Evaluation of Asian Hornet (Vespa velutina) Trappability in Alto-Minho, Portugal: Commercial vs. Artisanal Equipment, Human Factors, Geography, Climatology, and Vegetation
Abstract
1. Introduction
2. Materials and Methods
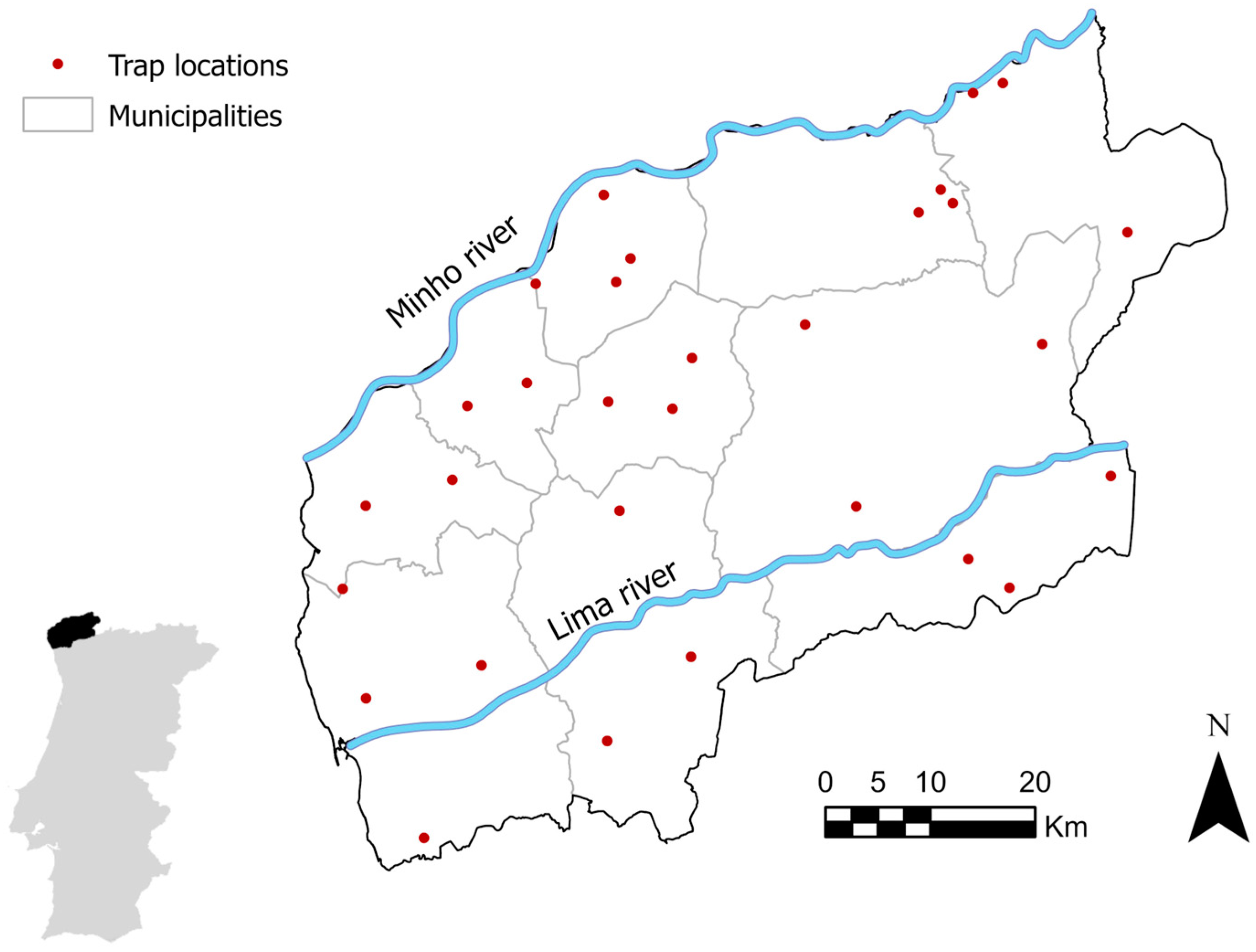
2.1. Sampling
2.2. Traps and Attractants
2.3. Human, Geographical, Climatological, and Vegetation Factors
2.4. Data Analysis
3. Results
3.1. Differences in Captures between the Different Combinations of Trap and Attractant
3.2. Temporal Variation of Captures
3.3. The Influence of Human Factors, Geography, Climate, and Vegetation on Captures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lima, C.G.; Vaz, A.S.; Honrado, J.P.; Aranha, J.; Crespo, N.; Vicente, J.R. The Invasion by the Yellow-Legged Hornet: A Systematic Review. J. Nat. Conserv. 2022, 67, 126173. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Salles, J.M.; Courchamp, F. The Economic Cost of Control of the Invasive Yellow-Legged Asian Hornet. NeoBiota 2020, 55, 11–25. [Google Scholar] [CrossRef]
- Castro, L.; Pagola-Carte, S. Vespa velutina Lepeletier, 1836 (Hymenoptera: Vespidae), Recolectada en la Península Ibérica. Heteropterus Rev. Entomol. 2010, 10, 193–196. [Google Scholar]
- Grosso-Silva, J.M.; Maia, M. Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae), New Species for Portugal. Arq. Entomolóxicos 2012, 6, 53–54. [Google Scholar]
- Barbier, Y.; Renneson, J.-L. Vespa velutina Lepeletier. 1836. Available online: http://www.atlashymenoptera.net/pagetaxon.asp?tx_id=3877 (accessed on 22 August 2024).
