Emergent and Known Honey Bee Pathogens through Passive Surveillance in the Republic of Kosovo
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Clinical Inspection of Samples
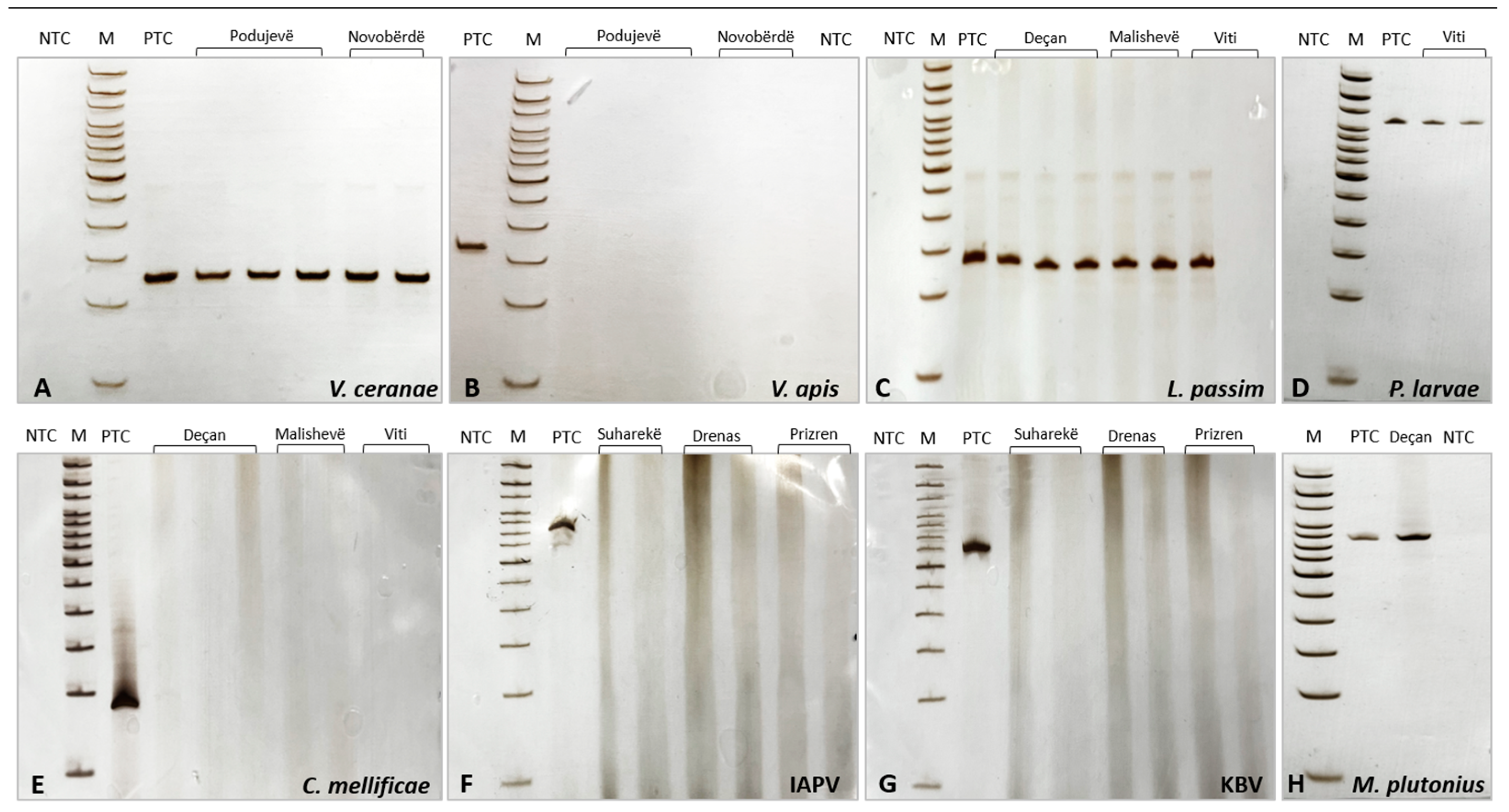
2.3. Vairimorpha spp. Spore Detection and Vairimorpha apis/Vairimorpha ceranae Species Identification
2.4. Lotmaria passim and Crithidia mellificae Detection
2.5. Honey Bee Viruses
2.6. Paenibacillus larvae (AFB) Detection by Field Tests, Isolation, and PCR
2.7. Genotyping of Paenibacillus larvae Isolates
2.8. Melissococcus plutonius Detection by Field Tests and PCR
3. Results
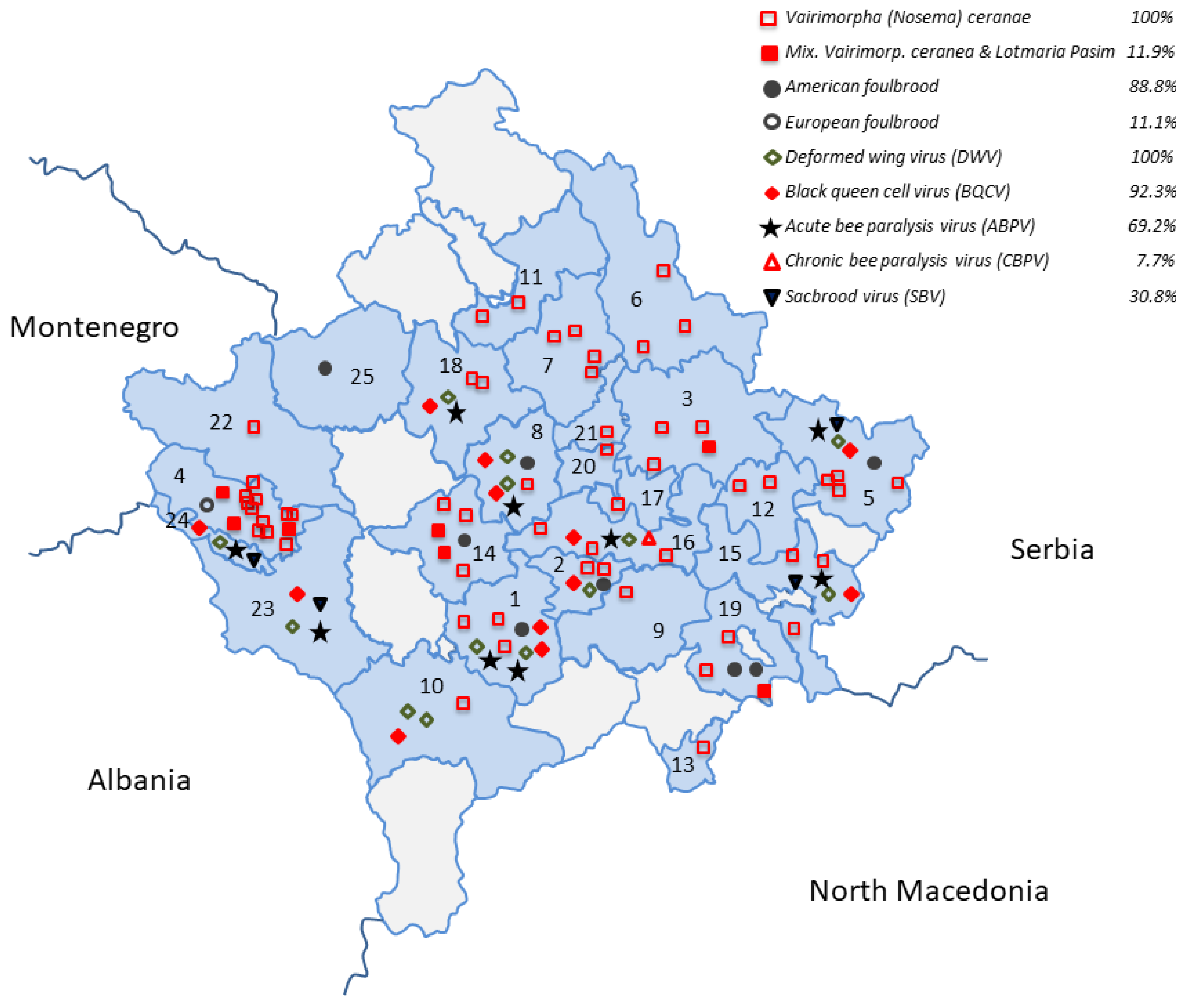
3.1. Group 1—Vairimorpha spp. and Trypanosomatids L. passim and C. mellificae
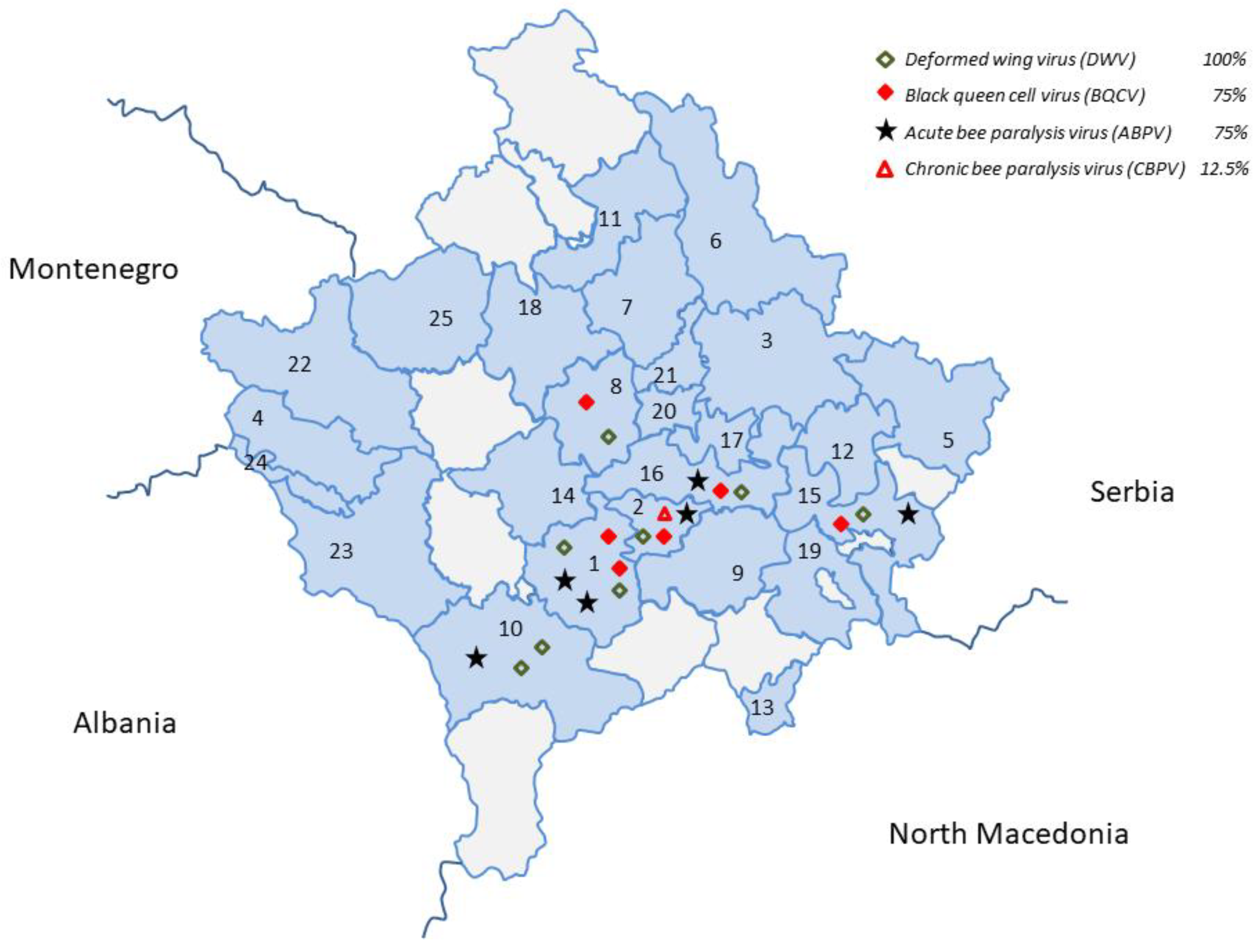
3.2. Group 2—Honey Bee Viruses (ABPV, CBPV, DWV, SBV, BQCV, KBV, and IAPV)
3.3. Group 3—Paenibacillus larvae and Melissococcus plutonius Detection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, Y.S.; Huang, W.-F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invert. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.; Zhou, L.; Boncristiani, H.; Kimura, K.; Xiao, T.; Litkowski, A.; Pettis, J.S. Asymmetrical coexistence of Nosema ceranae and Nosema apis in honey bees. J. Invert. Pathol. 2009, 101, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Galajda, R.; Valenčáková, A.; Sučik, M.; Kandráčová, P. Nosema Disease of European Honey Bees. J. Fungi 2021, 7, 714. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Garcia-Palencia, P.; Martin-Hernandez, R.; Meana, A. Experimental infection of Apis mellifera honey bees with Nosema ceranae (Microsporidia). J. Invert. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honey bee Apis mellifera from Nosema ceranae infection. J. Invert. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honey bees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Bauchan, G.R.; Murphy, C.A.; Ravoet, J.; de Graaf, D.C.; Evans, J.D. Characterization of Two Species of Trypanosomatidae from the Honey Bee Apis mellifera: Crithidia mellificae Langridge and McGhee, and Lotmaria passim n. gen., n. sp. J. Eukaryot. Microbiol. 2015, 62, 567–583. [Google Scholar] [CrossRef]
