An Evaluation of the Use of Coffee Silverskin Particles and Extracts as Additives in Wheat Flour/Glucose Mixtures to Produce Bioactive Films for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biocomposite Wheat Flour Films (WFFs)
2.3. Film Characterization
2.4. Statistical Analysis
3. Results and Discussion
3.1. Optical and Morphological Analysis
3.2. Color Measurements
3.3. FTIR Spectroscopy
3.4. Water Absorption (WA), Solubility, and Swelling Degree
3.5. Water Vapor and Gas Permeability
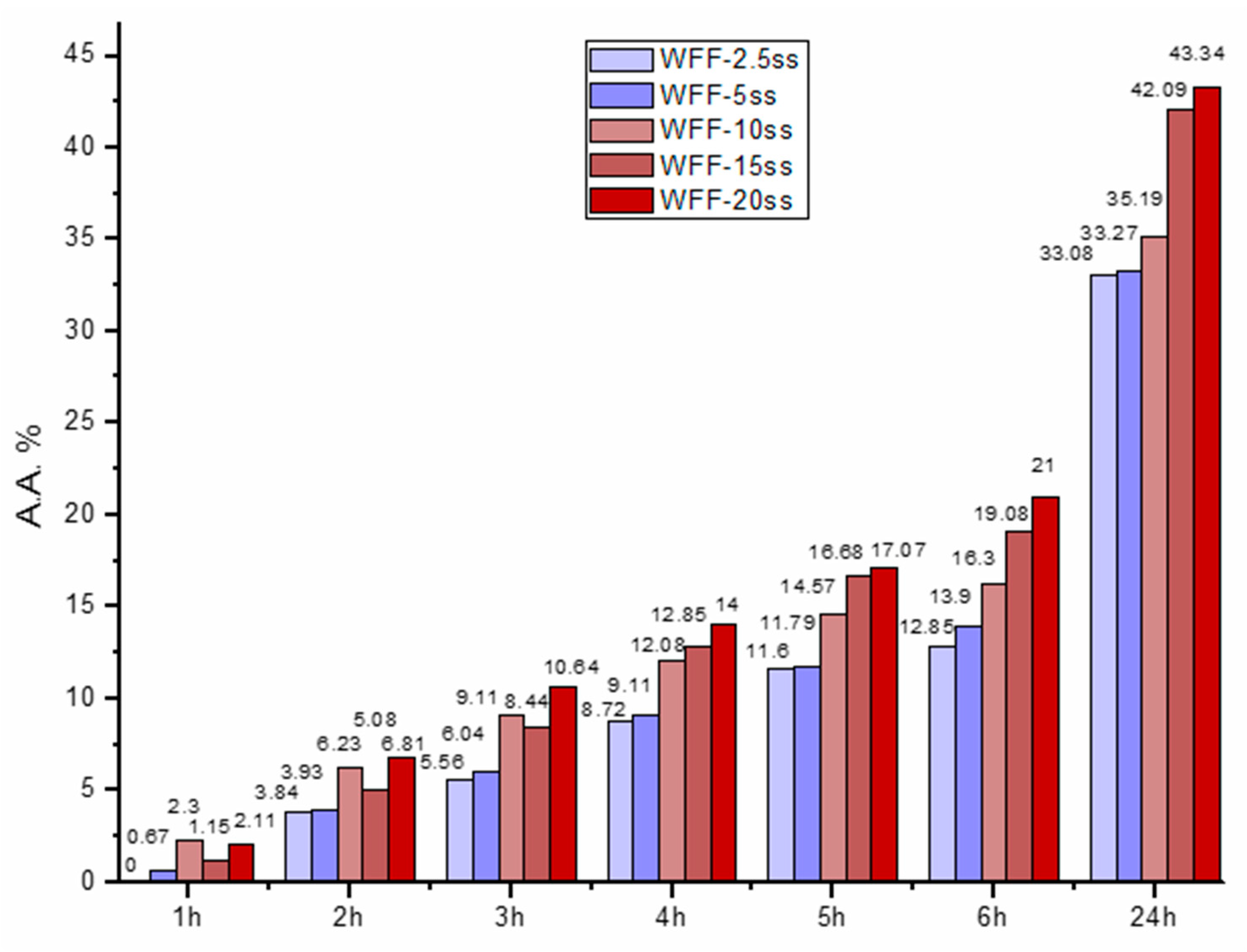
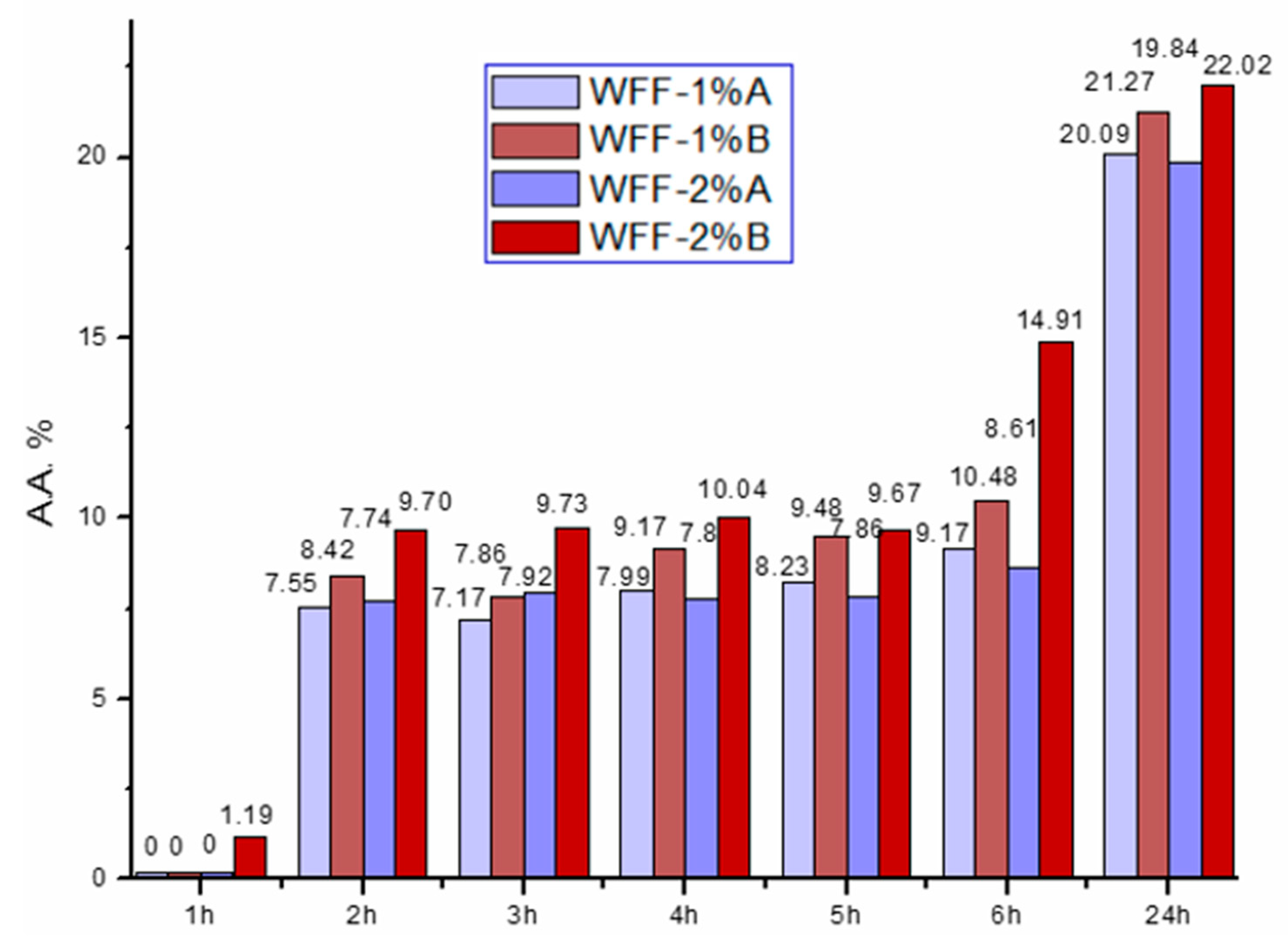
3.6. Antioxidant Activity
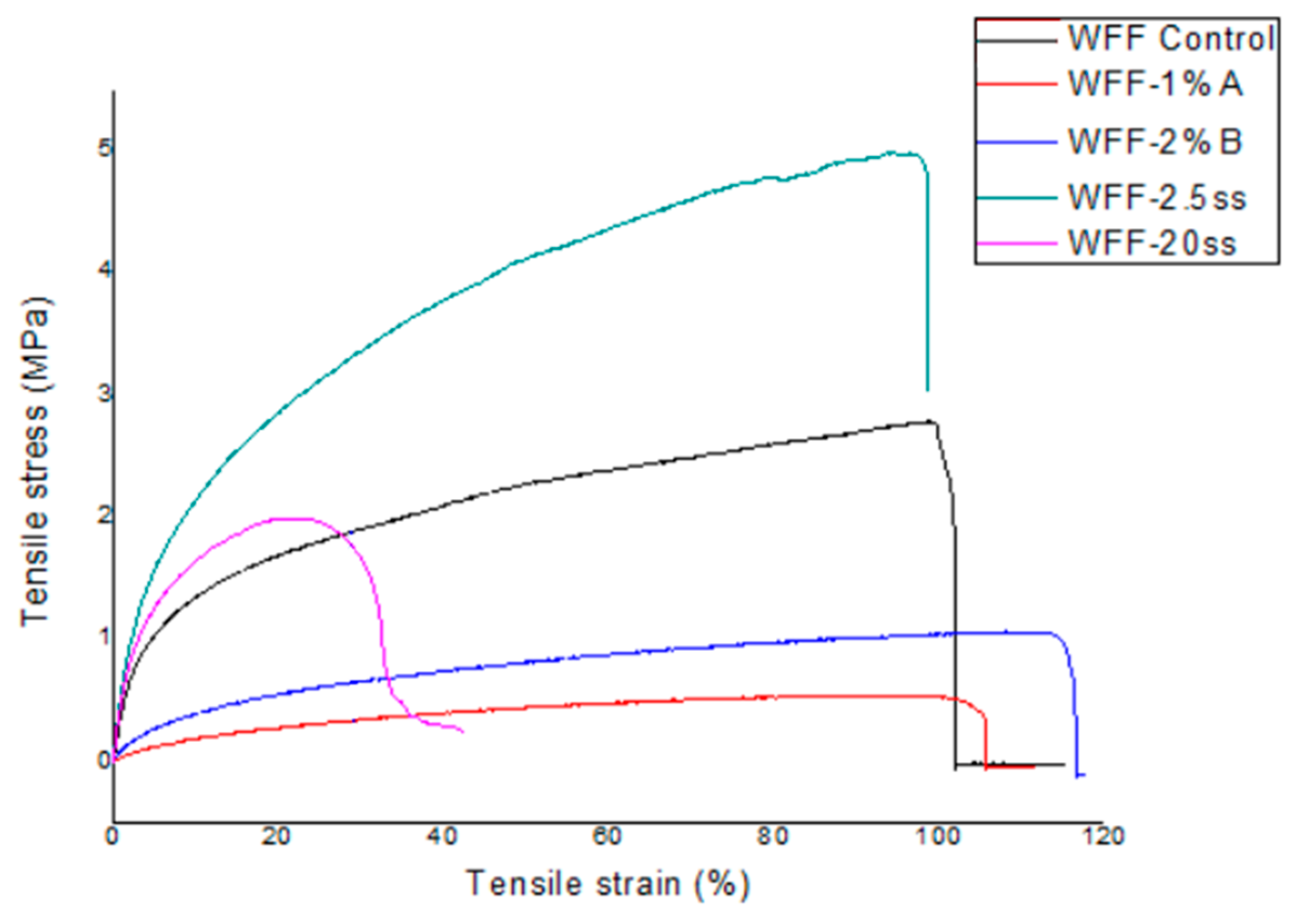
3.7. Mechanical Properties
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Le, T.; Maki, H.; Takahashi, K.; Okazaki, E.; Osako, K. Properties of Gelatin Film from Horse Mackerel (Trachurus japonicus) Scale. J. Food Sci. 2015, 80, E734–E741. [Google Scholar] [CrossRef] [PubMed]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Valdés, A.; Burgos, N.; Jiménez, A.; Garrigós, M.C. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Daudt, R.; Avena-Bustillos, R.; Williams, T.; Wood, D.; Külkamp-Guerreiro, I.; Marczak, L.; McHugh, T. Comparative study on properties of edible films based on pinhão (Araucaria angustifolia) starch and flour. Food Hydrocoll. 2016, 60, 279–287. [Google Scholar] [CrossRef]
- Orsuwan, A.; Sothornvit, R. Effect of banana and plasticizer types on mechanical, water barrier, and heat sealability of plasticized banana-based films. J. Food Process. Preserv. 2017, 42, e13380. [Google Scholar] [CrossRef]
- Benincasa, P.; Dominici, F.; Bocci, L.; Governatori, C.; Panfili, I.; Tosti, G.; Torre, L.; Puglia, D. Relationships between wheat flour baking properties and tensile characteristics of derived thermoplastic films. Ind. Crop. Prod. 2017, 100, 138–145. [Google Scholar] [CrossRef]
- Leblanc, N.; Saiah, R.; Beucher, E.; Gattin, R.; Castandet, M.; Saiter, J.-M. Structural investigation and thermal stability of new extruded wheat flour based polymeric materials. Carbohydr. Polym. 2008, 73, 548–557. [Google Scholar] [CrossRef]
