Molybdenum Recovery from the Copper Hydrometallurgical Extraction Route with High Content of Chloride Ions Using the Ion Exchange Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Development—Laboratory Tests
- (a)
- Resin preconditioning: Both resins need a HCl preconditioning to increase their affinity for molybdenum anions:
- (b)
- Molybdenum recovery from the copper PLS: Molybdenum can be recovered and exchanged during the elution stage. According to the Figure 2b, molybdenum, at pH values 1.0–2.5, was present mostly as Mo7O21(OH)33−. Equation (2) illustrates the ion exchange mechanism:
- (c)
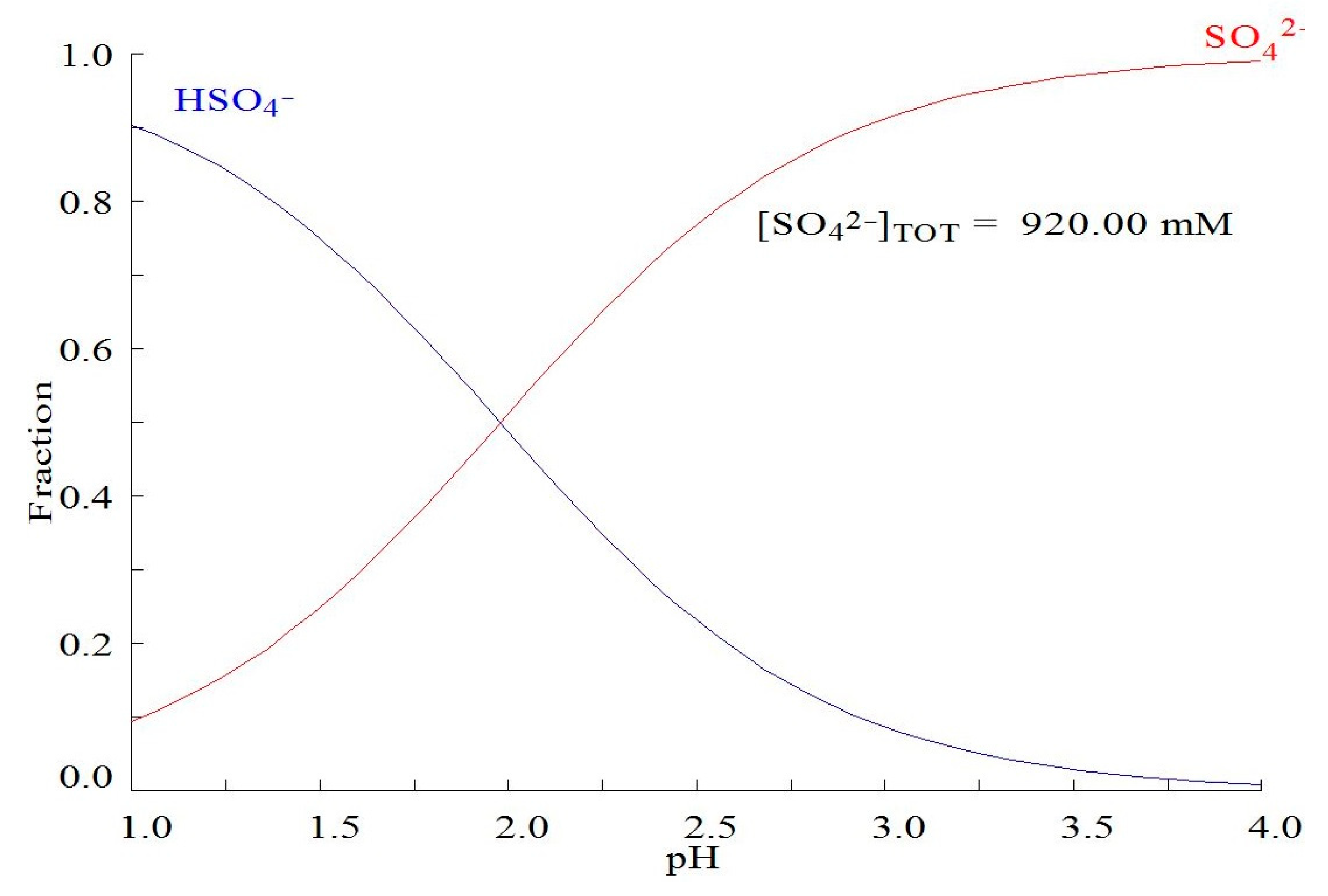
- Molybdenum elution: According to the Pourbaix diagram from Figure 2a, molybdenum can be effectively recovered from the resin phase using a NaOH solution, because in these conditions, the prevalent species are the anions. Equation (3) shows the ion exchange:
- (d)
- Resin regeneration: To carry out the process continuously, the resin conditioning must start again, as described in Equation (1).