- Arismendi, N.; Caro, S.; Castro, M.P.; Vargas, M.; Riveros, G.; Venegas, T. Impact of Mixed Infections of Gut Parasites Lotmaria passim and Nosema ceranae on the Lifespan and Immune-related Biomarkers in Apis mellifera. Insects 2020, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; De Risi, J.L. Temporal Analysis of the Honey Bee Microbiome Reveals Four Novel Viruses and Seasonal Prevalence of Known Viruses, Nosema, and Crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef]
- Ravoet, J.; Maharramov, J.; Meeus, I.; De Smet, L.; Wenseleers, T.; Smagghe, G.; de Graaf, D.C. Comprehensive Bee Pathogen Screening in Belgium Reveals Crithidia mellificae as a New Contributory Factor to Winter Mortality. PLoS ONE 2013, 8, e72443. [Google Scholar] [CrossRef]
- Schwarz, R.S.; Evans, J.D. Single and mixed-species trypanosome and microsporidia infections elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev. Comp. Immunol. 2013, 40, 300–310. [Google Scholar] [CrossRef]
- Strobl, V.; Yañez, O.; Straub, L.; Albrecht, M.; Neumann, P. Trypanosomatid parasites infecting managed honey bees and wild solitary bees. Int. J. Parasitol. 2019, 49, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.V.; Allen, M.F. The prevalence of pathogens in honey bee colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 1988, 113, 237–244. [Google Scholar] [CrossRef]
- Allen, M.; Ball, B. The incidence and world distribution of the honey bee viruses. Bee World 1996, 77, 141–162. [Google Scholar] [CrossRef]
- Martin, S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modeling approach. J. Appl. Ecol. 2001, 38, 1082–1093. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef]
- Shen, M.; Cui, L.; Ostiguy, N.; Cox-Foster, D. Intricate transmission routes and interactions between picorna-like viruses (Kashmir bee virus and sacbrood virus) with the honey bee host and the parasitic Varroa mite. J. Gen. Virol. 2005, 86, 2281–2289. [Google Scholar] [CrossRef]
- Chantawannakul, P.; Ward, L.; Boonham, N.; Brown, M. A scientific note on the detection of honey bee viruses using real-time PCR (TaqMan) in Varroa mites collected from a Thai honey bee (Apis mellifera) apiary. J. Invert. Pathol. 2006, 91, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Celle, O.; Blanchard, P.; Olivier, V.; Schurr, F.; Cougoule, N.; Faucon, J.-P.; Ribiere, M. Detection of Chronic bee paralysis virus (CBPV) genome and its replicative RNA form in various hosts and possible ways of spread. Virus Res. 2008, 133, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Nielsen, S.L.; Kryger, P. Varroa-virus interaction in collapsing honey bee colonies. PLoS ONE 2013, 8, e57540. [Google Scholar] [CrossRef] [PubMed]
