Structural Particularities, Prediction, and Synthesis Methods in High-Entropy Alloys
Abstract
:1. Introduction
2. Structural Particularities of HEAs, Prediction and Estimation Concepts
2.1. Definition and Composition
2.2. Microstructural Stability
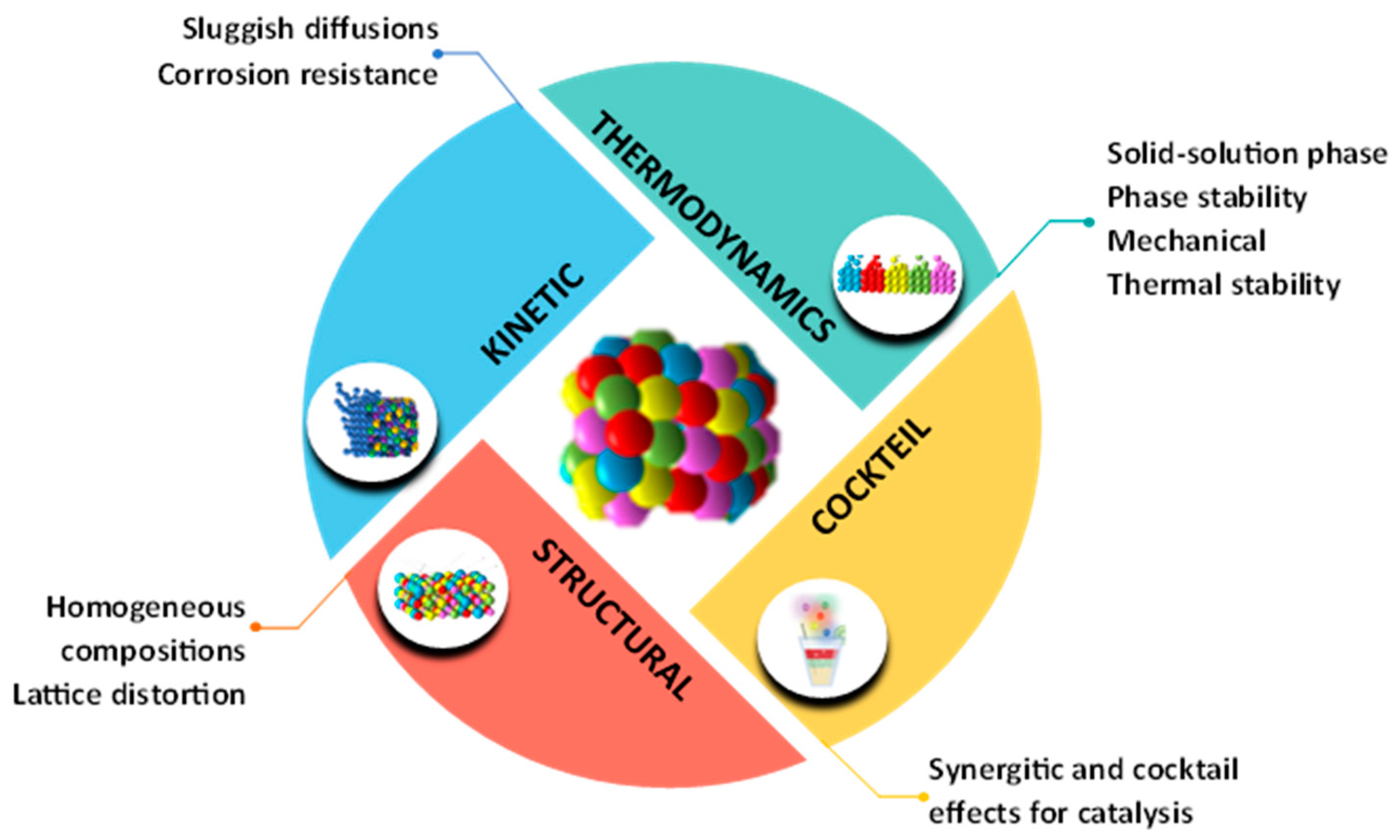
2.2.1. High-Entropy Effect
2.2.2. Severe Lattice Distortion
2.2.3. Slow Diffusion Effect
2.2.4. Cocktail Effect
2.3. Prediction and Estimation Concepts
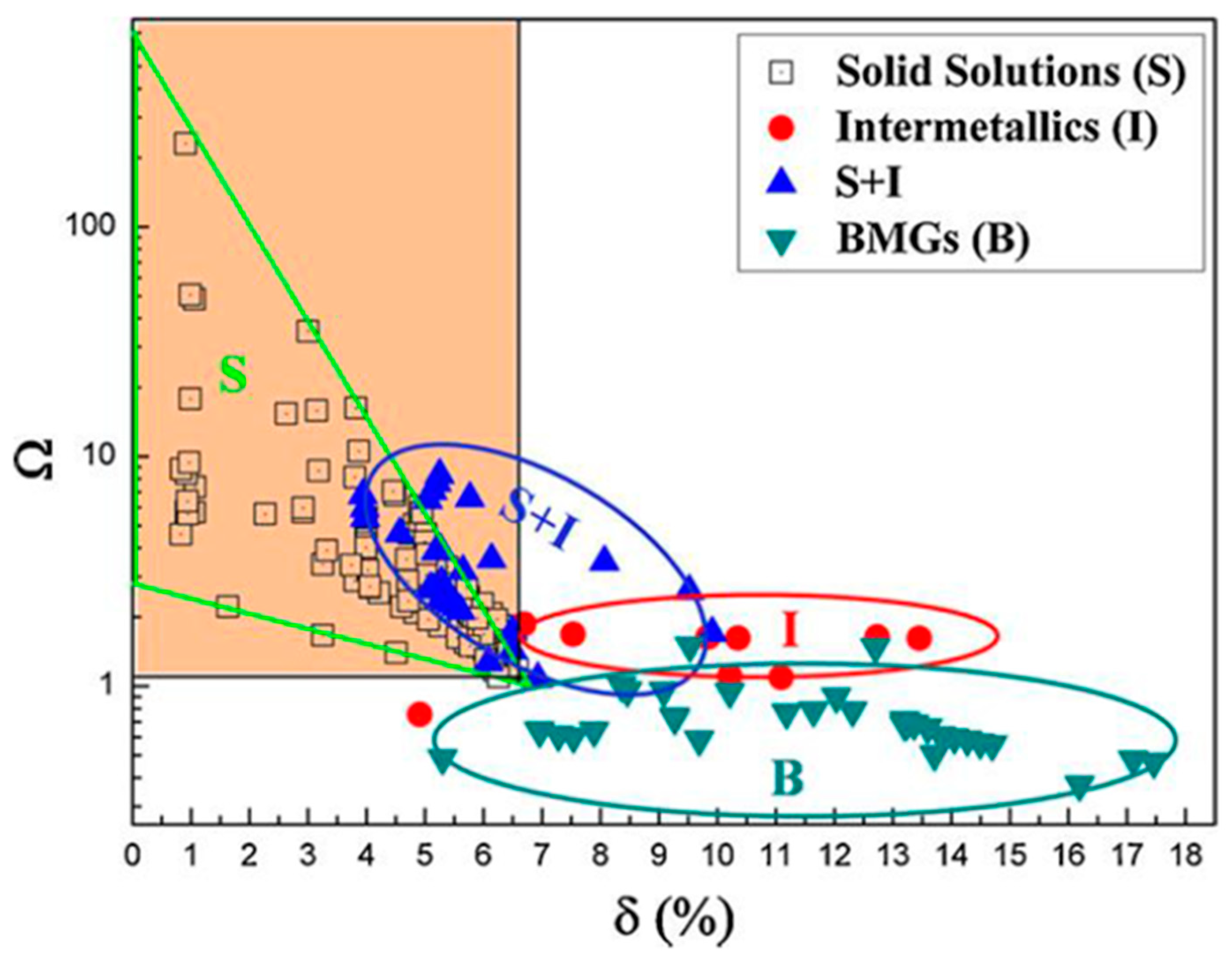
2.3.1. Gibbs Law
- ri—the atomic radius of each element;
