Status and Migration Activity of Lead, Cobalt and Nickel in Water and in Bottom Sediments of Lake Markakol, Kazakhstan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Analyses
- An aqueous solution of lead ions (SSO 7012-93) (2K-1) in concentrations CPb = 0.0125; 0.025; 0.05; 0.1 mg/L;
- An aqueous solution of cobalt ions (SSO 7880-2001) (NK-EC) in concentrations CCo = 0.1; 0.15; 0.2; 0.5 mg/L;
- An aqueous solution of nickel ions (SSO 7873-2000) (NK-EC) in concentrations CNi = 0.1; 0.15; 0.2; 0.5 mg/L.
2.4. Standards
2.5. GIS Analysis and Map Construction
3. Results and Discussion
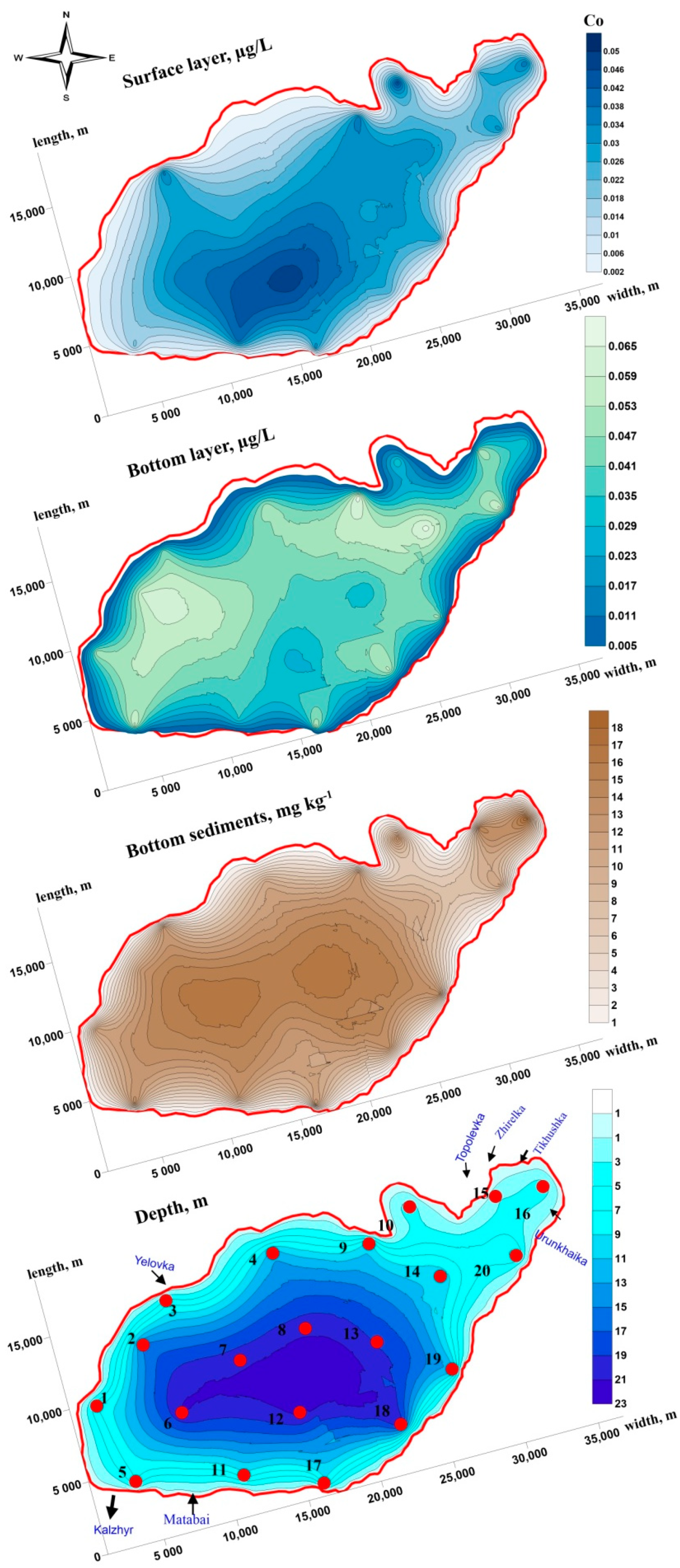
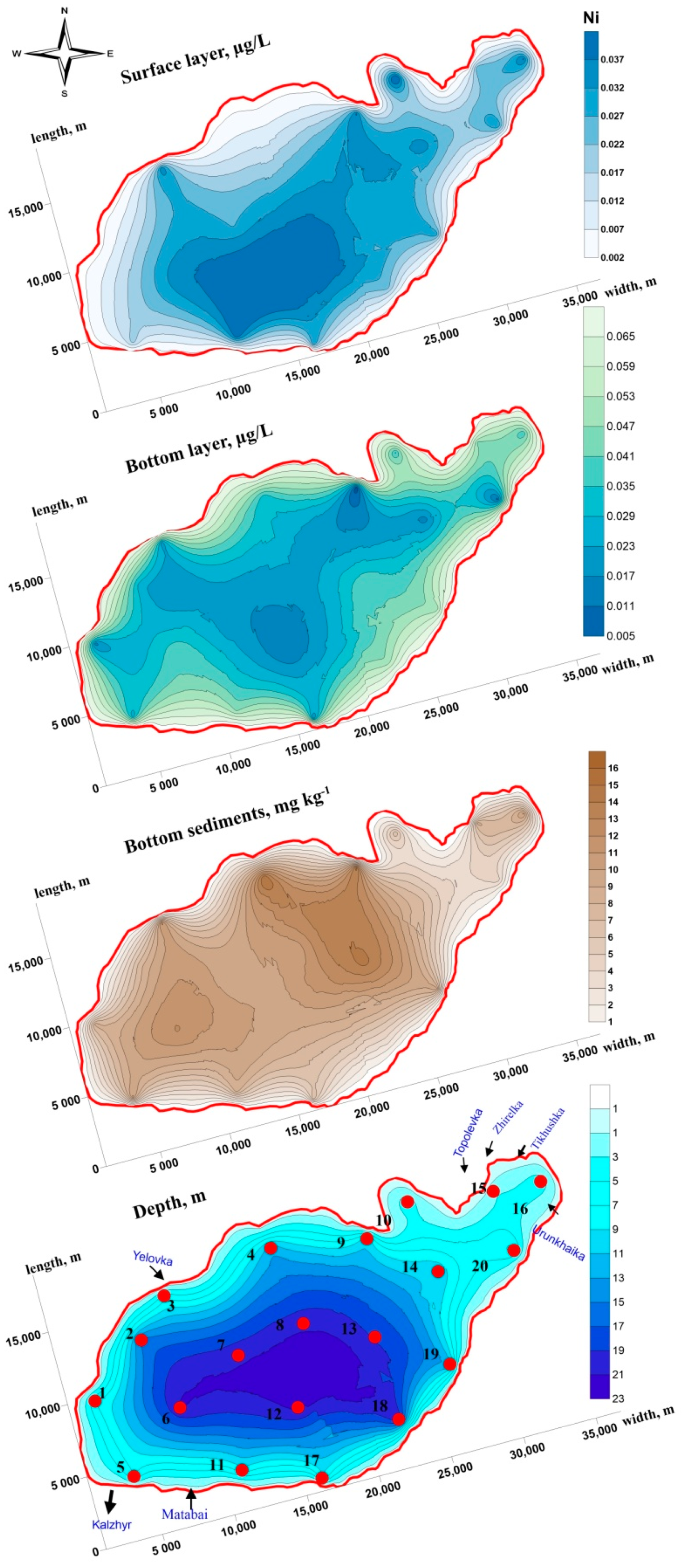
3.1. Lead, Cobalt and Nickel Concentrations in the Water Column
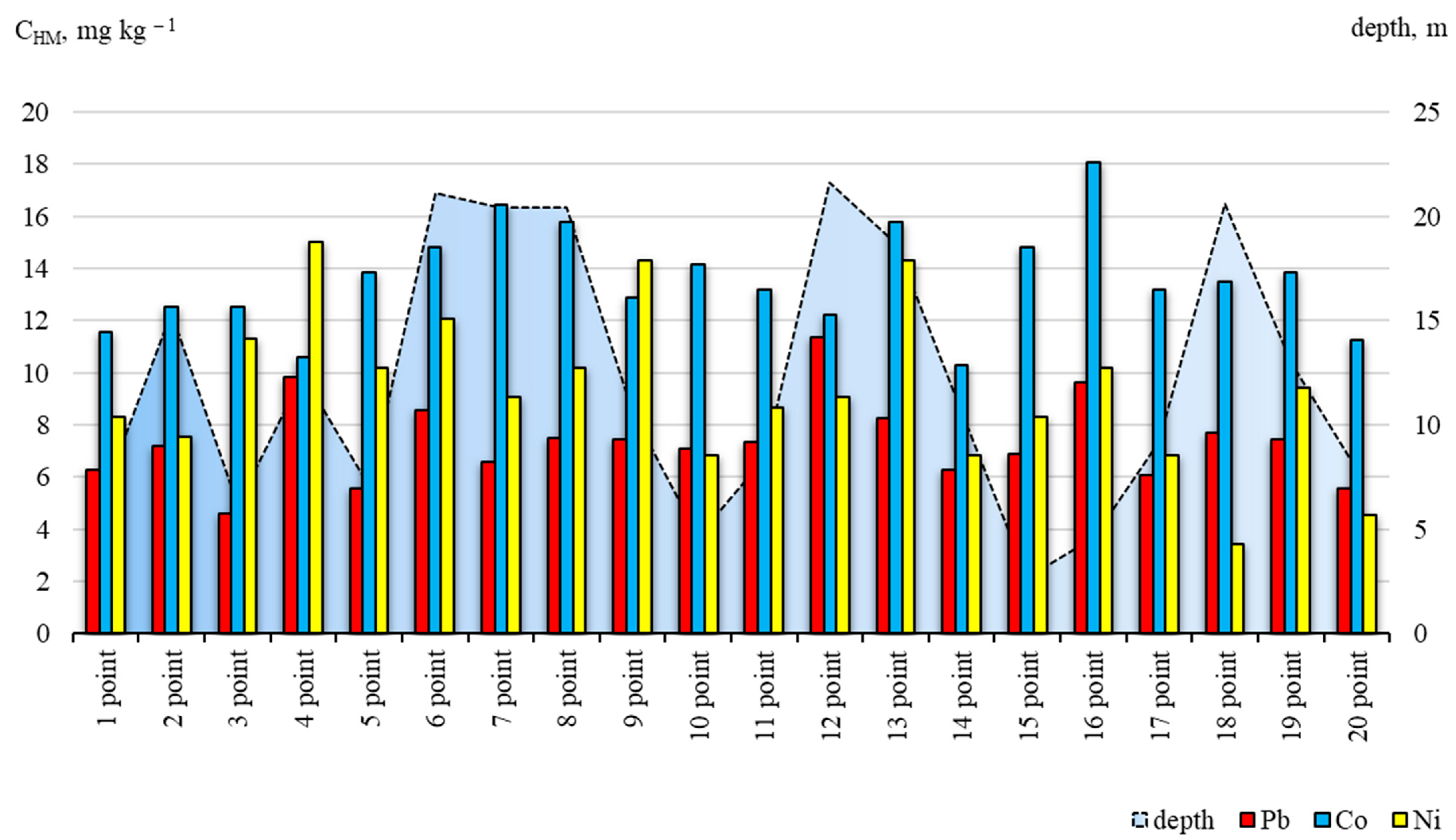
3.2. Lead, Cobalt and Nickel Concentrations in Bottom Sediments