- Mondet, F.; de Miranda, J.R.; Kretzschmar, A.; Le Conte, Y.; Mercer, A.R. On the front line: Quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathog. 2014, 10, e1004323. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Nat. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef]
- Valles, S.M.; Chen, Y.P.; Firth, A.E.; Guerin, D.M.A.; Hashimoto, Y.; Herrero, S.; de Miranda, J.R.; Ryabov, E.; ICTV Report Consortium. ICTV virus taxonomy profile: Iflaviridae. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef]
- McMenamin, A.J.; Flenniken, M.L. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect. Sci. 2018, 26, 120–129. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Two viruses from adult honey bees (Apis mellifera Linnaeus). Virology 1963, 21, 390–395. [Google Scholar] [CrossRef]
- Maori, E.; Lavi, S.; Mozes-Koch, R.; Gantman, Y.; Peretz, Y.; Edelbaum, O.; Tanne, E.; Sela, I. Isolation and characterization of Israeli acute paralysis virus, a dicistrovirus affecting honey bees in Israel: Evidence for diversity due to intra- and inter-species. J. Gen. Virol. 2007, 88, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Ribière, M.; Ball, B.; Aubert, M. Natural history and geographical distribution of honey bee viruses. In Virology and the Honey Bee; Aubert, M., Ball, B., Fries, I., Moritz, R., Milani, N., Bernardinelli, I., Eds.; European Communities: Luxembourg, 2008; pp. 15–84. [Google Scholar]
- Anderson, D.L.; Gibbs, A.J. Inapparent virus infections and their interactions in pupae of the honey bee (Apis mellifera L.) in Australia. J. Gen. Virol. 1988, 69, 1617–1625. [Google Scholar] [CrossRef]
- Dall, D.J. Inapparent infection of honey bee pupae by Kashmir and Sacbrood bee viruses in Australia. Ann. Appl. Biol. 1985, 106, 461–468. [Google Scholar] [CrossRef]
- De Miranda, J.; Cordoni, G.; Budge, G. The Acute bee paralysis virus–Kashmir bee virus–Israeli acute paralysis virus complex. J. Invert. Pathol. 2010, 103 (Suppl. S1), S30–S47. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. Recent research on honey bee viruses. Bee World 1975, 56, 55–64. [Google Scholar] [CrossRef]
