Studies of Fluorescence Lifetimes of Biological Warfare Agents Simulants and Interferers Using the Stroboscopic Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement Setups
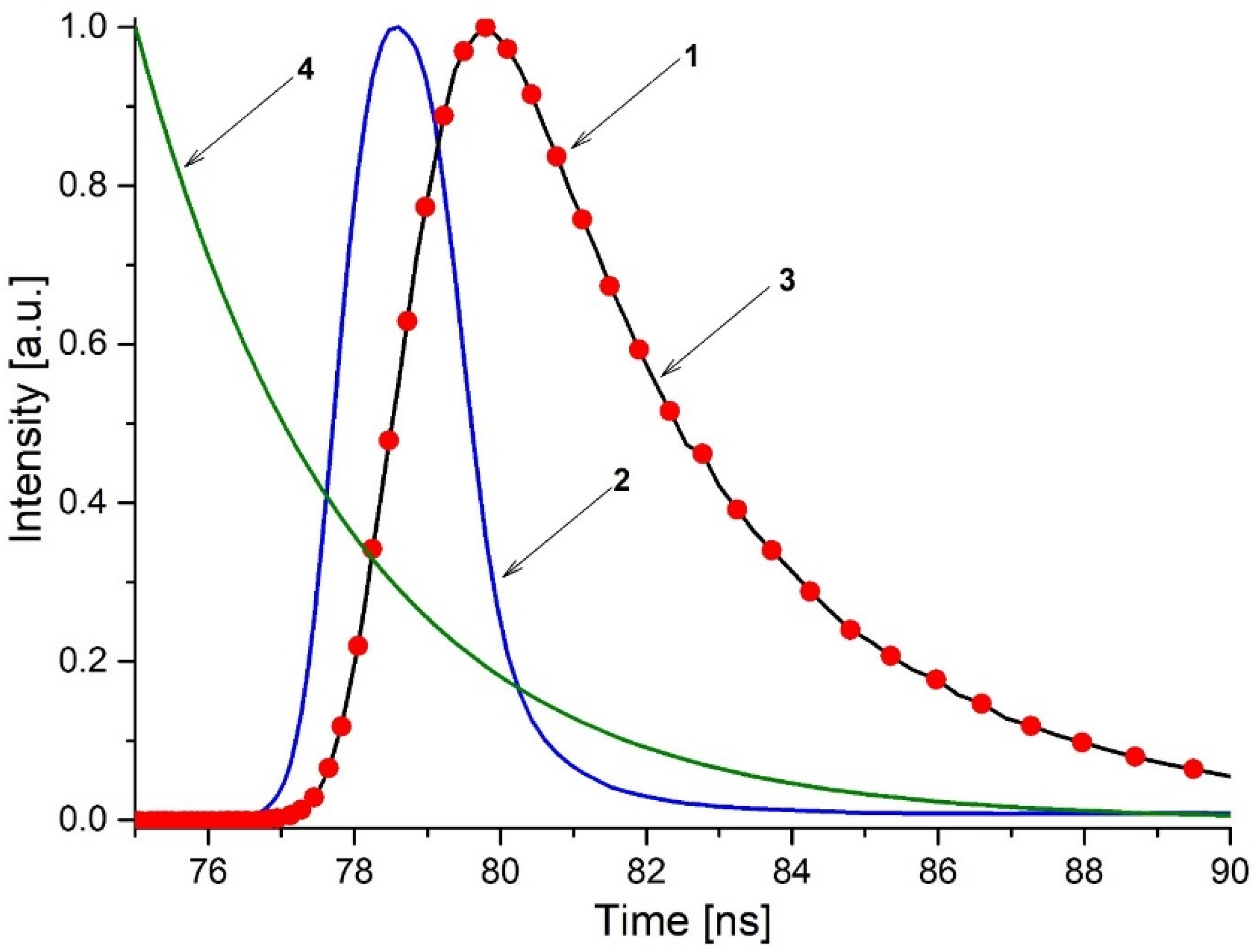
2.2. Fluorescence Lifetime Measurements
2.3. Data Analysis
2.4. Microorganisms
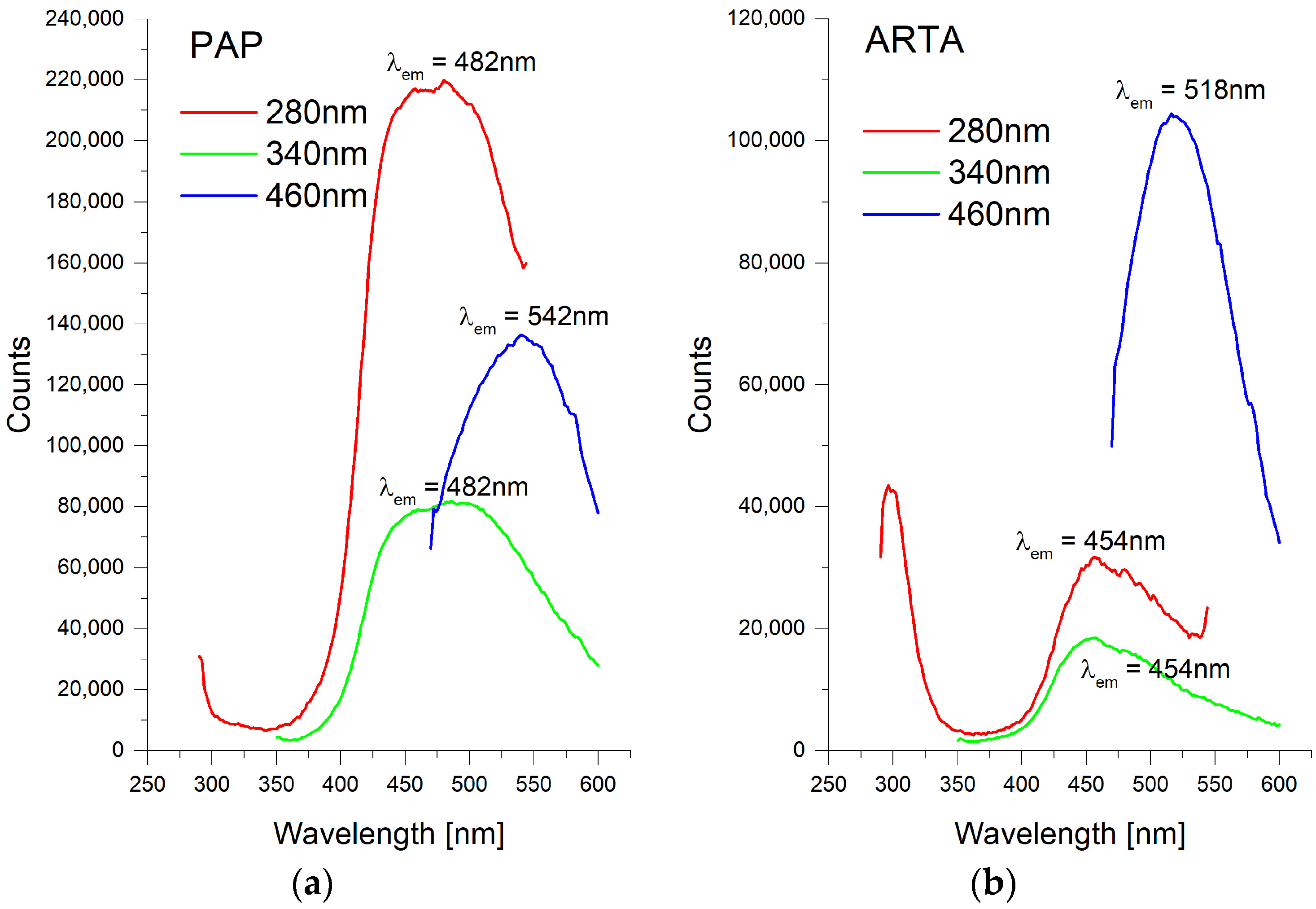
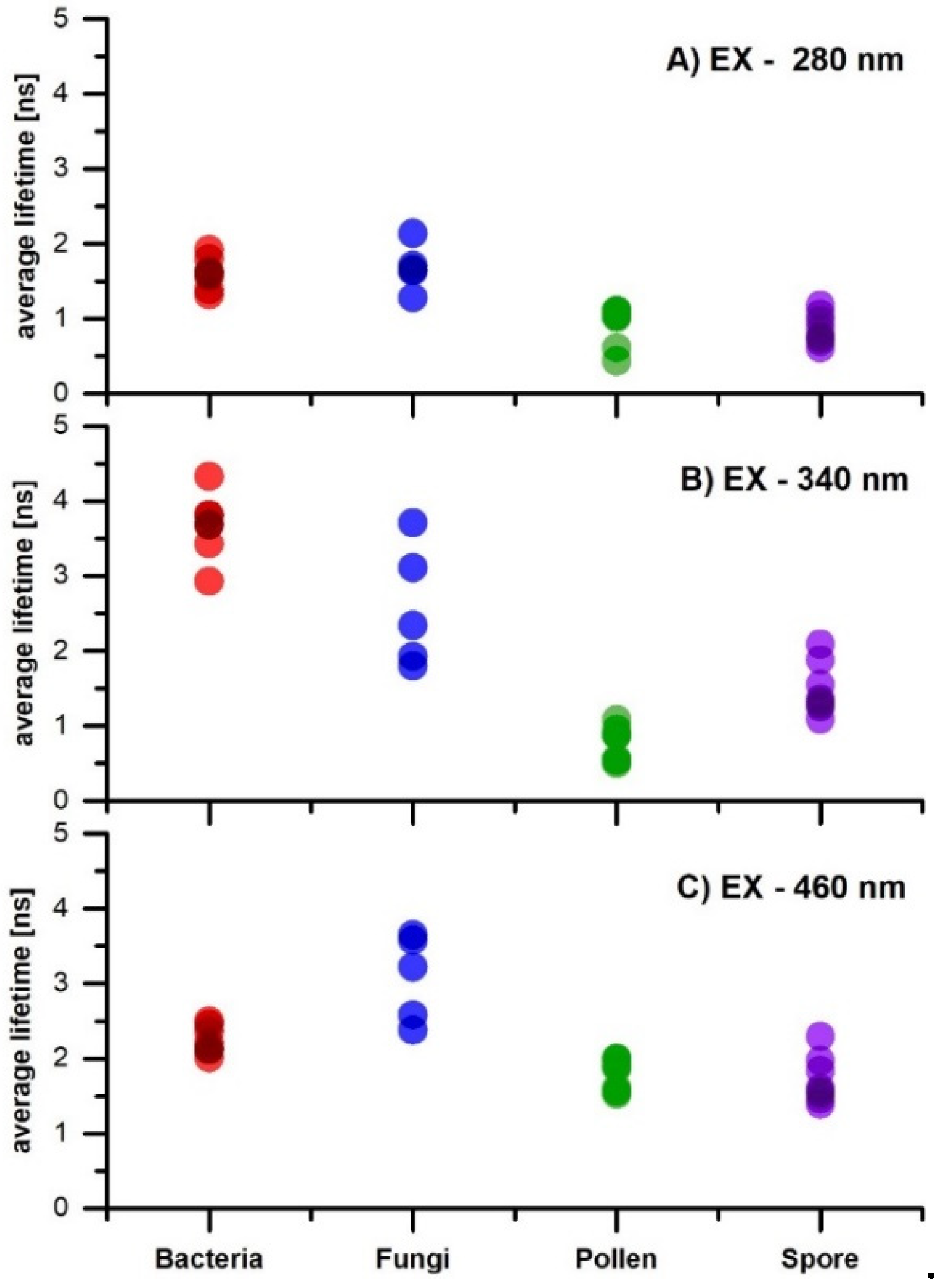
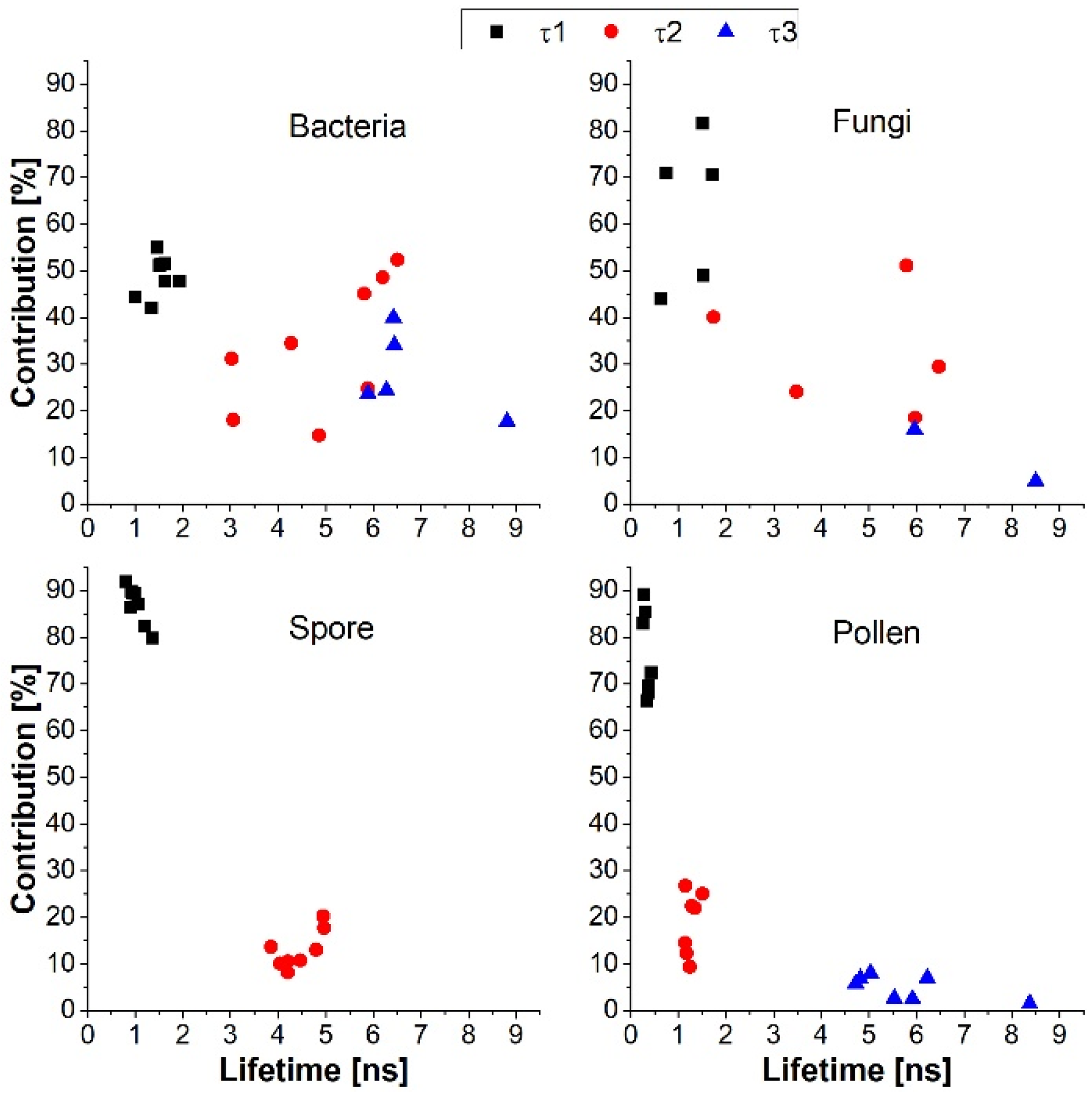
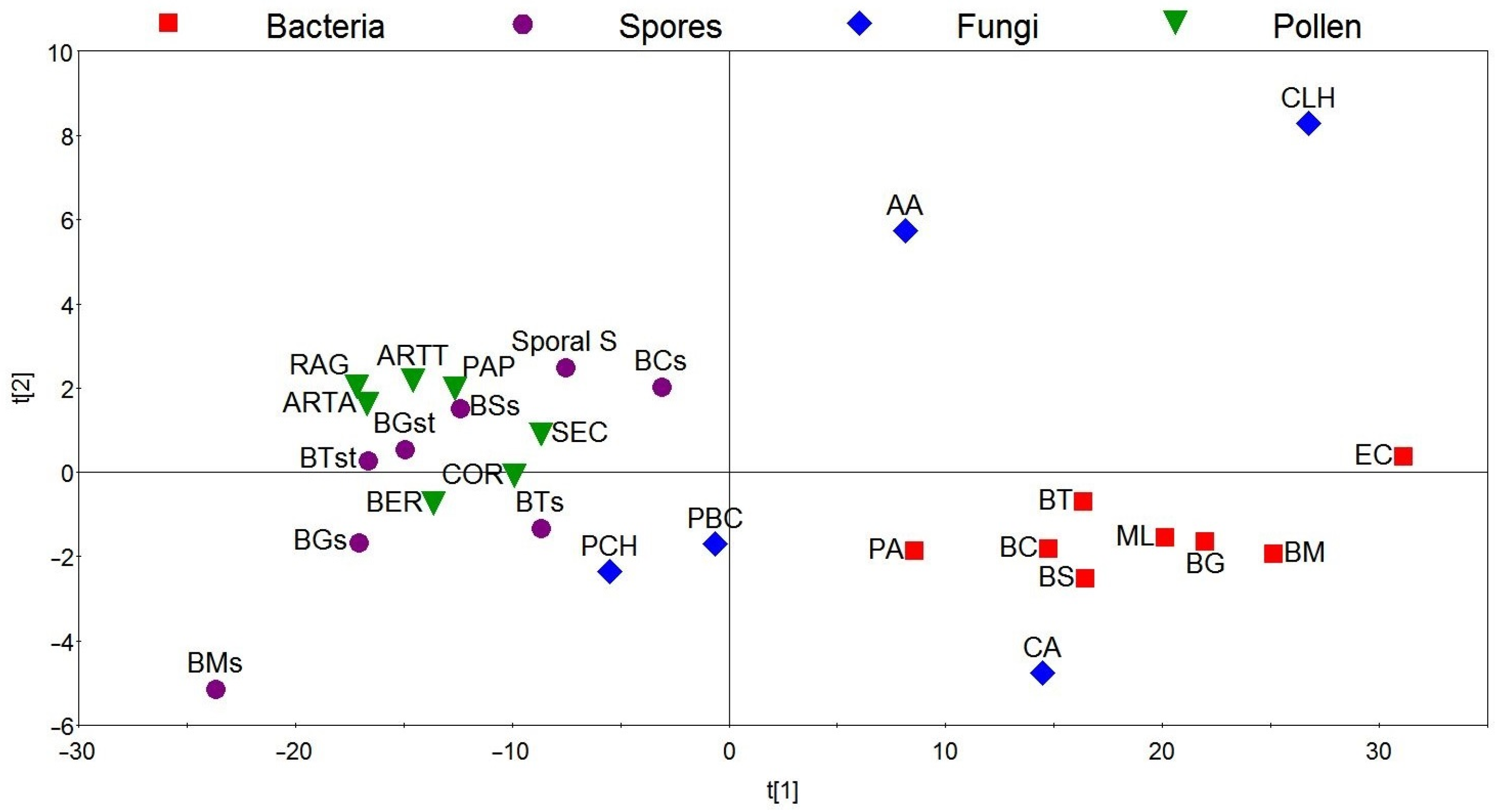
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Després, V.R.; Alex Huffman, J.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.O.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus Ser. B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Blais-Lecours, P.; Perrott, P.; Duchaine, C. Non-culturable bioaerosols in indoor settings: Impact on health and molecular approaches for detection. Atmos. Environ. 2015, 110, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef]
- Doron, S.; Gorbach, S.L. Bacterial Infections: Overview. Int. Encycl. Public Health 2008, 273–282. [Google Scholar] [CrossRef]
- CDC. Bioterrorism Agents/Diseases. Available online: https://emergency.cdc.gov/agent/agentlist-category.asp (accessed on 22 December 2023).
- Gabbarini, V.; Rossi, R.; Ciparisse, J.F.; Malizia, A.; Divizia, A.; De Filippis, P.; Anselmi, M.; Carestia, M.; Palombi, L.; Divizia, M.; et al. Laser-induced fluorescence (LIF) as a smart method for fast environmental virological analyses: Validation on Picornaviruses. Sci. Rep. 2019, 9, 12598. [Google Scholar] [CrossRef]
