Recovery of Saponins, Phenolic Compounds and Antioxidant Capacity from Curculigo orchioides Gaertn Rhizomes by Different Extraction Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Experimental Design
2.3.1. Single-Factor Investigation Experiments
2.3.2. Optimization of Extraction Conditions Using Response Surface Methodology (RSM)
2.3.3. Ultrasound-Assisted Extraction (UAE)
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
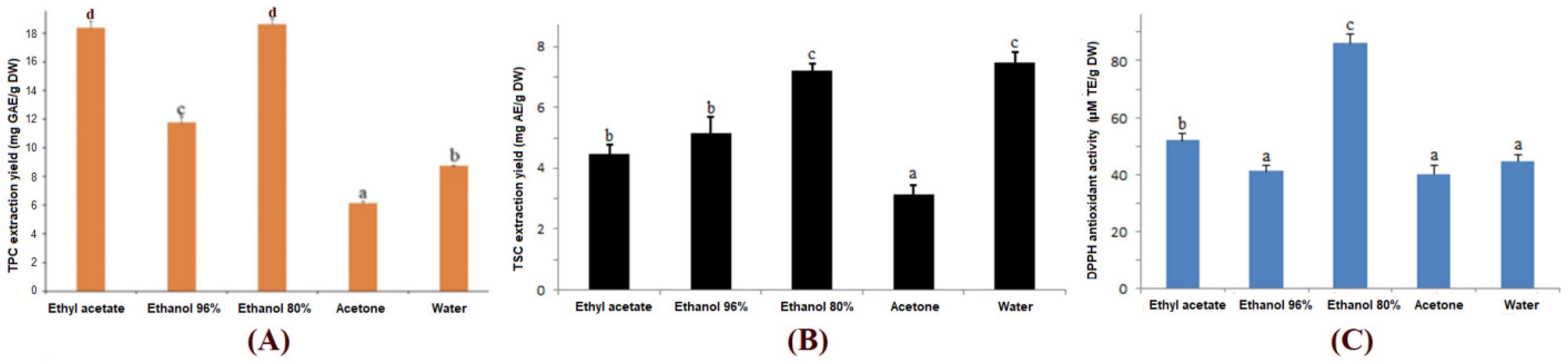
3.1. Effects of Solvents on Extraction Yield
3.2. Single-Factor Investigation of Extraction Conditions
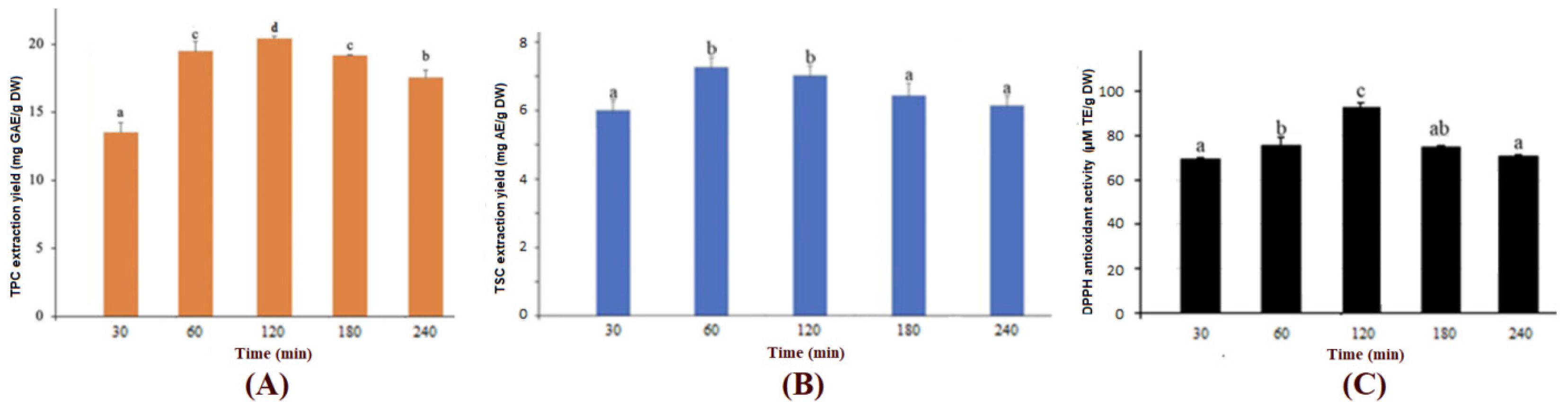
3.2.1. Effects of Extraction Time on Extraction Yield
3.2.2. Effects of Extraction Temperature on Extraction Yield
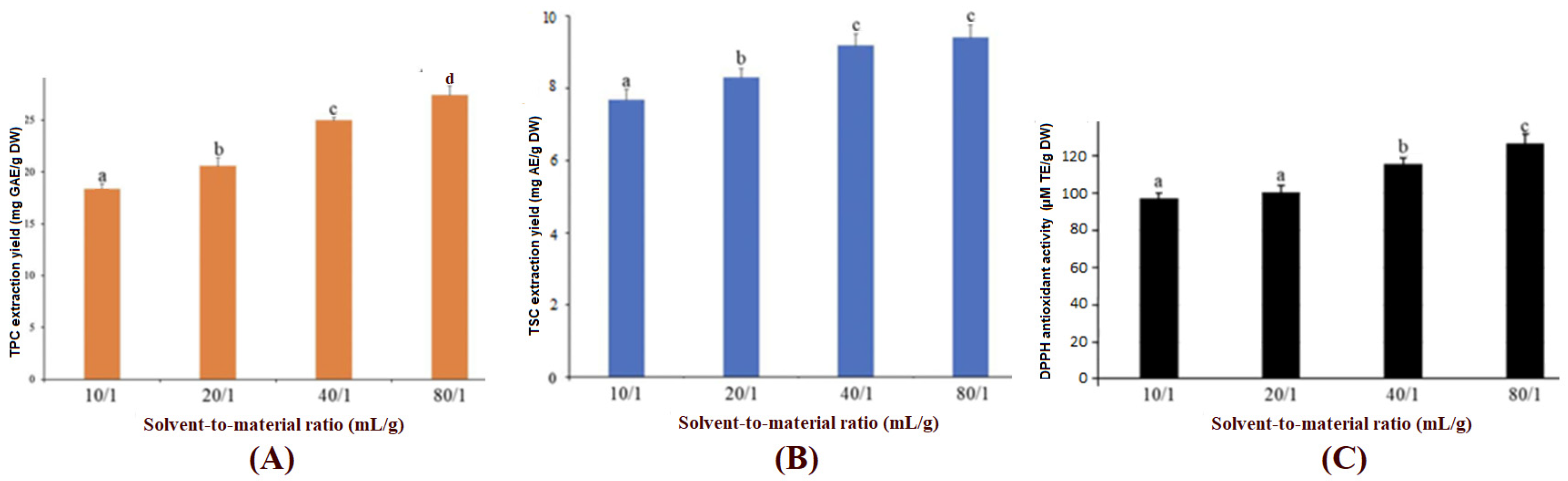
3.2.3. Influence of Solvent-to-Material Ratio on Extraction Yields of TPC, TSC and Antioxidant Activity
3.3. Optimization of Extraction Conditions Using Response Surface Methodology
3.3.1. Fitting Models for Prediction of TPC Extraction Yield (Y1), TSC Extraction Yield (Y2) and DPPH Antioxidant Activity (Y3)
− 0.000518 × X12 − 0.007908 × X22 − 0.009996 × X32
0.000018 × X12 − 0.007629 × X22 − 0.001257 × X32
0.000614 × X12 + 0.094392 × X22 + 0.044185 × X32
3.3.2. Statistical Analysis of Individual and Interacting Impacts of Technological Factors on Output Responses
3.3.3. Verification of Optimal Values through Experimental Validation
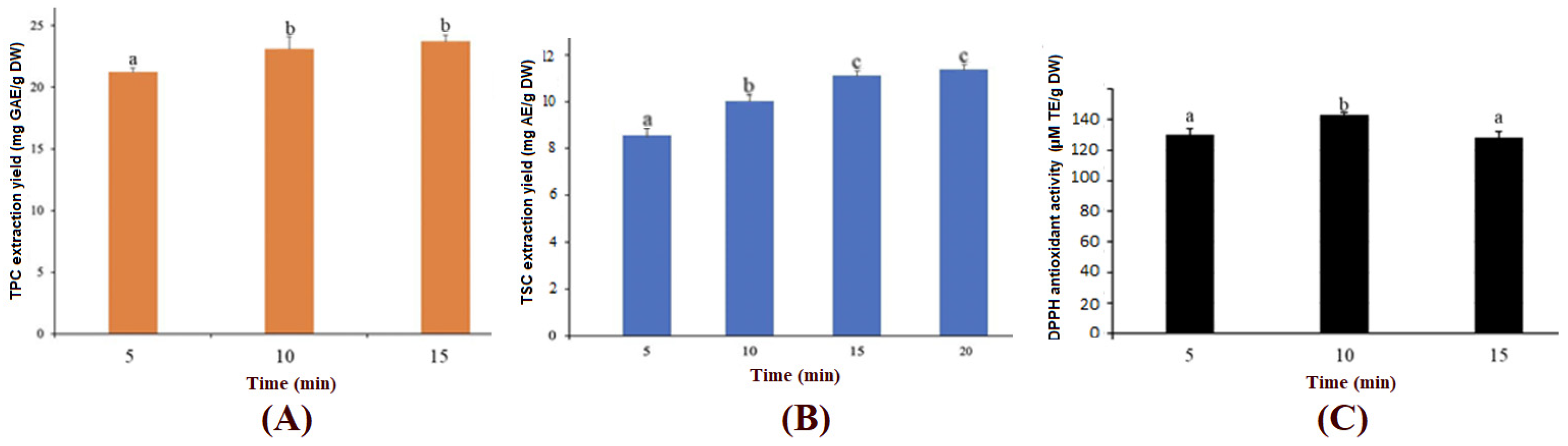
3.4. Ultrasound-Assisted Extraction of TPC, TSC and DPPH Antioxidant Activity from COG Rhizomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.-H.; Huang, J.; Ma, X.-C.; Li, G.-Y.; Ma, Y.-P.; Li, N.; Wang, J.-H. Phenolic glycosides from Curculigo orchioides Gaertn. Fitoterapia 2013, 86, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-C.; Wang, K.-J.; Wang, Z.-Y.; Li, N. Chemical constituents from rhizomes of Curculigo breviscapa. Bull. Korean Chem. Soc. 2010, 31, 224–226. [Google Scholar] [CrossRef]
- Brintha, S.; Rajesh, S.; Renuka, R.; Santhanakrishnan, V.; Gnanam, R. Phytochemical analysis and bioactivity prediction of compounds in methanolic extracts of Curculigo orchioides Gaertn. J. Pharmacogn. Phytochem. 2017, 6, 192–197. [Google Scholar]
- Theng, K.B.; Korpenwar, A.N. Preliminary phytochemical and physicochemical analysis of Curculigo orchioides Gaertn root tubers. Int. J. Bioassays 2014, 3, 3373–3375. [Google Scholar]