3.3. Spatial Distribution and Migration Activity of Lead, Cobalt and Nickel in the “Water-Bottom Sediments” System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Groll, M.; Opp, C. Lake-catchment interactions and their responses to hydrological extremes. Quat. Int. 2018, 475, 1–3. [Google Scholar] [CrossRef]
- Vinnå, L.R.; Medhaug, I.; Schmid, M.; Bouffard, D. The vulnerability of lakes to climate change along an altitudinal gradient. Nat. Commun. Earth Environ. 2021, 2. Available online: https://api.semanticscholar.org/CorpusID:231948782 (accessed on 24 July 2024).
- Nikanorov, A.M.; Zhulidov, A.V.; Pokarzhevsky, A.D. Biomonitoring of Heavy Metals in Freshwater Ecosystems; Hydrometeoizdat: Leningrad, Russia, 1985; p. 144. Available online: https://search.rsl.ru/ru/record/01001263159 (accessed on 9 February 2024).
- Official, Standardized and Recommended Methods of Analysis. Compiled and edited for the Analytical Methods Committee of the Society for Analytical Chemistry; Jolly, S.C., Heffer, W., Eds.; Sons, Ltd.: Cambridge, UK, 1963; p. 577. [Google Scholar]
- Research Report. Biological Substantiation under the Program: “Determination of Fish Capacity of Fishery Reservoirs and/or Their Sites, Development of Biological Substantiation of Fish and Other Aquatic Animals MPL (Maximum Permissible Level), Regime and Regulation of Fishing on Fishery Reservoirs of International, Republican Importance and Water Bodies of Special Protected Natural Areas of Yertis basin, as well as Assessment of Fish Resources Status on Reserve Water Bodies of Local Importance” Section: Water Body of Markakol State Natural Reserve (Lake Markakol). Ust-Kamenogorsk: Scientific and Production Center of Fisheries LLP. 2022, 53, No. of state registration 0122РК00005. Available online: https://ecoportal.kz/Disscusion/DisPublic/PublicHearingDetail?hearingId=7805 (accessed on 24 July 2024).
- Nemova, N.N. Biochemical Indication of Fish State. M. Nauka 2004, 215. Available online: https://f.eruditor.link/file/1234844/ (accessed on 24 July 2024).