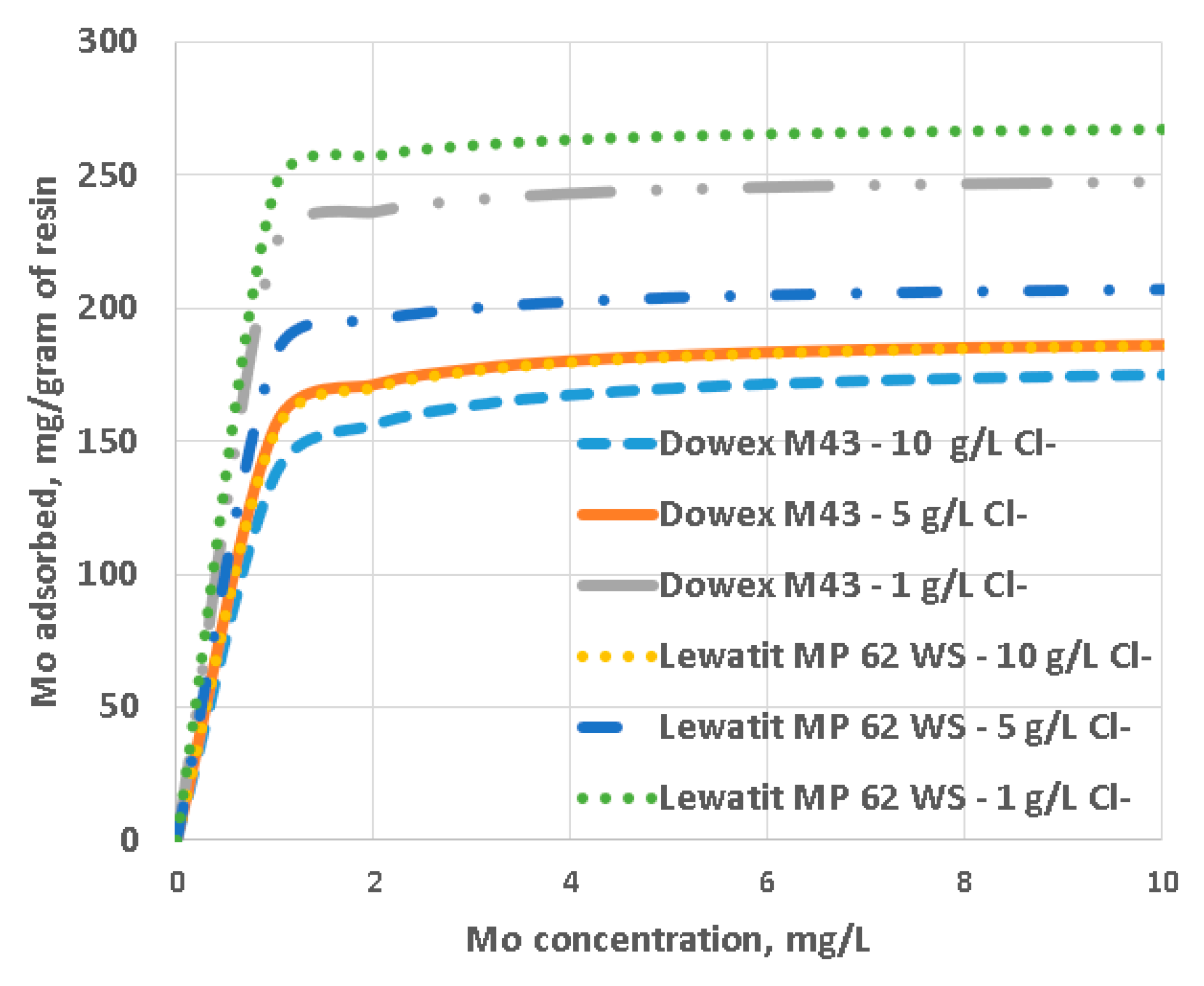
2.2. Langmuir Adsorption Analysis
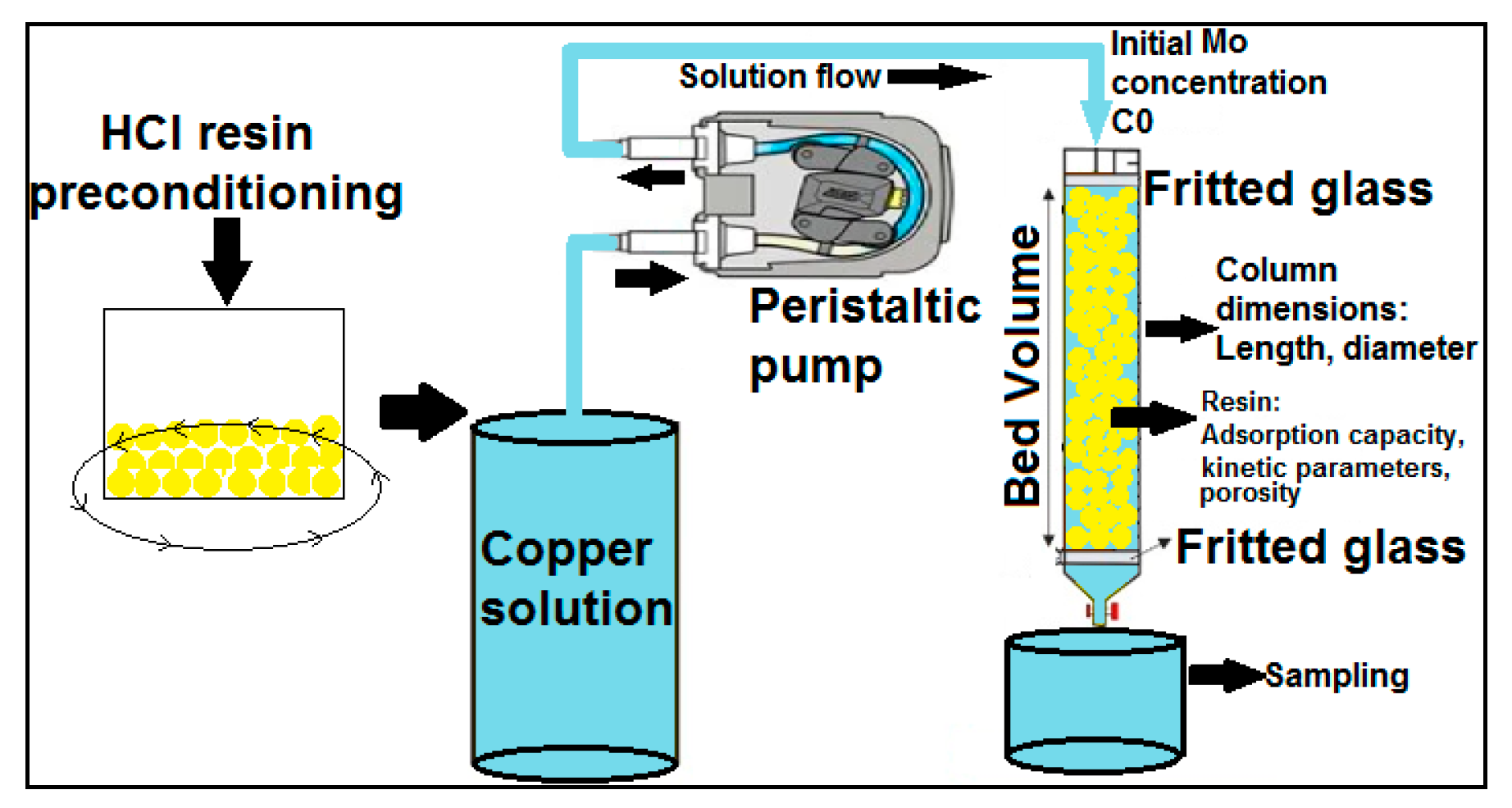
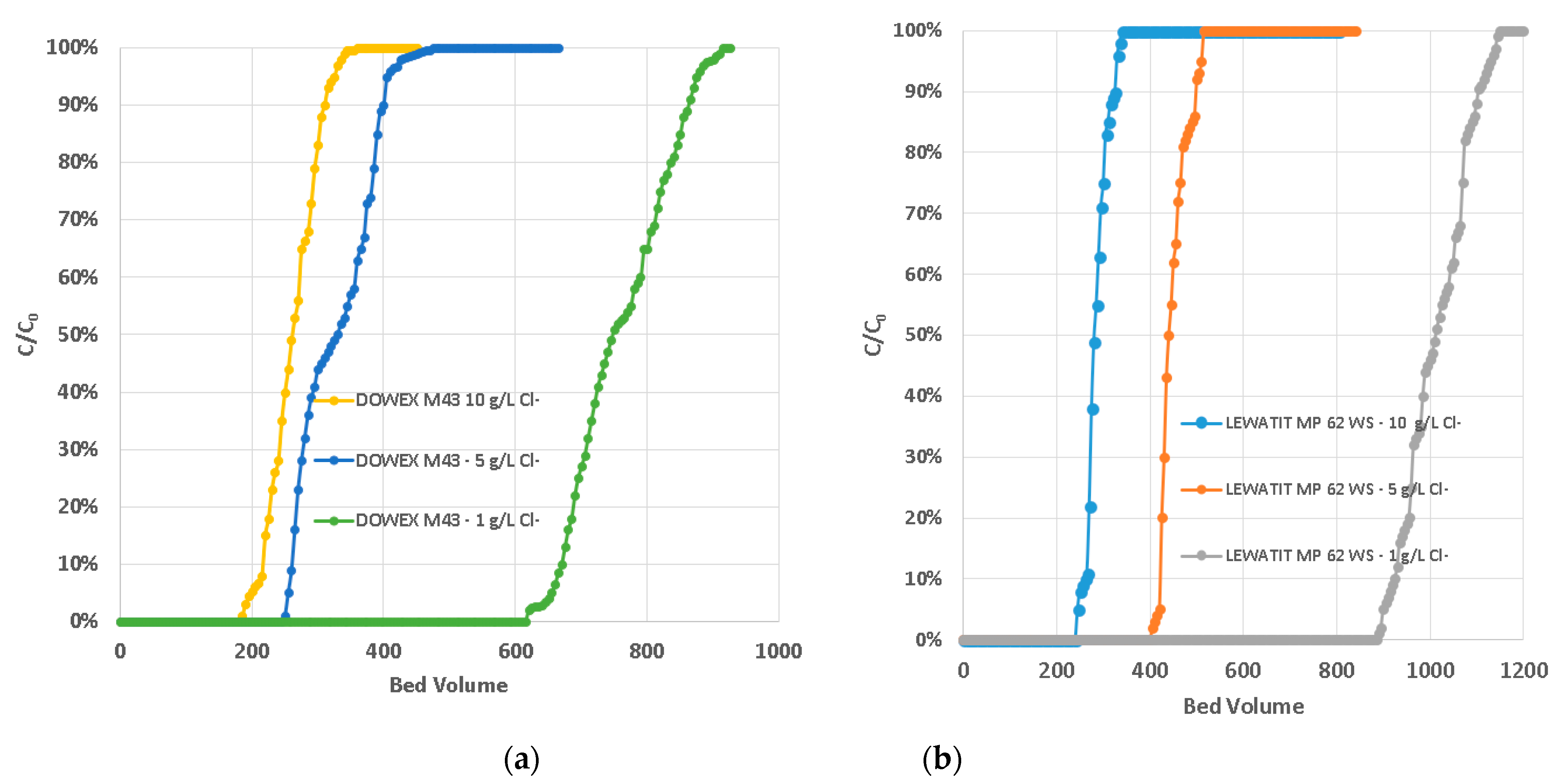
2.3. Experimental Development—Continuous Tests
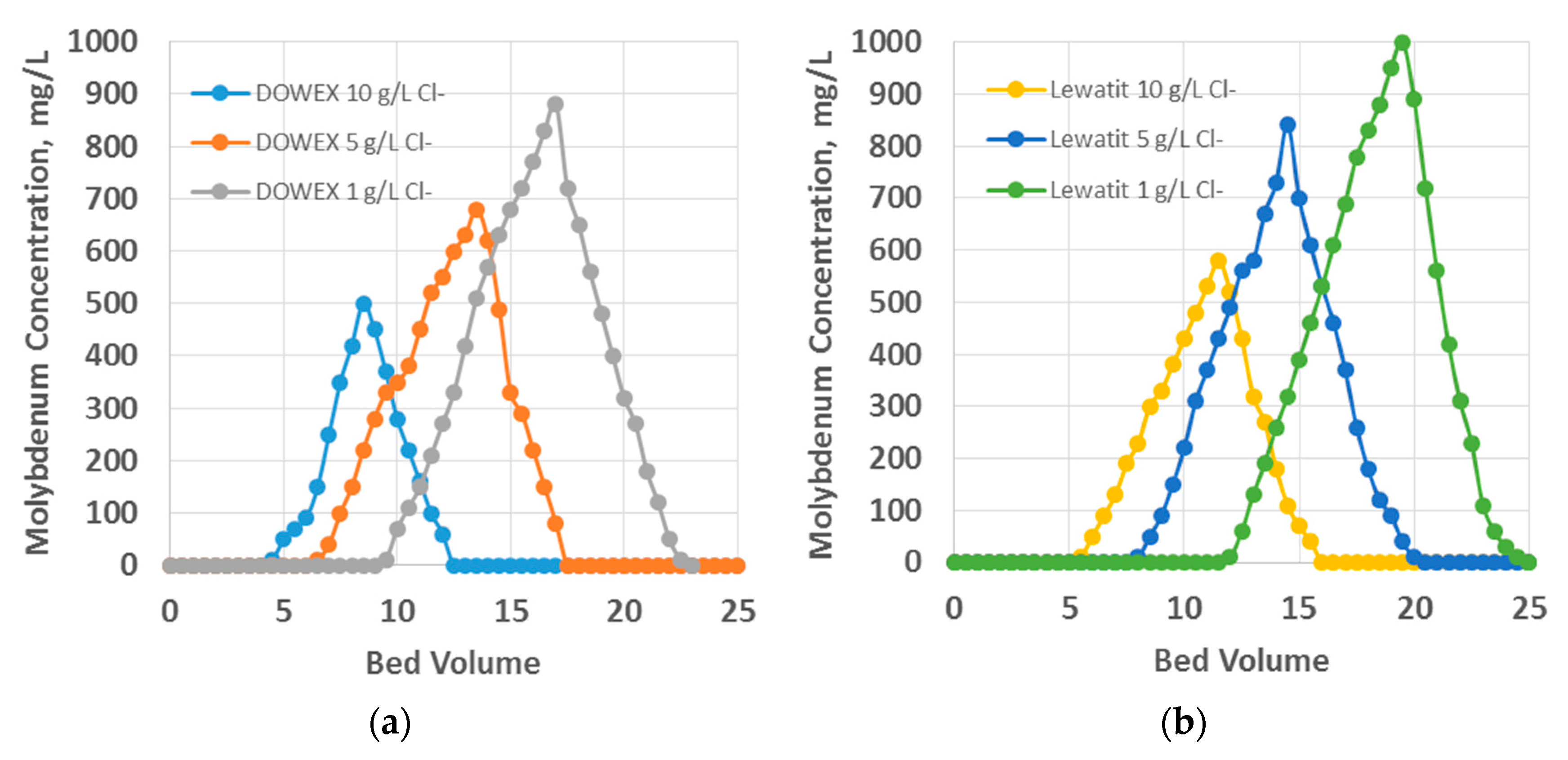
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IMOA. Global Molybdenum Production and Use Rises in 2023. International MOlybdenum Association. 3 April 2024. [En línea]. Available online: https://www.imoa.info/molybdenum-media-centre/latest-news/latest-news-details.php?objectID=749&lang=en (accessed on 7 August 2024).
- Gupta, C.K. Chapter 1 Principles and applications of molybdenum. In Extractive Metallurgy of Molybdenum; CRC Press: Boca Raton, FL, USA, 1992; pp. 1–15. [Google Scholar]
- Kholmogorov, A.; Kononova, O. Processing mineral raw materials in Siberia: Ores of molybdenum, tungsten, lead and gold. Hydrometallurgy 2005, 76, 37–54. [Google Scholar] [CrossRef]
- Gerhardt, N.; Palant, A.; Petrova, V.; Tagirov, R. Solvent extraction of molybdenum (VI), tungsten (VI) and rhenium (VII) by diisododecylamine from leach liquors. Hydrometallurgy 2001, 60, 1–5. [Google Scholar] [CrossRef]
- Karimova, L.; Kairalapov, Y.; Tussupbekova, T.; Oleinikova, T.; Makasheva, G. Hydrometallurgical processing of molybdenum middlings from Shatyrkul-Zhaysan cluster ore. J. Min. Metall. Sect. B Metall. 2024, 60, 71–83. [Google Scholar] [CrossRef]
- Olsson, C. Passivation of Stainless Steels and Other Chromium Bearing Alloys. In Encyclopedia of Interfacial Chemistry; Elsevier, 2018; pp. 357–364. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780124095472135851?via%3Dihub (accessed on 7 August 2024).
- IMOA. Applications of Molybdenum Metal and Its Alloys; IMOA: London, UK, 2013. [Google Scholar]