- Bailey, L.; Gibbs, A.J.; Woods, R.D. Sacbrood virus of the larval honey bee (Apis mellifera Linnaeus). Virology 1964, 23, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Siede, R. Honey bee viruses. Adv. Virus Res. 2007, 70, 33–80. [Google Scholar] [CrossRef]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Fürst, M.; Weging, S.; Brown, M.J.F.; Gogol-Döring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. Biol. Sci. 2016, 283, 20160811. [Google Scholar] [CrossRef]
- Paxton, R.J.; Schäfer, M.O.; Nazzi, F.; Zanni, V.; Annoscia, D.; Marroni, F.; Bigot, D.; Laws-Quinn, E.R.; Panziera, D.; Jenkins, C.; et al. Epidemiology of a major honey bee pathogen, deformed wing virus: Potential worldwide replacement of genotype A by genotype B. Int. J. Parasitol. Parasites Wildl. 2022, 18, 157–171. [Google Scholar] [CrossRef]
- Bailey, L. Ætiology of European foulbrood; a disease of the larval honey-bee. Nature 1956, 178, 1130. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V. Honey Bee Pathology; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Ellis, J.D.; Munn, P.A. The worldwide health status of honey bees. Bee World 2005, 86, 88–101. [Google Scholar] [CrossRef]
- Genersch, E. Honey bee pathology: Current threats to honey bees and beekeeping. Appl. Microbiol. Biot. 2010, 87, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hulaj, B.; Goga, I.; Cana, A.; Merovci, X.; Rossi, F.; Crudele, S.; Ricchiuti, L.; Mutinelli, F. Passive surveillance of American foulbrood in the Republic of Kosovo: Geographic distribution and genotype characterization. J. Apicul. Res. 2023, 62, 320–325. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef] [PubMed]
- Bordin, F.; Zulian, L.; Granato, A.; Caldon, M.; Colamonico, R.; Toson, M.; Trevisan, L.; Biasion, L.; Mutinelli, F. Presence of Known and Emerging Honey Bee Pathogens in Apiaries of Veneto Region (Northeast of Italy) during Spring 2020 and 2021. Appl. Sci. 2022, 12, 2134. [Google Scholar] [CrossRef]
- Bartolomé, C.; Buendía-Abad, M.; Benito, M.; De la Rua, P.; Ornosa, C.; Martín-Hernández, R.; Higes, M.; Maside, X. A new multiplex PCR protocol to detect mixed trypanosomatid infections in species of Apis and Bombus. J. Invert. Pathol. 2018, 154, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Martinello, M.; Baratto, C.; Manzinello, C.; Piva, E.; Borin, A.; Toson, M.; Granato, A.; Boniotti, M.B.; Gallina, A.; Mutinelli, F. Honey bee spring mortality in the north-east of Italy: Detection of pesticides and viruses in dead honey bees and other matrices. J. Apicult. Res. 2017, 56, 239–254. [Google Scholar] [CrossRef]