- Gao, X.; Tong, J.; Guo, L.; Yu, L.; Li, S.; Yang, B.; Wang, L.; Liu, Y.; Li, F.; Guo, J.; et al. Influence of gluten and starch granules interactions on dough mixing properties in wheat (Triticum aestivum L.). Food Hydrocoll. 2020, 106, 105885. [Google Scholar] [CrossRef]
- Chen, G.-X.; Zhou, J.-W.; Liu, Y.-L.; Lu, X.-B.; Han, C.-X.; Zhang, W.-Y.; Xu, Y.-H.; Yan, Y.-M. Biosynthesis and Regulation of Wheat Amylose and Amylopectin from Proteomic and Phosphoproteomic Characterization of Granule-binding Proteins. Sci. Rep. 2016, 6, 33111. [Google Scholar] [CrossRef]
- BeMiller, J.N. Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Mechanisms of starch gelatinization during heating of wheat flour and its effect on in vitro starch digestibility. Food Hydrocoll. 2018, 82, 370–378. [Google Scholar] [CrossRef]
- Godwin, A.L.E.D. PLASTICIZERS. In Applied Polymer Science: 21st Century; Elsevier: Amsterdam, The Netherlands, 2000; pp. 157–175. [Google Scholar]
- Lim, W.S.; Ock, S.Y.; Park, G.D.; Lee, I.W.; Lee, M.H.; Park, H.J. Heat-sealing property of cassava starch film plasticized with glycerol and sorbitol. Food Packag. Shelf Life 2020, 26, 100556. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A. Antimicrobial, mechanical and barrier properties of triticale protein films incorporated with oregano essential oil. Food Biosci. 2013, 1, 2–9. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y. Wheat Gluten–Based Coatings and Films: Preparation, Properties, and Applications; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Giyatmi; Melanie, S.; Fransiska, D.; Darmawan, M.; Irianto, H. Barrier and physical properties of arrowroot starch-carrageenan based biofilms. J. Bio-Sci. 2017, 25, 45–56. [Google Scholar] [CrossRef]
- Petaloti, A.-I.; Makri, S.; Achilias, D.S. Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications. Gels 2024, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Sung, S.H.; Chang, Y.; Han, J. Development of polylactic acid nanocomposite films reinforced with cellulose nanocrystals derived from coffee silverskin. Carbohydr. Polym. 2017, 169, 495–503. [Google Scholar] [CrossRef]