- Bertolino, S.; Lioy, S.; Laurino, D.; Manino, A.; Porporato, M. Spread of the Invasive Yellow-Legged Hornet Vespa velutina (Hymenoptera: Vespidae) in Italy. Appl. Entomol. Zool. 2016, 51, 589–597. [Google Scholar] [CrossRef]
- Witt, R. Erstfund Eines Nestes der Asiatischen Hornisse Vespa velutina Lepeletier, 1838 in Deutschland und Details Zum Nestbau (Hymenoptera, Vespinae). Ampulex 2015, 7, 42–53. [Google Scholar]
- Budge, G.E.; Hodgetts, J.; Jones, E.P.; Ostojá-Starzewski, J.C.; Hall, J.; Tomkies, V.; Semmence, N.; Brown, M.; Wakefield, M.; Stainton, K. The Invasion, Provenance and Diversity of Vespa velutina Lepeletier (Hymenoptera: Vespidae) in Great Britain. PLoS ONE 2017, 12, e0185172. [Google Scholar] [CrossRef]
- Smit, J.; Noordijk, J.; Zeegers, T. Will the Asian Hornet (Vespa velutina) Settle in the Nederlands? Nederlandse Entomologische Vereniging (NEV): Amsterdam, The Netherlands, 2018. [Google Scholar]
- Apiservice le Frelon Asiatique se Propage en Suisse 2023. Available online: https://abeilles.ch/le-frelon-asiatique-se-propage-en-suisse/ (accessed on 1 August 2024).
- Ries, C.; Schneider, N.; Vitali, F.; Weigand, A. First Records and Distribution of the Invasive Alien Hornet Vespa velutina nigrithorax Du Buysson, 1905 (Hymenoptera: Vespidae) in Luxembourg. Bull. Soc. Nat. Luxemb. 2021, 123, 181–193. [Google Scholar]
- Walter, J.; Görner, T.; Šulda, L.; Bureš, J.; Myslík, Z.; Milička, R.; Bartoňová, A.S.; Beneš, J.; Biemann, O.; Brus, J. First Czech Record of the Asian Hornet (Vespa velutina) and a Climatic Prediction of Its Spread in the Czech Republic. Res. Sq. 2024. preprints. [Google Scholar] [CrossRef]
- Schorkopf, D.L.P.; Steube, C.; Pisecker, G.; Morawetz, L. First Record of the Asian Yellow-Legged Hornet (Vespa velutina Lepeletier, 1836) in Austria. Entomol. Austriaca 2024, 3, 1–12. [Google Scholar]
- European Commission Comission Implementing Regulation (EU) 2016/1141 of 13 July 2016 Adopting a List of Invasive Alien Species of Union Concern Pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council; European Union: Brussels, Belgium, 2016.