- χi—electronegativity of each component element;
- VECi—valence electron concentration for each element i;
- ΔHi,j—binary enthalpy;
- Tm,i—melting point of element i;
2.3.2. CALPHAD Method
3. Synthesis Methods and Their Influence on the Structure and Properties of HEA Alloys
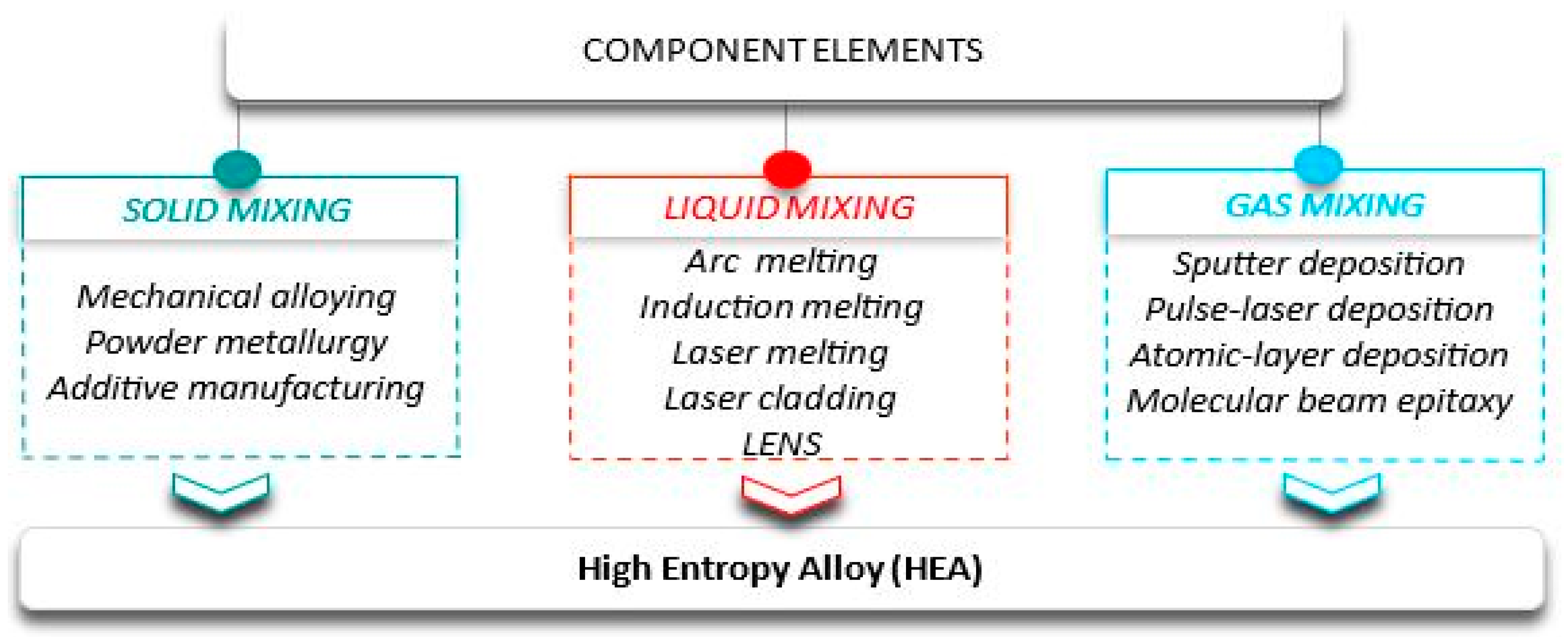
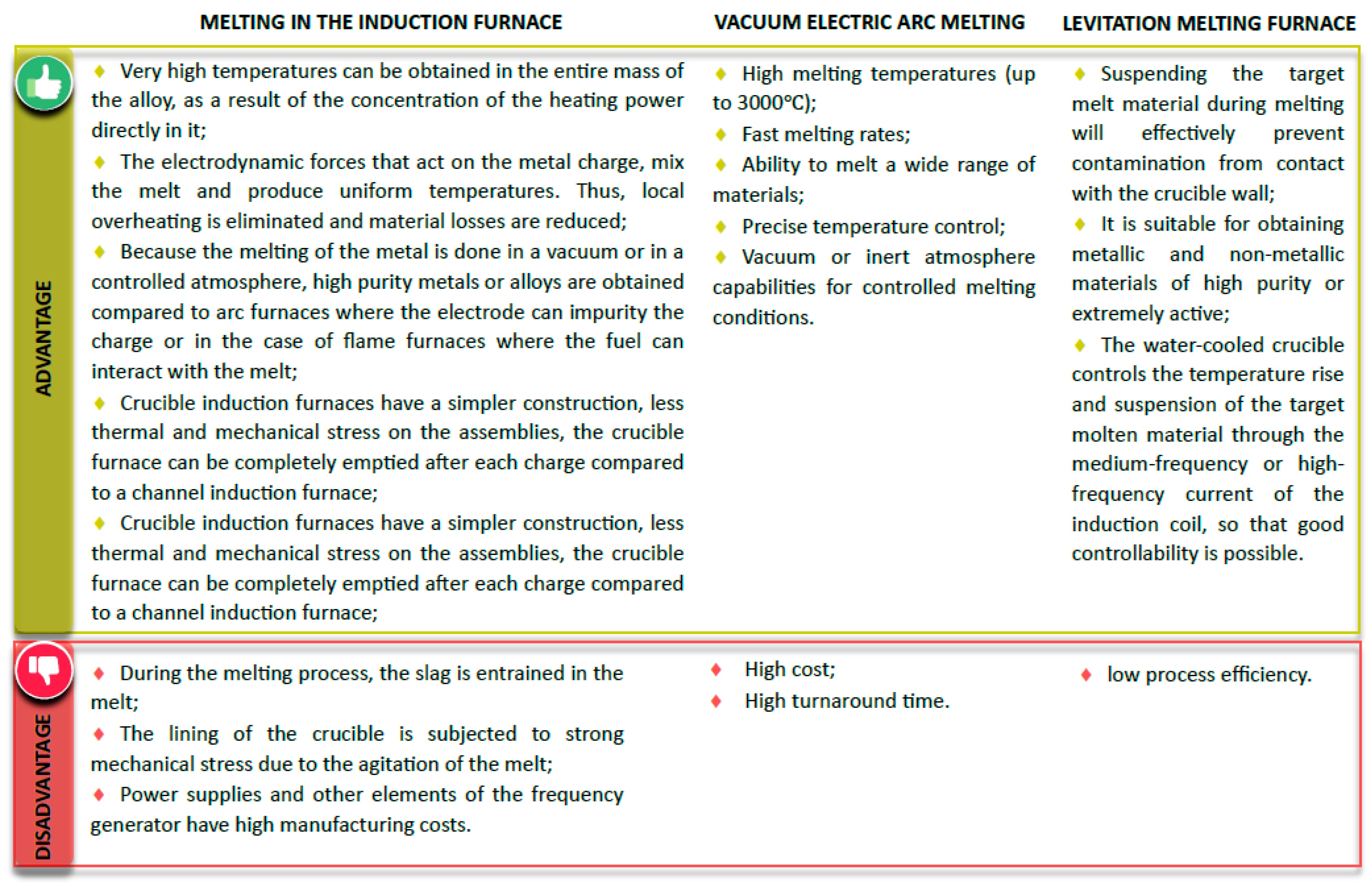
3.1. Synthesis Methods for HEA Alloys
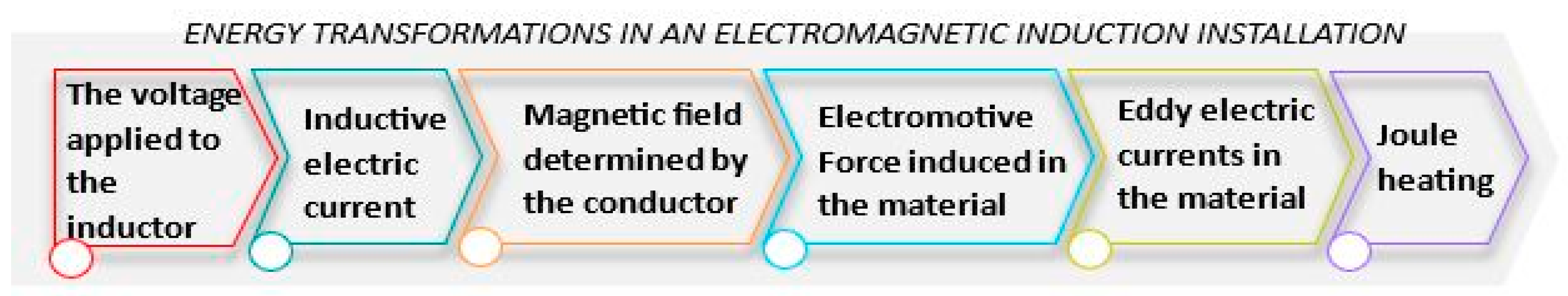
- The transfer of electromagnetic energy from the coil to the body to be heated;
- The generation of heat in the material as a result of the Joule effect;
- The transfer of heat through thermal conduction throughout the entire mass of the body.
- Easily fusible metals, which melt at temperatures below 500 °C (e.g., Sn, Pb, Cd, Zn, etc.)
- Metals with medium melting temperatures, ranging from 500 °C to 1000 °C (e.g., Al, Mg, Sb, Ag, etc.)
- Metals with high melting temperatures, ranging from 1000 °C to 1500 °C (e.g., Cu, Au, Ni, Co, Mn, etc.)
- Refractory metals, which have melting temperatures above 1500 °C (e.g., Cr, Zr, Hf, Ti, W, Mo, etc.)