- Nguyet, N.T.; Nhung, N.M.; Vu, H.X.A.; Man, N.Q.; Minh, T.T.; Van Thi, T.T. In vitro antioxidant activity and Content of compounds from Curculigo orchioides rhizome. Hue Univ. J. Sci. Nat. Sci. 2020, 129, 71–77. [Google Scholar]
- Asif, M. A Review on Phytochemical and Ethnopharmacological Activities of Curculigo orchioides. Mahidol Univ. J. Pharm. Sci. 2012, 39, 1–10. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. Polyphen. Plants 2019, 243–259. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Tao, L.; Zhang, X.; Hao, F.; Zhao, S.; Bai, C. Recent advances in separation and analysis of saponins in natural products. Separations 2022, 9, 163. [Google Scholar] [CrossRef]
- AOAC. Determination of Moisture, Ash, Protein and Fat. In Official Method of Analysis of the Association of Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Medica 1976, 29, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive folin-ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Li, B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels: II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Stoica, R.; Senin, R.M.; Ion, R.-M. Ethanol concentration effect on the extraction of phenolic compounds from Ribes nigrum assessed by spectrophotometric and HPLC-DAD methods. Rev. Chim. 2013, 64, 620–624. [Google Scholar]
- Nguyen, N.; Nguyen, M.; Nguyen, V.; Le, V.; Trieu, L.; Le, X.; Khang, T.; Giang, N.; Thach, N.; Hung, T. The effects of different extraction conditions on the polyphenol, flavonoids components and antioxidant activity of Polyscias fruticosa roots. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022067. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Villano, C.L.; Rosenblum, A.; Magura, S.; Fong, C.; Cleland, C.; Betzler, T.F. Prevalence and correlates of posttraumatic stress disorder and chronic severe pain in psychiatric outpatients. J. Rehabil. Res. Dev. 2007, 44, 167–178. [Google Scholar] [CrossRef]
- Thoo, Y.Y.; Ho, S.K.; Liang, J.Y.; Ho, C.W.; Tan, C.P. Effects of binary solvent extraction system, extraction time and extraction temperature on phenolic antioxidants and antioxidant capacity from mengkudu (Morinda citrifolia). Food Chem. 2010, 120, 290–295. [Google Scholar] [CrossRef]
