AI-Based Electroencephalogram Analysis in Rodent Models of Epilepsy: A Systematic Review
Abstract
:1. Introduction
- Interictal discharge: Interictal spikes are the intermittent neurological discharge observed between seizures in patients and animals with epilepsy [24,25]. Interictal spikes are noncoincidental [26] but relatable to seizures, and they are quantified as the most reliable epileptogenic biomarker [27,28].
- Preictal discharge: The preictal state is the abnormal neural discharge just before the manifestation of a seizure event, where a patient may feel the presence of seizure aura, which are physiological changes such as muscle twitches and gastrointestinal upset [29]. The temporal and spatial changes of the preictal discharge correlate with seizure onset; towards the onset of seizure, the amplitude of preictal discharge increases, while the interval decreases [30]. Thus, understanding and detecting the preictal spike mechanism can predict the next seizure episode [31].
- Ictal discharge: This is the most symptomatic and shortest phase of the epileptiform discharge. The ictal spike represents the critical event of a seizure that characterises an active epileptic condition.
- Postictal discharge: This state of the brain describes the abnormal neurological performance that begins at the end of a seizure episode; this state may last for hours before the resumption of the neural baseline activity [32]. Postictal discharge receives less attention than the interictal and preictal states. Nevertheless, the postictal characteristics may assist in distinguishing epileptic and non-epileptic seizures [33]
2. Fundamental Concepts and Background
2.1. Classification of Epilepsy
2.2. Rodent Models of Epilepsy
2.3. EEG Signal
2.4. Machine Learning and Deep Learning Techniques
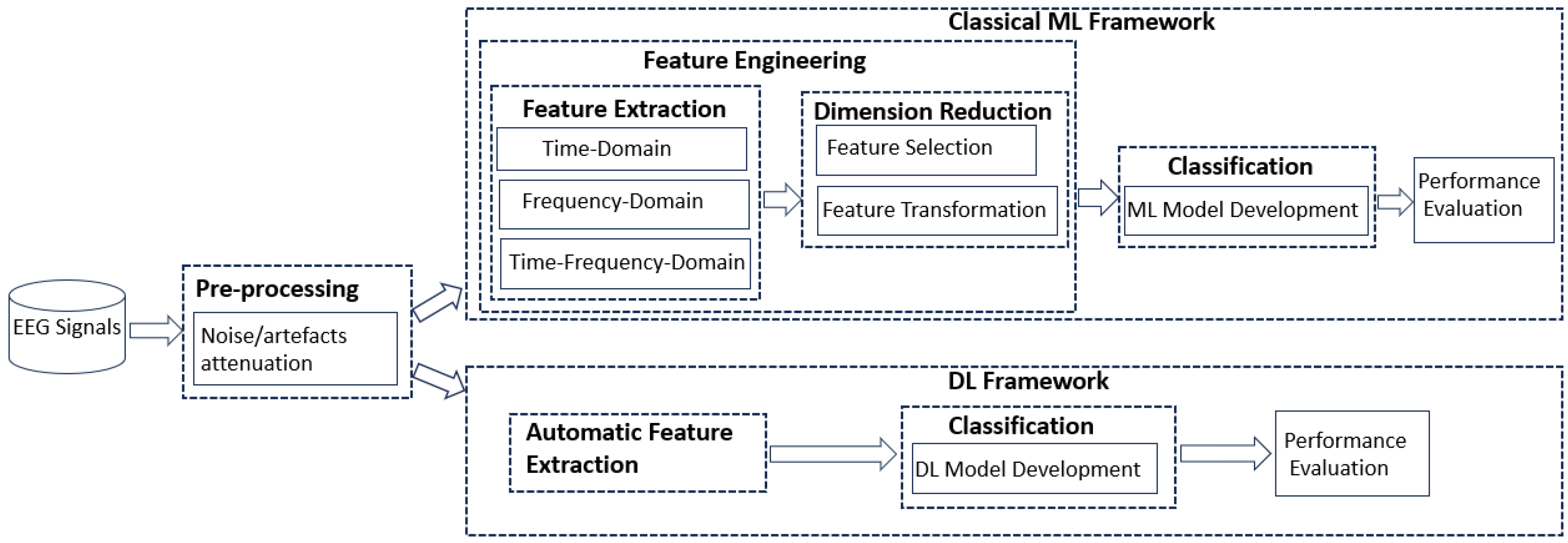
3. Materials and Methods
- Defining the research questions;
- Execution of article searches within specified databases;
- Filtering articles through the evaluation of their relevance;
- Data extraction;
- Synthesizing of results.
3.1. Research Questions
- RQ1: What rodent models of epilepsy and seizure/epilepsy types have been automatically analysed with ML or DL algorithms?
- RQ2: What features and feature engineering techniques have been considered in the classical machine learning detection and prediction of seizures in the rodent model of epilepsy?
- RQ3: What ML or DL methods have been exploited in detecting and predicting seizures from EEG of rodent models of epilepsy?
- RQ4: What training methodologies and evaluation metrics have been used in the rodent models of epilepsy, and which of the developed DL/ML models have been implemented?
3.2. Search Execution
- S1: ((EEG OR Electroencephalogram) AND (Seizure detection OR Machine learning seizure detection OR Deep learning seizure detection OR Interictal spike detection OR Ictal spike detection) AND (Rodent model of epilepsy OR Mouse model of epilepsy OR Rat model of epilepsy)).
- S2: ((EEG OR Electroencephalogram) AND (Seizure prediction OR Machine learning seizure prediction OR Deep learning seizure prediction OR Spike detection OR Preictal spike detection) AND (Rodent model of epilepsy OR Mouse model of epilepsy OR Rat model of epilepsy)).
- S3: ((“EEG” OR “Electroencephalogram” OR “iEEG”) AND (“Seizure detection” OR “Machine learning seizure detection” OR “Deep learning seizure detection” OR “Interictal spike detection” OR “Ictal spike detection”) AND (“Rodent model of epilepsy” OR “Mouse model of epilepsy” OR “Rat model of epilepsy”)).
- S4: ((“EEG” OR “Electroencephalogram” OR “iEEG”) AND (“Seizure prediction” OR “Machine learning seizure prediction” OR “Deep learning seizure prediction” OR “Spike detection” OR “Preictal spike detection”) AND (“Rodent model of epilepsy” OR “Mouse model of epilepsy” OR “Rat model of epilepsy”)).
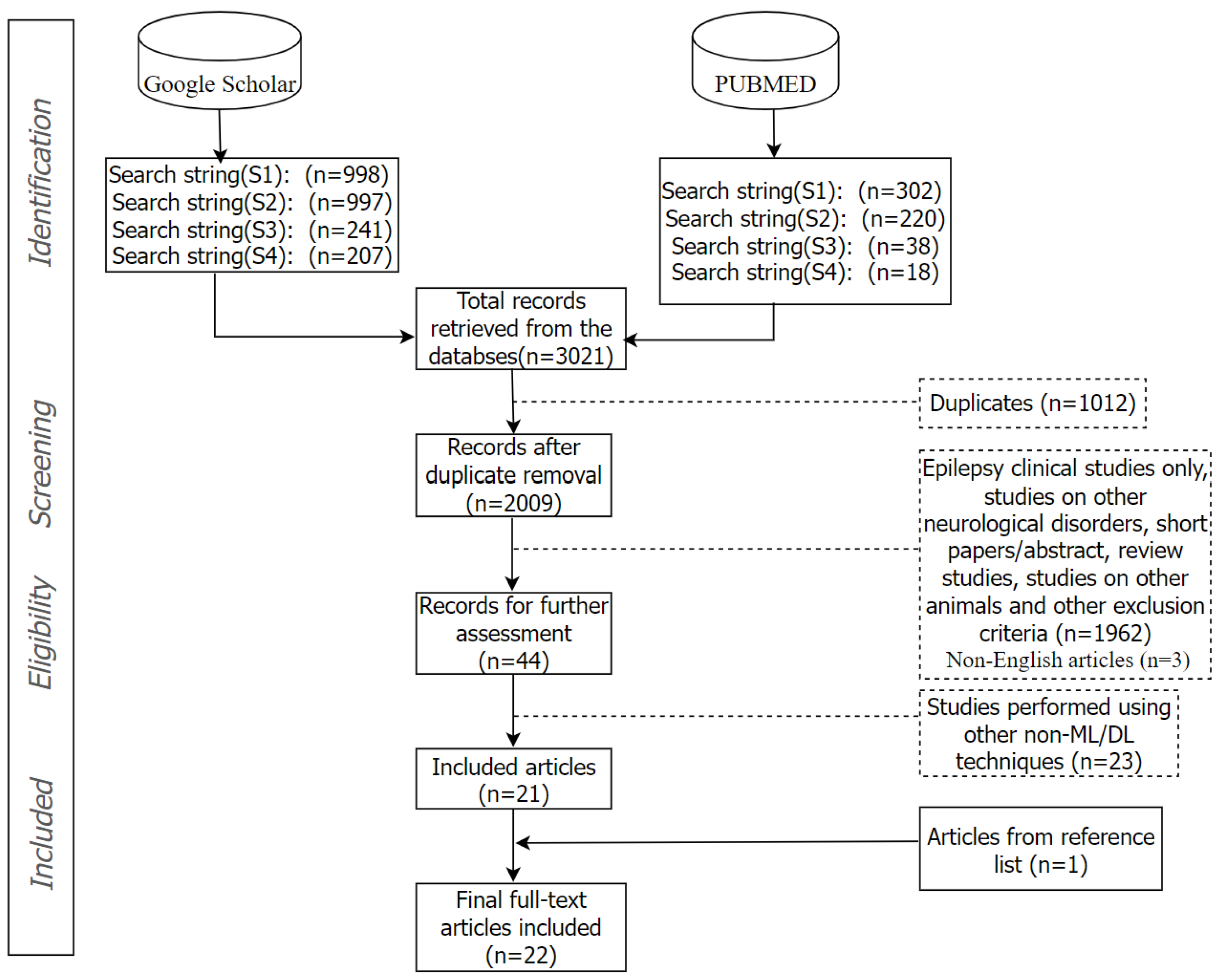
3.3. Article Selection and Filtering
- Inclusion criteria:
- Conference papers published by ACM, IEEE, or Springer;
- Published between 1 January 1994 and 1 January 2024;
- Focused on automatic seizure detection and prediction with machine learning and deep learning techniques;
- The study is conducted using EEG on rodent models of epilepsy;
- Full text available;
- The study reported in the article is empirical.