3.2. Influence of the Synthesis Method on the Structure and Properties of HEA Alloys
3.2.1. Influence on the Microstructure of Different HEA Alloys
3.2.2. Influence on the Mechanical Properties of Different HEA Alloys
4. Conclusions
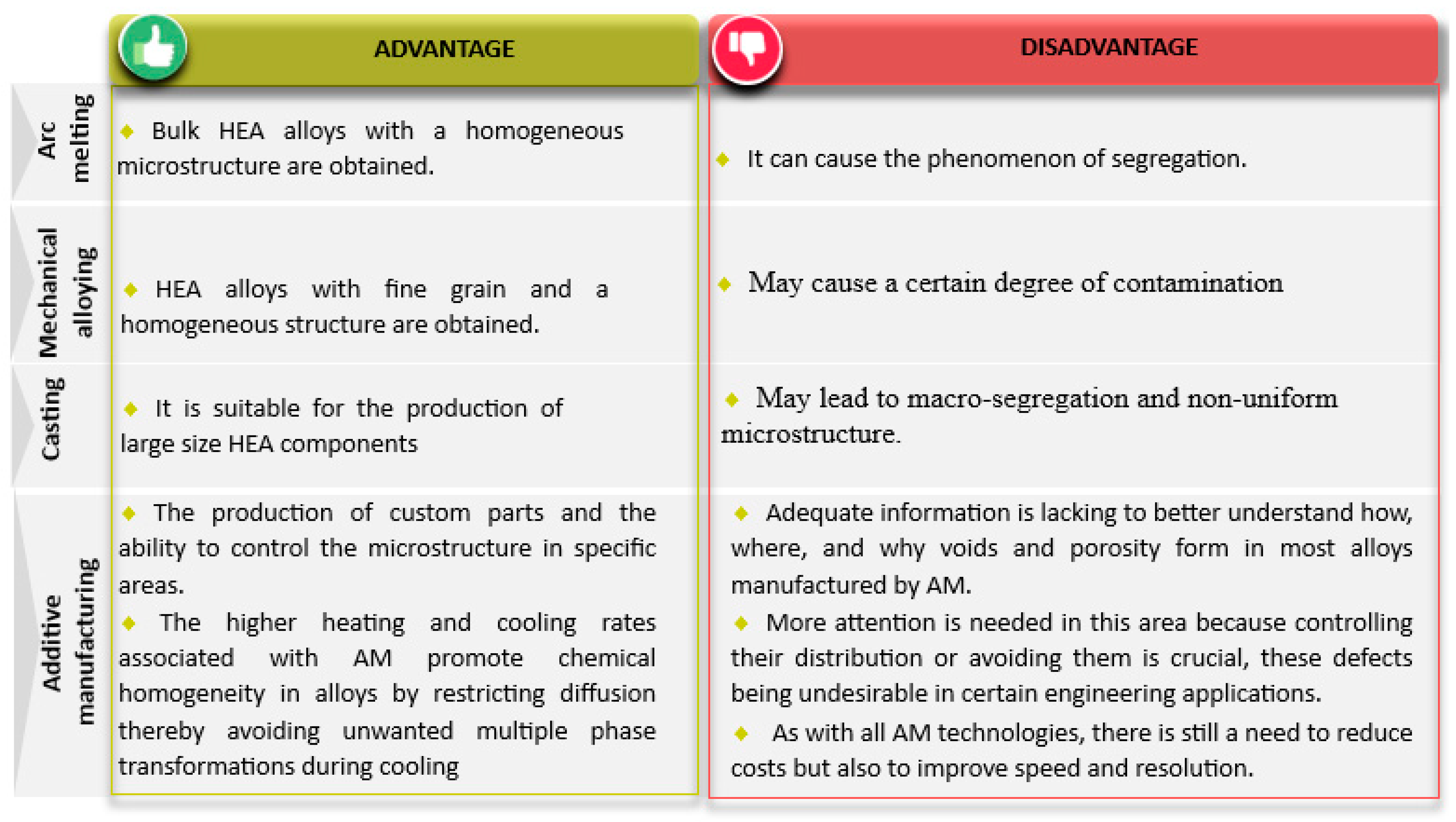
- Microstructural Impact: The choice of synthesis method leads to diverse microstructures. For example, direct laser synthesis and arc melting produce different textures and structures, such as dendritic and Widmanstätten, due to varying solidification conditions.
- Phase Evolution: The evolution of phases in HEAs is influenced by parameters like grinding duration, ball-to-powder ratio, initial structure, and mixing order during mechanical alloying and spark plasma sintering. Similarly, cooling rates and local atomic arrangements during melting and casting impact phase stability and microstructure.
- Mechanical Properties: The mechanical properties, including hardness, ductility, and tensile strength, are significantly influenced by the synthesis method. Additive manufacturing techniques like selective laser melting (SLM) and laser powder bed fusion (LPBF) enhance these properties through rapid cooling rates and refined microstructures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Overview of High-Entropy Alloys. In High-Entropy Alloys: Fundamentals and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–19. ISBN 9783319270135. [Google Scholar]
- Anamu, U.S.; Ayodele, O.O.; Olorundaisi, E.; Babalola, B.J.; Odetola, P.I.; Ogunmefun, A.; Ukoba, K.; Jen, T.C.; Olubambi, P.A. Fundamental Design Strategies for Advancing the Development of High Entropy Alloys for Thermo-Mechanical Application: A Critical Review. J. Mater. Res. Technol. 2023, 27, 4833–4860. [Google Scholar] [CrossRef]
- Kattner, U.R. The CALPHAD Method and Its Role in Material and Process Development. Tecnol. Metal. Mater. Mineração 2016, 13, 3–15. [Google Scholar] [CrossRef]
- Onawale, O.T.; Cobbinah, P.V.; Nzeukou, R.A.; Matizamhuka, W.R. Synthesis Route, Microstructural Evolution, and Mechanical Property Relationship of High-Entropy Alloys (HEAs): A Review. Materials 2021, 14, 3065. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.; Li, X. Recent Advances on Environmental Corrosion Behavior and Mechanism of High-Entropy Alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Postolnyi, B.; Buranich, V.; Smyrnova, K.; Araújo, J.P.; Rebouta, L.; Pogrebnjak, A.; Rogoz, V. Multilayer and High-Entropy Alloy-Based Protective Coatings for Solving the Issue of Critical Raw Materials in the Aerospace Industry. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1024, 012009. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent Progress in High-Entropy Alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Ranganathan, S. Alloyed Pleasures: Multimetallic Cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Gondhalekar, A.A. Design and Development of Light Weight High Entropy Alloys. Master’s Thesis, JÖNKÖPING University, Jönköping, Sweden, 2019. [Google Scholar]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and Development of High Entropy Alloys for Structural Applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous Decrease in X-Ray Diffraction Intensities of Cu-Ni-Al-Co-Cr-Fe-Si Alloy Systems with Multi-Principal Elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Tsai, K.Y.; Tsai, M.H.; Yeh, J.W. Sluggish Diffusion in Co-Cr-Fe-Mn-Ni High-Entropy Alloys. Acta Mater. 2013, 61, 4887–4897. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent Progress in High-Entropy Alloys. In Proceedings of the Presentation at Changsha Meeting, Changsha, China, 2011. [Google Scholar]
- Paul, T.R.; Belova, I.V.; Murch, G.E. Analysis of Diffusion in High Entropy Alloys. Mater. Chem. Phys. 2018, 210, 301–308. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Danielewski, M. State-of-the-Art Diffusion Studies in the High Entropy Alloys. Metals 2020, 10, 347. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Bagdasaryan, A.A.; Yakushchenko, I.V.; Beresnev, V.M. The Structure and Properties of High-Entropy Alloys and Nitride Coatings Based on Them. Russ. Chem. Rev. 2014, 83, 1027–1061. [Google Scholar] [CrossRef]
- Sonar, T.; Ivanov, M.; Trofimov, E.; Tingaev, A.; Suleymanova, I. An Overview of Microstructure, Mechanical Properties and Processing of High Entropy Alloys and Its Future Perspectives in Aeroengine Applications. Mater. Sci. Energy Technol. 2024, 7, 35–60. [Google Scholar] [CrossRef]