- Chan, S.; Lee, C.; Yap, C.; Wan Aida, W.; Ho, C. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int. Food Res. J. 2009, 16, 203–213. [Google Scholar]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhandl, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Bouterfas, K.; Mehdadi, Z.; Latreche, A.; Aouad, L. Antimicrobial power of the flavonoids extracted from the leaves of Marrubium vulgare L. coming from the mount of Tessala (western Algeria). Phytothérapie 2014, 12, 6–14. [Google Scholar] [CrossRef]
- Bertoldo, M.; Ciardelli, F. Water extraction and degradation of a sterically hindered phenolic antioxidant in polypropylene films. Polymer 2004, 45, 8751–8759. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Chuyen, H.V. Extraction of saponins, total soluble solids and antioxidant activity from Polyscias fruticosa roots. Food Res. 2023, 7, 42–47. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- d’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Che Sulaiman, I.S.; Basri, M.; Fard Masoumi, H.R.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, D.-X.; Hou, A.-J.; Lei, G.-Q.; Liu, Z.-J.; Chen, J.-K.; Zhou, T.-S. Antioxidative phenols and phenolic glycosides from Curculigo orchioides. Chem. Pharm. Bull. 2005, 53, 1065–1067. [Google Scholar] [CrossRef]
- Réblová, Z. Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 2012, 30, 171–175. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.d.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of phenolic compounds from citrus by-products using pressurized liquids—An application to orange peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Martinez de la Ossa, E.J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Khoang, L.T.; Huyen, H.T.T.; Chung, H.V.; Duy, L.X.; Toan, T.Q.; Bich, H.T.; Minh, P.T.H.; Pham, D.T.N.; Hien, T.T. Optimization of total saponin extraction from Polyscias fruticosa roots using the ultrasonic-assisted method and response surface methodology. Processes 2022, 10, 2034. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.; Mason, T.J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Diz, P.; Prieto, M.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C. Optimization of ultrasound-assisted extraction to obtain mycosterols from Agaricus bisporus L. by response surface methodology and comparison with conventional Soxhlet extraction. Food Chem. 2016, 197, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cui, J.; Zhang, H.; Duan, Y.; Zhang, D.; Cai, M.; Chen, G. Ultrasound assisted extraction of polyphenolic compounds from red sorghum (Sorghum bicolor L.) bran and their biological activities and polyphenolic compositions. Ind. Crops Prod. 2018, 112, 296–304. [Google Scholar] [CrossRef]