- Dominici, F.; García, D.G.; Fombuena, V.; Luzi, F.; Puglia, D.; Torre, L.; Balart, R. Bio-polyethylene-based composites reinforced with alkali and palmitoyl chloride-treated coffee silverskin. Molecules 2019, 24, 3113. [Google Scholar] [CrossRef]
- Hejna, A.; Korol, J.; Kosmela, P.; Kuzmin, A.; Piasecki, A.; Kulawik, A.; Chmielnicki, B. By-products from food industry as a promising alternative for the conventional fillers for wood–polymer composites. Polymers 2021, 13, 893. [Google Scholar] [CrossRef]
- Totaro, G.; Sisti, L.; Fiorini, M.; Lancellotti, I.; Andreola, F.N.; Saccani, A. Formulation of Green Particulate Composites from PLA and PBS Matrix and Wastes Deriving from the Coffee Production. J. Polym. Environ. 2019, 27, 1488–1496. [Google Scholar] [CrossRef]
- Pagliarini, E.; Totaro, G.; Saccani, A.; Gaggìa, F.; Lancellotti, I.; Di Gioia, D.; Sisti, L. Valorization of coffee wastes as plant growth promoter in mulching film production: A contribution to a circular economy. Sci. Total. Environ. 2023, 871, 162093. [Google Scholar] [CrossRef] [PubMed]
- Conde, T.; Mussatto, S.I. Isolation of polyphenols from spent coffee grounds and silverskin by mild hydrothermal pretreatment. Prep. Biochem. Biotechnol. 2016, 46, 406–409. [Google Scholar] [CrossRef]
- Drakos, A.; Pelava, E.; Evageliou, V. Properties of flour films as affected by the flour’s source and particle size. Food Res. Int. 2018, 107, 551–558. [Google Scholar] [CrossRef] [PubMed]
- An Outline of Standard ASTM E96 for Cup Method Water Vapor Permeability Testing. [Online]. Available online: www.labthinkinternational.com.cn (accessed on 5 December 2022).
- Petaloti, A.-I.; Achilias, D.S. The Development of Sustainable Biocomposite Materials Based on Poly(lactic acid) and Silverskin, a Coffee Industry By-Product, for Food Packaging Applications. Sustainability 2024, 16, 5075. [Google Scholar] [CrossRef]