- Balaji, V.; Anthony Xavior, M. Development of High Entropy Alloys (HEAs): Current Trends. Heliyon 2024, 10, e26464. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Yang, T.; Zhang, T.; Zhang, J.; Lin, J.; Yan, Y.; Li, C.; Si, X.; Cao, J.; et al. Characteristics, Applications and Perspective of High Entropy Alloys for Interfacial Joining: A Review. J. Manuf. Process. 2024, 110, 303–317. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S.; Yeh, J.W.; Bhattacharjee, P.P. High-Entropy Alloys; Elsevier: Amsterdam, The Netherland, 2019; pp. 1–363. [Google Scholar] [CrossRef]
- Buranich, V.; Rogoz, V.; Postolnyi, B.; Pogrebnjak, A. Predicting the Properties of the Refractory High-Entropy Alloys for Additive Manufacturing-Based Fabrication and Mechatronic Applications. In Proceedings of the 2020 IEEE 10th International Conference on “Nanomaterials: Applications and Properties” (NAP), Sumy, Ukraine, 9–13 November 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Gao, M.C.; Qiao, J. High-Entropy Alloys (Heas). Metals 2018, 8, 108. [Google Scholar] [CrossRef]
- Takeuchi, A.; Amiya, K.; Wada, T.; Yubuta, K.; Zhang, W. High-Entropy Alloys with a Hexagonal Close-Packed Structure Designed by Equi-Atomic Alloy Strategy and Binary Phase Diagrams. JOM 2014, 66, 1984–1992. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Lin, S.J.; Chang, S.Y. Mechanical Performance of the AlxCoCrCuFeNi High-Entropy Alloy System with Multiprincipal Elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Zhu, J.M.; Fu, H.M.; Zhang, H.F.; Wang, A.M.; Li, H.; Hu, Z.Q. Microstructures and Compressive Properties of Multicomponent AlCoCrFeNiMox Alloys. Mater. Sci. Eng. A 2010, 527, 6975–6979. [Google Scholar] [CrossRef]
- William Hume-Rothery, B.; Powell, H.M. On the Theory of Super-Lattice Structures in Alloys. Z. Krist. Cryst. Mater. 1935, 91, 23–47. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Selection Rules for Complex Multi-Component Alloys with Equiatomic or Close-to-Equiatomic Compositions. Chin. J. Nat. 2013, 35, 85–96. [Google Scholar]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.X.; Li, D.M.; Shi, L.; Cai, B.; Wang, M.X. Effect of Elemental Interaction on Microstructure of CuCrFeNiMn High Entropy Alloy System. J. Alloys Compd. 2010, 493, 148–153. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B. A New Thermodynamic Parameter to Predict Formation of Solid Solution or Intermetallic Phases in High Entropy Alloys. J. Alloys Compd. 2016, 658, 603–607. [Google Scholar] [CrossRef]
- Poletti, M.G.; Battezzati, L. Electronic and Thermodynamic Criteria for the Occurrence of High Entropy Alloys in Metallic Systems. Acta Mater. 2014, 75, 297–306. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of Electro-Negativity on the Stability of Topologically Close-Packed Phase in High Entropy Alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in High-Entropy Alloys. JOM 2014, 66, 1964–1965. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Salishchev, G.A.; Tikhonovsky, M.A.; Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Kolodiy, I.V.; Tortika, A.S.; Senkov, O.N. Effect of Mn and v on Structure and Mechanical Properties of High-Entropy Alloys Based on CoCrFeNi System. J. Alloys Compd. 2014, 591, 11–21. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Otto, F.; Pharr, G.M.; George, E.P. Recovery, Recrystallization, Grain Growth and Phase Stability of a Family of FCC-Structured Multi-Component Equiatomic Solid Solution Alloys. Intermetallics 2014, 46, 131–140. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and Mechanical Property of As-Cast, -Homogenized, and -Deformed AlxCoCrFeNi (0≤ x≤ 2) High-Entropy Alloys. J. Alloys Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical Properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 Refractory High Entropy Alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.N.; Scott, J.M.; Senkova, S.V.; Miracle, D.B.; Woodward, C.F. Microstructure and Room Temperature Properties of a High-Entropy TaNbHfZrTi Alloy. J. Alloys Compd. 2011, 509, 6043–6048. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Tsai, K.-Y.; Tsai, C.-W.; Lee, C.; Juan, C.-C.; Yeh, J.-W. Criterion for Sigma Phase Formation in Cr- and V-Containing High-Entropy Alloys. Mater. Res. Lett. 2013, 1, 207–212. [Google Scholar] [CrossRef]