- Rakybaeva, A.A.; Dzhantasova, A.S.; Baimukanov, M.T. Towards assessment of the current state of zooplankton of Lake Markakol. Bull. KazNU. Biol. Ser. 2011, 4, 98–102. [Google Scholar]
- Unified Ecological Internet resource of the Ministry of Ecology, Geology and Natural Resources of the Republic of Kazakhstan. National Reports of the Convention on Biodiversity Conservation. Fourth National Report of the Republic of Kazakhstan on Biological Diversity. 2008. 110. Available online: https://ecogosfond.kz/orhusskaja-konvencija/dostup-k-jekologicheskoj-informacii/haly-araly-yntyma-tasty/haly-araly-konvencijalardy-ltty-bajandamalary/nacionalnye-doklady-konvencii-po-sohraneniju-bioraznoobrazija/ (accessed on 8 February 2024).
- Proceedings of the Institute of Hydrobiology and Ecology. Volume II State of the Hydrobionts of Water Bodies of Specially Protected Natural Territories of Republican Importance of East Kazakhstan and Almaty Oblast of Kazakhstan (Information-Analytical Manual). In Part 2. Markakol State Nature Reserve; Almaty, Kazakhstan, 2017; p. 55. Available online: https://ihe.kz/images/ige/2/2.pdf (accessed on 10 February 2024).
- Baimukanov, M.T. History of fishing on Lake Markakol, problems of maturation of fish resources, fish gene pool and ways to solve them. Proc. Markakol Reserve Ust-Kamenogorsk 2009, 1, 90–101. [Google Scholar]
- Report on the research work “Monitoring of the condition and assessment of the micro- and macroplastic pollution level of the Lake Markakol aquatic environment” (interim). Performed under Grant Funding No. AP14870595. State registration number 0122РК00391. Almaty. 2023, 85.
- Opp, C. Schwermetalle. Analyse und Ökologische Bewertung der Landschaft, 2nd ed.; Bastian, O., Schreiber, K.-F., Eds.; Spektrum Akademischer Verlag: Berlin, Germany, 1999; pp. 239–246. [Google Scholar]
- He, Y.; Li, B.B.; Zhang, K.N.; Li, Z.; Chen, Y.G.; Ye, W.M. Experimental and numerical study on heavy metal contaminant migration and retention behavior of engineered barrier in tailings pond. Environ. Pollut. 2019, 252, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Yozukmaz, A.; Yabanlı, M. Heavy metal contamination and potential ecological risk assessment in sediments of Lake Bafa (Turkey). Sustainability 2023, 15, 9969. [Google Scholar] [CrossRef]
- Uddin, M.M.; Peng, G.; Wang, Y.; Huang, J.; Huang, L. Pollution status, spatial distribution and ecological risk of heavy metals in sediments of a drinking water lake in South Eastern China. Environ. Pollut. Bioavailab. 2021, 33, 19–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Dai, R.; Leszek, S.; Wang, X.; Xiao, L. Adsorption and migration of heavy metals between sediments and overlying water in the Xinhe River in central China. Water Sci. Technol. 2021, 84, 1257–1269. [Google Scholar] [CrossRef]
- Ismukhanova, L.; Choduraev, T.; Opp, C.; Madibekov, A. Accumulation of heavy metals in bottom sediment and their migration in the water ecosystem of Kapshagay Reservoir in Kazakhstan. Appl. Sci. 2022, 12, 11474. [Google Scholar] [CrossRef]
- Amirgaliyev, N.A.; Ismukhanova, L.T. The level of anthropogenic pollution of the Kapshagay Water Reservoir, Republic of Kazakhstan. In Water Resources Management in Central Asia. The Handbook of Environmental Chemistry; Zonn, I., Zhiltsov, S., Kostianoy, A., Semenov, A., Eds.; Springer: Cham, Switzerland, 2020; Volume 105, pp. 143–162. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, D.; Yue, W.; Wang, B.; Huo, L.; Liu, K.; Zhang, B.T. Heavy metals in sediments of Hulun Lake in Inner Mongolia; Spatial–temporal distributions, contamination assessment and source apportionment. Water 2023, 15, 1329. [Google Scholar] [CrossRef]
- Ekeanyanwu, C.R.; Ogbuinyi, C.A.; Etienajirhevwe, O.F. Trace metals distribution in fish tissues, bottom sediments and water from Okumeshi River in Delta State, Nigeria. Ethiop. J. Environ. Stud. Manag. 2010, 3, 12–17. [Google Scholar] [CrossRef]