- Savage, N.J.; Krentz, C.E.; Könemann, T.; Han, T.T.; Mainelis, G.; Pöhlker, C.; Alex Huffman, J. Systematic characterization and fluorescence threshold strategies for the wideband integrated bioaerosol sensor (WIBS) using size-resolved biological and interfering particles. Atmos. Meas. Tech. 2017, 10, 4279–4302. [Google Scholar] [CrossRef]
- Huffman, J.A.; Treutlein, B.; Pöschl, U. Fluorescent biological aerosol particle concentrations and size distributions measured with an Ultraviolet Aerodynamic Particle Sizer (UV-APS) in Central Europe. Atmos. Chem. Phys. 2010, 10, 3215–3233. [Google Scholar] [CrossRef]
- Wojtanowski, J.; Zygmunt, M.; Muzal, M.; Knysak, P.; Młodzianko, A.; Gawlikowski, A.; Drozd, T.; Kopczyński, K.; Mierczyk, Z.; Kaszczuk, M.; et al. Performance verification of a LIF-LIDAR technique for stand-off detection and classification of biological agents. Opt. Laser Technol. 2015, 67, 25–32. [Google Scholar] [CrossRef]
- Hernandez, M.; Perring, A.E.; McCabe, K.; Kok, G.; Granger, G.; Baumgardner, D. Chamber catalogues of optical and fluorescent signatures distinguish bioaerosol classes. Atmos. Meas. Tech. 2016, 9, 3283–3292. [Google Scholar] [CrossRef]
- Duschek, F.; Fellner, L.; Gebert, F.; Grünewald, K.; Köhntopp, A.; Kraus, M.; Mahnke, P.; Pargmann, C.; Tomaso, H.; Walter, A. Standoff detection and classification of bacteria by multispectral laser-induced fluorescence. Adv. Opt. Technol. 2017, 6, 75–83. [Google Scholar] [CrossRef]
- Taketani, F.; Kanaya, Y.; Nakamura, T.; Koizumi, K.; Moteki, N.; Takegawa, N. Measurement of fluorescence spectra from atmospheric single submicron particle using laser-induced fluorescence technique. J. Aerosol Sci. 2013, 58, 1–8. [Google Scholar] [CrossRef]