- Mitrica, D.; Badea, I.C.; Olaru, M.T.; Serban, B.A.; Vonica, D.; Burada, M.; Geanta, V.; Rotariu, A.N.; Stoiciu, F.; Badilita, V.; et al. Modeling and Experimental Results of Selected Lightweight Complex Concentrated Alloys, before and after Heat Treatment. Materials 2020, 13, 4330. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.; Yang, Y.; Juan, C.; Chin, T.; Tsai, C.; Yeh, J. A Light-Weight High-Entropy Alloy Al20Be20Fe10Si15Ti35. Sci. China Tech. Sci. 2018, 61, 184–188. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Vicario, I.; Albizuri, J.; Guraya, T.; Garcia, J.C. Phase Prediction, Microstructure and High Hardness of Novel Light-Weight High Entropy Alloys. J. Mater. Res. Technol. 2018, 8, 795–803. [Google Scholar] [CrossRef]
- Feng, R.; Gao, M.C.; Lee, C.; Mathes, M.; Zuo, T.; Chen, S.; Hawk, J.A.; Zhang, Y.; Liaw, P.K. Design of Light-Weight High-Entropy Alloys. Entropy 2016, 18, 333. [Google Scholar] [CrossRef]
- Jung, I.H.; Van Ende, M.A. Computational Thermodynamic Calculations: FactSage from CALPHAD Thermodynamic Database to Virtual Process Simulation. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, F.; Chen, S.; Cao, W. Computational Thermodynamics Aided High-Entropy Alloy Design. JOM 2012, 64, 839–845. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Chen, S.L.; Zhu, J.; Cao, W.S.; Kattner, U.R. An Understanding of High Entropy Alloys from Phase Diagram Calculations. Calphad Comput. Coupling Phase Diagr. Thermochem. 2014, 45, 1–10. [Google Scholar] [CrossRef]
- Gorsse, S.; Senkov, O.N. About the Reliability of CALPHAD Predictions in Multicomponent Systems. Entropy 2018, 20, 899. [Google Scholar] [CrossRef]
- Raghavan, R.; Hari Kumar, K.C.; Murty, B.S. Analysis of Phase Formation in Multi-Component Alloys. J. Alloys Compd. 2012, 544, 152–158. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miller, J.D.; Miracle, D.B.; Woodward, C. Accelerated Exploration of Multi-Principal Element Alloys for Structural Applications. Calphad Comput. Coupling Phase Diagr. Thermochem. 2015, 50, 32–48. [Google Scholar] [CrossRef]
- Chang, Y.A.; Chen, S.; Zhang, F.; Yan, X.; Xie, F.; Schmid-Fetzer, R.; Oates, W.A. Phase Diagram Calculation: Past, Present and Future. Prog. Mater. Sci. 2004, 49, 313–345. [Google Scholar] [CrossRef]
- Chou, K.; Chang, Y.A. A Study of Ternary Geometrical Models. Berichte Bunsenges. Physikalische Chem. 1989, 93, 735–741. [Google Scholar] [CrossRef]
- Sun, W.; Huang, X.; Luo, A.A. Phase Formations in Low Density High Entropy Alloys. Calphad Comput. Coupling Phase Diagr. Thermochem. 2017, 56, 19–28. [Google Scholar] [CrossRef]
- Chen, H.L.; Mao, H.; Chen, Q. Database Development and Calphad Calculations for High Entropy Alloys: Challenges, Strategies, and Tips. Mater. Chem. Phys. 2018, 210, 279–290. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Yeh, J.W. Phases, Microstructure and Mechanical Properties of AlxCoCrFeNi High-Entropy Alloys at Elevated Temperatures. J. Alloys Compd. 2014, 589, 143–152. [Google Scholar] [CrossRef]
- Luan, H.W.; Shao, Y.; Li, J.F.; Mao, W.L.; Han, Z.D.; Shao, C.; Yao, K.F. Phase Stabilities of High Entropy Alloys. Scr. Mater. 2020, 179, 40–44. [Google Scholar] [CrossRef]
- Alam, I.; Adaan-Nyiak, M.A.; Tiamiyu, A.A. Revisiting the Phase Stability Rules in the Design of High-Entropy Alloys: A Case Study of Quaternary Alloys Produced by Mechanical Alloying. Intermetallics 2023, 159, 107919. [Google Scholar] [CrossRef]
- Yamabe-Mitarai, Y.; Yanao, K.; Toda, Y.; Ohnuma, I.; Matsunaga, T. Phase Stability of Ti-Containing High-Entropy Alloys with a Bcc or Hcp Structure. J. Alloys Compd. 2022, 911, 164849. [Google Scholar] [CrossRef]
- Gorsse, S.; Tancret, F. Current and Emerging Practices of CALPHAD toward the Development of High Entropy Alloys and Complex Concentrated Alloys. J. Mater. Res. 2018, 33, 2899–2923. [Google Scholar] [CrossRef]
- Zhang, Y. Preparation Methods of High-Entropy Materials. In High-Entropy Materials; Springer: Singapore, 2019; pp. 65–75. [Google Scholar]
- Zhilyaev, A.P.; Langdon, T.G. Using High-Pressure Torsion for Metal Processing: Fundamentals and Applications. Prog. Mater. Sci. 2008, 53, 893–979. [Google Scholar] [CrossRef]
- El-Hadad, S. High Entropy Alloys: The Materials of Future. Int. J. Mater. Technol. Innov. 2022, 2, 67–84. [Google Scholar] [CrossRef]