- ASTM D 882. Tensile Testing of Thin Plastic Sheeting. Available online: https://www.instron.com/es-es/-/media/literature-library/applications/2006/02/astm-d-882---tensile-testing-of-thin-plastic-sheeting.pdf (accessed on 15 November 2020).
- Thiagamani, S.M.K.; Nagarajan, R.; Jawaid, M.; Anumakonda, V.; Siengchin, S. Utilization of chemically treated municipal solid waste (spent coffee bean powder) as reinforcement in cellulose matrix for packaging applications. Waste Manag. 2017, 69, 445–454. [Google Scholar] [CrossRef]
- Mossavi, M.P.; Kashiri, M.; Maghsoudlou, Y.; Khomiri, M.; Alami, M. Development and characterization of a novel multifunctional film based on wheat filter flour incorporated with carvacrol: Antibacterial, antifungal, and insecticidal potentials. Food Sci. Technol. Int. 2022, 28, 603–612. [Google Scholar] [CrossRef]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of a hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Characterization and in vitro antimicrobial study of soy protein isolate films incorporating carvacrol. Food Hydrocoll. 2021, 122, 107091. [Google Scholar] [CrossRef]
- Kemsley, E.K.; Ruault, S.; Wilson, R.H. Discrimination between Coffea arabica and Coffea canephora variant robusta beans using infrared spectroscopy. Food Chem. 1995, 54, 321–326. [Google Scholar] [CrossRef]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Discrimination between roasted coffee, roasted corn and coffee husks by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. LWT 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Irudayaraj, J. Rapid determination of caffeine content in soft drinks using FTIR–ATR spectroscopy. Food Chem. 2002, 78, 261–266. [Google Scholar] [CrossRef]
- Cremer, D.R.; Kaletunç, G. Fourier transform infrared microspectroscopic study of the chemical microstructure of corn and oat flour-based extrudates. Carbohydr. Polym. 2003, 52, 53–65. [Google Scholar] [CrossRef]