- Kwaśny, M.; Bombalska, A.; Kaliszewski, M.; Włodarski, M.; Kopczyński, K. Fluorescence Methods for the Detection of Bioaerosols in Their Civil and Military Applications. Sensors 2023, 23, 3339. [Google Scholar] [CrossRef] [PubMed]
- Włodarski, M.; Kaliszewski, M.; Trafny, E.A.; Szpakowska, M.; Lewandowski, R.; Bombalska, A.; Kwaśny, M.; Kopczyński, K.; Mularczyk-Oliwa, M. Fast, reagentless and reliable screening of “white powders” during the bioterrorism hoaxes. Forensic Sci. Int. 2015, 248, 71–77. [Google Scholar] [CrossRef]
- Trafny, E.A.; Lewandowski, R.; Stȩpińska, M.; Kaliszewski, M. Biological threat detection in the air and on the surface: How to define the risk. Arch. Immunol. Ther. Exp. 2014, 62, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Babichenko, S.; Bentahir, M.; Piette, A.S.; Poryvkina, L.; Rebane, O.; Smits, B.; Sobolev, I.; Soboleva, N.; Gala, J.L. Non-Contact, Real-Time Laser-Induced Fluorescence Detection and Monitoring of Microbial Contaminants on Solid Surfaces Before, During and After Decontamination. J. Biosens. Bioelectron. 2018, 2, 255. [Google Scholar]
- Walczak, W.R. Lab-on-a-chip fluorescence detection with image sensor and software-based image conditioning. Bull. Polish Acad. Sci. Tech. Sci. 2011, 59, 157–165. [Google Scholar] [CrossRef]