- World Organisation for Animal Health (WOAH). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2023. American Foulbrood of Honey Bees (Infection of Honey Bees with Paenibacillus larvae), Chapter 3.2.2 (Version Adopted in 2023). Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.02.02_AMERICAN_FOULBROOD.pdf (accessed on 18 May 2023).
- Dobbelaere, W.; de Graaf, D.C.; Peeters, J.E. Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 2001, 32, 363–370. [Google Scholar] [CrossRef]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef]
- Govan, V.A.; Brozel, V.; Allsopp, M.H.; Davison, S. A PCR detection method for rapid identification of Melissococcus pluton in honey bee larvae. Appl. Environ. Microbiol. 1998, 64, 1983–1985. [Google Scholar] [CrossRef]
- Genersch, E.; Von Der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S. The German Bee Monitoring Project: A Long Term Study to Understand Periodically High Winter Losses of Honey Bee Colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Genersch, E.; Aubert, M. Emerging and Re-Emerging Viruses of the Honey Bee (Apis mellifera L.). Vet. Res. 2010, 41, 54. [Google Scholar] [CrossRef] [PubMed]
- Papini, R.; Mancianti, F.; Canovai, R.; Cosci, F.; Rocchigiani, G.; Benelli, G.; Canale, A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017, 24, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Vugrek, O.; Grilec, D.; Petrinec, Z. Prevalence and distribution of Nosema ceranae in Croatian honey bee colonies. Vet. Med. 2010, 55, 457–462. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Genersch, E.; Kovacevic, S.R.; Ljubenkovic, J.; Radakovic, M.; Aleksic, N. Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie 2011, 42, 49–58. [Google Scholar] [CrossRef]
- Stevanovic, J.; Simeunovic, P.; Gajic, B.; Lakic, N.; Radovic, D.; Fries, I.; Stanimirovic, Z. Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 2013, 44, 522–536. [Google Scholar] [CrossRef]
- Shumkova, R.; Georgieva, A.; Radoslavov, G.; Sirakova, D.; Dzhebir, G.; Neov, B.; Bouga, M.; Hristov, P. The first report of the prevalence of Nosema ceranae in Bulgaria. PeerJ 2018, 6, e4252. [Google Scholar] [CrossRef]