- Monceau, K.; Bonnard, O.; Moreau, J.; Thiéry, D. Spatial Distribution of Vespa velutina Individuals Hunting at Domestic Honeybee Hives: Heterogeneity at a Local Scale. Insect Sci. 2014, 21, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Laurino, D.; Lioy, S.; Carisio, L.; Manino, A.; Porporato, M. Vespa velutina: An Alien Driver of Honey Bee Colony Losses. Diversity 2019, 12, 5. [Google Scholar] [CrossRef]
- Requier, F.; Fournier, A.; Pointeau, S.; Rome, Q.; Courchamp, F. Economic Costs of the Invasive Yellow-Legged Hornet on Honey Bees. Sci. Total Environ. 2023, 898, 165576. [Google Scholar] [CrossRef]
- Feás, X.; Vidal, C.; Remesar, S. What We Know about Sting-Related Deaths? Human Fatalities Caused by Hornet, Wasp and Bee Stings in Europe (1994–2016). Biology 2022, 11, 282. [Google Scholar] [CrossRef]
- Cobey, S.; Lawrence, T.; Jensen, M. The Asian Giant Hornet: What the Public and Beekeepers Need to Know; Washington State University Extension: Pullman, WA, USA, 2020. [Google Scholar]
- Van Itterbeeck, J.; Feng, Y.; Zhao, M.; Wang, C.; Tan, K.; Saga, T.; Nonaka, K.; Jung, C. Rearing Techniques for Hornets with Emphasis on Vespa velutina (Hymenoptera: Vespidae): A Review. J. Asia Pac. Entomol. 2021, 24, 103–117. [Google Scholar] [CrossRef]
- Feás Sánchez, X.; Charles, R.J. Notes on the Nest Architecture and Colony Composition in Winter of the Yellow-Legged Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae), in Its Introduced Habitat in Galicia (NW Spain). Insects 2019, 10, 237. [Google Scholar] [CrossRef]
- Diéguez-Antón, A.; Escuredo, O.; Seijo, M.C.; Rodríguez-Flores, M.S. Embryo, Relocation and Secondary Nests of the Invasive Species Vespa velutina in Galicia (NW Spain). Animals 2022, 12, 2781. [Google Scholar] [CrossRef]
- Perrard, A.; Haxaire, J.; Rortais, A.; Villemant, C. Observations on the Colony Activity of the Asian Hornet Vespa velutina Lepeletier 1836 (Hymenoptera: Vespidae: Vespinae) in France. Ann. Société Entomol. Fr. 2009, 45, 119–127. [Google Scholar] [CrossRef]
- Sorvari, J. Social Wasp (Hymenoptera: Vespidae) Beer Trapping in Finland 2008-2012: A German Surprise. Entomol. Fenn. 2013, 24, 156–164. [Google Scholar] [CrossRef][Green Version]
- Wegner, G.S.; Jordan, K.K. Comparison of Three Liquid Lures for Trapping Social Wasps (Hymenoptera: Vespidae). J. Econ. Entomol. 2005, 98, 664–666. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the Biological and Physical Control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A Review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Bacandritsos, N.; Papanastasiou, I.; Saitanis, C.; Roinioti, E. Three Non-Toxic Insect Traps Useful in Trapping Wasps Enemies of Honey Bees. Bull. Insectology 2006, 59, 135–145. [Google Scholar]
- Monceau, K.; Bonnard, O.; Thiéry, D. Chasing the Queens of the Alien Predator of Honeybees: A Water Drop in the Invasiveness Ocean. Open J. Ecol. 2012, 2, 183–191. [Google Scholar] [CrossRef]
- Sánchez, O.; Arias, A. All That Glitters Is Not Gold: The Other Insects That Fall into the Asian Yellow-Legged Hornet Vespa velutina ‘Specific’Traps. Biology 2021, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Center for International Earth Science Information Network—CIESIN. Gridded Population of the World, Version 4 (GPWv4): Population Density Adjusted to Match 2015 Revision UN WPP Country Totals, Revision 11. 2020. Available online: https://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-density-adjusted-to-2015-unwpp-country-totals-rev11 (accessed on 1 August 2024).