- Jafarabadi, A.R.; Bakhtiari, A.R.; Spanò, N.; Cappelloc, T. First report of geochemical fractionation distribution, bioavailability and risk assessment of potentially toxic inorganic elements in sediments of coral reef islands of the Persian Gulf, Iran. Mar. Pollut. Bull. 2018, 137, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Zhang, W. Experimental study on release of heavy metals in sediment under hydrodynamic conditions. IOP Conf. Ser. Earth Environ. Sci. 2018, 208, 012040. [Google Scholar] [CrossRef]
- Klake, R.K.; Nartey, V.K.; Doamekpor, L.K.; Edor, K.F. Correlation between heavy metals in fish and sediment in Sakumo and Kpeshie Lagoons, Ghana. J. Environ. Prot. 2012, 3, 1070–1077. [Google Scholar] [CrossRef]
- Afzaal, M.; Hameed, S.; Liaqat, I.; Khan, A.A.A.; Manan, H.A.; Shahid, R.; Altaf, M. Heavy metals contamination in water, sediments and fish of freshwater ecosystems in Pakistan. Water Pract. Technol. 2022, 17, 1253–1272. [Google Scholar] [CrossRef]
- Khan, B.N.; Ullah, H.; Ashfaq, Y.; Hussain, N.; Atique, U.; Aziz, T.; Alharbi, M.; Albekairi, T.H.; Alasmari, A.F. Elucidating the effects of heavy metals contamination on vital organ of fish and migratory birds found at fresh water ecosystem. Heliyon 2023, 9, e20968. [Google Scholar] [CrossRef]
- Chan, W.S.; Routh, J.; Luo, C.; Dario, M.; Miao, Y.; Luo, D.; Wei, L. Metal accumulations in aquatic organisms and health risks in an acid mine-affected site in South China. Env. Geochem. Health 2021, 43, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef]
- Kudryavtseva, V.A.; Shigaeva, T.D.; Pankratova, N.M. Features of migration of heavy metals in the system ‘‘Bottom Water—Pore Water—Surface layer of Bottom sediments’’ of the Coastal zone in the Eastern part of the Gulf of Finland. Bull. Tomsk. Polytech. Univ. Geo Аssets Eng. 2022, 333, 95–104. [Google Scholar] [CrossRef]
- Berezovikov, N.N.; Zinchenko, Y.K.; Zinchenko, E.S. Markakol Reserve. Reserves of the USSR; Reserves of Central Asia and Kazakhstan: Moscow, Russia, 1990; pp. 114–128. Available online: https://www.undp.org/sites/g/files/zskgke326/files/migration/kz/7059-16397.pdf (accessed on 10 December 2023).
- Surface Water Resources of the USSR. Altai and Western Siberia. Mountain Altai and Upper Irtysh, Part 1; Semenova, V.A., Ed.; Leningrad State University: Leningrad, Russia, 1969; Volume 15, p. 318. Available online: https://disk.yandex.kz/d/UMicA14_a2Y5F (accessed on 3 April 2024).
- Filonets, P.P. Essays on the Geography of Internal Waters of Central, Southern and Eastern Kazakhstan: (Lakes, Reservoirs and Glaciers); Nauka: Alma-Ata, Russia, 1981; p. 232. Available online: https://search.rsl.ru/ru/record/01001070860 (accessed on 6 March 2024).
- On Approval of the Unified System of Water Quality Classification in Water Bodies. Order of the Chairman of the Committee on Water Resources of the Ministry of Agriculture of the Republic of Kazakhstan dated November 9, 2016; No.151. Registered with the Ministry of Justice of the Republic of Kazakhstan on December 13, No. 14513. Official Website of Adlet zan. Available online: https://adilet.zan.kz/rus/docs/V1600014513 (accessed on 9 February 2024).
- ST RK ISO 8288-2005; Water Quality. Determination of Cobalt, Nickel, Copper, Zinc, Cadmium and Lead. Flame Atomic Absorption Spectrometric Methods (ISO 8288:1986). Committee on Technical Regulation and Metrology of the Ministry of Industry and Trade of the Republic of Kazakhstan: Astana, Kazakhstan, 2005; p. 23. Available online: https://files.stroyinf.ru/Data2/1/4293741/4293741232.pdf (accessed on 8 February 2024).
- RD 52.18.289-90; Guiding Document. Methodical Instructions. Methodology for Measuring the Mass Fraction of Mobile Forms of Metals (Copper, Lead, Zinc, Nickel, Cadmium, Cobalt, Chromium, Manganese) in Soil Samples by Atomic Absorption Analysis. USSR State Committee on Hydrometeorology: Moscow, Russia, 1990; p. 37. Available online: https://files.stroyinf.ru/Data2/1/4293783/4293783539.pdf (accessed on 8 February 2024).