- Rennie, M.Y.; Dunham, D.; Lindvere-Teene, L.; Raizman, R.; Hill, R.; Linden, R. Understanding real-time fluorescence signals from bacteria and wound tissues observed with the MolecuLight i:XTM. Diagnostics 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Kwaśny, M.; Bombalska, A. Applications of Laser-Induced Fluorescence in Medicine. Sensors 2022, 22, 2956. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, V.; Huston, A.L.; Scotto, C.; Eversole, J.D. Multiple UV wavelength excitation and fluorescence of bioaerosols. Opt. Express 2004, 12, 4457. [Google Scholar] [CrossRef]
- Pan, Y.-L.; Hill, S.C.; Pinnick, R.G.; Huang, H.; Bottiger, J.R.; Chang, R.K. Fluorescence spectra of atmospheric aerosol particles measured using one or two excitation wavelengths: Comparison of classification schemes employing different emission and scattering results. Opt. Express 2010, 18, 12436–12457. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-L. Detection and characterization of biological and other organic-carbon aerosol particles in atmosphere using fluorescence. J. Quant. Spectrosc. Radiat. Transf. 2015, 150, 12–35. [Google Scholar] [CrossRef]
- Feugnet, G.; Lallier, E.; Grisard, A.; McIntosh, L.; Hellström, J.E.; Jelger, P.; Laurell, F.; Albano, C.; Kaliszewski, M.; Wlodarski, M.; et al. Improved laser-induced fluorescence method for bio-attack early warning detection system. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 1–4 August 2008; Volume 7116. [Google Scholar]
- Žukauskas, A.; Vitta, P.; Kurilčik, N.; Juršenas, S.; Bakiene, E. Characterization of biological materials by frequency-domain fluorescence lifetime measurements using ultraviolet light-emitting diodes. Opt. Mater. 2008, 30, 800–805. [Google Scholar] [CrossRef]