- Exclusion criteria:
- The study uses EEG from humans or other animals, not rodents;
- Full text is inaccessible;
- Literature review studies, books, short papers/abstracts;
- The study is focused on other neurological disorders (Alzheimer’s, dementia, sleep disorder, etc.);
- The study uses other clinical methods of determining epilepsy (e.g., genomics);
- Studies not written in English;
- Studies focusing on clinical studies only;
- Studies performed using other nonmachine or deep learning techniques;
- Journals not listed in Journal Citation Report (JCR).
- Data ExtractionRelevant data were gathered and analysed to synthesise and answer the research questions from the included studies. In the process, the following data fields were extracted:
- –
- D1: The aim of the article.
- –
- D2: The rodent models of epilepsy analysed.
- –
- D3: The epileptiform discharge recognition task: detection or prediction of seizures.
- –
- D4: The feature engineering techniques adopted.
- –
- D5: Employed ML or DL technique, either classical or a combination of techniques and evaluation result.
- –
- D6: The implementation of the ML or DL models.
4. Results
4.1. Ml and DL Article Distributions
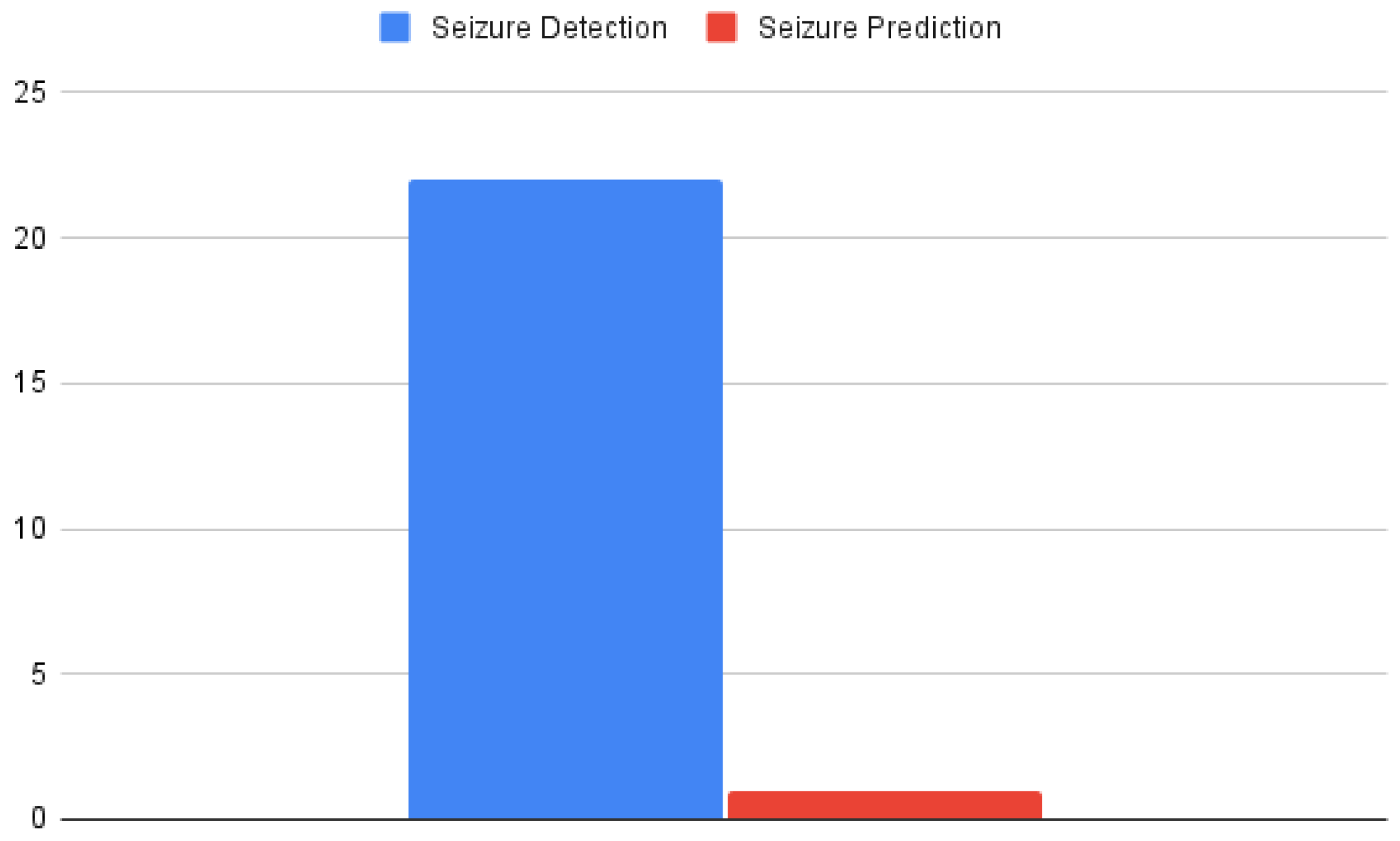
4.2. Distribution of Articles among Epileptiform Discharge Tasks
4.3. RQ 1: What Rodent Models of Epilepsy and Seizure/Epilepsy Types Have Been Automatically Analysed with ML or DL Algorithms?
4.4. RQ2: What Features and Feature Engineering Techniques Have Been Considered in the Classical Machine Learning Detection and Prediction of Seizures in Rodent Models of Epilepsy?
4.5. RQ3: What ML or DL Methods Have Been Exploited in Detecting and Predicting Seizures from EEG of Rodent Models of Epilepsy?
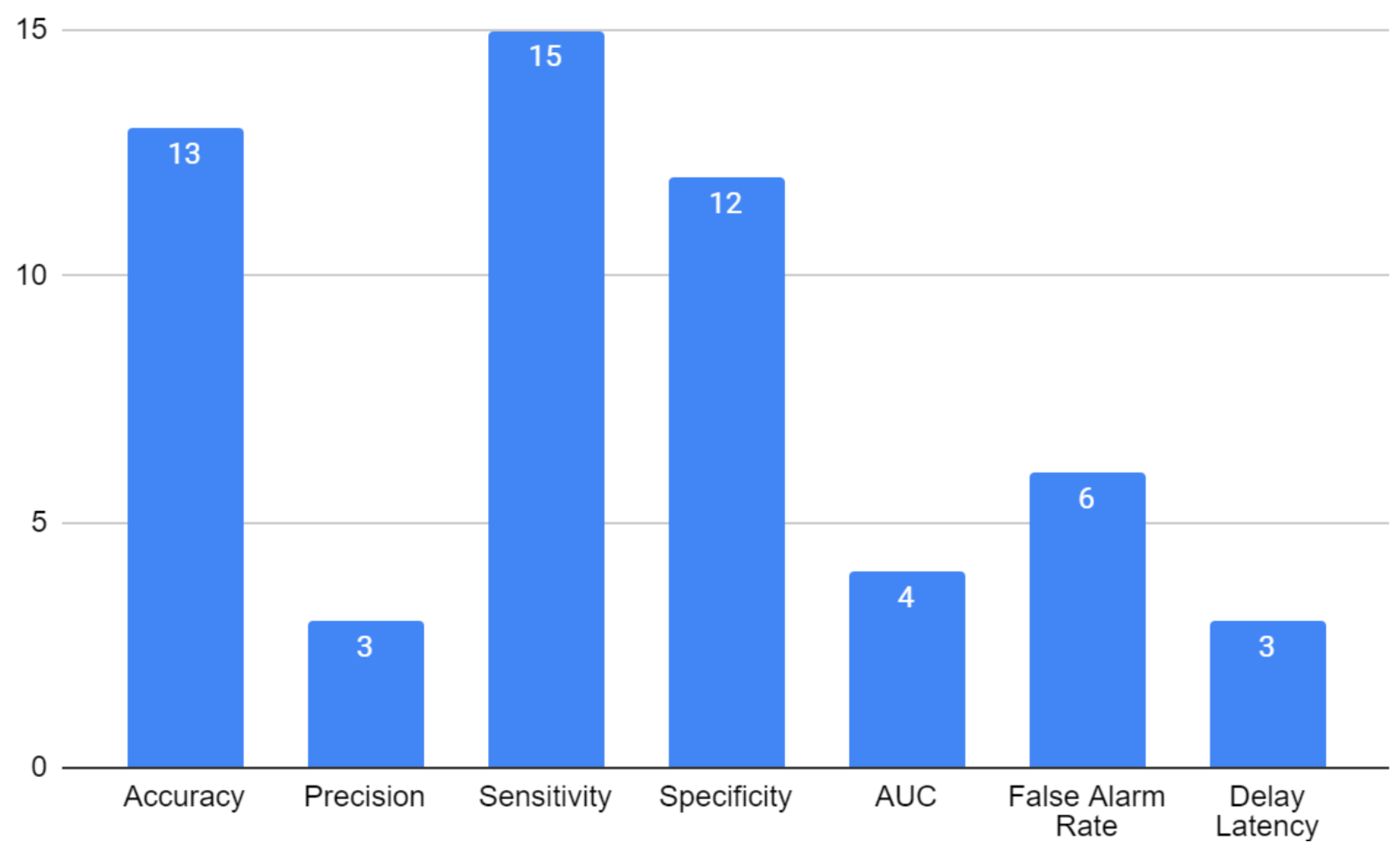
4.6. RQ4: What Training Methodologies and Evaluation Metrics Have Been Used in the Rodent Models of Epilepsy, and Which of the Developed DL/ML Models Have Been Implemented?