- Bożym, M.; Rajmund, A. The study of cobalt leaching from soils, sewage sludges and composts using a one-step extraction. Ochr. Srodowiska Zasobów Nat. 2015, 26, 1–6. [Google Scholar] [CrossRef]
- Eliseeva, N.V.; Chekhovich, E.E.; Zubkova, T.A. Content and group composition of cobalt compounds in soils of rice fields of Kuban and other soils of Russia. Bull. Altai State Agrar. Univ. 2013, 2, 32–36. [Google Scholar]
- Kashintseva, M.L.; Chernikova, O.A.; Shilenko, N.A.; Sokolova, S.A.; Anisova, S.N. List of Fishery Standards, Maximum Permissible Concentrations (MPC) and Approximate Safe Impact Levels (ASIL) of Harmful Substances for Water Bodies of Fishery Importance; VNIRO Publishing House: Moscow, Russia, 1999; p. 304. Available online: https://docs.cntd.ru/document/1200044750 (accessed on 6 May 2023).
- Kozhakhmetov, S.M.; Kvyatkovsky, S.A.; Sadykov, S.B.; Chekimbayev, A.F.; Sadykov, T.S. Smelting process of oxidized cobalt-nickel ores from Gornostayevskoye deposit for ferronickel. Metallurgy. Complex Util. Miner. Raw Mater. 2015, 1, 25–30. [Google Scholar]
- Amralinova, B.B. Regularities of Formation and Assessment of Prospects of Nickel-Cobalt Weathering Crusts of East Kazakhstan. Ph.D. Thesis, on Specialty 6D070600—Geology and Exploration of Mineral Deposits. Ust-Kamenogorsk, Kazakhstan, 2017; p. 145. Available online: https://www.geokniga.org/bookfiles/geokniga-zakonomernosti-formirovaniya-i-ocenka-perspektiv-nikel-kobaltovyh-kor.pdf (accessed on 11 February 2024).
- Production of Nickel-Cobalt Products in the Republic of Kazakhstan. LLP "DAMU RESEARCH", Astana, Kazakhstan. 2015, p. 113. Available online: https://damu.kz/upload/iblock/51c/MarketingovoeIssledovanie_ProizvodstvoNikel_kobaltovovoyProduktsiiVKazakhstane.docx (accessed on 2 May 2024).
- Dyachkov, B.A.; Mochalkina, L.N.; Kuzmina, O.N.; Bochkova, O.I.; Kravchenko, M.M. Types of weathering crust deposits in Eastern Kazakhstan. Bull. EKSTU. Geol. Min. Metall. 2005, 4, 6–27. [Google Scholar]
- Boguta, P.; Skic, K.; Baran, A.; Szara-Bqk, M. The influence of the physicochemical properties of sediment on the content and ecotoxicity of trace elements in bottom sediments. Chemosphere 2022, 287, 4. [Google Scholar] [CrossRef]
- Buyang, S.; Yi, Q.; Cui, H.; Wan, K.; Zhang, S. Distribution and adsorption of metals on different particle size fractions of sediments in a hydrodynamically disturbed canal. Sci. Total Environ. 2019, 670, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Namieśnik, J.; Rabajczyk, А. The speciation and physicochemical forms of metals in surface waters and sediments. Chem. Speciat. Bioavailab. 2010, 22, 1–24. [Google Scholar] [CrossRef]
- Abdallah, M.A.M. Accumulation and distribution of heavy metals in surface sediments from the continental shelf adjacent to Abu Qir Bay, Egypt, as a function of grain size. Geo-Mar. Lett. 2023, 43, 2. [Google Scholar] [CrossRef]
- Miao, X.; Hao, Y.; Liu, H.; Xie, Z.; Miao, D.; He, X. Effects of heavy metals speciation’s in sediments on their bioaccumulation in wild fish in rivers in Liuzhou—A typical karst catchment in southwest China. Ecotoxicol. Environ. Saf. 2021, 214, 112099. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Lares, M.; Rentería-Villalobos, M.; Mendieta-Mendoza, A.; Ortíz-Caballero, Z.; Montero-Cabrera, E.; Vioque, I. Partitioning and availability of metals from water suspended sediments: Potential pollution risk assessment. Water 2022, 14, 980. [Google Scholar] [CrossRef]
- Leonova, G.A.; Bobrov, V.A.; Krivonogov, S.K.; Bogush, A.A.; Bychinskii, V.A.; Mal’tsev, A.E.; Anoshin, G.N. Biogeochemical specifics of sapropel formation in Cisbaikalian undrained Lakes (by the example of Lake Ochki). Geol. Geophys. 2015, 56, 949–969. [Google Scholar] [CrossRef]
- Strakhovenko, V.D.; Taran, O.P.; Ermolaeva, N.I. Geochemical characteristics of the sapropel sediments of small lakes in the Ob’—Irtysh interfluence. Geol. Geophys. 2014, 55, 1466–1477. [Google Scholar] [CrossRef]