- Matović, K.; Vidanović, D.; Manić, M.; Stojiljković, M.; Radojićić, S.; Debeljak, Z.; Šekler, M.; Ćirić, J. Twenty-five-year study of Nosema spp. in honey bees (Apis mellifera) in Serbia. Saudi J. Biol. Sci. 2020, 27, 518–523. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invert. Pathol. 2010, 103 (Suppl. S1), 73. [Google Scholar] [CrossRef]
- Bromenshenk, J.J.; Henderson, C.B.; Wick, C.H.; Stanford, M.F.; Zulich, A.W.; Jabbour, R.E.; Deshpande, S.V.; McCubbin, P.E.; Seccomb, R.A.; Welch, P.M.; et al. Iridovirus and microsporidian linked to honey bee colony decline. PLoS ONE 2010, 5, e13181. [Google Scholar] [CrossRef]
- Alaux, C.; Folschweiller, M.; McDonnell, C.; Beslay, D.; Cousin, M.; Dussaubat, C.; Brunet, J.L.; Le Conte, Y. Pathological effects of the microsporidium Nosema ceranae on honey bee queen physiology (Apis mellifera). J. Invert. Pathol. 2011, 106, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Grupe, A.C.; Quandt, C.A. A Growing Pandemic: A Review of Nosema Parasites in Globally Distributed Domesticated and Native Bees. PLoS Pathog. 2020, 16, e1008580. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Shutler, D.; Burgher-MacLellan, K.L.; Rogers, R.E.L. Infra-Population and community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS ONE 2014, 9, e99465. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Schwarz, R.S.; Vejnovic, B.; Evans, J.D.; Irwin, R.E.; Glavinic, U.; Stanimirovic, Z. Species-specific diagnostics of Apis mellifera trypanosomatids: A nine-year survey (2007–2015) for trypanosomatids and microsporidians in Serbian honey bees. J. Invert. Pathol. 2016, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Berényi, O.; Bakonyi, T.; Derakhshifar, I.; Köglberger, H.; Nowotny, N. Occurrence of Six Honey bee Viruses in Diseased Austrian Apiaries. Appl. Environ. Microbiol. 2006, 72, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Bacandritsos, N.; Granato, A.; Budge, G.; Papanastasiou, I.; Roinioti, E.; Caldon, M.; Falcaro, C.; Gallina, A.; Mutinelli, F. Sudden Deaths and Colony Population Decline in Greek Honey Bee Colonies. J. Invert. Pathol. 2010, 105, 335–340. [Google Scholar] [CrossRef]
- Cirkovic, D.; Stevanovic, J.; Glavinic, U.; Aleksic, N.; Djuric, S.; Aleksic, J.; Stanimirovic, Z. Honey bee viruses in Serbian colonies of different strength. PeerJ 2018, 6, e5887. [Google Scholar] [CrossRef]