- Xiao, Q.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. The role of heating time on the characteristics, functional properties and antioxidant activity of enzyme-hydrolyzed rice proteins-glucose Maillard reaction products. Food Biosci. 2021, 43, 101225. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Salva, T.J.; Ferreira, M.M. Chemometric studies for quality control of processed brazilian coffees using drifts. J. Food Qual. 2010, 33, 212–227. [Google Scholar] [CrossRef]
- Luo, S.; Chen, J.; He, J.; Li, H.; Jia, Q.; Hossen, A.; Dai, J.; Qin, W.; Liu, Y. Preparation of corn starch/rock bean protein edible film loaded with d-limonene particles and their application in glutinous rice cake preservation. Int. J. Biol. Macromol. 2022, 206, 313–324. [Google Scholar] [CrossRef]
- Figueiró, S.; Góes, J.C.; Moreira, R.; Sombra, A. On the physico-chemical and dielectric properties of glutaraldehyde crosslinked galactomannan–collagen films. Carbohydr. Polym. 2004, 56, 313–320. [Google Scholar] [CrossRef]
- Peron-Schlosser, B.; Carpiné, D.; Jorge, R.M.M.; Spier, M.R. Optimization of wheat flour by product films: A technological and sustainable approach for bio-based packaging material. J. Food Sci. 2021, 86, 4522–4538. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Sobral, P.J.; Menegalli, F.C. Development and characterization of biofilms based on Amaranth flour (Amaranthus caudatus). J. Food Eng. 2005, 67, 215–223. [Google Scholar] [CrossRef]
- Balan, G.C.; Paulo, A.F.S.; Correa, L.G.; Alvim, I.D.; Ueno, C.T.; Coelho, A.R.; Ströher, G.R.; Yamashita, F.; Sakanaka, L.S.; Shirai, M.A. Production of Wheat Flour/PBAT Active Films Incorporated with Oregano Oil Microparticles and Its Application in Fresh Pastry Conservation. Food Bioprocess Technol. 2021, 14, 1587–1599. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Sarebanha, S.; Farhan, A. Eco-friendly Composite Films Based on Polyvinyl Alcohol and Jackfruit Waste Flour. J. Packag. Technol. Res. 2018, 2, 181–190. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2018, 89, 143–152. [Google Scholar] [CrossRef]