- Gadjalova, A.; Mihaylova, D. Ultrasound-assisted extraction of medicinal plants and evaluation of their biological activity. Food Res. 2019, 3, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Roach, P.D.; Golding, J.B.; Parks, S.E.; Nguyen, M.H. Ultrasound-assisted extraction of GAC peel: An optimization of extraction conditions for recovering carotenoids and antioxidant capacity. Processes 2019, 8, 8. [Google Scholar] [CrossRef]



| No | Coded Levels | Actual Variables | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Time (min) | Temperature (°C) | Solvent-to-Material Ratio (mL/g) | |
| 1 | −1 | −1 | 0 | 60 | 40 | 60 |
| 2 | −1 | 0 | −1 | 60 | 50 | 40 |
| 3 | −1 | 0 | +1 | 60 | 50 | 80 |
| 4 | −1 | +1 | 0 | 60 | 60 | 60 |
| 5 | 0 | −1 | −1 | 120 | 40 | 40 |
| 6 | 0 | −1 | +1 | 120 | 40 | 80 |
| 7 | 0 | 0 | 0 | 120 | 50 | 60 |
| 8 | 0 | 0 | 0 | 120 | 50 | 60 |
| 9 | 0 | 0 | 0 | 120 | 50 | 60 |
| 10 | 0 | +1 | −1 | 120 | 60 | 40 |
| 11 | 0 | +1 | +1 | 120 | 60 | 80 |
| 12 | +1 | −1 | 0 | 180 | 40 | 60 |
| 13 | +1 | 0 | −1 | 180 | 50 | 40 |
| 14 | +1 | 0 | +1 | 180 | 50 | 80 |
| 15 | +1 | +1 | 0 | 180 | 60 | 60 |
| No | X1 | X2 | X3 | Y1 (mg GAE/g DW) | Y2 (mg AE/g DW) | Y3 (µM TE/g DW) |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 15.98 | 8.05 | 90.14 |
| 2 | −1 | 0 | −1 | 15.26 | 7.04 | 69.58 |
| 3 | −1 | 0 | +1 | 14.62 | 10.87 | 90.65 |
| 4 | −1 | +1 | 0 | 19.07 | 9.46 | 68.40 |
| 5 | 0 | −1 | −1 | 16.98 | 7.20 | 95.48 |
| 6 | 0 | −1 | +1 | 20.81 | 8.40 | 109.26 |
| 7 | 0 | 0 | 0 | 22.35 | 8.66 | 67.28 |
| 8 | 0 | 0 | 0 | 22.48 | 9.63 | 66.31 |
| 9 | 0 | 0 | 0 | 24.07 | 9.95 | 75.29 |
| 10 | 0 | +1 | −1 | 17.08 | 6.85 | 76.10 |
| 11 | 0 | +1 | +1 | 17.84 | 10.14 | 106.12 |
| 12 | +1 | −1 | 0 | 22.80 | 8.18 | 104.15 |
| 13 | +1 | 0 | −1 | 16.29 | 7.30 | 73.13 |
| 14 | +1 | 0 | +1 | 22.24 | 10.70 | 124.69 |
| 15 | +1 | +1 | 0 | 23.39 | 9.18 | 62.42 |
| Regression Coefficient | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|
| Estimated Value | Prob > |t| | Estimated Value | Prob > |t| | Estimated Value | Prob > |t| | |
