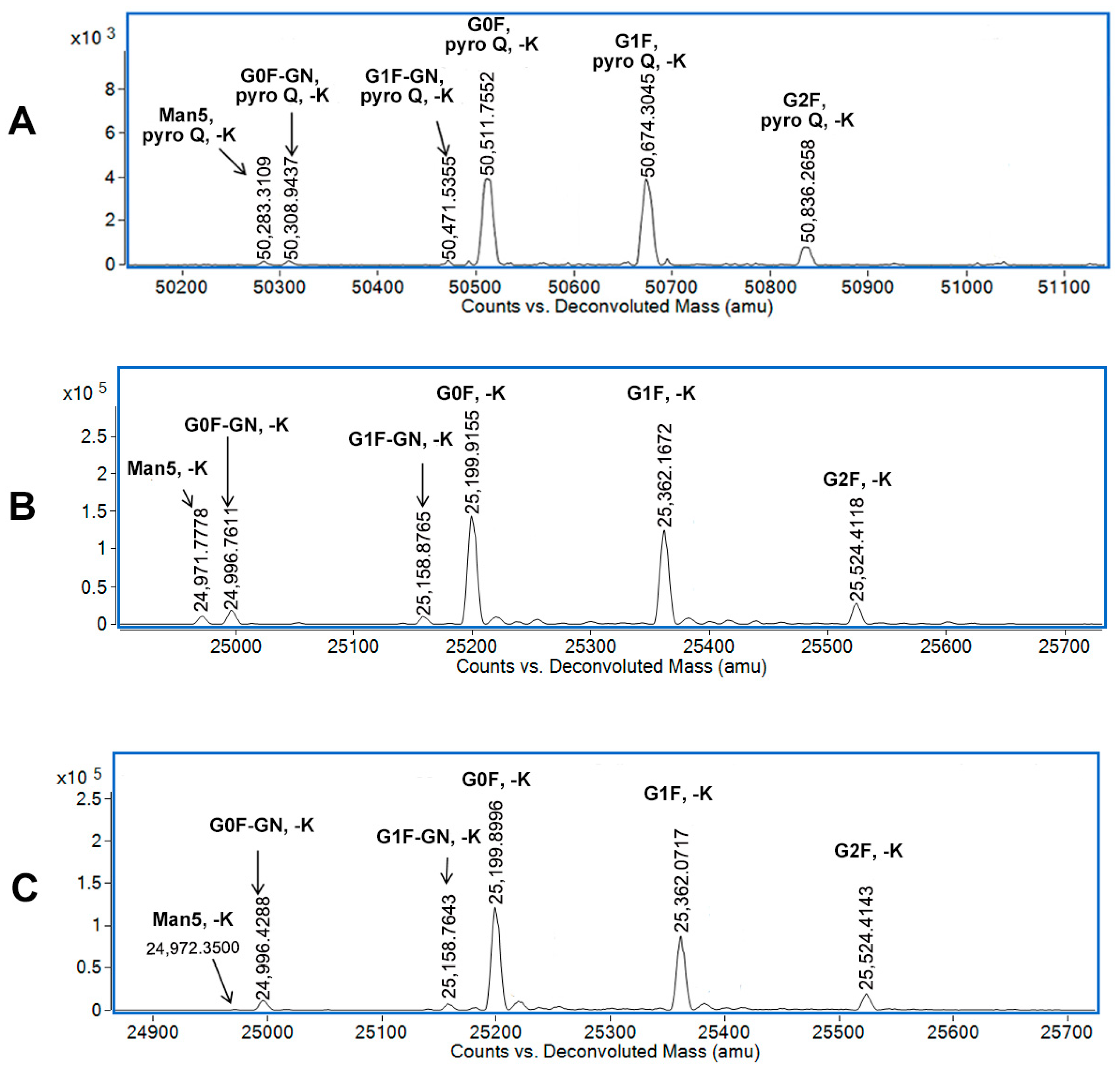
3.2. N-Glycan Data from the Reduction Method
3.3. N-Glycan Data from the Off-Line IdeS Digestion Method
3.4. N-Glycan Data from the 2D-LC-MS Method
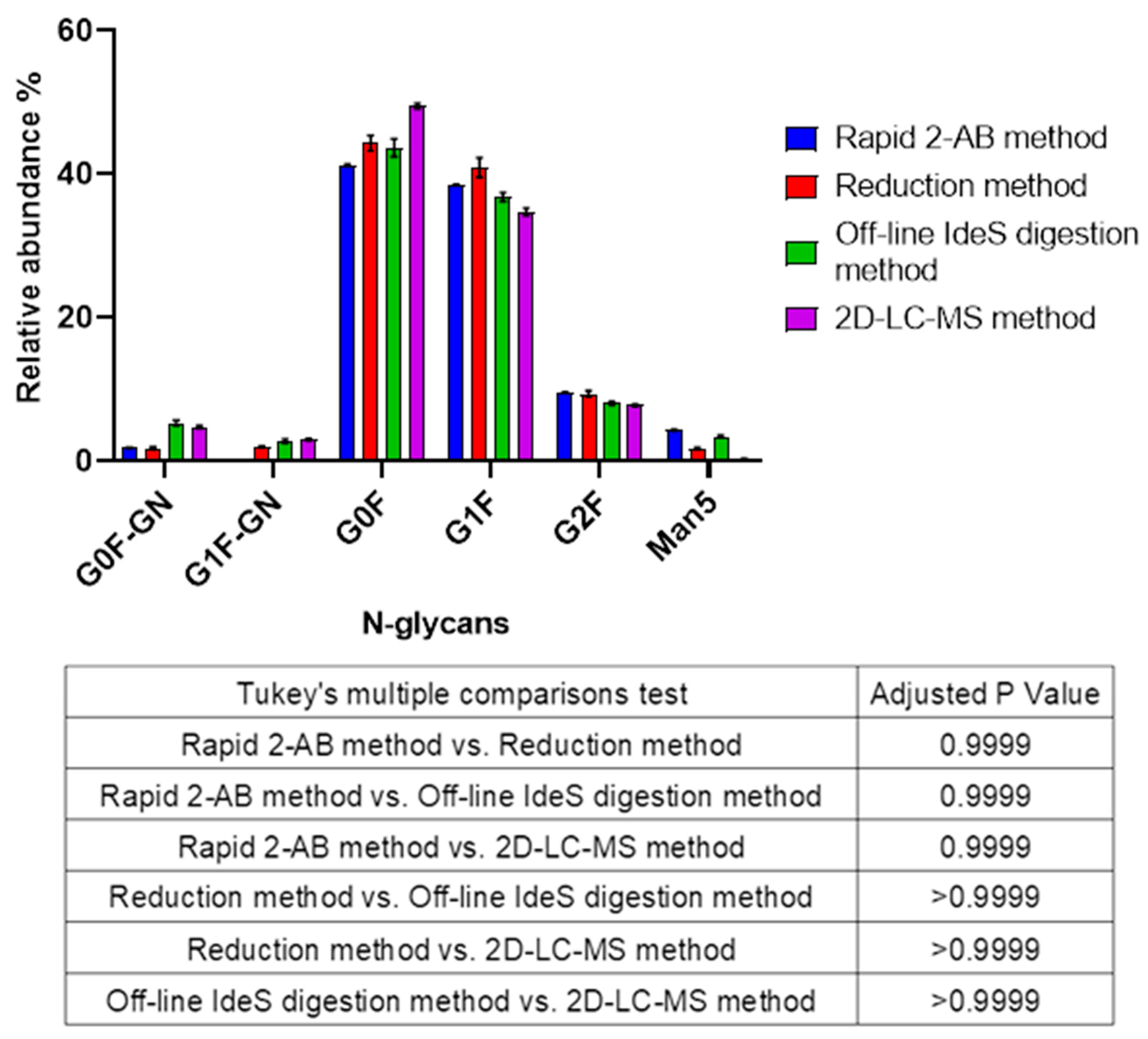
3.5. Comparison of Four Rapid N-Glycan Analytical Methods
3.6. Great Application Potential of These Four Rapid N-Glycan Analytical Methods in Cell Line Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaozhi. Top10 Best-Selling Drugs in 2023. Available online: https://m.baidu.com/bh/m/detail/ar_9971613961676379877 (accessed on 22 February 2024).