- Silva-Rodrigues, H.C.; Silveira, M.P.; Helm, C.V.; Jorge, L.M.d.M.; Jorge, R.M.M. Gluten free edible film based on rice flour reinforced by guabiroba (Campomanesia xanthocarpa) pulp. J. Appl. Polym. Sci. 2020, 137, 49254. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Camiletti, O.F.; Riveros, C.G.; Aguirre, A.; Grosso, N.R. Sunflower oil preservation by using chickpea flour film as bio-packaging material. J. Food Sci. 2021, 86, 61–67. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Tessaro, L.; Lucas, A.A.; Tapia-Blácido, D.R. Bioactive films based on babassu mesocarp flour and starch. Food Hydrocoll. 2017, 70, 383–391. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Martorell, P.; Genovés, S.; Ramón, D.; Stamatakis, K.; Fresno, M.; Molina, A.; Del Castillo, M.D. Coffee silverskin extract protects against accelerated aging caused by oxidative Agents. Molecules 2016, 21, 721. [Google Scholar] [CrossRef]
- Lv, J.; Yu, L.; Lu, Y.; Niu, Y.; Liu, L.; Costa, J.; Yu, L. Phytochemical compositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chem. 2012, 135, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Favaro, S.L.; Pereira, A.G.B.; Fernandes, J.R.; Baron, O.; da Silva, C.T.P.; Moisés, M.P.; Radovanovic, E. Outstanding Impact Resistance of Post-Consumer HDPE/Multilayer Packaging Composites. Mater. Sci. Appl. 2017, 8, 15–25. [Google Scholar] [CrossRef]
- Puglia, D.; Dominici, F.; Kenny, J.M.; Santulli, C.; Governatori, C.; Tosti, G.; Benincasa, P. Tensile Behavior of Thermoplastic Films from Wheat Flours as Function of Raw Material Baking Properties. J. Polym. Environ. 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Sreekumar, P.A.; Leblanc, N.; Saiter, J.M. Effect of Glycerol on the Properties of 100% Biodegradable Thermoplastic Based on Wheat Flour. J. Polym. Environ. 2013, 21, 388–394. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Rokosa, M.; Pieczykolan, M.; Antosik, A.K.; Skórczewska, K. Biocomposites Based on Wheat Flour with Urea-Based Eutectic Plasticizer and Spent Coffee Grounds: Preparation, Physicochemical Characterization, and Study of their Influence on Plant Growth. Materials 2024, 17, 1212. [Google Scholar] [CrossRef]