- Carrer, D.; Roujean, J.-L.; Meurey, C. Comparing Operational MSG/SEVIRI Land Surface Albedo Products from Land SAF with Ground Measurements and MODIS. IEEE Trans. Geosci. Remote Sens. 2009, 48, 1714–1728. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R. Global Aridity Index and Potential Evapotranspiration (ET0) Climate Database V2. CGIAR Consort. Spat. Inf. 2019, 10, m9. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Davis, N.N.; Badger, J.; Hahmann, A.N.; Hansen, B.O.; Mortensen, N.G.; Kelly, M.; Larsén, X.G.; Olsen, B.T.; Floors, R.; Lizcano, G.; et al. The Global Wind Atlas: A High-Resolution Dataset of Climatologies and Associated Web-Based Application. Bull. Am. Meteorol. Soc. 2023, 104, E1507–E1525. [Google Scholar] [CrossRef]
- EEA. EU-Hydro River Network Database 2006–2012 (Vector); Europe—Version 1.3; EEA: Copenhagen, Denmark, 2020. [Google Scholar] [CrossRef]
- EEA. Copernicus DEM—Global and European Digital Elevation Model. 2019. Available online: https://spacedata.copernicus.eu/collections/copernicus-digital-elevation-model (accessed on 1 August 2024).
- EEA. Normalised Difference Vegetation Index 2020-Present (Raster 300 m), Global, 10-Daily—Version 2; 2024. Available online: https://land.copernicus.eu/en/products/vegetation/normalised-difference-vegetation-index-v2-0-300m (accessed on 1 August 2024).
- Lioy, S.; Laurino, D.; Capello, M.; Romano, A.; Manino, A.; Porporato, M. Effectiveness and Selectiveness of Traps and Baits for Catching the Invasive Hornet Vespa velutina. Insects 2020, 11, 706. [Google Scholar] [CrossRef]
- Rojas-Nossa, S.V.; Novoa, N.; Serrano, A.; Calviño-Cancela, M. Performance of Baited Traps Used as Control Tools for the Invasive Hornet Vespa velutina and Their Impact on Non-Target Insects. Apidologie 2018, 49, 872–885. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Seijo-Rodríguez, A.; Escuredo, O.; Seijo-Coello, M.d.C. Spreading of Vespa velutina in Northwestern Spain: Influence of Elevation and Meteorological Factors and Effect of Bait Trapping on Target and Non-Target Living Organisms. J. Pest Sci. 2019, 92, 557–565. [Google Scholar] [CrossRef]
- Wen, P.; Cheng, Y.-N.; Dong, S.-H.; Wang, Z.-W.; Tan, K.; Nieh, J.C. The Sex Pheromone of a Globally Invasive Honey Bee Predator, the Asian Eusocial Hornet, Vespa velutina. Sci. Rep. 2017, 7, 12956. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-N.; Wen, P.; Tan, K.; Darrouzet, E. Designing a Sex Pheromone Blend for Attracting the Yellow-Legged Hornet (Vespa velutina), a Pest in Its Native and Invasive Ranges Worldwide. Entomol. Gen. 2022, in press. [Google Scholar] [CrossRef]
- Dong, S.; Sun, A.; Tan, K.; Nieh, J.C. Identification of Giant Hornet Vespa Mandarinia Queen Sex Pheromone Components. Curr. Biol. 2022, 32, R211–R212. [Google Scholar] [CrossRef] [PubMed]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A New Invasive Predator of Honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar] [CrossRef]
- Cappa, F.; Cini, A.; Bortolotti, L.; Poidatz, J.; Cervo, R. Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats. Insects 2021, 12, 1037. [Google Scholar] [CrossRef]
- Rome, Q.; Perrard, A.; Muller, F.; Fontaine, C.; Quilès, A.; Zuccon, D.; Villemant, C. Not Just Honeybees: Predatory Habits of Vespa velutina (Hymenoptera: Vespidae) in France. In Proceedings of the Annales de la Société Entomologique de France (NS); Taylor & Francis: Abingdon, UK, 2021; Volume 57, pp. 1–11. [Google Scholar]