- Polyak, D.E. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2024. [Google Scholar]
- Zelikman, A.N.; Krein, O.E.; Samsonov, G.V. Metallurgy of rare metals. Isr. Sci. Transl. 1966, 60–98. [Google Scholar]
- Prasad, P.M.; Mankhand, T.R.; Prasad, A.J.K. Molybdenum extraction process: An overview. In Proceedings of the Extraction of Non-Ferrous Metals from Ores and Byproducts; National Metallurgical Laboratory: Jamshedpur, Indian, 1997. [Google Scholar]
- Keilholz, S.; Uhlenhut, H.; Gabke, A.; García-Schollenbruch, M.; Kohlmann, H. In situ studies on the industrial production process for molybdenum dioxide, MoO2. Z. Für Anorg. Und Allg. Chem. 2023, 649, e202300111. [Google Scholar] [CrossRef]
- Liu, R.; Cao, J.; Zhao, Z.; Li, Y. Enhanced molybdenum extraction and purification from acidic lixivium using organophosphorus extractants. Sep. Purif. Technol. 2024, 337, 126387. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, Z.; Li, Y. Acid leaching–extraction–circulation process based on Mo(VI) coordination with H3PO4 to efficiently extract molybdenum from different components of molybdenum calcine. Sep. Purif. Technol. 2023, 322, 124269. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.; Wen, J.; Li, W.; Yang, H.; Liu, W. Selective anion exchange adsorption of molybdenum(VI) at low concentrations from an acid leached vanadium shale solution containing aluminium and phosphorus. Hydrometallurgy 2024, 229, 106366. [Google Scholar] [CrossRef]
- Peng, M.; Tan, Y.; Shen, K.; Hu, Z.; Liu, C.; Li, F.; Liu, Z.; Yan, W.; Hua, J. Thermodynamics of Separating Molybdenum(VI) Over Iron(III) from High Acid Leach Solutions with Mixtures of P507 and N235. Recent Dev. Met. Energy Extr. Waste Stream 2023, 76, 1497–1507. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, Y.; Wang, Z.; Chen, X.; Chen, A.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Molybdenum Separation from Sodium-Tungstate-Molybdenum Aqueous Solutions Using an Anionic Gel-Based 201 × 7 Resin. J. Sustain. Metall. 2024, 10, 184–194. [Google Scholar] [CrossRef]
- Liu, X.; Cao, Z.; Chen, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Research on removal of iron and copper from low-grade molybdenum calcine in strong acid system. J. Ind. Eng. Chem. 2024, 135, 505–512. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. Recovery of molybdenum and vanadium with high purity from sulfuric acid leach solution of spent hydrodesulfurization catalysts by ion exchange. Hydrometallurgy 2014, 147–148, 142–147. [Google Scholar] [CrossRef]
- Kholmogorov, A.G.; Kononova, O.N.; Panchenko, O.N. A review of the use of ion exchange for molybdenum recovery in russia. Can. Metall. Q. 2004, 43, 297–304. [Google Scholar] [CrossRef]
- Bhappu, R.B.; Reynolds, D.H.; Schwab; Schwab, D.A. Hydrometallurgical Recovery of Molybdenum from the Questa Mine; State Bureau of Mines and Mineral Resources, New Mexico Institute of Mining and Technology: Socorro, Mexico, 1965. [Google Scholar]
- Dardel, F.D. Ion Exchange Basics. Ion Exchange. 25 October 2023. [En línea]. Available online: http://dardel.info/IX/IX_Intro.html (accessed on 15 July 2024).
- Orrego, P.; Hernández, J.; Reyes, A. Uranium and molybdenum recovery from copper leaching solutions using ion exchange. Hydrometallurgy 2019, 184, 116–121. [Google Scholar] [CrossRef]
- Elfeghe, S.; Sheng, Q.; Zhang, Y. Separation of Lead and Copper Ions in Acidic Media Using an Ion-Exchange Resin with a Thiourea Functional Group. ACS Omega 2022, 7, 13042–13049. [Google Scholar] [CrossRef]