- Milićević, V.; Radojičić, S.; Kureljušić, J.; Šekler, M.; Nešić, M.; Veljović, L.; Zorić, J.M.; Radosavljević, V. Molecular detection of black queen cell virus and Kashmir bee virus in honey. AMB Express 2018, 8, 128. [Google Scholar] [CrossRef]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of deformed wing virus between honey bees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invert. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Pior, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Shafiey, H.; Paxton, R.J. Transcriptomic Responses Underlying the High Virulence of Black Queen Cell Virus and Sacbrood Virus following a Change in Their Mode of Transmission in Honey Bees (Apis mellifera). Viruses 2023, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
| Municipality | Group 1 | Group 2 | Group 3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vairimorpha spp. and Trypanosomatids | Honey Bee Viruses | American and European Foulbrood | ||||||||||||||||
| N. of Sampled Apiaries | N. of Apiaries with Positive Results | N. of Sampled Apiaries | N. of Apiaries with Positive Results (Honey Bee Samples at Different Stages of Development) | N. of Sampled Apiaries | N. of Apiaries with Positive Results (Varroa Mite Samples) | N. of Sampled Apiaries | N. of Apiaries with Positive Results | |||||||||||
| Vairimorpha ceranae | Lotmaria passim | ABPV | CBPV | DWV | BQCV | SBV | ABPV | CBPV | DWV | BQCV | AFB | EFB | ||||||
| 1 | Suharekë | 3 | 3 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 1 | 1 | nt |
| 2 | Shtime | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | nt |
| 3 | Prishtinë | 4 | 4 | 1 | ns | ns | ns | |||||||||||
| 4 | Deҫan | 14 | 14 | 3 | ns | ns | 1 | nt | 1 | |||||||||
| 5 | Kamenicë | 4 | 4 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | ns | 1 | 1 | nt | ||||
| 6 | Podujevë | 3 | 3 | 0 | ns | ns | ns | |||||||||||
| 7 | Vushtri | 4 | 4 | 0 | ns | ns | ns | |||||||||||
| 8 | Drenas | 1 | 1 | 0 | 2 | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | nt |
| 9 | Ferizaj | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 10 | Prizren | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | ns | ||
| 11 | Mitrovicë | 2 | 2 | 0 | ns | ns | ns | |||||||||||
| 12 | Novobërdë | 2 | 2 | 0 | ns | ns | ns | |||||||||||
| 13 | Hani i Elezit | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 14 | Malishevë | 3 | 3 | 2 | ns | ns | 1 | 1 | nt | |||||||||