- Thiéry, D.; Doblas-Bajo, M.; Tourrain, Z.; Le Provost, G.; Núñez-Pérez, E. Electrical Traps, so Called Harps, Efficient and Selective against Vespa velutina Workers Predating on Hives. Entomol. Gen. 2023, 43, 5. [Google Scholar] [CrossRef]
- Oke, T.R. The Energetic Basis of the Urban Heat Island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Bessa, A.S.; Carvalho, J.; Gomes, A.; Santarém, F. Climate and Land-Use Drivers of Invasion: Predicting the Expansion of Vespa velutina nigrithorax into the Iberian Peninsula. Insect Conserv. Divers. 2016, 9, 27–37. [Google Scholar] [CrossRef]
- Vinson, M.R.; Hawkins, C.P. Broad-scale Geographical Patterns in Local Stream Insect Genera Richness. Ecography 2003, 26, 751–767. [Google Scholar] [CrossRef]
- Bailey, S.; Horner-Devine, M.C.; Luck, G.; Moore, L.A.; Carney, K.M.; Anderson, S.; Betrus, C.; Fleishman, E. Primary Productivity and Species Richness: Relationships among Functional Guilds, Residency Groups and Vagility Classes at Multiple Spatial Scales. Ecography 2004, 27, 207–217. [Google Scholar] [CrossRef]
- Herrera, C.; Jurado-Rivera, J.A.; Leza, M. Ensemble of Small Models as a Tool for Alien Invasive Species Management Planning: Evaluation of Vespa velutina (Hymenoptera: Vespidae) under Mediterranean Island Conditions. J. Pest Sci. 2023, 96, 359–371. [Google Scholar] [CrossRef]
- Requier, F.; Nürnberger, F.; Rojas-Nossa, S.V.; Rome, Q. Spatial Distribution of Vespa velutina-Mediated Beekeeping Risk in France and Germany. J. Pest Sci. 2024. [Google Scholar] [CrossRef]
- Huld, T.A.; Šúri, M.; Dunlop, E.D.; Micale, F. Estimating Average Daytime and Daily Temperature Profiles within Europe. Environ. Model. Softw. 2006, 21, 1650–1661. [Google Scholar] [CrossRef]
- Spångmyr, M.; Global Effects of Albedo Change Due to Urbanization. Lunds Universitets Naturgeografiska Institution-Seminarieuppsatser 2010. Available online: https://lup.lub.lu.se/luur/download?func=downloadFile&recordOId=1856904&fileOId=1856935 (accessed on 1 August 2024).



| Trap/Bait | 1 | 2 | 3 | 4 | F Test p-Value | |
|---|---|---|---|---|---|---|
| Fortnight | ||||||
| 1 February | 1.83 | 1.70 | 1.80 | 1.30 | >0.05 | |
| 2 February | 4.67 | 4.77 | 4.80 | 4.20 | >0.05 | |
| 1 March | 8.73 | 7.97 | 8.40 | 7.67 | >0.05 | |
| 2 March | 18.67 | 17.93 | 17.87 | 16.90 | >0.05 | |
| 1 April | 23.40 | 22.60 | 23.20 | 22.20 | >0.05 | |
| 2 April | 14.27 a | 16.77 b | 14.47 a | 16.17 b | >0.05 | |
| 1 May | 7.23 | 6.73 | 7.83 | 7.10 | >0.05 | |
| 2 May | 3.23 a,b | 3.03 a | 4.30 c | 3.90 b,c | >0.05 | |
| 1 June | 2.07 | 1.97 | 2.40 | 1.80 | >0.05 | |
| 2 June | 7.13 | 6.63 | 7.97 | 7.37 | >0.05 | |
| 1 July | 14.13 | 15.33 | 14.60 | 14.83 | >0.05 | |
| 2 July | 32.17 | 31.27 | 29.97 | 30.33 | >0.05 | |
| 1 August | 75.67 | 75.03 | 72.97 | 71.23 | >0.05 | |
| 2 August | 114.43 | 112.20 | 112.87 | 109.93 | >0.05 | |
| DV in the Model | Primary Workers | Secondary Workers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IV in the Model | |||||||||
| p-Value | AIC | β | Exp(β) | p-Value | AIC | β | Exp(β) | ||
| Ner of beehives | <0.001 | 11,399 | 0.058 *** | 1.060 | <0.001 | 82,827 | 0.075 *** | 1.078 | |
| Albedo | <0.001 | 462 | 0.003 *** | 1.003 | <0.001 | 3095 | 0.004 *** | 1.004 | |
| Aridity index | <0.001 | 477 | 3.444 *** | 31.326 | <0.001 | 2140 | 4.649 *** | 104.464 | |