5. Discussion
5.1. Research Gaps
5.2. Limitations of the Study
5.3. Future Works
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, R.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.; Elger, C.; Engel, J., Jr.; Forsgren, L.; French, J.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- Shimizu, H.; Morimoto, Y.; Yamamoto, N.; Tayama, T.; Ozawa, H.; Imamura, A. Overlap between epilepsy and neurodevelopmental disorders: Insights from clinical and genetic studies. Epilepsy 2022. [Google Scholar] [CrossRef]
- McNamara, J. Emerging insights into the genesis of epilepsy. Nature 1999, 399, A15–A22. [Google Scholar] [CrossRef]
- Fisher, R.; Cross, J.; French, J.; Higurashi, N.; Hirsch, E.; Jansen, F.; Lagae, L.; Moshé, S.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Benbadis, S.; Agrawal, V.; Tatum, W. How many patients with psychogenic nonepileptic seizures also have epilepsy? Neurology 2001, 57, 915–917. [Google Scholar] [CrossRef]
- Xu, Y.; Nguyen, D.; Mohamed, A.; Carcel, C.; Li, Q.; Kutlubaev, M.; Anderson, C.; Hackett, M. Frequency of a false positive diagnosis of epilepsy: A systematic review of observational studies. Seizure 2016, 41, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wei, C.; Fu, M.; Li, X.; Zhang, H.; Yao, B. MCC950 alleviates seizure severity and angiogenesis by inhibiting NLRP3/IL-1β signaling pathway-mediated pyroptosis in mouse model of epilepsy. Int. Immunopharmacol. 2024, 126, 111236. [Google Scholar] [CrossRef]
- Laxer, K.; Trinka, E.; Hirsch, L.; Cendes, F.; Langfitt, J.; Delanty, N.; Resnick, T.; Benbadis, S. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. 2014, 37, 59–70. [Google Scholar] [CrossRef]
- Beghi, E.; Giussani, G.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.; Adib, M.; Agrawal, S.; Alahdab, F.; et al. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 19, 357–375. [Google Scholar] [CrossRef]
- Fiest, K.; Sauro, K.; Wiebe, S.; Patten, S.; Kwon, C.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H.; Sisodiya, S.; Vezzani, A. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Kandratavicius, L.; Balista, P.; Lopes-Aguiar, C.; Ruggiero, R.; Umeoka, E.; Garcia-Cairasco, N.; Bueno-Junior, L.; Leite, J. Animal models of epilepsy: Use and limitations. Neuropsychiatr. Dis. Treat. 2014, 10, 1693–1705. [Google Scholar]
- Singh, R.; Farooq, S.; Mannan, A.; Singh, T.; Najda, A.; Grażyna, Z.; Albadrani, G.; Sayed, A.; Abdel-Daim, M. Animal models of diabetic microvascular complications: Relevance to clinical features. Biomed. Pharmacother. 2022, 145, 112305. [Google Scholar] [CrossRef] [PubMed]
- Edoho, M.; Mamad, O.; Henshall, D.C.; Mooney, C.; Wei, L. Prediction of Epilepsy Phenotype in Intra-amygdala Kainic Acid Mouse Model of Epilepsy. In Proceedings of the 2023 10th International Conference on Biomedical and Bioinformatics Engineering, Kyoto, Japan, 9–12 November 2023; pp. 196–200. [Google Scholar]
- Dawson, T.; Golde, T.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Grone, B.; Baraban, S. Animal models in epilepsy research: Legacies and new directions. Nat. Neurosci. 2015, 18, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal models of epilepsy: A phenotype-oriented review. Aging Dis. 2022, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. The validity of animal models of depression. Psychopharmacology 1984, 83, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Garner, J. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? Ilar J. 2014, 55, 438–456. [Google Scholar] [CrossRef]
- Minguillon, J.; Lopez-Gordo, M.; Pelayo, F. Trends in EEG-BCI for daily-life: Requirements for artifact removal. Biomed. Signal Process. Control 2017, 31, 407–418. [Google Scholar] [CrossRef]
- Slimen, I.; Boubchir, L.; Seddik, H. Epileptic seizure prediction based on EEG spikes detection of ictal-preictal states. J. Biomed. Res. 2020, 34, 162. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, U.; Boston, R.; Cook, M.; D’Souza, W. Characteristics of epileptiform discharge duration and interdischarge interval in genetic generalized epilepsies. Front. Neurol. 2018, 9, 36. [Google Scholar] [CrossRef]
- Smith, E.; Liou, J.; Merricks, E.; Davis, T.; Thomson, K.; Greger, B.; House, P.; Emerson, R.; Goodman, R.; McKhann, G.; et al. Human interictal epileptiform discharges are bidirectional traveling waves echoing ictal discharges. Elife 2022, 11, e73541. [Google Scholar] [CrossRef]
- Tatum, W.; Rubboli, G.; Kaplan, P.; Mirsatari, S.; Radhakrishnan, K.; Gloss, D.; Caboclo, L.; Drislane, F.; Koutroumanidis, M.; Schomer, D.; et al. Clinical utility of EEG in diagnosing and monitoring epilepsy in adults. Clin. Neurophysiol. 2018, 129, 1056–1082. [Google Scholar] [CrossRef]
- Sablok, S.; Gururaj, G.; Shaikh, N.; Shiksha, I.; Choudhary, A. Interictal spike detection in EEG using time series classification. In Proceedings of the 2020 4th International Conference on Intelligent Computing and Control Systems (ICICCS), Madurai, India, 13–15 May 2020; pp. 644–647. [Google Scholar]
- Heers, M.; Böttcher, S.; Kalina, A.; Katletz, S.; Altenmüller, D.; Baroum, A.; Strobbe, G.; Mierlo, P.; Oertzen, T.; Marusic, P.; et al. Detection of interictal epileptiform discharges in an extended scalp EEG array and high-density EEG—A prospective multicenter study. Epilepsia 2022, 63, 1619–1629. [Google Scholar] [CrossRef]
- Prasanth, T.; Thomas, J.; Yuvaraj, R.; Jing, J.; Cash, S.; Chaudhari, R.; Leng, T.; Rathakrishnan, R.; Rohit, S.; Saini, V.; et al. Deep learning for interictal epileptiform spike detection from scalp EEG frequency sub bands. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montréal, QC, Canada, 20–24 July 2020; pp. 3703–3706. [Google Scholar]
- Selim, S.; Elhinamy, E.; Othman, H.; Abouelsaadat, W.; Salem, M. A review of machine learning approaches for epileptic seizure prediction. In Proceedings of the 2019 14th International Conference on Computer Engineering and Systems (ICCES), Cairo, Egypt, 17–18 December 2019; pp. 239–244. [Google Scholar]
- Chen, J.; Li, L.; Wu, D.; Li, X.; Xue, Q.; Wang, L.; Du, J.; Wang, D.; Hu, M.; Ren, L.; et al. Dynamic preictal discharges in patients with mesial temporal lobe epilepsy. J. Clin. Neurophysiol. 2018, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Nazari, J.; Motie Nasrabadi, A.; Menhaj, M.; Raiesdana, S. Epilepsy seizure prediction with few-shot learning method. Brain Inform. 2022, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rémi, J.; Noachtar, S. Clinical features of the postictal state: Correlation with seizure variables. Epilepsy Behav. 2010, 19, 114–117. [Google Scholar] [CrossRef]
- Ettinger, A.; Weisbrot, D.; Nolan, E.; Devinsky, O. Postictal symptoms help distinguish patients with epileptic seizures from those with non-epileptic seizures. Seizure 1999, 8, 149–151. [Google Scholar] [CrossRef]
- Wei, L.; Boutouil, H.; Gerbatin, R.; Mamad, O.; Heiland, M.; Reschke, C.; Del Gallo, F.; Fabene, P.; Henshall, D.; Lowery, M.; et al. Detection of spontaneous seizures in EEGs in multiple experimental mouse models of epilepsy. J. Neural Eng. 2021, 18, 056060. [Google Scholar] [CrossRef]
- Angeles, D. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia 1981, 22, 489–501. [Google Scholar]
- Fisher, R. The new classification of seizures by the International League Against Epilepsy 2017. Curr. Neurol. Neurosci. Rep. 2017, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.; Angus-Leppan, H. Epilepsy: Diagnosis, classification and management. Medicine 2020, 48, 522–528. [Google Scholar] [CrossRef]
- Wirrell, E.; Tinuper, P.; Perucca, E.; Moshé, S. Introduction to the epilepsy syndrome papers. Epilepsia 2022, 63, 1330–1332. [Google Scholar] [CrossRef]
- Milligan, T. Epilepsy: A clinical overview. Am. J. Med. 2021, 134, 840–847. [Google Scholar] [CrossRef]
- Rusina, E.; Bernard, C.; Williamson, A. The kainic acid models of temporal lobe epilepsy. Eneuro 2021, 8, 1–24. [Google Scholar] [CrossRef]
- Marshall, G.; Gonzalez-Sulser, A.; Abbott, C. Modelling epilepsy in the mouse: Challenges and solutions. Dis. Model. Mech. 2021, 14, dmm047449. [Google Scholar] [CrossRef]