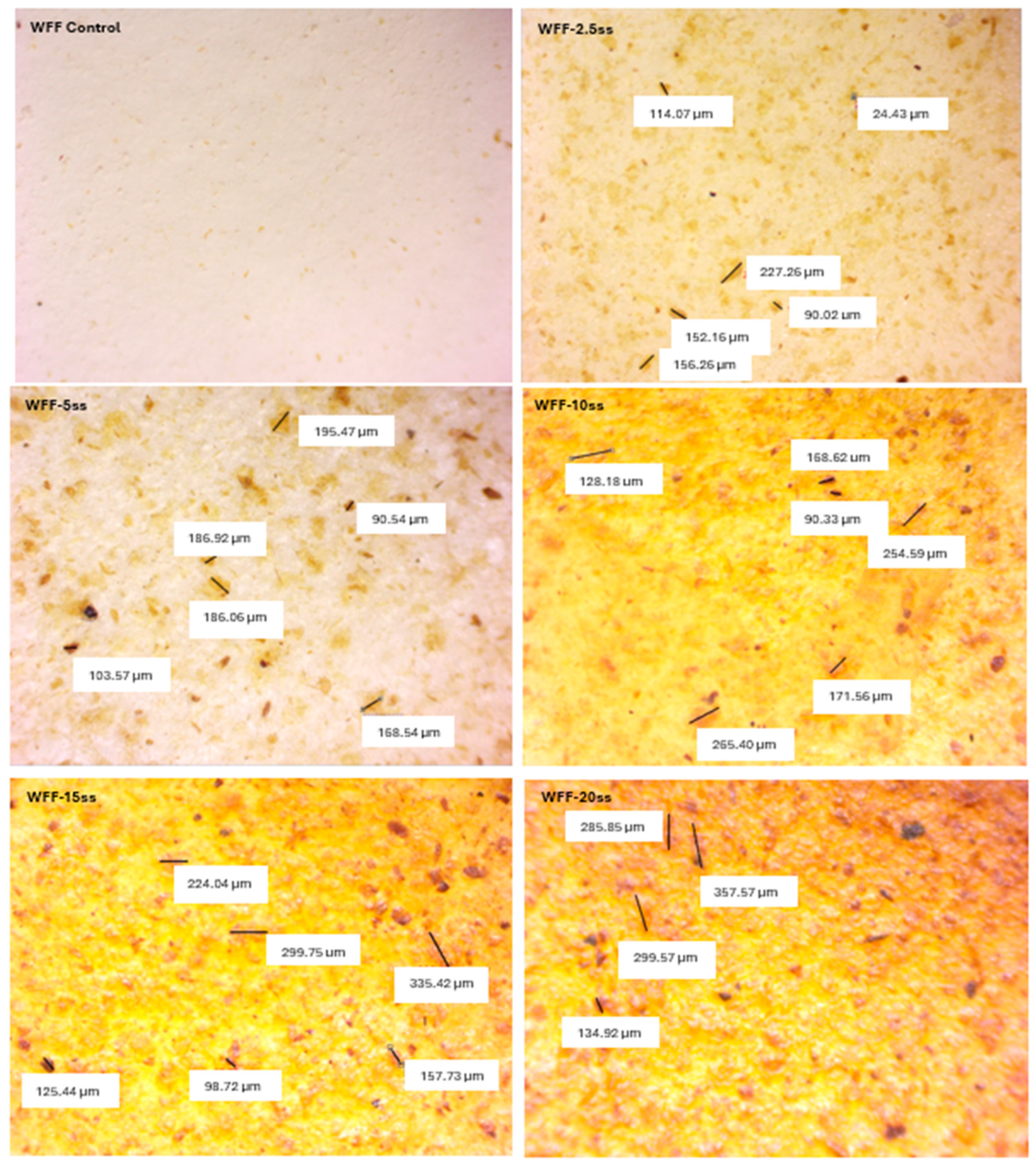
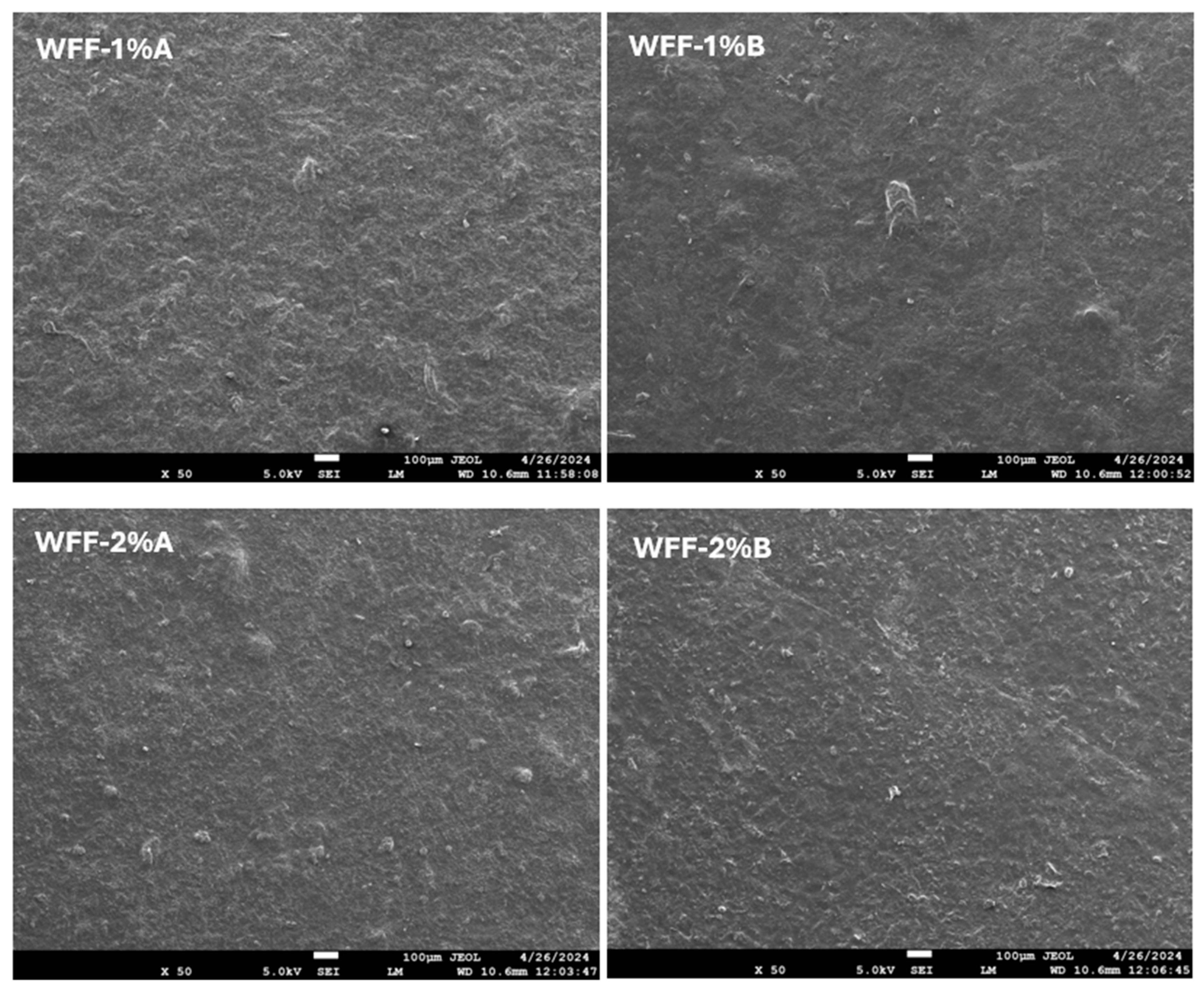
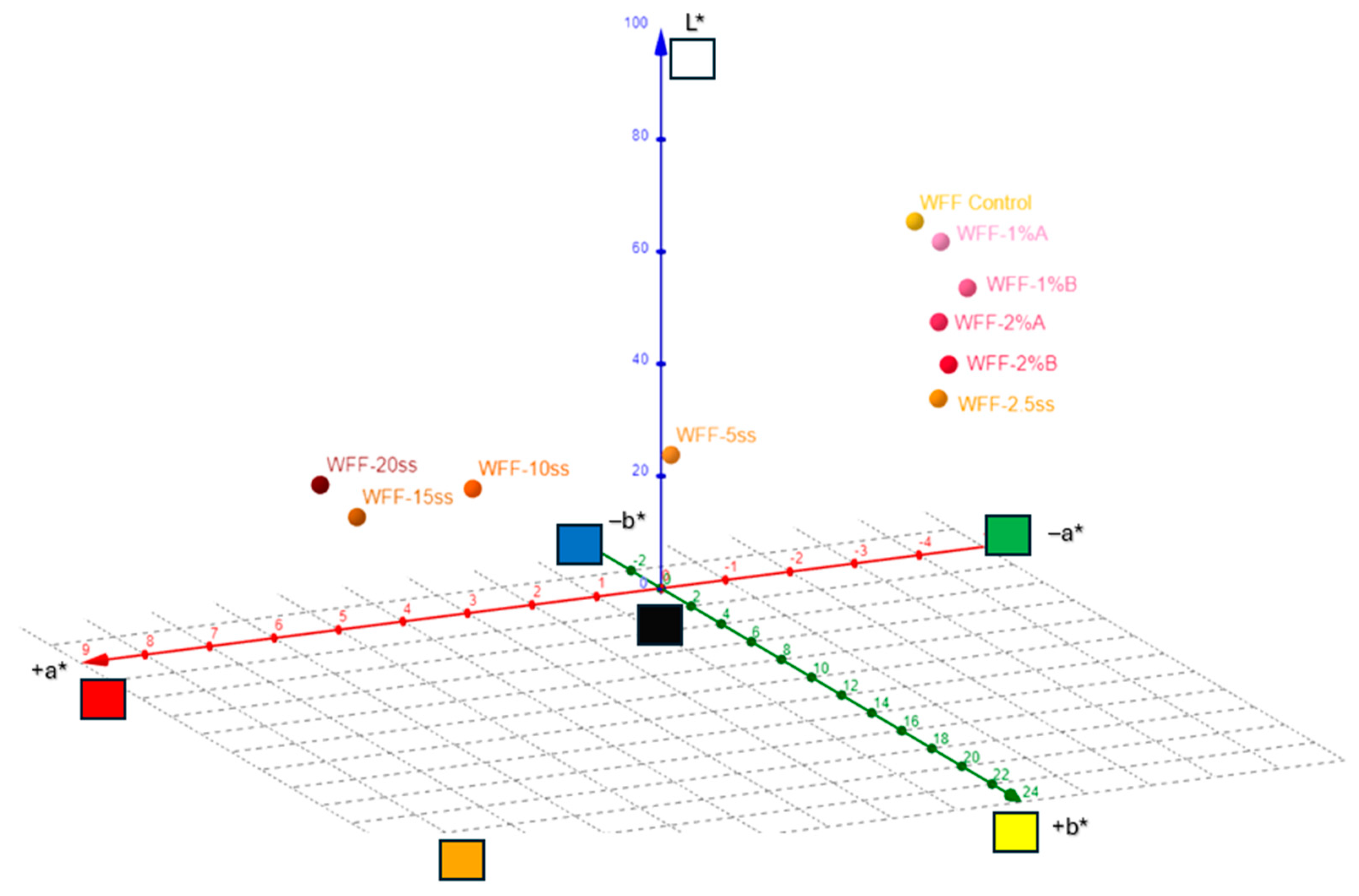



| L* | a* | b* | c* | h | R% (400nm) | K/S | |
|---|---|---|---|---|---|---|---|
| WFF | 81.56 | −1.28 | 11.39 | 11.46 | 96.43 | 36.72 | 0.55 |
| WFF-2.5ss | 72.98 | 1.20 | 23.59 | 24.04 | 86.07 | 19.01 | 1.73 |
| WFF-5ss | 63.22 | 4.64 | 20.56 | 21.72 | 78.87 | 13.01 | 2.91 |
| WFF-10ss | 55.93 | 7.01 | 17.55 | 18.79 | 71.16 | 11.98 | 3.23 |
| WFF-15ss | 51.91 | 8.61 | 16.71 | 17.59 | 62.73 | 11.75 | 3.31 |
| WFF-20ss | 48.00 | 7.91 | 11.27 | 13.77 | 54.95 | 12.04 | 3.21 |
| WFF-1%A | 80.73 | −1.27 | 13.15 | 13.21 | 95.53 | 34.72 | 0.61 |
| WFF-1%B | 79.46 | −0.77 | 17.07 | 17.09 | 92.60 | 23.89 | 1.21 |
| WFF-2%A | 77.17 | 0.05 | 18.69 | 18.69 | 89.85 | 21.88 | 1.39 |
| WFF-2%B | 75.34 | 0.62 | 21.79 | 21.79 | 88.35 | 19.81 | 1.62 |
| Water Absorption (%) | Solubility (%) | Swelling Degree (%) | |
|---|---|---|---|
| WFF Control | 15.2 ± 0.68 a | 54.55 ± 0.89 a | 124.12 ± 3.45 a |
| WFF-2.5ss | 9.31 ± 0.03 b | 48.04 ± 1.35 b | 129.35 ± 3.15 a |
| WFF-5ss | 9.88 ± 0.34 c | 47.66 ± 1.48 b | 147.12 ± 1.12 b |
| WFF-10ss | 11.79 ± 0.17 d | 51.79 ± 0.24 c | 165.81 ± 2.75 c |
| WFF-15ss | 12.53 ± 0.1 e | 51.13 ± 0.34 c | 167.29 ± 0.86 c |
| WFF-20ss | 12.51 ± 0.7 e | 50.11 ± 1.15 b,c | 176.96 ± 1.15 d |
| WFF-1%A | 13.81 ± 0.06 f | 63.15 ± 1.55 d | 185.79 ± 0.98 e |
| WFF-1%B | 11.78 ± 0.09 d | 57.40 ± 1.64 e | 179.95 ± 0.72 f |
| WFF-2%A | 10.56 ± 0.25 g | 62.08 ± 0.34 d | 143.30 ± 2.98 b |
| WFF-2%B | 9.32 ± 0.14 b | 59.80 ± 1.04 e | 134.64 ± 1.36 g |
| WVTR (g/m2∙d) | WVP (10−6) (g/m∙d∙Pa) | OTR (cm3/(m2∙d∙0.1 MPa) | |
|---|---|---|---|
| WFF control | 20.21 | 2.33 | 11.58 |
| WFF-2.5ss | 14.12 | 1.54 | 7.63 |
| WFF-5ss | 18.26 | 1.31 | 10.47 |
| WFF-10ss | 18.79 | 2.41 | 13.56 |
| WFF-15ss | 18.75 | 2.02 | 14.97 |
| WFF-20ss | 13.84 | 1.54 | 19.93 |
| WFF-1%A | 14.43 | 1.43 | 9.9 |
| WFF-1%B | 12.27 | 1.34 | 9.42 |
| WFF-2%A | 10.57 | 1.08 | 9.35 |
| WFF-2%B | 10.47 | 1.04 | 8.33 |