- Kurilcik, N.; Vitta, P.; Zukauskas, A.; Gaska, R.; Ramanavicius, A.; Kausaite, A.; Jursenas, S. Fluorescence detection of biological objects with ultraviolet and visible light-emitting diodes. Opt. Appl. 2006, XXXVI, 193–198. [Google Scholar]
- Thomas, A.; Sands, D.; Baum, D.; To, L.; Rubel, G.O. Emission wavelength dependence of fluorescence lifetimes of bacteriological spores and pollens. Appl. Opt. 2006, 45, 6634–6639. [Google Scholar] [CrossRef]
- James, D.R.; Siemiarczuk, A.; Ware, W.R. Stroboscopic optical boxcar technique for the determination of fluorescence lifetimes. Rev. Sci. Instrum. 1992, 63, 1710–1716. [Google Scholar] [CrossRef]
- Kaliszewski, M.; Włodarski, M.; Młyńczak, J.; Leśkiewicz, M.; Bombalska, A.; Mularczyk-Oliwa, M.; Kwaśny, M.; Buliński, D.; Kopczyński, K. A new real-time bio-aerosol fluorescence detector based on semiconductor CW excitation UV laser. J. Aerosol Sci. 2016, 100, 14–25. [Google Scholar] [CrossRef]
- Eaton, D.F. Recommended methods for fluorescence decay analysis. Pure Appl. Chem. 1990, 62, 1631–1648. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, Y.; Liao, X.; Yang, W.; Zhu, Y.; Huang, H. Development and calibration of a single UV LED based bioaerosol monitor. Opt. Express 2013, 21, 26303–26310. [Google Scholar] [CrossRef] [PubMed]
- Davitt, K.; Song, Y.K.; Nurmikko, A.V.; Pan, Y.L.; Chang, R.K.; Ren, Z.; Jeon, S.R.; Gherasimova, M.; Han, J. UV-LEDs and linear arrays at 340 nm and 280 nm for fluorescence detection of bioaerosols. In Proceedings of the Photonic Applications Systems Technologies Conference, Baltimore, MD, USA, 23–28 May 2005; ISBN 1-55752-770-9. [Google Scholar]