- Leite, J.; Garcia-Cairasco, N.; Cavalheiro, E. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002, 50, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Goddard, G.; McIntyre, D.; Leech, C. A permanent change in brain function resulting from daily electrical stimulation. Exp. Neurol. 1969, 25, 295–330. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.; Thomas, S. Status epilepticus. Ann. Indian Acad. Neurol. 2009, 12, 140. [Google Scholar]
- Turski, W.; Cavalheiro, E.; Schwarz, M.; Czuczwar, S.; Kleinrok, Z.; Turski, L. Limbic seizures produced by pilocarpine in rats: Behavioural, electroencephalographic and neuropathological study. Behav. Brain Res. 1983, 9, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Chauviere, L.; Doublet, T.; Ghestem, A.; Siyoucef, S.; Wendling, F.; Huys, R.; Jirsa, V.; Bartolomei, F.; Bernard, C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann. Neurol. 2012, 71, 805–814. [Google Scholar] [CrossRef]
- Henderson, K.; Gupta, J.; Tagliatela, S.; Litvina, E.; Zheng, X.; Van Zandt, M.A.; Woods, N.; Grund, E.; Lin, D.; Royston, S.; et al. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J. Neurosci. 2014, 34, 13492–13504. [Google Scholar] [CrossRef]
- Lévesque, M.; Avoli, M.; Bernard, C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J. Neurosci. Methods 2016, 260, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.; Hamling, K.; Hong, S.; Anvar, M.; Lee, L.; Baraban, S. Preclinical animal models for Dravet syndrome: Seizure phenotypes, comorbidities and drug screening. Front. Pharmacol. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016, 126, 157–184. [Google Scholar] [CrossRef]
- Irizarry, R.; Sukato, D.; Kollmar, R.; Schild, S.; Silverman, J.; Sundaram, K.; Stephenson, S.; Stewart, M. Seizures induce obstructive apnea in DBA/2J audiogenic seizure-prone mice: Lifesaving impact of tracheal implants. Epilepsia 2020, 61, e13–e16. [Google Scholar] [CrossRef]
- Bosco, F.; Guarnieri, L.; Leo, A.; Tallarico, M.; Gallelli, L.; Rania, V.; Citraro, R.; De Sarro, G. Audiogenic epileptic DBA/2 mice strain as a model of genetic reflex seizures and SUDEP. Front. Neurol. 2023, 14, 1223074. [Google Scholar] [CrossRef] [PubMed]
- Depaulis, A.; David, O.; Charpier, S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J. Neurosci. Methods 2016, 260, 159–174. [Google Scholar] [CrossRef]
- Valassina, N.; Brusco, S.; Salamone, A.; Serra, L.; Luoni, M.; Giannelli, S.; Bido, S.; Massimino, L.; Ungaro, F.; Mazzara, P.; et al. Scn1a gene reactivation after symptom onset rescues pathological phenotypes in a mouse model of Dravet syndrome. Nat. Commun. 2022, 13, 161. [Google Scholar] [CrossRef]
- Gerbatin, R.; Augusto, J.; Boutouil, H.; Reschke, C.; Henshall, D. Life-span characterization of epilepsy and comorbidities in Dravet syndrome mice carrying a targeted deletion of exon 1 of the Scn1a gene. Exp. Neurol. 2022, 354, 114090. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gersbacher, M.; Inquimbert, P.; Kovacs, D. Reduced sodium channel Nav1.1 levels in BACE1-null mice. J. Biol. Chem. 2011, 286, 8106–8116. [Google Scholar] [CrossRef] [PubMed]
- Lachaux, J.; Axmacher, N.; Mormann, F.; Halgren, E.; Crone, N. High-frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog. Neurobiol. 2012, 98, 279–301. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004, 7, 446–451. [Google Scholar] [CrossRef] [PubMed]
- White, H.; Smith, M.; Wilcox, K. Mechanisms of action of antiepileptic drugs. Int. Rev. Neurobiol. 2007, 81, 85–110. [Google Scholar] [PubMed]
- Shah, A.; Mittal, S. Invasive electroencephalography monitoring: Indications and presurgical planning. Ann. Indian Acad. Neurol. 2014, 17, S89. [Google Scholar] [CrossRef] [PubMed]
- Ball, T.; Kern, M.; Mutschler, I.; Aertsen, A.; Schulze-Bonhage, A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage 2009, 46, 708–716. [Google Scholar] [CrossRef]
- Fisher, R.; Scharfman, H.; DeCurtis, M. How can we identify ictal and interictal abnormal activity? In Issues in Clinical Epileptology: A View from the Bench; Springer: Dordrecht, The Netherlands, 2014; pp. 3–23. [Google Scholar]
- Engel, A.; Moll, C.; Fried, I.; Ojemann, G. Invasive recordings from the human brain: Clinical insights and beyond. Nat. Rev. Neurosci. 2005, 6, 35–47. [Google Scholar] [CrossRef]
- Brienza, M.; Davassi, C.; Mecarelli, O. Artifacts. Clin. Electroencephalogr. 2019, 109–130. [Google Scholar] [CrossRef]
- Millán, J.; Carmena, J. Invasive or noninvasive: Understanding brain-machine interface technology. IEEE Eng. Med. Biol. Mag. 2010, 29, 16–22. [Google Scholar] [CrossRef]
- Wang, Q.; Prisco, N.; Tang, J.; Gennarino, V. Protocol for recording epileptiform discharges of EEG and behavioral seizures in freely moving mice. STAR Protoc. 2022, 3, 101245. [Google Scholar] [CrossRef]
- Moyer, J.; Gnatkovsky, V.; Ono, T.; Otáhal, J.; Wagenaar, J.; Stacey, W.; Noebels, J.; Ikeda, A.; Staley, K.; Curtis, M.; et al. Standards for data acquisition and software-based analysis of in vivo electroencephalography recordings from animals. A TASK 1-WG 5 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 2017, 58, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Nayak, C.; Anilkumar, A. EEG Normal Waveforms. Updated 31 July 2020. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020; Available online: http://www.ncbi.nlm.nih.gov (accessed on 8 March 2024).
- Sucholeiki, R.; Louis, S. Normal EEG waveforms. E-Med. 2008, 1–7. [Google Scholar]
- Huang, Z.; Wang, M. A review of electroencephalogram signal processing methods for brain-controlled robots. Cogn. Robot. 2021, 1, 111–124. [Google Scholar] [CrossRef]
- Zijlmans, M.; Jiruska, P.; Zelmann, R.; Leijten, F.; Jefferys, J.; Gotman, J. High-frequency oscillations as a new biomarker in epilepsy. Ann. Neurol. 2012, 71, 169–178. [Google Scholar] [CrossRef]
- Islam, M.; Zhao, X.; Miao, Y.; Sugano, H.; Tanaka, T. Epileptic seizure focus detection from interictal electroencephalogram: A survey. Cogn. Neurodyn. 2023, 17, 1–23. [Google Scholar] [CrossRef]
- Engel, J., Jr.; Bragin, A.; Staba, R.; Mody, I. High-frequency oscillations: What is normal and what is not? Epilepsia 2009, 50, 598–604. [Google Scholar] [CrossRef]
- Alarcon, G.; Binnie, C.; Elwes, R.; Polkey, C. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr. Clin. Neurophysiol. 1995, 94, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawas, S.; Poothrikovil, R.; Abdelbasit, K.; Delamont, R. The correlation between electroencephalography amplitude and interictal abnormalities: Audit study. Sultan Qaboos Univ. Med. J. 2014, 14, e473. [Google Scholar]
- Wei, L.; Mchugh, J.C.; Mooney, C. Transfer Learning for the Identification of Paediatric EEGs with Interictal Epileptiform Abnormalities. IEEE Access 2024, 12, 86073–86082. [Google Scholar] [CrossRef]
- Wei, L.; Mchugh, J.C.; Mooney, C. Interictal Epileptiform Discharge Classification for the Prediction of Epilepsy Type in Children. In Proceedings of the 2023 10th International Conference on Biomedical and Bioinformatics Engineering, Kyoto, Japan, 9–12 November 2023. [Google Scholar]
- Nayak, C.; Anilkumar, A. EEG normal waveforms. Updated 24 July 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hosseini, M.; Hosseini, A.; Ahi, K. A review on machine learning for EEG signal processing in bioengineering. IEEE Rev. Biomed. Eng. 2020, 14, 204–218. [Google Scholar] [CrossRef]
- Radüntz, T.; Scouten, J.; Hochmuth, O.; Meffert, B. Automated EEG artifact elimination by applying machine learning algorithms to ICA-based features. J. Neural Eng. 2017, 14, 046004. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Yang, Q.; Wang, R.; Lin, P.; Gao, J.; Leng, Y.; Yang, Y.; Wang, H. A brain-computer interface based on a few-channel EEG-fNIRS bimodal system. IEEE Access 2017, 5, 208–218. [Google Scholar] [CrossRef]
- Fatourechi, M.; Bashashati, A.; Ward, R.; Birch, G. EMG and EOG artifacts in brain computer interface systems: A survey. Clin. Neurophysiol. 2007, 118, 480–494. [Google Scholar] [CrossRef]
- Mannan, M.; Kamran, M.; Kang, S.; Jeong, M. Effect of EOG signal filtering on the removal of ocular artifacts and EEG-based brain-computer interface: A comprehensive study. Complexity 2018, 2018, 4853741. [Google Scholar] [CrossRef]