- ChinaTungsten. «ChinaTungsten Online (Xiamen) Manu. & Sales Corp». 20 May 2020. [En línea]. Available online: http://www.molybdenum.com.cn/molybdenum-production-processes.html (accessed on 25 June 2024).
- Gao, Z.; Zhang, B.; Liu, H.; Wang, W.; Cao, Y. Recovery of molybdenum from alkali leaching solution of low-grade molybdenum concentrate by ion exchange. Green Process. Synth. 2015, 4, 317–321. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, B.; Han, G.; Wang, M.; Huang, Y.; Su, S.; Xue, Y.; Wang, Y. Clean separation and purification for strategic metals of molybdenum and rhenium from minerals and waste alloy scraps–A review. Esources Conserv. Recycl. 2022, 181, 106232. [Google Scholar] [CrossRef]
- Dydo, P. Industrial-Scale Technology for Molybdic Acid Production from Waste Petrochemical Catalysts. Materials 2023, 16, 5762. [Google Scholar] [CrossRef]
- Liu, B.; Xu, S.; Huang, Y.; Han, G.; Sun, H.; Yang, S.; Zhang, L. Selective extraction of molybdenum from an industrial powdery tungsten-molybdenum mixture scraps through ultrasonic-assisted electrochemical dissolution mechanism. Sep. Purif. Technol. 2024, 352, 128162. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Olayiwola, A.; Yang, N.; Liu, B.; Weigand, J.J.; Wenzel, M.; Du, H. Recovering valuable metals from spent hydrodesulfurization catalyst via blank roasting and alkaline leaching. J. Hazard. Mater. 2021, 416, 125849. [Google Scholar] [CrossRef]
- Dash, B.; Bhattacharya, I.N.; Ramanamurthy, B.V.; Paramguru, R.K. Preparation and characterization of molybdenum trioxide from spent hydrodesulfurization catalist. Korean J. Chem. Eng. 2011, 28, 1546–1549. [Google Scholar] [CrossRef]