- Kneissl, M.; Seong, T.Y.; Han, J.; Amano, H. The emergence and prospects of deep-ultraviolet light-emitting diode technologies. Nat. Photonics 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 2007; ISBN 9780387463124. [Google Scholar]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on förster resonance energy transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef]
- Pan, C.P.; Muiño, P.L.; Barkley, M.D.; Callis, P.R. Correlation of tryptophan fluorescence spectral shifts and lifetimes arising directly from heterogeneous environment. J. Phys. Chem. B 2011, 115, 3245–3253. [Google Scholar] [CrossRef]
- Demchenko, A. Ultraviolet Spectroscopy of Proteins, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 9783642708497. [Google Scholar]
- Pöhlker, C.; Huffman, J.A.; Pöschl, U. Autofluorescence of atmospheric bioaerosols—Fluorescent biomolecules and potential interferences. Atmos. Meas. Tech. 2012, 5, 37–71. [Google Scholar] [CrossRef]
- Cannon, T.M.; Lagarto, J.L.; Dyer, B.T.; Garcia, E.; Kelly, D.J.; Peters, N.S.; Lyon, A.R.; French, P.M.W.; Dunsby, C. Characterization of NADH fluorescence properties under one-photon excitation with respect to temperature, pH, and binding to lactate dehydrogenase. OSA Contin. 2021, 4, 1610. [Google Scholar] [CrossRef]
- Gonnella, T.P.; Leedahl, T.S.; Karlstad, J.P.; Picklo, M.J. NADH fluorescence lifetime analysis of the effect of magnesium ions on ALDH2. Chem. Biol. Interact. 