- Arun, S.; Radhika, N.; Saleh, B. Advances in Vacuum Arc Melting for High Entropy Alloys: A Review. Vacuum 2024, 226, 113314. [Google Scholar] [CrossRef]
- Dewangan, S.K.; Mangish, A.; Kumar, S.; Sharma, A.; Ahn, B.; Kumar, V. A Review on High-Temperature Applicability: A Milestone for High Entropy Alloys. Eng. Sci. Technol. Int. J. 2022, 35, 101211. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yang, B.; Sahul, M. Nanocomposite and Nanocrystalline Materials and Coatings; Springer: Singapore, 2024; Volume 214. [Google Scholar] [CrossRef]
- Porter, D.A.; Kenneth, E.; Easterling, A.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9780429112256. [Google Scholar]
- Joseph, J.; Jarvis, T.; Wu, X.; Stanford, N.; Hodgson, P.; Fabijanic, D.M. Comparative Study of the Microstructures and Mechanical Properties of Direct Laser Fabricated and Arc-Melted AlxCoCrFeNi High Entropy Alloys. Mater. Sci. Eng. A 2015, 633, 184–193. [Google Scholar] [CrossRef]
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and Corrosion Properties of AlCoCrFeNi High-Entropy Alloy Fabricated with Selective Electron Beam Melting. Addit. Manuf. 2018, 23, 264–271. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. First Demonstration of Promising Selective Electron Beam Melting Method for Utilizing High-Entropy Alloys as Engineering Materials. Mater. Lett. 2015, 159, 12–15. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, G.; Yin, K.; Wang, W.; Cheng, W.; Wang, Y. The Strengthening Effects of Relatively Lightweight AlCoCrFeNi High Entropy Alloy. Mater. Charact. 2019, 151, 302–309. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical Alloying Synthesis and Spark Plasma Sintering Consolidation of CoCrFeNiAl High-Entropy Alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Jung, H.Y.; Peter, N.J.; Gärtner, E.; Dehm, G.; Uhlenwinkel, V.; Jägle, E.A. Bulk Nanostructured AlCoCrFeMnNi Chemically Complex Alloy Synthesized by Laser-Powder Bed Fusion. Addit. Manuf. 2020, 35, 101337. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, W.H.; Wang, H.; Wu, Y.; Liu, X.J.; Nieh, T.G.; Lu, Z.P. Effects of Al Addition on Structural Evolution and Tensile Properties of the FeCoNiCrMn High-Entropy Alloy System. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Wang, C.; Ji, W.; Fu, Z. Mechanical Alloying and Spark Plasma Sintering of CoCrFeNiMnAl High-Entropy Alloy. In Proceedings of the Advanced Powder Technology; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 25, pp. 1334–1338. [Google Scholar]
- Gao, X.; Lu, Y. Laser 3D Printing of CoCrFeMnNi High-Entropy Alloy. Mater. Lett. 2019, 236, 77–80. [Google Scholar] [CrossRef]
- Kucza, W.; Dąbrowa, J.; Cieślak, G.; Berent, K.; Kulik, T.; Danielewski, M. Studies of “Sluggish Diffusion” Effect in Co-Cr-Fe-Mn-Ni, Co-Cr-Fe-Ni and Co-Fe-Mn-Ni High Entropy Alloys; Determination of Tracer Diffusivities by Combinatorial Approach. J. Alloys Compd. 2018, 731, 920–928. [Google Scholar] [CrossRef]
- Pickering, E.J.; Muñoz-Moreno, R.; Stone, H.J.; Jones, N.G. Precipitation in the Equiatomic High-Entropy Alloy CrMnFeCoNi. Scr. Mater. 2016, 113, 106–109. [Google Scholar] [CrossRef]
- Yao, M.J.; Pradeep, K.G.; Tasan, C.C.; Raabe, D. A Novel, Single Phase, Non-Equiatomic FeMnNiCoCr High-Entropy Alloy with Exceptional Phase Stability and Tensile Ductility. Scr. Mater. 2014, 72–73, 5–8. [Google Scholar] [CrossRef]
- Joo, S.H.; Kato, H.; Jang, M.J.; Moon, J.; Tsai, C.W.; Yeh, J.W.; Kim, H.S. Tensile Deformation Behavior and Deformation Twinning of an Equimolar CoCrFeMnNi High-Entropy Alloy. Mater. Sci. Eng. A 2017, 689, 122–133. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Zheng, H.; Li, H.; Liu, S.; Cheng, G.J. A Review on Microstructures and Properties of High Entropy Alloys Manufactured by Selective Laser Melting. Int. J. Extrem. Manuf. 2020, 2, 032003. [Google Scholar] [CrossRef]
- Ji, W.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F.; Fu, Z. Alloying Behavior and Novel Properties of CoCrFeNiMn High-Entropy Alloy Fabricated by Mechanical Alloying and Spark Plasma Sintering. Intermetallics 2015, 56, 24–27. [Google Scholar] [CrossRef]