- Bocharnikov, V.S.; Borovikov, A.A. To the Question about Sapropels and Their Influence on Water Physical Properties in Mixtures with Sand during Construction and Operation of Engineering and Reclamation Systems; Lower Volga Agro-University Complex: Volgograd, Russia, 2021; Volume 4, pp. 324–334. [Google Scholar]
- Valette-Silver, N.J. The use of sediment cores to reconstruct historical trends in contamination of estuarine and coastal sediments. Estuaries 1993, 16, 577–588. [Google Scholar] [CrossRef]
- Förstner, U.J.N. Lake sediments as indicators of heavy-metal pollution. Naturwissenschaften 1976, 63, 465–470. [Google Scholar] [CrossRef]
- Costa-Böddeker, S.; Thuyên, L.X.; Hoelzmann, P.; de Stigter, H.C.; van Gaever, P.; Huy, H.Đ.; Schwalb, A. The hidden threat of heavy metal pollution in high sedimentation and highly dynamic environment: Assessment of metal accumulation rates in the Thi Vai Estuary, Southern Vietnam. Environ. Pollut. 2018, 242, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ayrault, S.; Meybeck, M.; Mouchel, J.-M.; Gaspéri, J.; Lestel, L.; Lorgeoux, C.; Boust, D.; Flipo, N.; Labadie, P.; Lestel, L. Sedimentary archives reveal the concealed history of micropollutant contamination in the Seine River basin. The Seine River Basin. In The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2020; Volume 90. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskuła, J.; Siepak, M. Heavy metals in bottom sediments of reservoirs in the lowland area of western Poland: Concentrations, distribution, sources and ecological risk. Water 2019, 11, 56. [Google Scholar] [CrossRef]
- Mrozińska, N.; Bąkowska, M. Effects of heavy metals in lake water and sediments on bottom invertebrates inhabiting the brackish coastal Lake Łebsko on the southern Baltic coast. Int. J. Environ. Res. Public Health 2020, 17, 6848. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Rodríguez, G.; Castañeda-Chávez, M.D.R.; Lango-Reynoso, F. Geoacumulation of heavy metals in sediment of the fluvial-lagoon-deltaic system of the Palizada River, Campeche, Mexico. Int. J. Environ. Res. Public Health 2020, 17, 969. [Google Scholar] [CrossRef]
- Juśkiewicz, W.; Gierszewski, P. Toxic metal pollution of aquatic ecosystems of European Union nature protection areas in a region of intensive agriculture (Lake Gopło, Poland). Aquat Sci. 2022, 84, 52. [Google Scholar] [CrossRef]
- Krasnenko, A.S.; Pechkin, A.S.; Kobelev, V.O.; Agbalyan, E.V.; Shinkaruk, E.V. Lake Yantarnoye—Status, problems, prospects. Sci. Bull. Yamalo-Nenets Auton. Dist. 2018, 4, 37–43. [Google Scholar]
- Moiseenko, T.I. Theoretical Bases of Anthropogenic Load Rationing on Subarctic Water Bodies; Kola Scientific Center of the RAS: Apatity, Russia, 1997; p. 261. Available online: https://www.aquaticecology.ru/books_english.html (accessed on 6 April 2024).
- Shahid, S.; Sultana, T.; Sultana, S.; Hussain, B.; Al-Ghanim, K.A.; Al-Bashir, F.; Riaz, M.N.; Mahboob, S. Detecting aquatic pollution using histological investigations of the gills, liver, kidney, and muscles of Oreochromis niloticus. Toxics 2022, 10, 564. [Google Scholar] [CrossRef] [PubMed]
- Ylikörkkö, J.; Zueva, M.; Kashulin, N.; Kashulina, T.; Sandimirov, S.; Christensen, G.; Jelkänen, E. Pasvik water quality until 2013: Environmental monitoring programme in the Norwegian, Finnish and Russian border area. Cent. Econ. Dev. Transp. Environ. Lapland 2014, 96, 43. [Google Scholar]
- Ylikörkkö, J.; Christensen, G.; Kashulin, N.; Denisov, D.; Andersen, H.J.; Jelkänen, E. Environmental Challenges in the Joint Border Area of Norway, Finland and Russia. 2015, 41, 165. Available online: https://www.doria.fi/handle/10024/104779 (accessed on 24 July 2024).
- Burdina, N.F. Chemical and toxicological assessment of fish (literature review). Young Sci. 2019, 25, 69–72. Available online: https://moluch.ru/archive/263/61009/ (accessed on 8 February 2024).