| Tensile Strain at Break (%) | Tensile Stress at Break (MPa) | Young’s Modulus (MPa) | |
|---|---|---|---|
| WFF Control | 118.97 ± 6.30 a | 2.64 ± 0.13 a | 41.22 ± 4.48 a |
| WFF-2.5ss | 104.16 ± 4.01 b | 4.73 ± 0.24 b | 74.80 ± 0.71 b |
| WFF-5ss | 96.59 ± 1.37 c | 3.53 ± 0.02 c | 74.02 ± 1.21 b |
| WFF-10ss | 74.95 ± 2.90 d | 2.28 ± 0.21 a | 66.52 ± 1.42 c |
| WFF-15ss | 66.18 ± 1.4 e | 3.53 ± 0.12 c | 69.48 ± 1.51 c |
| WFF-20ss | 39.61 ± 2.90 f | 2.82 ± 0.18 a | 67.37 ± 4.31 c |
| WFF-1%A | 119.56 ± 8.61 a | 1.16 ± 0.19 d | 12.17 ± 0.83 d |
| WFF-2%A | 118.15 ± 7.79 a | 0.95 ± 0.01 e | 13.33 ± 0.99 d |
| WFF-1%B | 121.33 ± 0.12 a | 0.99 ± 0.05 d,e,f | 22.63 ± 3.22 e |
| WFF-2%B | 120.61 ± 11.09 a | 1.19 ± 0.21 d,f | 17.04 ± 0.90 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petaloti, A.-I.; Valtopoulou, A.; Gkogkou, C.; Achilias, D.S. An Evaluation of the Use of Coffee Silverskin Particles and Extracts as Additives in Wheat Flour/Glucose Mixtures to Produce Bioactive Films for Food Packaging. Appl. Sci. 2024, 14, 7563. https://doi.org/10.3390/app14177563
Petaloti A-I, Valtopoulou A, Gkogkou C, Achilias DS. An Evaluation of the Use of Coffee Silverskin Particles and Extracts as Additives in Wheat Flour/Glucose Mixtures to Produce Bioactive Films for Food Packaging. Applied Sciences. 2024; 14(17):7563. https://doi.org/10.3390/app14177563
Chicago/Turabian StylePetaloti, Argyri-Ioanna, Anastasia Valtopoulou, Christina Gkogkou, and Dimitris S. Achilias. 2024. "An Evaluation of the Use of Coffee Silverskin Particles and Extracts as Additives in Wheat Flour/Glucose Mixtures to Produce Bioactive Films for Food Packaging" Applied Sciences 14, no. 17: 7563. https://doi.org/10.3390/app14177563
APA StylePetaloti, A.-I., Valtopoulou, A., Gkogkou, C., & Achilias, D. S. (2024). An Evaluation of the Use of Coffee Silverskin Particles and Extracts as Additives in Wheat Flour/Glucose Mixtures to Produce Bioactive Films for Food Packaging. Applied Sciences, 14(17), 7563. https://doi.org/10.3390/app14177563