| Intercept | ||||||
| 22.967 | <0.0001 * | 9.413 | <0.0001 * | 69.627 | <0.0001 * | |
| Linear | ||||||
| 2.474 | 0.0027 * | −0.008 | 0.9744 | 5.703 | 0.1576 | |
| 0.101 | 0.8302 | 0.475 | 0.0858 | −10.749 | 0.0259 * | |
| 1.238 | 0.0398 * | 1.465 | 0.0012 * | 14.554 | 0.0082 * | |
| Interaction | ||||||
| −0.625 | 0.3694 | −0.103 | 0.7577 | −4.998 | 0.3505 | |
| 1.648 | 0.0483 * | −0.108 | 0.7464 | 7.623 | 0.1772 | |
| −0.768 | 0.2801 | 0.523 | 0.1575 | 4.060 | 0.4411 | |
| Quadratic | ||||||
| −1.866 | 0.0368 * | 0.067 | 0.8457 | 2.212 | 0.6799 | |
| −0.791 | 0.2844 | −0.763 | 0.0672 | 9.439 | 0.1207 | |
| −3.998 | 0.0018 * | −0.503 | 0.1850 | 17.674 | 0.0173 * | |
| Output Responses | Optimal Conditions | Predicted Values | Actual Values |
|---|---|---|---|
| TPC (mg GAE/g DW) | 178 min 45 °C 68 mL/g | 24.32 ± 2.15 b | 23.58 ± 2.24 b |
| TSC (mg AE/g DW) | 60 min 57 °C 80 mL/g | 10.95 ± 1.72 a | 11.33 ± 1.20 a |
| Antioxidant activity (µM TE/g DW) | 180 min 40 °C 80 mL/g | 138.52 ± 29.49 c | 133.45 ± 19.95 c |
| Output Responses | Parameters | Maceration | UAE |
|---|---|---|---|
| TPC (mg GAE/g DW) | Extraction time | 178 min | 10 min |
| Recovery yield | 23.73 ± 0.77 a | 23.58 ± 2.24 a | |
| TSC (mg AE/g DW) | Extraction time | 60 min | 15 min |
| Recovery yield | 11.27 ± 0.82 b | 11.33 ± 1.20 b | |
| Antioxidant activity (µM TE/g DW) | Extraction time | 178 min | 10 min |
| Recovery yield | 141.85 ± 10.12 c | 133.45 ± 19.95 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.D.; Do, L.T.K.; Dang, T.N.D.; Pham, V.D.; Hoang, V.C. Recovery of Saponins, Phenolic Compounds and Antioxidant Capacity from Curculigo orchioides Gaertn Rhizomes by Different Extraction Methods. Appl. Sci. 2024, 14, 7535. https://doi.org/10.3390/app14177535
Bui TD, Do LTK, Dang TND, Pham VD, Hoang VC. Recovery of Saponins, Phenolic Compounds and Antioxidant Capacity from Curculigo orchioides Gaertn Rhizomes by Different Extraction Methods. Applied Sciences. 2024; 14(17):7535. https://doi.org/10.3390/app14177535
Chicago/Turabian StyleBui, Thuc Duyen, Linh Thuy Khanh Do, Thi Ngoc Dung Dang, Van Doanh Pham, and Van Chuyen Hoang. 2024. "Recovery of Saponins, Phenolic Compounds and Antioxidant Capacity from Curculigo orchioides Gaertn Rhizomes by Different Extraction Methods" Applied Sciences 14, no. 17: 7535. https://doi.org/10.3390/app14177535