- Martin, K.P.; Grimaldi, C.; Grempler, R.; Hansel, S.; Kumar, S. Trends in industrialization of biotherapeutics: A survey of product characteristics of 89 antibody-based biotherapeutics. MAbs 2023, 15, 2191301. [Google Scholar] [CrossRef]
- Khetan, R.; Curtis, R.; Deane, C.M.; Hadsund, J.T.; Kar, U.; Krawczyk, K.; Kuroda, D.; Robinson, S.A.; Sormanni, P.; Tsumoto, K.; et al. Current advances in biopharmaceutical informatics: Guidelines, impact and challenges in the computational developability assessment of antibody therapeutics. MAbs 2022, 14, 2020082. [Google Scholar] [CrossRef]
- Farid, S.S.; Baron, M.; Stamatis, C.; Nie, W.; Coffman, J. Benchmarking biopharmaceutical process development and manufacturing cost contributions to R&D. MAbs 2020, 12, 1754999. [Google Scholar] [CrossRef]
- Higel, F.; Seidl, A.; Sorgel, F.; Friess, W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Joshi, S.; Guttman, A.; Rathore, A.S. N-Glycosylation of monoclonal antibody therapeutics: A comprehensive review on significance and characterization. Anal. Chim. Acta 2022, 1209, 339828. [Google Scholar] [CrossRef] [PubMed]
- Carillo, S.; Pérez-Robles, R.; Jakes, C.; Ribeiro da Silva, M.; Millán Martín, S.; Farrell, A.; Navas, N.; Bones, J. Comparing different domains of analysis for the characterisation of N-glycans on monoclonal antibodies. J. Pharm. Anal. 2020, 10, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Majewska, N.I.; Tejada, M.L.; Betenbaugh, M.J.; Agarwal, N. N-Glycosylation of IgG and IgG-Like Recombinant Therapeutic Proteins: Why Is It Important and How Can We Control It? Annu. Rev. Chem. Biomol. Eng. 2020, 11, 311–338. [Google Scholar] [CrossRef]
- Matsumiya, S.; Yamaguchi, Y.; Saito, J.; Nagano, M.; Sasakawa, H.; Otaki, S.; Satoh, M.; Shitara, K.; Kato, K. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J. Mol. Biol. 2007, 368, 767–779. [Google Scholar] [CrossRef]
- Upton, R.; Bell, L.; Guy, C.; Caldwell, P.; Estdale, S.; Barran, P.E.; Firth, D. Orthogonal Assessment of Biotherapeutic Glycosylation: A Case Study Correlating N-Glycan Core Afucosylation of Herceptin with Mechanism of Action. Anal. Chem. 2016, 88, 10259–10265. [Google Scholar] [CrossRef]
- Raju, T.S.; Jordan, R.E. Galactosylation variations in marketed therapeutic antibodies. MAbs 2012, 4, 385–391. [Google Scholar] [CrossRef]
- Thomann, M.; Reckermann, K.; Reusch, D.; Prasser, J.; Tejada, M.L. Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Mol. Immunol. 2016, 73, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Flynn, G.C. Effect of high mannose glycan pairing on IgG antibody clearance. Biologicals 2016, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H. Characterization of glycosylation in monoclonal antibodies and its importance in therapeutic antibody development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Duivelshof, B.L.; Jiskoot, W.; Beck, A.; Veuthey, J.L.; Guillarme, D.; D’Atri, V. Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications. Anal. Chim. Acta 2019, 1089, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Eckard, A.D.; Dupont, D.R.; Young, J.K. Development of Two Analytical Methods Based on Reverse Phase Chromatographic and SDS-PAGE Gel for Assessment of Deglycosylation Yield in N-Glycan Mapping. Biomed. Res. Int. 2018, 2018, 3909674. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Tan, C.; Gupta, S.; Guhan, T.; Barkhordarian, H.; Lull, J.; Stevens, J.; Munro, T. A novel mammalian cell line development platform utilizing nanofluidics and optoelectro positioning technology. Biotechnol. Prog. 2018, 34, 1438–1446. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Albanetti, T.; Miro-Quesada, G.; Flack, L.; Li, L.; Klover, J.; Burson, K.; Evans, K.; Ivory, W.; Bowen, M.; et al. High-throughput screening of antibody-expressing CHO clones using an automated shaken deep-well system. Biotechnol. Prog. 2018, 34, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, R.; Trbojevic-Akmacic, I.; Greville, G.; Rudd, P.M.; Lauc, G. The sweet spot for biologics: Recent advances in characterization of biotherapeutic glycoproteins. Expert Rev. Proteom. 2018, 15, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Reusch, D. Monoclonal Antibodies Physicochemical Analysis; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Camperi, J.; Dai, L.; Guillarme, D.; Stella, C. Development of a 3D-LC/MS Workflow for Fast, Automated, and Effective Characterization of Glycosylation Patterns of Biotherapeutic Products. Anal. Chem. 2020, 92, 4357–4363. [Google Scholar] [CrossRef] [PubMed]
- Marquet, F.; D’Atri, V.; Guillarme, D.; Borchard, G. A New Practice to Monitor the Fabrication Process of Fab-Targeting Ligands from Bevacizumab by LC-MS: Preparation and Analytical Characterization. Sci. Pharm. 2022, 90, 5. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, Y.; Zhang, L.; Xu, Y.; Zhang, Z. In-Depth Characterization of mAb Charge Variants by On-Line Multidimensional Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2023, 95, 7977–7984. [Google Scholar] [CrossRef] [PubMed]
- Jaag, S.; Shirokikh, M.; Lammerhofer, M. Charge variant analysis of protein-based biopharmaceuticals using two-dimensional liquid chromatography hyphenated to mass spectrometry. J. Chromatogr. A 2021, 1636, 461786. [Google Scholar] [CrossRef]
- Agilent. Agilent AdvanceBio Gly-X N-Glycan Prep with 2-AB Express Kit. Available online: https://www.agilent.com.cn/cs/library/usermanuals/public/5994-1228EN.pdf (accessed on 1 July 2019).
- Jarasch, A.; Koll, H.; Regula, J.T.; Bader, M.; Papadimitriou, A.; Kettenberger, H. Developability assessment during the selection of novel therapeutic antibodies. J. Pharm. Sci. 2015, 104, 1885–1898. [Google Scholar] [CrossRef]
- Foster, S.W.; Parker, D.; Kurre, S.; Boughton, J.; Stoll, D.R.; Grinias, J.P. A review of two-dimensional liquid chromatography approaches using parallel column arrays in the second dimension. Anal. Chim. Acta 2022, 1228, 340300. [Google Scholar] [CrossRef]
- Rautenberg, T.A.; Ng, S.K.A.; Downes, M. A cross-sectional study of symptoms and health-related quality of life in menopausal-aged women in China. BMC Womens Health 2023, 23, 563. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Kasahara, M.; Takemoto, S.; Hattori, M. The Wear Behavior of Glass-Ceramic CAD/CAM Blocks against Bovine Enamel. Materials 2023, 16, 6839. [Google Scholar] [CrossRef]