- Toroyan, R.A.; Bedanokov, M.K.; Takh, I.P. Migration of heavy metals in water ecosystems (using the example of the Belaya River in the North-Western Caucasus). Russ. J. Earth Sci. 2022, 22, ES01SI12. [Google Scholar] [CrossRef]
- Sharipova, O.A. Distribution of heavy metals in bottom sediments of Lake Balkhash depending on natural and anthropogenic factors. Bull. Tomsk. State Univ. 2015, 390, 225–230. [Google Scholar] [CrossRef]
- Dauwalter, V.A.; Dauwalter, M.V. Geoecological assessment of natural waters in the zone of influence of the Severonickel Combine. In Textbook on the Disciplines “Environmental Geochemistry”, “Hydrogeology”, “Geoecology” for Students of the Direction 022000.62 “Ecology and Nature Management” and Speciality 020804.65 “Geoecology”; Publishing House of Murmansk State Technical University: Murmansk, Russia, 2012; p. 216. [Google Scholar]
- Dauwalter, V.A. Geoecology of Bottom Sediments of Lakes; Publishing House of Murmansk State Technical University: Murmansk, Russia, 2012; p. 242. Available online: https://inep.ksc.ru/documents/%D0%94%D0%B0%D1%83%D0%B2%D0%B0%D0%BB%D1%8C%D1%82%D0%B5%D1%80%20%D0%92.%D0%90.%20%D0%93%D0%B5%D0%BE%D1%8D%D0%BA%D0%BE%D0%BB%D0%BE%D0%B3%D0%B8%D1%8F%20%D0%B4%D0%BE%D0%BD%D0%BD%D1%8B%D1%85%20%D0%BE%D1%82%D0%BB%D0%BE%D0%B6%D0%B5%D0%BD%D0%B8%D0%B9%20%D0%BE%D0%B7%D0%B5%D1%80.pdf (accessed on 1 July 2024).
- Kluska, M.; Jabłońska, J. Variability and heavy metal pollution levels in water and bottom sediments of the Liwiec and Muchawka Rivers (Poland). Water 2023, 15, 2833. [Google Scholar] [CrossRef]
- Myasnikova, N.A.; Potakhin, M.S. Granulometric composition of the bottom sediments in the Torosjarvi Lake (White Sea Basin). Vestn. Voronezh State Univ. Ser. Geogr. Geoecology 2021, 1, 45–56. [Google Scholar] [CrossRef]
- Hahn, J.; Opp, C.; Evgrafova, A.; Groll, M.; Zitzer, N.; Laufenberg, G. Impact of dam draining on the mobility of heavy metals and arsenic in water and basin bottom sediments of three studied dams in Germany. Sci. Total Environ. 2018, 640–641, 1072–1081. [Google Scholar] [CrossRef]
- Alekin, O.A. Fundamentals of Hydrochemistry; Publishing House Hydrometeorological: Leningrad, Russia, 1970; p. 442. Available online: https://www.geokniga.org/bookfiles/geokniga-osnovy-gidrohimii.pdf (accessed on 2 September 2023).
- Karapetyants, M.K.; Drakin, S.I. General and Inorganic Chemistry; Publishing House Chemistry: Moscow, Russia, 1993; p. 592. Available online: https://www.nntu.ru/frontend/web/ngtu/files/org_structura/library/resurvsy/pervokursnik/inel/xim/osnovn/2.pdf (accessed on 10 July 2023).
- Perelman, A.I. Geochemistry of Natural Waters; Nauka: Moscow, Russia, 1982; p. 154. Available online: http://booksshare.net/index.php?id1=4&category=biol&author=perelman-ai&book=1982 (accessed on 10 February 2024).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismukhanova, L.; Madibekov, A.; Opp, C.; Zhadi, A.; Sultanbekova, B.; Zhumatayev, S. Status and Migration Activity of Lead, Cobalt and Nickel in Water and in Bottom Sediments of Lake Markakol, Kazakhstan. Appl. Sci. 2024, 14, 7487. https://doi.org/10.3390/app14177487
Ismukhanova L, Madibekov A, Opp C, Zhadi A, Sultanbekova B, Zhumatayev S. Status and Migration Activity of Lead, Cobalt and Nickel in Water and in Bottom Sediments of Lake Markakol, Kazakhstan. Applied Sciences. 2024; 14(17):7487. https://doi.org/10.3390/app14177487
Chicago/Turabian StyleIsmukhanova, Laura, Azamat Madibekov, Christian Opp, Askhat Zhadi, Botakoz Sultanbekova, and Serik Zhumatayev. 2024. "Status and Migration Activity of Lead, Cobalt and Nickel in Water and in Bottom Sediments of Lake Markakol, Kazakhstan" Applied Sciences 14, no. 17: 7487. https://doi.org/10.3390/app14177487