- Kar, B.B.; Murthy, B.V.R.; Misra, V.N. Extraction of molybdenum from spent catalyst by salt-roasting. Int. J. Miner. Process. 2005, 76, 143–147. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, C.Y. A literature review of the recovery of molybdenum and vanadium from spent hydrodesulphurisation catalysts: Part I: Metallurgical processes. Hydrometallurgy 2009, 98, 1–9. [Google Scholar] [CrossRef]
- Ojeda, M.; Rivarola, J.; Quiroga, O. Study on chlorination of molybdenum trioxide mixed with carbon black. Miner. Eng. 2002, 15, 585–591. [Google Scholar] [CrossRef]
- Nair, K.; Sathiyamoorthy, D.; Bose, D.; Gupta, C. Processing of low-grade Indian molybdenite concentrate by chlorination in a fluidised-bed reactor. Int. J. Miner. Process. 1988, 23, 171–180. [Google Scholar] [CrossRef]
- Cicconi, F.; Ubaldini, A.; Fiore, A.; Rizzo, A.; Cataldo, S.; Agostini, P.; Pietropaolo, A.; Salvi, S.; Cuzzola, V. Dissolution of Molybdenum in Hydrogen Peroxide: A Thermodynamic, Kinetic and Microscopic Study of a Green Process for 99mTc Productio. Molecules 2023, 28, 2090. [Google Scholar] [CrossRef] [PubMed]
- Kar, B.; Datta, P.; Misra, V. Spent catalyst: Secondary source for molybdenum recovery. Hydrometallurgy 2004, 72, 87–92. [Google Scholar] [CrossRef]
- Zhang, M.; Song, H.; Zheng, C.; Lin, Z.; Liu, Y.; Wu, W.; Gao, X. Highly efficient recovery of molybdenum from spent catalyst by an optimized process. J. Air Waste Manag. Assoc. 2020, 70, 971–979. [Google Scholar] [CrossRef]
- Vollprecht, D.; Plessl, K.; Neuhold, S.; Kittinger, F.; Öfner, W.; Müller, P.; Mischitz, R.; Sedlazeck, K. Recovery of Molybdenum, Chromium, Tungsten, Copper, Silver, and Zinc from Industrial Waste Waters Using Zero-Valent Iron and Tailored Beneficiation Processes. Processes 2020, 8, 279. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Uranium 2016: Resources, Production and Demand; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Prajitno, M.; Taufiqurrakhman, M.; Harbottle, D.; Hunter, T. Kinetic Studies of Cs+ and Sr2+ Ion Exchange Using Clinoptilolite in Static Columns and an Agitated Tubular Reactor (ATR). Chemengineering 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Saji, V.; Lee, C.W. Molybdenum, molybdenum oxides, and their electrochemistry. Mater. Sci. Chem. 2012, 5, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- Man-Seung, L.; Seong-Ho, S.; Myung-Ho, L. Ionic Equilibria and Ion Exchange of Molybdenum(VI) from Strong Acid Solution. Bull. Korean Chem. Soc. 2011, 32, 3687–3691. [Google Scholar]
- Le, M.N.; Son, S.H.; Lee, M.S. Solvent Extraction of Mo(VI) and W(VI) from Dilute Chloride Solution by Amine and Neutral Extractants. J. Korean Inst. Resour. Recycl. 2019, 28, 55–61. [Google Scholar]
- Gluszcz, P.; Jamroz, T.; Sencio, B.; Ledakowicz, S. Equilibrium and dynamic investigations of organic acids adsorption onto ion-exchange resins. Bioprocess Biosyst. Eng. 2004, 26, 185–190. [Google Scholar]
- Lemaire, J.; Blanc, C.; Lutin, F.; Theoleyre, M.; Stambouli, M.; Pareau, D. Purification of organic acids by chromatography with strong anionic resins: Investigation of uptake mechanisms. J. Chromatogr. A 2016, 1458, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Alchin, D.; Wansbrough, H. Ion Exchange Resins; XIII-Water-D-Ion Exchange Resins; the Department of Cheminstry of the University of California: Irvine, CA, USA, 2012. [Google Scholar]
- Kopeliovich, D. Substech-Substances and Technology. 13 December 2023. [En línea]. Available online: http://www.substech.com/dokuwiki/doku.php?id=ion_exchange_resins (accessed on 25 January 2024).