- Tamburro, G.; Fiedler, P.; Stone, D.; Haueisen, J.; Comani, S. A new ICA-based fingerprint method for the automatic removal of physiological artifacts from EEG recordings. PeerJ 2018, 6, e4380. [Google Scholar] [CrossRef]
- Sanei, S.; Chambers, J. EEG Signal Processing; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Azlan, W.; Low, Y. Feature extraction of Electroencephalogram (EEG) signal—A review. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences (IECBES), Kuala Lumpur, Malaysia, 8–10 December 2014; pp. 801–806. [Google Scholar]
- Yi, Y.; Billor, N.; Liang, M.; Cao, X.; Ekstrom, A.; Zheng, J. Classification of EEG signals: An interpretable approach using functional data analysis. J. Neurosci. Methods 2022, 376, 109609. [Google Scholar] [CrossRef]
- Tong, S.; Thankor, N. Quantitative EEG Analysis Methods and Clinical Applications; Artech House: Norwood, MA, USA, 2009. [Google Scholar]
- Rodriguez-Bermudez, G.; Garcia-Laencina, P. Analysis of EEG signals using nonlinear dynamics and chaos: A review. Appl. Math. Inf. Sci. 2015, 9, 2309. [Google Scholar]
- Li, J.; Cheng, K.; Wang, S.; Morstatter, F.; Trevino, R.; Tang, J.; Liu, H. Feature selection: A data perspective. ACM Comput. Surv. CSUR 2017, 50, 1–45. [Google Scholar] [CrossRef]
- Priyanka, S.; Dema, D.; Jayanthi, T. Feature selection and classification of Epilepsy from EEG signal. In Proceedings of the 2017 International Conference on Energy, Communication, Data Analytics and Soft Computing (ICECDS), Chennai, India, 1–2 August 2017; pp. 2404–2406. [Google Scholar]
- Sánchez-Hernández, S.; Salido-Ruiz, R.; Torres-Ramos, S.; Román-Godinez, I. Evaluation of feature selection methods for classification of epileptic seizure EEG signals. Sensors 2022, 22, 3066. [Google Scholar] [CrossRef]
- Boubchir, L.; Daachi, B.; Pangracious, V. A review of feature extraction for EEG epileptic seizure detection and classification. In Proceedings of the 2017 40th International Conference on Telecommunications and Signal Processing (TSP), Barcelona, Spain, 5–7 July 2017; pp. 456–460. [Google Scholar]
- Rasheed, K.; Qayyum, A.; Qadir, J.; Sivathamboo, S.; Kwan, P.; Kuhlmann, L.; O’Brien, T.; Razi, A. Machine learning for predicting epileptic seizures using EEG signals: A review. IEEE Rev. Biomed. Eng. 2020, 14, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Martin-Martin, A.; Orduna-Malea, E.; Thelwall, M.; López-Cózar, E. Google Scholar, Web of Science, and Scopus: A systematic comparison of citations in 252 subject categories. J. Inf. 2018, 12, 1160–1177. [Google Scholar] [CrossRef]
- Gusenbauer, M. Google Scholar to overshadow them all? Comparing the sizes of 12 academic search engines and bibliographic databases. Scientometrics 2019, 118, 177–214. [Google Scholar] [CrossRef]
- Misra, D.; Ravindran, V. An overview of the functionalities of PubMed. J. R. Coll. Physicians Edinb. 2022, 52, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Smith, D.; Swiniarski, R.; Dudek, F.; White, A.; Staley, K.; Cios, K. Analysis of EEG epileptic signals with rough sets and support vector machines. In Proceedings of the Artificial Intelligence in Medicine: 12th Conference on Artificial Intelligence in Medicine, AIME 2009, Verona, Italy, 18–22 July 2009; Proceedings 12. pp. 325–334. [Google Scholar]
- Wang, Y.; Liang, S.; Shaw, F.; Huang, Y.; Chen, Y. An energy efficient real-time seizure detection method in rats with spontaneous temporal lobe epilepsy. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 29–35. [Google Scholar]
- Fumeaux, N.; Ebrahim, S.; Coughlin, B.; Kadambi, A.; Azmi, A.; Xu, J.; Abou Jaoude, M.; Nagaraj, S.; Thomson, K.; Newell, T.; et al. Accurate detection of spontaneous seizures using a generalized linear model with external validation. Epilepsia 2020, 61, 1906–1918. [Google Scholar] [CrossRef]
- Buteneers, P.; Verstraeten, D.; Van Nieuwenhuyse, B.; Stroobandt, D.; Raedt, R.; Vonck, K.; Boon, P.; Schrauwen, B. Real-time detection of epileptic seizures in animal models using reservoir computing. Epilepsy Res. 2013, 103, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Bauer, S.; Neubert, V.; Costard, L.; Rosenow, F.; Triesch, J. A deep residual neural network based framework for epileptogenesis detection in a rodent model with single-channel EEG recordings. In Proceedings of the 2019 12th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Suzhou, China, 19–21 October 2019; pp. 1–6. [Google Scholar]
- Baser, O.; Yavuz, M.; Ugurlu, K.; Onat, F.; Demirel, B. Automatic detection of the spike-and-wave discharges in absence epilepsy for humans and rats using deep learning. Biomed. Signal Process. Control 2022, 76, 103726. [Google Scholar] [CrossRef]
- Li, R.; Millist, L.; Foster, E.; Yuan, X.; Guvenc, U.; Radfar, M.; Marendy, P.; Ni, W.; O’Brien, T.; Casillas-Espinosa, P. Spike and wave discharges detection in Genetic Absence Epilepsy Rat from Strasbourg and Patients with Genetic Generalized Epilepsy. Epilepsy Res. 2023, 194, 107181. [Google Scholar] [CrossRef]
- Liang, S.; Chang, W.; Chiueh, H. EEG-based absence seizure detection methods. In Proceedings of the 2010 International Joint Conference on Neural Networks (IJCNN), Barcelona, Spain, 18–23 July 2010; pp. 1–4. [Google Scholar]
- Chen, T.; Chiueh, H.; Liang, S.; Chang, S.; Jeng, C.; Hsu, Y.; Chien, T. The implementation of a low-power biomedical signal processor for real-time epileptic seizure detection on absence animal models. IEEE J. Emerg. Sel. Top. Circuits Syst. 2011, 1, 613–621. [Google Scholar] [CrossRef]
- Buteneers, P.; Verstraeten, D.; Mierlo, P.; Wyckhuys, T.; Stroobandt, D.; Raedt, R.; Hallez, H.; Schrauwen, B. Automatic detection of epileptic seizures on the intra-cranial electroencephalogram of rats using reservoir computing. Artif. Intell. Med. 2011, 53, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Pfammatter, J.; Maganti, R.; Jones, M. An automated, machine learning–based detection algorithm for spike-wave discharges (SWDs) in a mouse model of absence epilepsy. Epilepsia Open 2019, 4, 110–122. [Google Scholar] [CrossRef]
- Pan, Y.; Ge, S.; Tang, F.; Al Mamun, A. Detection of epileptic spike-wave discharges using SVM. In Proceedings of the 2007 IEEE International Conference on Control Applications, Singapore, 1–3 October 2007; pp. 467–472. [Google Scholar]
- Besné, G.; Horrillo-Maysonnial, A.; Nicolás, M.; Capell-Pascual, F.; Urrestarazu, E.; Artieda, J.; Valencia, M. An interactive framework for the detection of ictal and interictal activities: Cross-species and stand-alone implementation. Comput. Methods Programs Biomed. 2022, 218, 106728. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Jang, H. Comparison of different input modalities and network structures for deep learning-based seizure detection. Sci. Rep. 2020, 10, 122. [Google Scholar] [CrossRef]
- Jang, H.; Cho, K. Dual deep neural network-based classifiers to detect experimental seizures. Korean J. Physiol. Pharmacol. 2019, 23, 131–139. [Google Scholar] [CrossRef]
- Mohammadpoory, Z.; Nasrolahzadeh, M.; Mahmoodian, N.; Sayyah, M.; Haddadnia, J. Complex network based models of ECoG signals for detection of induced epileptic seizures in rats. Cogn. Neurodyn. 2019, 13, 325–339. [Google Scholar] [CrossRef]
- De, A.; Konar, A.; Samanta, A.; Biswas, S.; Basak, P. Seizure prediction using low frequency EEG wavesfrom WAG/Rij rats. In Proceedings of the 2017 2nd International Conference for Convergence in Technology (I2CT), Mumbai, India, 7–9 April 2017; pp. 244–249. [Google Scholar]
- Kotloski, R. A Machine Learning Approach to Seizure Detection in a Rat Model of Post-Traumatic Epilepsy. Sci. Rep. 2023, 13, 15807. [Google Scholar] [CrossRef]
- Pan, Y.; Ge, S.; Al Mamun, A.; Tang, F. Detection of seizures in EEG signal using weighted locally linear embedding and SVM classifier. In Proceedings of the 2008 IEEE Conference on Cybernetics and Intelligent Systems, Chengdu, China, 21–24 September 2008; pp. 358–363. [Google Scholar]
- Nan, M.; Talathi, S.; Myers, S.; Ditto, W.; Khargonekar, P.; Carney, P. Support vector machines for seizure detection in an animal model of chronic epilepsy. J. Neural Eng. 2010, 7, 036001. [Google Scholar] [CrossRef]