| 15 | Gjilan | 3 | 3 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ns | ||
| 16 | Lipjan | 3 | 3 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | ns | ||
| 17 | Graqanicë | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 18 | Skenderaj | 2 | 2 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | ns | ns | ||||||
| 19 | Viti | 2 | 2 | 1 | ns | ns | 2 | 2 | nt | |||||||||
| 20 | Fushë Kosovë | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 21 | Obiliq | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 22 | Pejë | 1 | 1 | 0 | ns | ns | ns | |||||||||||
| 23 | Gjakovë | ns | 1 | 1 | 0 | 1 | 1 | 1 | ns | ns | ||||||||
| 24 | Junik | ns | 1 | 1 | 0 | 1 | 1 | 1 | ns | ns | ||||||||
| 25 | Istog | ns | ns | ns | 1 | 1 | nt | |||||||||||
| Total apiaries sampled per group | 59 | 13 | 8 | 9 | ||||||||||||||
| Total pathogens detected per group | 59 | 7 | 9 | 1 | 13 | 12 | 4 | 6 | 1 | 8 | 6 | 8 | 1 | |||||
| % of infections | 100 | 11.9 | 69.2 | 7.7 | 100 | 92.3 | 30.8 | 75 | 12.5 | 100 | 75 | |||||||
| % of infection in honey bees and Varroa together | 84.6 | 15.4 | 100 | 92.3 | 30.8 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulaj, B.; Granato, A.; Bordin, F.; Goga, I.; Merovci, X.; Caldon, M.; Cana, A.; Zulian, L.; Colamonico, R.; Mutinelli, F. Emergent and Known Honey Bee Pathogens through Passive Surveillance in the Republic of Kosovo. Appl. Sci. 2024, 14, 987. https://doi.org/10.3390/app14030987
Hulaj B, Granato A, Bordin F, Goga I, Merovci X, Caldon M, Cana A, Zulian L, Colamonico R, Mutinelli F. Emergent and Known Honey Bee Pathogens through Passive Surveillance in the Republic of Kosovo. Applied Sciences. 2024; 14(3):987. https://doi.org/10.3390/app14030987
Chicago/Turabian StyleHulaj, Beqë, Anna Granato, Fulvio Bordin, Izedin Goga, Xhavit Merovci, Mauro Caldon, Armend Cana, Laura Zulian, Rosa Colamonico, and Franco Mutinelli. 2024. "Emergent and Known Honey Bee Pathogens through Passive Surveillance in the Republic of Kosovo" Applied Sciences 14, no. 3: 987. https://doi.org/10.3390/app14030987
APA StyleHulaj, B., Granato, A., Bordin, F., Goga, I., Merovci, X., Caldon, M., Cana, A., Zulian, L., Colamonico, R., & Mutinelli, F. (2024). Emergent and Known Honey Bee Pathogens through Passive Surveillance in the Republic of Kosovo. Applied Sciences, 14(3), 987. https://doi.org/10.3390/app14030987