- Okeola, F.; Odebunm, E. Freundlich and Langmuir isotherms parameters for adsorption of methylene blue by activated carbon derived from agrowastes. Adv. Nat. Appl. Sci. 2010, 4, 281–288. [Google Scholar]
- Meroufel, B.; Benali, O.; Benyahia, M.; Benmoussa, Y.; Zenasni, M.A. Adsorptive Removal of Anionic Dye from Aqueous Solutions by Algerian Kaolin: Characteristics, Isotherm, Kinetic and Thermodynamic Studies. J. Mater. Environ. Sci. 2013, 4, 482–491. [Google Scholar]
- Wołowicz, A.; Wawrzkiewicz, M. Screening of Ion Exchange Resins for Hazardous Ni(II) Removal From Aqueous Solutions: Kinetic and Equilibrium Batch Adsorption Method. Processes 2021, 9, 285. [Google Scholar] [CrossRef]
- Beres, M. Chapter 9—Expanding the boundaries of SFC: Analysis of biomolecules. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 257–297. [Google Scholar]
- Ullah Khan, H. Ion Exchange Technologies; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Brodsky, F.M. Clathrin and Clathrin-Dependent Endocytosis. In Encyclopedia of Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 384–393. [Google Scholar]
- Ardö, Y.; Chatterton, D.E.W.; Varming, C. Analytical Methods|Chromatographic Methods. In Encyclopedia of Dairy Sciences; Elsevier: Mississippi State, MS, USA, 2011; pp. 169–176. [Google Scholar]



| Mine Production (Tons) | Reserves | ||
|---|---|---|---|
| 2022 | 2023 | (thousand MTs) | |
| USA | 34,600 | 34,000 | 3500 |
| Argentina | − | − | 100 |
| Armenia | 7800 | 7800 | 150 |
| Australia | 277 | 500 | 690 |
| Canada | 1390 | 970 | 72 |
| Chile | 45,600 | 46,000 | 1400 |
| China | 106,000 | 110,000 | 5800 |
| Iran | 3700 | 3700 | 43 |
| Korea, North | 700 | 700 | NA |
| Korea, Republic of | 367 | 400 | 8 |
| Mexico | 15,500 | 15,000 | 130 |
| Mongolia | 3000 | 3100 | NA |
| Peru | 31,600 | 37,000 | 1500 |
| Russia | 1700 | 1700 | 1100 |
| Turkey | − | − | 52 |
| Uzbekistan | 1700 | 1700 | 21 |
| World total | 253,000 | 260,000 | 15,000 |
| Cu (g/L) | Mo (mg/L) | Fe (g/L) | Zn (g/L) | Cl (g/L) | SO42− (g/L) | pH | Eh |
|---|---|---|---|---|---|---|---|
| 2.5 | 50 | 5 | 0.5 | 1.0−5.0−10.0 | 91.8 | 1.0–1.5 | 0.3–0.5 |
| Ion Exchange Column | |
|---|---|
| Diameter of the column (D) | 2 cm |
| Height of the column (h) | 100 cm |
| Time (t) | 2 h |
| Volumetric flow rate (f) | 5 BV/h |
| Linear flow rate (u) | 2 × 10−3 m/s |
| Resin porosity (e) | 85% |
| Resin | Cl− (g/L) | RL | KL, L/mg | Mo Yields | Zn Yields | Fe Yields | |
|---|---|---|---|---|---|---|---|
| DOWEX M43 | 1 | 250 | 2.4E−03 | 8.2 | 83% | 47% | 19% |
| DOWEX M43 | 5 | 190 | 4.4E−03 | 4.5 | 63% | 36% | 13% |
| DOWEX M43 | 10 | 180 | 6.2E−03 | 3.2 | 60% | 22% | 7% |
| Lewatit MP 62−WS | 1 | 270 | 2.0E−03 | 10.0 | 90% | 53% | 15% |
| Lewatit MP 62−WS | 5 | 210 | 3.1E−03 | 6.5 | 70% | 42% | 8% |
| Lewatit MP 62−WS | 10 | 190 | 4.7E−03 | 4.2 | 63% | 30% | 2% |
| Resin | Cl− (g/L) | Mo Yields | Zn Yields | Fe Yields |
|---|---|---|---|---|
| DOWEX M43 | 1 | 89% | 0.3% | 0% |
| DOWEX M43 | 5 | 85% | 0.2% | 0% |
| DOWEX M43 | 10 | 78% | 0.15% | 0% |
| Lewatit MP 62−WS | 1 | 95% | 0.32% | 0% |
| Lewatit MP 62−WS | 5 | 89% | 0.23% | 0% |
| Lewatit MP 62−WS | 10 | 83% | 0.2% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, J.; Quintriqueo, A.; Hernández, J. Molybdenum Recovery from the Copper Hydrometallurgical Extraction Route with High Content of Chloride Ions Using the Ion Exchange Technique. Appl. Sci. 2024, 14, 7477. https://doi.org/10.3390/app14177477
Tapia J, Quintriqueo A, Hernández J. Molybdenum Recovery from the Copper Hydrometallurgical Extraction Route with High Content of Chloride Ions Using the Ion Exchange Technique. Applied Sciences. 2024; 14(17):7477. https://doi.org/10.3390/app14177477
Chicago/Turabian StyleTapia, Jaime, Angélica Quintriqueo, and José Hernández. 2024. "Molybdenum Recovery from the Copper Hydrometallurgical Extraction Route with High Content of Chloride Ions Using the Ion Exchange Technique" Applied Sciences 14, no. 17: 7477. https://doi.org/10.3390/app14177477