- Ramirez-Vélez, M.; Staba, R.; Barth, D.; Meyer, F. Nonlinear classification of EEG data for seizure detection. In Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: Nano to Macro, Arlington, VA, USA, 6–9 April 2006; pp. 956–959. [Google Scholar]
- Mascott, C.; Gotman, J.; Beaudet, A. Automated EEG monitoring in defining a chronic epilepsy model. Epilepsia 1994, 35, 895–902. [Google Scholar] [CrossRef]
- Niknazar, M.; Mousavi, S.; Motaghi, S.; Dehghani, A.; Vahdat, B.; Shamsollahi, M.; Sayyah, M.; Noorbakhsh, S. A unified approach for detection of induced epileptic seizures in rats using ECoG signals. Epilepsy Behav. 2013, 27, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Hussain, M.; Aboalsamh, H. An automated system for epilepsy detection using EEG brain signals based on deep learning approach. Expert Syst. Appl. 2018, 107, 61–71. [Google Scholar]
- Esmaeilpour, A.; Tabarestani, S.; Niazi, A. Deep learning-based seizure prediction using EEG signals: A comparative analysis of classification methods on the CHB-MIT dataset. Eng. Rep. 2024, e12918. [Google Scholar] [CrossRef]
- Abhishek, S.; Kumar, S.; Mohan, N.; Soman, K. EEG based automated detection of seizure using machine learning approach and traditional features. Expert Syst. Appl. 2024, 251, 123991. [Google Scholar]
- Chandel, G.; Aggarwal, T.; Singh, T.; Singh, S.; Singh, K.; Singh, H. Analysis of EEG Signals Using Machine Learning Algorithms. In Proceedings of the 2024 International Conference on Advances in Modern Age Technologies for Health and Engineering Science (AMATHE), Shivamogga, India, 16–17 May 2024; pp. 1–5. [Google Scholar]
- Kode, H.; Elleithy, K.; Almazedah, L. Epileptic Seizure detection in EEG signals using Machine Learning and Deep Learning Techniques. IEEE Access 2024, 12, 80657–80668. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Yang, X.; Wang, X.; Yin, J. A Robust Automatic Epilepsy Seizure Detection Algorithm Based on Interpretable Features and Machine Learning. Electronics 2024, 13, 2727. [Google Scholar] [CrossRef]
- Kunekar, P.; Gupta, M.; Gaur, P. Detection of epileptic seizure in EEG signals using machine learning and deep learning techniques. J. Eng. Appl. Sci. 2024, 71, 21. [Google Scholar] [CrossRef]
- Srinivas, P.; Arulprakash, M.; Vadivel, M.; Anusha, N.; Rajasekar, G.; Srinivasan, C. Support Vector Machines Based Predictive Seizure Care using IoT-Wearable EEG Devices for Proactive Intervention in Epilepsy. In Proceedings of the 2024 2nd International Conference on Computer, Communication and Control (IC4), Indore, India, 8–10 February 2024; pp. 1–5. [Google Scholar]
- Urbina Fredes, S.; Dehghan Firoozabadi, A.; Adasme, P.; Zabala-Blanco, D.; Palacios Játiva, P.; Azurdia-Meza, C. Enhanced Epileptic Seizure Detection through Wavelet-Based Analysis of EEG Signal Processing. Appl. Sci. 2024, 14, 5783. [Google Scholar] [CrossRef]
- Hermawan, A.; Zaeni, I.; Wibawa, A.; Gunawan, G.; Hendrawan, W.; Kristian, Y. A multi representation deep learning approach for epileptic seizure detection. J. Robot. Control JRC 2024, 5, 187–204. [Google Scholar] [CrossRef]
- Abdulwahhab, A.; Abdulaal, A.; Al-Ghrairi, A.; Mohammed, A.; Valizadeh, M. Detection of epileptic seizure using EEG signals analysis based on deep learning techniques. Chaos Solitons Fractals 2024, 181, 114700. [Google Scholar] [CrossRef]
- Trbalić, A.; Hasić, A.; Skejić, E.; Demirović, N. Seizure Detection Based on EEG Signals and Deep Learning. In Proceedings of the 2024 47th MIPRO ICT and Electronics Convention (MIPRO), Opatija, Croatia, 20–24 May 2024; pp. 1145–1150. [Google Scholar]
- Shafiezadeh, S.; Pozza, M.; Testolin, A. A Comparison of Recurrent and Convolutional Deep Learning Architectures for EEG Seizure Forecasting. BIOSTEC (1) 2024, 1, 583–590. [Google Scholar]
- Das, S.; Mumu, S.; Akhand, M.A.H.; Salam, A.; Kamal, M. Epileptic Seizure Detection from Decomposed EEG Signal through 1D and 2D Feature Representation and Convolutional Neural Network. Information 2024, 15, 256. [Google Scholar] [CrossRef]
- Georgis-Yap, Z.; Popovic, M.; Khan, S. Supervised and unsupervised deep learning approaches for EEG seizure prediction. J. Healthc. Inform. Res. 2024, 8, 286–312. [Google Scholar] [CrossRef]
- Abderrahim, N.; Echtioui, A.; Khemakhem, R.; Zouch, W.; Ghorbel, M.; Ben Hamida, A. Epileptic Seizures Detection Using iEEG Signals and Deep Learning Models. Circuits Syst. Signal Process. 2024, 43, 1597–1626. [Google Scholar] [CrossRef]
- Shah, S.; Larijani, H.; Gibson, R.; Liarokapis, D. Epileptic seizure classification based on random neural networks using discrete wavelet transform for electroencephalogram signal decomposition. Appl. Sci. 2024, 14, 599. [Google Scholar] [CrossRef]
- Sadam, S.; Nalini, N. Epileptic seizure detection using scalogram-based hybrid CNN model on EEG signals. Signal Image Video Process. 2024, 18, 1577–1588. [Google Scholar] [CrossRef]
- Anita, M.; Kowshalya, A. Automatic epileptic seizure detection using MSA-DCNN and LSTM techniques with EEG signals. Expert Syst. Appl. 2024, 238, 121727. [Google Scholar] [CrossRef]
- Martinez, C.; Niediek, J.; Mormann, F.; Andrzejak, R. Seizure onset zone lateralization using a non-linear analysis of micro vs. macro electroencephalographic recordings during seizure-free stages of the sleep-wake cycle from epilepsy patients. Front. Neurol. 2020, 11, 553885. [Google Scholar] [CrossRef]
- Shoeb, A. Application of Machine Learning to Epileptic Seizure Onset Detection and Treatment; Massachusetts Institute of Technology: Cambridge, MA, USA, 2009. [Google Scholar]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, J.; Ivanov, P.; Mark, R.; Mietus, J.; Moody, G.; Peng, C.; Stanley, H.; et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Cook, M.; O’Brien, T.; Berkovic, S.; Murphy, M.; Morokoff, A.; Fabinyi, G.; D’Souza, W.; Yerra, R.; Archer, J.; Litewka, L.; et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study. Lancet Neurol. 2013, 12, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Obeid, I.; Picone, J. The temple university hospital EEG data corpus. Front. Neurosci. 2016, 10, 195498. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, N.; Tapani, K.; Lauronen, L.; Vanhatalo, S. A dataset of neonatal EEG recordings with seizure annotations. Sci. Data 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Wong, S.; Simmons, A.; Rivera-Villicana, J.; Barnett, S.; Sivathamboo, S.; Perucca, P.; Ge, Z.; Kwan, P.; Kuhlmann, L.; Vasa, R.; et al. EEG datasets for seizure detection and prediction—A review. Epilepsia Open 2023, 8, 252–267. [Google Scholar] [CrossRef]
- Briggs, K. Is preclinical data sharing the new norm? Drug Discov. Today 2018, 23, 499–502. [Google Scholar] [CrossRef]
- Schulze-Bonhage, A.; Kühn, A. Unpredictability of seizures and the burden of epilepsy. In Seizure Prediction in Epilepsy: From Basic Mechanisms to Clinical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–10. [Google Scholar]
- Fisher, R. Epilepsy from the patient’s perspective: Review of results of a community-based survey. Epilepsy Behav. 2000, 1, S9–S14. [Google Scholar] [CrossRef]
- Usman, S.; Usman, M.; Fong, S. Epileptic seizures prediction using machine learning methods. Comput. Math. Methods Med. 2017, 2017, 9074759. [Google Scholar] [CrossRef] [PubMed]
- Gowers, W. Epilepsy and Other Chronic Convulsive Diseases: Their Causes, Symptoms, and Treatment; Old Hickory Bookshop: Brinklow, MD, USA, 1901. [Google Scholar]
- Haut, S. Can patients with epilepsy predict their seizures? Ann. Neurol. 2006, 60, S17. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Duan, L.; Qiao, Y.; Xiao, Y. Learning EEG synchronization patterns for epileptic seizure prediction using bag-of-wave features. J. Ambient. Intell. Humaniz. Comput. 2018, 14, 15557–15572. [Google Scholar] [CrossRef]
- Peng, P.; Song, Y.; Yang, L.; Wei, H. Seizure prediction in EEG signals using STFT and domain adaptation. Front. Neurosci. 2022, 15, 825434. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, T.; Leal, A.; Lopes, F.; Dourado, A.; Martins, P.; Teixeira, C. Interpretable EEG seizure prediction using a multiobjective evolutionary algorithm. Sci. Rep. 2022, 12, 4420. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Khalid, S.; Aslam, M. Epileptic seizures prediction using deep learning techniques. IEEE Access 2020, 8, 39998–40007. [Google Scholar] [CrossRef]