2011, 191, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Berezin, M.M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2011, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Drössler, P.; Holzer, W.; Penzkofer, A.; Hegemann, P. Fluoresence quenching of riboflavin in aqueous solution by methionin and cystein. Chem. Phys. 2003, 286, 409–420. [Google Scholar] [CrossRef]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Pokhrel, M.R.; Braun, A.M.; Oliveros, E. Fluorescence of pterin, 6-formylpterin, 6-carboxypterin and folic acid in aqueous solution: Pp effects. Photochem. Photobiol. Sci. 2002, 1, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Dalterio, R.A.; Nelson, W.H.; Britt, D.; Sperry, J.F.; Tanguay, J.F.; Suib, S.L. The Steady-State and Decay Characteristics of Primary Fluorescence from Live Bacteria. Appl. Spectrosc. 1987, 41, 234–241. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Lee, D.; Pang, Y. Metal-enhanced fluorescence and excited state dynamics of carotenoids in thin polymer films. Sci. Rep. 2019, 9, 3551. [Google Scholar] [CrossRef]
- Feirer, N.; Fuqua, C. Pterin function in bacteria. Pteridines 2017, 28, 23–36. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in Plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; García-García, D.; San Andrés, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
- Davies Matthew Lloyd. Photophysical Studies of Triarylamine Dyes and an Investigation into Polyelectrolyte-DNA Interactions; Swansea University: Wales, UK, 2010. [Google Scholar]
- EasyLife X User’s Manual. 2015. Available online: https://www.chem.purdue.edu/ric/docs/EasyLife-X-Users-Manual.pdf (accessed on 22 December 2023).