| Distance to a main river | <0.001 | 8323 | 0.412 *** | 1.509 | <0.001 | 60,074 | 0.539 *** | 1.715 | |
| Elevation | <0.001 | 10,093 | 0.008 *** | 1.008 | <0.001 | 78,921 | 0.010 *** | 1.010 | |
| Mean annual precipitation | <0.001 | 273 | 0.003 *** | 1.003 | <0.001 | 1292 | 0.004 *** | 1.004 | |
| Precipitation seasonality | <0.001 | 253 | 0.900 *** | 1.094 | <0.001 | 647 | 0.122 *** | 1.130 | |
| Mean annual temperature | <0.001 | 340 | 0.034 *** | 1.034 | <0.001 | 1200 | 0.046 *** | 1.047 | |
| Temperature seasonality | <0.001 | 493 | 0.001 *** | 1.001 | <0.001 | 6098 | 0.001 *** | 1.001 | |
| NDVI | <0.001 | 375 | 0.025 *** | 1.025 | <0.001 | 2222 | 0.034 *** | 1.034 | |
| Population density | <0.001 | 15,129 | 0.09 *** | 1.009 | <0.001 | 108,247 | 0.012 *** | 1.012 | |
| Slope | <0.001 | 4126 | 0.294 *** | 1.342 | <0.001 | 32,493 | 0.386 *** | 1.471 | |
| Wind speed | <0.001 | 1359 | 0.738 *** | 2.091 | <0.001 | 11,346 | 0.983 *** | 2.674 | |
| N | Minimum | Maximum | Mean | Standard Deviation | |
|---|---|---|---|---|---|
| Ner | 30 | 1 | 100 | 33.830 | 28.122 |
| A | 30 | 1199.360 | 1692.380 | 1465.333 | 109.575 |
| AI | 30 | 1.091 | 1.420 | 1.291 | 0.096 |
| DR | 30 | 0.385 | 14.266 | 6.271 | 3.986 |
| E | 30 | 16.674 | 848.089 | 281.473 | 191.900 |
| MP | 30 | 1315.680 | 1446.880 | 1387.384 | 35.542 |
| MPS | 30 | 47.962 | 51.773 | 50.017 | 1.057 |
| MT | 30 | 116.157 | 140.953 | 132.790 | 7.398 |
| MTS | 30 | 3616.450 | 4668.230 | 4100.121 | 347.627 |
| NDVI | 30 | 147.664 | 195.797 | 180.142 | 10.265 |
| PD | 30 | 0.047 | 665.646 | 107.748 | 131.344 |
| S | 30 | 2.075 | 20.159 | 12.176 | 4.303 |
| WS | 30 | 3.922 | 7.549 | 5.704 | 0.912 |
| Ner | A | AI | DR | E | MP | MPS | MT | MTS | |
|---|---|---|---|---|---|---|---|---|---|
| DR | 0.447 * | ||||||||
| E | 0.471 ** | ||||||||
| MP | 0.449 * | ||||||||
| MPS | 0.672 ** | −0.431 * | −0.384 * | ||||||
| MT | −0.370 * | 0.662 ** | −0.665 ** | −0.434 * | 0.800 ** | ||||
| MTS | −0.801 ** | 0.540 ** | −0.894 ** | −0.903 ** | |||||
| PD | 0.418 * | −0.416 * | −0.367 * | ||||||
| WS | 0.401 * | 0.574 ** | 0.385 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, F.; Alonso, J.M.; Cano-Díaz, C. Evaluation of Asian Hornet (Vespa velutina) Trappability in Alto-Minho, Portugal: Commercial vs. Artisanal Equipment, Human Factors, Geography, Climatology, and Vegetation. Appl. Sci. 2024, 14, 7571. https://doi.org/10.3390/app14177571
Mata F, Alonso JM, Cano-Díaz C. Evaluation of Asian Hornet (Vespa velutina) Trappability in Alto-Minho, Portugal: Commercial vs. Artisanal Equipment, Human Factors, Geography, Climatology, and Vegetation. Applied Sciences. 2024; 14(17):7571. https://doi.org/10.3390/app14177571
Chicago/Turabian StyleMata, Fernando, Joaquim M. Alonso, and Concha Cano-Díaz. 2024. "Evaluation of Asian Hornet (Vespa velutina) Trappability in Alto-Minho, Portugal: Commercial vs. Artisanal Equipment, Human Factors, Geography, Climatology, and Vegetation" Applied Sciences 14, no. 17: 7571. https://doi.org/10.3390/app14177571
APA StyleMata, F., Alonso, J. M., & Cano-Díaz, C. (2024). Evaluation of Asian Hornet (Vespa velutina) Trappability in Alto-Minho, Portugal: Commercial vs. Artisanal Equipment, Human Factors, Geography, Climatology, and Vegetation. Applied Sciences, 14(17), 7571. https://doi.org/10.3390/app14177571