- Grasse, D.; Karunakaran, S.; Moxon, K. Closed-loop seizure prediction and prevention in rats with kainate-induced seizures. In Proceedings of the 2011 5th International IEEE/EMBS Conference on Neural Engineering, Cancun, Mexico, 27 April–1 May 2011; pp. 426–429. [Google Scholar]
- Howbert, J.; Patterson, E.; Stead, S.; Brinkmann, B.; Vasoli, V.; Crepeau, D.; Vite, C.; Sturges, B.; Ruedebusch, V.; Mavoori, J.; et al. Forecasting seizures in dogs with naturally occurring epilepsy. PLoS ONE 2014, 9, e81920. [Google Scholar] [CrossRef]
- Singh, N.; Vayer, P.; Tanwar, S.; Poyet, J.; Tsaioun, K.; Villoutreix, B. Drug discovery and development: Introduction to the general public and patient groups. Front. Drug Discov. 2023, 3, 1201419. [Google Scholar] [CrossRef]
- Nair, S.; Shiau, D.; Iasemidis, L.; Norman, W.; Pardalos, P.; Sackellares, J.; Carney, P. Seizure predictability in an experimental model of epilepsy. Data Min. Biomed. 2007, 7, 535–558. [Google Scholar]
- Sarmast, S.; Abdullahi, A.; Jahan, N. Current classification of seizures and epilepsies: Scope, limitations and recommendations for future action. Cureus 2020, 12, e10549. [Google Scholar] [CrossRef]
- Hauser, W.; Annegers, J.; Kurland, L.T. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993, 34, 453–458. [Google Scholar] [CrossRef]
- Wirrell, E.; Grossardt, B.; Wong-Kisiel, L.; Nickels, K. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: A population-based study. Epilepsy Res. 2011, 95, 110–118. [Google Scholar] [CrossRef]
- Camfield, P.; Camfield, C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015, 17, 117–123. [Google Scholar] [CrossRef]
- Beghi, E. The epidemiology of epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sultana, B.; Panzini, M.; Veilleux Carpentier, A.; Comtois, J.; Rioux, B.; Gore, G.; Bauer, P.; Kwon, C.; Jetté, N.; Josephson, C.; et al. Incidence and prevalence of drug-resistant epilepsy: A systematic review and meta-analysis. Neurology 2021, 96, 805–817. [Google Scholar] [CrossRef]
- Biset, G.; Abebaw, N.; Gebeyehu, N.; Estifanos, N.; Birrie, E.; Tegegne, K. Prevalence, incidence, and trends of epilepsy among children and adolescents in Africa: A systematic review and meta-analysis. BMC Public Health 2024, 24, 771. [Google Scholar] [CrossRef]
- Wei, L.; Mooney, C. Pediatric and Adolescent Seizure Detection: A Machine Learning Approach Exploring the Influence of Age and Sex in Electroencephalogram Analysis. BioMedInformatics 2024, 4, 796–810. [Google Scholar] [CrossRef]
- Rozensztrauch, A.; Kołtuniuk, A. The Quality of Life of Children with Epilepsy and the Impact of the Disease on the Family Functioning. Int. J. Environ. Res. Public Health 2022, 19, 2277. [Google Scholar] [CrossRef] [PubMed]
- Brigo, F.; Trinka, E.; Lattanzi, S.; Bragazzi, N.; Nardone, R.; Martini, M. A brief history of typical absence seizures—Petit mal revisited. Epilepsy Behav. 2018, 80, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Klonowski, W. Everything you wanted to ask about EEG but were afraid to get the right answer. Nonlinear Biomed. Phys. 2009, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Q.; Tan, S.; Chan, R. High-resolution time-frequency analysis of EEG signals using multiscale radial basis functions. Neurocomputing 2016, 195, 96–103. [Google Scholar] [CrossRef]
- Tzallas, A.; Tsipouras, M.; Fotiadis, D. Automatic seizure detection based on time-frequency analysis and artificial neural networks. Comput. Intell. Neurosci. 2007, 80510. [Google Scholar]
- Janecek, A.; Gansterer, W.; Demel, M.; Ecker, G. On the relationship between feature selection and classification accuracy. New Chall. Feature Sel. Data Min. Knowl. Discov. 2008, 4, 90–105. [Google Scholar]
- Torkkola, K.; Campbell, W. Mutual information in learning feature transformations. ICML 2000, 1015–1022. [Google Scholar]
- Jović, A.; Brkić, K.; Bogunović, N. A review of feature selection methods with applications. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 25–29 May 2015; pp. 1200–1205. [Google Scholar]
- Tang, J.; Alelyani, S.; Liu, H. Feature selection for classification: A review. Data Classif. Algorithms Appl. 2014, 37. [Google Scholar]
- Kumar, V.; Minz, S. Feature selection. SmartCR 2014, 4, 211–229. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, T.; Li, W. An efficient hybrid feature selection method using the artificial immune algorithm for high-dimensional data. Comput. Intell. Neurosci. 2022, 2022, 1452301. [Google Scholar] [PubMed]
- Liu, X.; Liang, Y.; Wang, S.; Yang, Z.; Ye, H. A hybrid genetic algorithm with wrapper-embedded approaches for feature selection. IEEE Access 2018, 6, 22863–22874. [Google Scholar] [CrossRef]
- Lu, H.; Chen, J.; Yan, K.; Jin, Q.; Xue, Y.; Gao, Z. A hybrid feature selection algorithm for gene expression data classification. Neurocomputing 2017, 256, 56–62. [Google Scholar] [CrossRef]
- Ma, W.; Zhou, X.; Zhu, H.; Li, L.; Jiao, L. A two-stage hybrid ant colony optimization for high-dimensional feature selection. Pattern Recognit. 2021, 116, 107933. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, C.; Zhou, X.; Huang, T. A hybrid feature selection method based on binary state transition algorithm and ReliefF. IEEE J. Biomed. Health Inform. 2018, 23, 1888–1898. [Google Scholar] [CrossRef]
- Cadenas, J.; Garrido, M.; MartiNez, R. Feature subset selection filter–wrapper based on low quality data. Expert Syst. Appl. 2013, 40, 6241–6252. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, J.; Ming, W.; Wang, S.; Sawan, M. Shorter latency of real-time epileptic seizure detection via probabilistic prediction. Expert Syst. Appl. 2024, 236, 121359. [Google Scholar] [CrossRef]
- Mormann, F.; Andrzejak, R.; Elger, C.; Lehnertz, K. Seizure prediction: The long and winding road. Brain 2007, 130, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Roy, Y.; Banville, H.; Albuquerque, I.; Gramfort, A.; Falk, T.; Faubert, J. Deep learning-based electroencephalography analysis: A systematic review. J. Neural Eng. 2019, 16, 051001. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Morales-Menendez, R.; Huang, X.; Hussain, N. A review of epileptic seizure detection using machine learning classifiers. Brain Inform. 2020, 7, 5. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Hu, S. A learning method for the class imbalance problem with medical data sets. Comput. Biol. Med. 2010, 40, 509–518. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhou, W.; Zhang, L.; Zhang, F.; Xu, F.; Leng, Y.; Wei, D.; Chen, M. Epileptic seizure detection based on imbalanced classification and wavelet packet transform. Seizure 2017, 50, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.; Bowyer, K.; Hall, L.; Kegelmeyer, W. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Drummond, C.; Holte, R. C4.5, class imbalance, and cost sensitivity: Why under-sampling beats over-sampling. Workshop Learn. Imbalanced Datasets II 2003, 11, 1–8. [Google Scholar]
- Ling, C.; Li, C. Data mining for direct marketing: Problems and solutions. KDD 1998, 98, 73–79. [Google Scholar]
- Chen, C.; Liaw, A.; Breiman, L. Using random forest to learn imbalanced data. J. Univ. Calif. 2004, 110, 24. [Google Scholar]
- Burez, J.; Poel, D. Handling class imbalance in customer churn prediction. Expert Syst. Appl. 2009, 36, 4626–4636. [Google Scholar] [CrossRef]
- He, H.; Garcia, E. Learning from imbalanced data. IEEE Trans. Knowl. Data Eng. 2009, 21, 1263–1284. [Google Scholar]
- Japkowicz, N.; Stephen, S. The class imbalance problem: A systematic study. Intell. Data Anal. 2002, 6, 429–449. [Google Scholar] [CrossRef]
- Vale, D.; El-Sharif, A.; Ali, M. Explainable artificial intelligence (XAI) post-hoc explainability methods: Risks and limitations in non-discrimination law. AI Ethics 2022, 2, 815–826. [Google Scholar] [CrossRef]
- Vellido, A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput. Appl. 2020, 32, 18069–18083. [Google Scholar] [CrossRef]
- Teng, Q.; Liu, Z.; Song, Y.; Han, K.; Lu, Y. A survey on the interpretability of deep learning in medical diagnosis. Multimed. Syst. 2022, 28, 2335–2355. [Google Scholar] [CrossRef] [PubMed]