- Grieb, N.; Oltrup, T.; Bende, T.; Leitritz, M.A. The Cosine Similarity Technique: A new method for smart EXCIMER laser control. Z. Med. Phys. 2020, 30, 253–258. [Google Scholar] [CrossRef]
- Zhou, S.; Veillon, L.; Dong, X.; Huang, Y.; Mechref, Y. Direct comparison of derivatization strategies for LC-MS/MS analysis of N-glycans. Analyst 2017, 142, 4446–4455. [Google Scholar] [CrossRef]
- Schiel, J.E.; Rogstad, S.M.; Boyne, M.T., 2nd. Comparison of Traditional 2-AB Fluorescence LC-MS/MS and Automated LC-MS for the Comparative Glycan Analysis of Monoclonal Antibodies. J. Pharm. Sci. 2015, 104, 2464–2472. [Google Scholar] [CrossRef]
| Glycan Name | Percentage/% | |||
|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | RSD | |
| G0F-GN | 1.92 | 1.76 | 1.86 | 4.19% |
| G0F | 41.33 | 41.06 | 41.27 | 0.34% |
| Man5 | 4.22 | 4.40 | 4.29 | 2.09% |
| G1F | 29.05 | 29.10 | 29.02 | 0.14% |
| G1F’ | 9.40 | 9.46 | 9.41 | 0.38% |
| G2F | 9.58 | 9.55 | 9.46 | 0.68% |
| Method | Analytical Principle | Pre-Treatment | Column | Detector |
|---|---|---|---|---|
| Rapid 2-AB method | N-glycans is released from the antibody by PNGase F, labelled by 2-AB, and tested by a fluorescence detector. | Denaturation, N-glycan release, labelling and purification. | HILIC column | Fluorescence detector |
| Reduction method | The antibody is reduced to generate HC and analyzed N-glycans by MS at the subunit level. | Off-line DTT reduction. | RP column | Q-TOF MS detector |
| Off-line IdeS digestion method | The antibody is off-line digested by IdeS to generate Fc/2 and analyzed N-glycans by MS at the subunit level. | Off-line IdeS digestion. | RP column | Q-TOF MS detector |
| 2D-LC-MS method | The antibody is on-line digested by the column with immobilized IdeS to generate Fc/2 and analyzed N-glycans by MS at the subunit level. | No pre-treatment. | Immobilized IdeS column and RP column | Q-TOF MS detector |
| Method | Protein Sample Requirement per Analysis | Total Testing Time per Analysis | Throughput | Total Testing Time for 100 Samples | Time Saving for 100 Samples | Time Saving Ratio for 100 Samples |
|---|---|---|---|---|---|---|
| Conventional 2-AB method | 1 mg [33] | 1~3 days [17] | 24 samples per 3 days * | 12.5 days | NA | NA |
| Rapid 2-AB method | 40 µg | 4 h | 16 samples per day # | 6.25 days | 6.25 days | 50% |
| Reduction method | 50 µg | 74 min | ~19 samples per day | ~5.26 days | ~7.24 days | ~58% |
| Off-line IdeS digestion method | 50 µg | 104 min | ~13 samples per day | ~7.69 days | ~4.81 days | ~38% |
| 2D-LC-MS method | 2 µg | 40 min | 36 samples per day | ~2.78 days | ~9.72 days | ~78% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Chu, J.; He, B. Comparison of Four Rapid N-Glycan Analytical Methods and Great Application Potential in Cell Line Development. Appl. Sci. 2024, 14, 7320. https://doi.org/10.3390/app14167320
Jin X, Chu J, He B. Comparison of Four Rapid N-Glycan Analytical Methods and Great Application Potential in Cell Line Development. Applied Sciences. 2024; 14(16):7320. https://doi.org/10.3390/app14167320
Chicago/Turabian StyleJin, Xiaoqing, Jianlin Chu, and Bingfang He. 2024. "Comparison of Four Rapid N-Glycan Analytical Methods and Great Application Potential in Cell Line Development" Applied Sciences 14, no. 16: 7320. https://doi.org/10.3390/app14167320