- O’Connor, D.J.; Lovera, P.; Iacopino, D.; O’Riordan, A.; Healy, D.A.; Sodeau, J.R. Using spectral analysis and fluorescence lifetimes to discriminate between grass and tree pollen for aerobiological applications. Anal. Methods 2014, 6, 1633–1639. [Google Scholar] [CrossRef]
- Awad, F.; Ramprasath, C.; Mathivanan, N.; Aruna, P.R.; Ganesan, S. Optical Fiber-Based Steady State and Fluorescence Lifetime Spectroscopy for Rapid Identification and Classification of Bacterial Pathogens Directly from Colonies on Agar Plates. Int. Sch. Res. Not. 2014, 2014, 430412. [Google Scholar] [CrossRef]
- Boens, N.; Qin, W.; Basarić, N.; Hofkens, J.; Ameloot, M.; Pouget, J.; Lefèvre, J.P.; Valeur, B.; Gratton, E.; VandeVen, M.; et al. Fluorescence lifetime standards for time and frequency domain fluorescence spectroscopy. Anal. Chem. 2007, 79, 2137–2149. [Google Scholar] [CrossRef]
- Jošth, R.; Antikainen, J.; Havel, J.; Herout, A.; Zemčík, P.; Hauta-Kasari, M. Real-time PCA calculation for spectral imaging (using SIMD and GP-GPU). J. Real-Time Image Process. 2012, 7, 97–103. [Google Scholar] [CrossRef]


| Compound | EX [nm] | Em [nm] | Lifetime [ns] | Reference |
|---|---|---|---|---|
| Phe | 257–260 | 282 | 6.4−6.8 | [33,34] |
| Tyr | 274−275 | 303−304 | 3.6 | [33,34] |
| Trp | 280−295 | 348−353 | 3.1 | [33,34] |
| NADH | 346 | 457 | 0.48 | [33,37,38,39,40] |
| FAD | 450 | 520 | 2.3−2.9 | [40] |
| Riboflavin | 370, 450 | 518 | 5.06 | [20,41,47] |
| Folic acid | 361 | 442 | t1 = 1.6 (12%) t2 = 4.78 (88%) | [42] |
| FMN | 451 | 520 | 4.66 | [40,43] |
| B-Carotene | 471, 498 | 568 | t1 = 0.16 (75%) t2 = 1.07 (25%) | [44,46] |
| Pterine | 353 | 440−450 | 5.0−7.6 | [45] |
| Biological Sample | Source | Abbrev. |
|---|---|---|
| Bacillus cereus | ATCC 14579 | BC |
| Bacillus cereus spores | ATCC 14579 | BCs |
| Bacillus atrophaeus var. globibii | ATCC 9372 | BG |
| Bacillus atrophaeus var. globigi spores | ATCC 9372 | BGs |
| Bacillus atrophaeus spores technical | MIHE (Warsaw, Poland) | BGst |
| Bacillus megaterium | PCM (Wrocław, Poland) 2006 | BM |
| Bacillus megaterium spores | PCM (Wrocław, Poland) 2006 | BMs |
| Bacillus subtilis | ATCC 6633 | BS |
| Bacillus subtilis spores | ATCC 6633 | BSs |
| Bacillus thuringiensis | ATCC 10792 | BT |
| Bacillus thuringiensis spores | ATCC 10792 | BTs |
| Bacillus thuringiensis spores technical | MIHE (Warsaw, Poland) | BTst |
| Testing spores | MIHE (Warsaw, Poland) | Sporal S |
| Micrococcus luteus | ATCC 4698 | ML |
| Escherichia coli | ATCC 25922 | EC |
| Alternaria alternata | ATCC 6663 | AA |
| Candida albicans | ATCC 18804 | CA |
| Cladosporum herbarum | ATCC 28987 | CLH |
| Penicillium chrysogenum | ATCC 9197 | PCH |
| Penicillium brevicompactum | ATCC 9056 | PBC |
| Bermuda grass pollen | Duke Scientific Corp. (Palo Alto, CA, USA) | BER |
| Corn pollen | Duke Scientific Corp. (Palo Alto, CA, USA) | COR |
| Ragweed pollen | Duke Scientific Corp. (Palo Alto, CA, USA) | RAG |
| Secale cereale pollen | Sigma-Aldrich (St. Louis, MO, USA) | SEC |
| Paper mulberry pollen | Duke Scientific Corp. (Palo Alto, CA, USA) | PAP |
| Artemisia tridentata pollen | Sigma-Aldrich (St. Louis, MO, USA) | ARTT |
| Artemisia absinthium pollen | Sigma-Aldrich (St. Louis, MO, USA) | ARTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliszewski, M.; Kwaśny, M.; Bombalska, A.; Włodarski, M.; Trafny, E.A.; Kopczyński, K. Studies of Fluorescence Lifetimes of Biological Warfare Agents Simulants and Interferers Using the Stroboscopic Method. Appl. Sci. 2024, 14, 7332. https://doi.org/10.3390/app14167332
Kaliszewski M, Kwaśny M, Bombalska A, Włodarski M, Trafny EA, Kopczyński K. Studies of Fluorescence Lifetimes of Biological Warfare Agents Simulants and Interferers Using the Stroboscopic Method. Applied Sciences. 2024; 14(16):7332. https://doi.org/10.3390/app14167332
Chicago/Turabian StyleKaliszewski, Miron, Mirosław Kwaśny, Aneta Bombalska, Maksymilian Włodarski, Elżbieta Anna Trafny, and Krzysztof Kopczyński. 2024. "Studies of Fluorescence Lifetimes of Biological Warfare Agents Simulants and Interferers Using the Stroboscopic Method" Applied Sciences 14, no. 16: 7332. https://doi.org/10.3390/app14167332