- Antoniadi, A.; Du, Y.; Guendouz, Y.; Wei, L.; Mazo, C.; Becker, B.; Mooney, C. Current challenges and future opportunities for XAI in machine learning-based clinical decision support systems: A systematic review. Appl. Sci. 2021, 11, 5088. [Google Scholar] [CrossRef]
- Jaotombo, F.; Adorni, L.; Ghattas, B.; Boyer, L. Finding the best trade-off between performance and interpretability in predicting hospital length of stay using structured and unstructured data. PLoS ONE 2023, 18, e0289795. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, U.; Piatetsky-Shapiro, G.; Smyth, P. From data mining to knowledge discovery in databases. AI Mag. 1996, 17, 37. [Google Scholar]
- Rudin, C.; Chen, C.; Chen, Z.; Huang, H.; Semenova, L.; Zhong, C. Interpretable machine learning: Fundamental principles and 10 grand challenges. Stat. Surv. 2022, 16, 1–85. [Google Scholar] [CrossRef]
- Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef]
- Tan, S.; Caruana, R.; Hooker, G.; Lou, Y. Distill-and-compare: Auditing black-box models using transparent model distillation. In Proceedings of the 2018 AAAI/ACM Conference on AI, Ethics, and Society, New Orleans, LA, USA, 1–3 February 2018; pp. 303–310. [Google Scholar]
- Ribeiro, M.; Singh, S.; Guestrin, C. Model-agnostic interpretability of machine learning. arXiv 2016, arXiv:1606.05386. [Google Scholar]
- Ribeiro, M.; Singh, S.; Guestrin, C. “Why should i trust you?” Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Camburu, O.; Giunchiglia, E.; Foerster, J.; Lukasiewicz, T.; Blunsom, P. Can I trust the explainer? Verifying post-hoc explanatory methods. arXiv 2019, arXiv:1910.02065. [Google Scholar]
- Visani, G.; Bagli, E.; Chesani, F.; Poluzzi, A.; Capuzzo, D. Statistical stability indices for LIME: Obtaining reliable explanations for machine learning models. J. Oper. Res. Soc. 2022, 73, 91–101. [Google Scholar] [CrossRef]
- Molnar, C.; König, G.; Herbinger, J.; Freiesleben, T.; Dandl, S.; Scholbeck, C.; Casalicchio, G.; Grosse-Wentrup, M.; Bischl, B. General pitfalls of model-agnostic interpretation methods for machine learning models. In International Workshop on Extending Explainable AI beyond Deep Models and Classifiers; Springer: Cham, Switzerland, 2020; pp. 39–68. [Google Scholar]
- Zhang, Q.; Wu, Y.; Zhu, S. Interpretable convolutional neural networks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–22 June 2018; pp. 8827–8836. [Google Scholar]
- Chen, C.; Li, O.; Tao, D.; Barnett, A.; Rudin, C.; Su, J. This looks like that: Deep learning for interpretable image recognition. Adv. Neural Inf. Process. Syst. 2019, 32. [Google Scholar]
- Koh, P.; Nguyen, T.; Tang, Y.; Mussmann, S.; Pierson, E.; Kim, B.; Liang, P. Concept bottleneck models. In Proceedings of the International Conference on Machine Learning, Virtual, 13–18 July 2020; Volume 119, pp. 5338–5348. [Google Scholar]
- Chen, Z.; Bei, Y.; Rudin, C. Concept whitening for interpretable image recognition. Nat. Mach. Intell. 2020, 2, 772–782. [Google Scholar] [CrossRef]
- Angelov, P.; Soares, E. Towards explainable deep neural networks (xDNN). Neural Netw. 2020, 130, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Nauta, M.; Van Bree, R.; Seifert, C. Neural prototype trees for interpretable fine-grained image recognition. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Nashville, TN, USA, 20–25 June 2021; pp. 14933–14943. [Google Scholar]
- Joseph, V.; Vakayil, A. SPlit: An optimal method for data splitting. Technometrics 2022, 64, 166–176. [Google Scholar] [CrossRef]
- Bouthillier, X.; Delaunay, P.; Bronzi, M.; Trofimov, A.; Nichyporuk, B.; Szeto, J.; Mohammadi Sepahvand, N.; Raff, E.; Madan, K.; Voleti, V.; et al. Accounting for variance in machine learning benchmarks. Proc. Mach. Learn. Syst. 2021, 3, 747–769. [Google Scholar]
- Zhang, Y.; Guo, Y.; Yang, P.; Chen, W.; Lo, B. Epilepsy seizure prediction on EEG using common spatial pattern and convolutional neural network. IEEE J. Biomed. Health Inform. 2019, 24, 465–474. [Google Scholar] [CrossRef] [PubMed]


| Ref | Rodent Model | Seizure/Epilepsy Type Modelled |
|---|---|---|
| [110] | -aminobutyric acid A (GABAA) receptor mutation (2R43Q) | Absence epilepsy |
| [102] | Intrahippocampal kainic acid | Mesial temporal lobe epilepsy |
| [112] | High temperature | Dravet syndrome |
| [109] | Genetic and kainate systemic injection | Absence and tonic–clonic seizures |
| [103] | Genetic and kainate systemic injection | Absence and limbic seizures |
| [108] | Genetic | Absence seizures |
| [107] | Genetic | Absence seizures |
| [120] | - | - |
| [34] | Intra-amygdala kainic acid, Dravet, Pilocarpine | Temporal lope epilepsy, Dravet syndrome |
| [115] | Pentylenetetrazole | Clonic seizures |
| [106] | GAER | Absence seizures |
| [104] | Perforant pathway stimulation | Mesial temporal lobe epilepsy with hippocampal sclerosis |
| [114] | Pilocarpine hydrochloride | Convulsive seizures |
| [113] | Pilocarpine injection | Generalized tonic–clonic seizures |
| [105] | GAERS | Absence seizures |
| [117] | Perforant path kindling susceptible | Post-traumatic epilepsy |
| [116] | Kindling | Frontal lobe epilepsy |
| Author | EEG Domain | Feature Extraction | Feature Type | Feature Selection | Feature Transformation |
|---|---|---|---|---|---|
| [110] | FD | Wavelet | 12 linear | - | - |
| [102] | TD, FD | - | 141 linear | - | PCA |
| [112] | TD, FD | Gabor transform | 9 linear | Neighborhood component analysis | - |
| [109] | FD | Wavelet | 12 linear | Forward feature selection | - |
| [103] | FD | Wavelet | 2 linear | Forward feature selection | - |
| [108] | TD, FD | FFT | 2 linear, 1 nonlinear | - | - |
| [107] | FD | FFT | 1 linear, 1 nonlinear | - | PCA |
| [120] | TD | PCA, Laplacian Eigenmap manifold | linear | - | PCA and Laplacian Eigenmap manifold |
| [34] | TD, FD | Wavelet | 19 linear | ||
| [115] | TD | Natural visibility algorithm, Markov–binary visibility graph, | 14 linear | Sequential forward selection | - |
| [106] | FD | FFT | 2 linear | - | - |
| [116] | FD, TFD | FFT, wavelet transform | 1 linear, 1 nonlinear | - | - |
| Author | Number of Rodents | Classifier | Result | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Sensitivity (%) | Specificity (%) | AUC (%) | False Alarm (%) | Delay Latency (s) | |||
| [110] | 11 | SVM | - | 59.00 | 68.00 | - | - | - | - |
| [102] | 11 | GLM | - | - | - | - | 99.50 | - | - |
| [112] | 13 | RF | 92.80–97.20 | 77.30–97.40 | 93.30–95.80 | 91.90–98.90 | - | - | - |
| [103] | 45 | RC-BRR | - | - | 96.20 | 98.20 | - | 9.10 | 0.97 |
| [107] | 3 | RBF-SVM | 97.50 | - | 97.03 | 97.83 | - | - | - |
| [120] | - | KRR | 73.43–97.76 | - | - | - | - | - | - |
| [34] | 26 | XGBoost | 93.1–98.8 | - | 76.30–98.8 | 93.10–98.80 | - | - | - |
| [115] | 27 | MLP | 92.13 | - | 98.94 | - | 86.03 | - | - |
| [106] | 18 | GNN | - | - | 98.01 | - | 98.90 | 95.90 | - |
| Author | Number of Rodents | Classifier | Result | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Sensitivity (%) | Specificity (%) | AUC (%) | False Alarm (%) | Delay Latency (s) | |||
| [104] | 7 | Deep residual neural network | - | - | 83.00 | 83.00 | 90.00 | - | - |
| [114] | - | Sequential dual deep neural networks | - | 98.00 | 100.00 | - | - | 6.00 | - |
| [113] | 32 | Fully connected neural network | 99.60–99.90 | - | 96.20–96.70 | 99.60–99.90 | 98.90–99.30 | 0.9–1.1 | - |
| [105] | 11 | CNN | 94.28 | - | 96.60 | 90.40 | - | - | - |
| [117] | 8 | GoogleNet | 81.10–95.60 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edoho, M.; Mooney, C.; Wei, L. AI-Based Electroencephalogram Analysis in Rodent Models of Epilepsy: A Systematic Review. Appl. Sci. 2024, 14, 7398. https://doi.org/10.3390/app14167398
Edoho M, Mooney C, Wei L. AI-Based Electroencephalogram Analysis in Rodent Models of Epilepsy: A Systematic Review. Applied Sciences. 2024; 14(16):7398. https://doi.org/10.3390/app14167398
Chicago/Turabian StyleEdoho, Mercy, Catherine Mooney, and Lan Wei. 2024. "AI-Based Electroencephalogram Analysis in Rodent Models of Epilepsy: A Systematic Review" Applied Sciences 14, no. 16: 7398. https://doi.org/10.3390/app14167398