- Li, R.; Niu, P.; Yuan, T.; Cao, P.; Chen, C.; Zhou, K. Selective Laser Melting of an Equiatomic CoCrFeMnNi High-Entropy Alloy: Processability, Non-Equilibrium Microstructure and Mechanical Property. J. Alloys Compd. 2018, 746, 125–134. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, H.; Jiao, J.; Zhou, W.; Yang, Y.; Ren, X. Improving the Strength and Ductility of Laser Directed Energy Deposited CrMnFeCoNi High-Entropy Alloy by Laser Shock Peening. Addit. Manuf. 2020, 35, 101417. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Wang, J.; Wang, W.Y.; Liu, Y.; Luo, X.; Kou, H.; Beaugnon, E. Microstructure and Properties of Bulk Al0.5CoCrFeNi High-Entropy Alloy by Cold Rolling and Subsequent Annealing. Mater. Sci. Eng. A 2018, 729, 141–148. [Google Scholar] [CrossRef]
- Lin, C.M.; Tsai, H.L. Evolution of Microstructure, Hardness, and Corrosion Properties of High-Entropy Al0.5CoCrFeNi Alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Peyrouzet, F.; Hachet, D.; Soulas, R.; Navone, C.; Godet, S.; Gorsse, S. Selective Laser Melting of Al0.3CoCrFeNi High-Entropy Alloy: Printability, Microstructure, and Mechanical Properties. JOM 2019, 71, 3443–3451. [Google Scholar] [CrossRef]
- Sathiyamoorthi, P.; Basu, J.; Kashyap, S.; Pradeep, K.G.; Kottada, R.S. Thermal Stability and Grain Boundary Strengthening in Ultrafine-Grained CoCrFeNi High Entropy Alloy Composite. Mater. Des. 2017, 134, 426–433. [Google Scholar] [CrossRef]
- Lin, D.; Xu, L.; Jing, H.; Han, Y.; Zhao, L.; Minami, F. Effects of Annealing on the Structure and Mechanical Properties of FeCoCrNi High-Entropy Alloy Fabricated via Selective Laser Melting. Addit. Manuf. 2020, 32, 101058. [Google Scholar] [CrossRef]
- Zhang, K.B.; Fu, Z.Y.; Zhang, J.Y.; Shi, J.; Wang, W.M.; Wang, H.; Wang, Y.C.; Zhang, Q.J. Annealing on the Structure and Properties Evolution of the CoCrFeNiCuAl High-Entropy Alloy. J. Alloys Compd. 2010, 502, 295–299. [Google Scholar] [CrossRef]
- Ganji, R.S.; Sai Karthik, P.; Bhanu Sankara Rao, K.; Rajulapati, K.V. Strengthening Mechanisms in Equiatomic Ultrafine Grained AlCoCrCuFeNi High-Entropy Alloy Studied by Micro- and Nanoindentation Methods. Acta Mater. 2017, 125, 58–68. [Google Scholar] [CrossRef]
- Welk, B.A.; Williams, R.E.A.; Viswanathan, G.B.; Gibson, M.A.; Liaw, P.K.; Fraser, H.L. Nature of the Interfaces between the Constituent Phases in the High Entropy Alloy CoCrCuFeNiAl. Ultramicroscopy 2013, 134, 193–199. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Chen, X.H.; Wang, T.; Si, J.J.; Wang, L.; Wang, Y.D.; Hui, X.D. Phase Composition and Solid Solution Strengthening Effect in TiZrNbMoV High-Entropy Alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Kunce, I.; Polanski, M.; Bystrzycki, J. Microstructure and Hydrogen Storage Properties of a TiZrNbMoV High Entropy Alloy Synthesized Using Laser Engineered Net Shaping (LENS). Int. J. Hydrogen Energy 2014, 39, 9904–9910. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructures and Mechanical Properties of Multiprincipal Component CoCrFeNiTix Alloys. Mater. Sci. Eng. A 2012, 556, 170–174. [Google Scholar] [CrossRef]
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and Mechanical Properties of Ni1,5Co1,5CrFeTi0,5 High Entropy Alloy Fabricated by Mechanical Alloying and Spark Plasma Sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
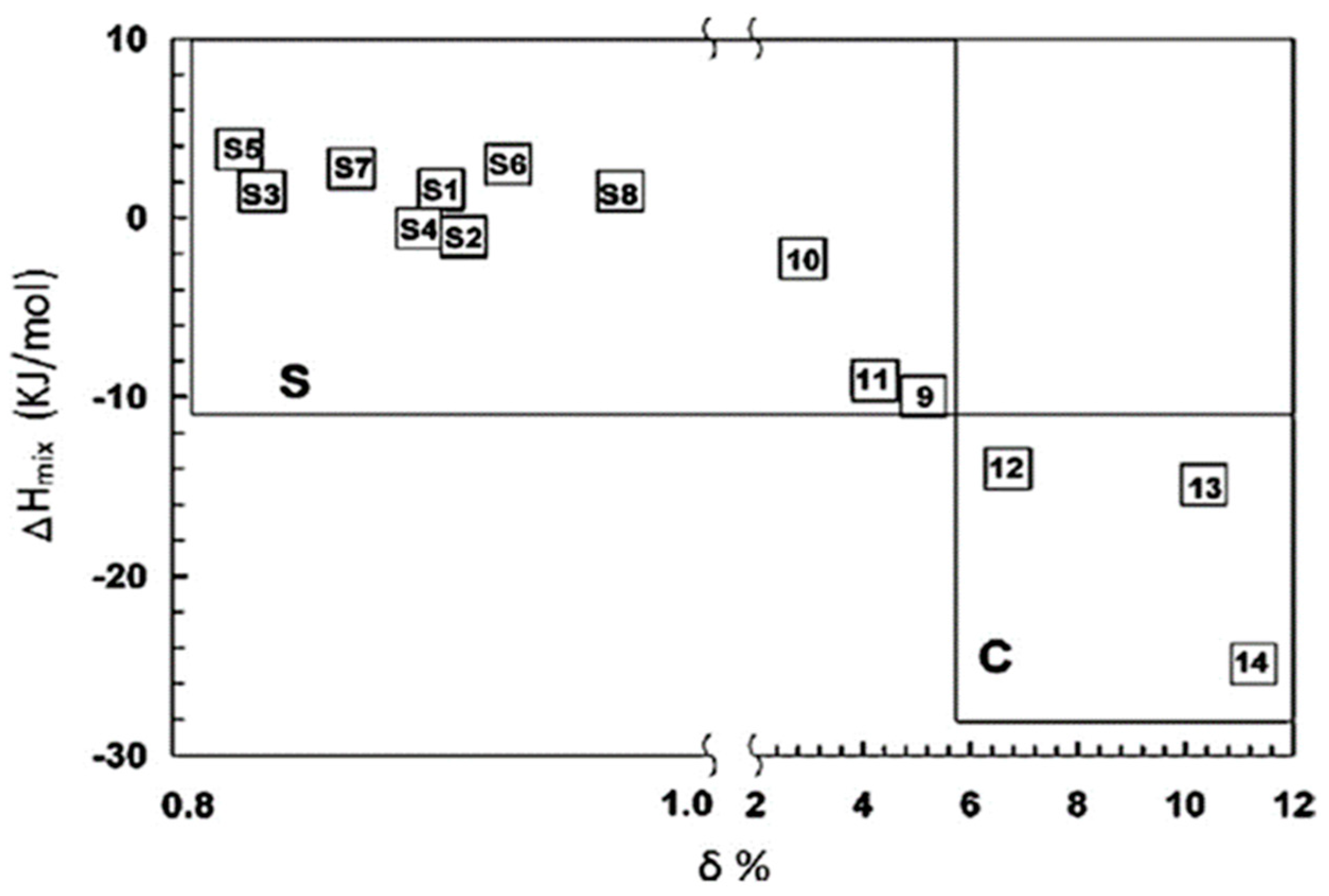
| Material | Parameters | Phase Type | Experimental [Reference] | |||||
|---|---|---|---|---|---|---|---|---|
| ΔSmix, J/molK | ΔHmix, kJ/mol | δ, % | Ω | Δχ, % | VEC | |||
| CoCrFeMnNi | 13.38 | −4.16 | 3.27 | 5.79 | 4.465 | 8 | FCC | FCC [42] |
| CoCrFeNi | 11.53 | −3.75 | 0.3 | 5.75 | 4.853 | 8.25 | FCC | FCC [43] |
| CoCrFeNiAl0.25 | 12.71 | −6.75 | 3.47 | 3.42 | 5.315 | 7.94 | BCC + FCC | FCC [44] |
| CrFeMnCuCo | 13.38 | 4.16 | 3.14 | 5.56 | 4.108 | 8.2 | FCC | FCC [42] |
| WMoNbTa | 11.53 | −6.5 | 2.32 | 5.6 | 3.765 | 5.5 | BCC | BCC [45] |
| WMoNbTaV | 13.38 | −4.64 | 3.15 | 8.54 | 4.458 | 5.4 | BCC | BCC [45] |
| NbTaHfZrTi | 13.38 | 2.72 | 4.98 | 12.41 | 6.567 | 4.4 | BCC | BCC [46] |
| CrMnFe1.5Ni0.5Al0.5 | 12.66 | −7.26 | 5.15 | 3 | 4.822 | 7 | BCC + FCC | BCC + IM [47] |
| CrMnFe1.5Ni0.5Al1.2 | 12.94 | −11.29 | 6.02 | 1.85 | 5.244 | 6.46 | BCC | BCC [47] |
| Cr0.5Fe0.5NiAlCo0.5MnV | 15.75 | −15.74 | 5.29 | 1.68 | 7.355 | 6.64 | BCC | BCC [47] |
| CrNiCoMnV | 13.38 | −8.8 | 3.33 | 2.85 | 7.375 | 7.4 | BCC + FCC | FCC + IM [42] |
| CrNiMnFeTi | 13.38 | −12.32 | 6.58 | 1.99 | 10.219 | 7 | BCC + FCC | BCC + FCC + IM [42] |
| Al3.4Cu0.5Si0.2Zn0.5Mg0.2 | 8.15 | −3.38 | 5.7 | 2.37 | 7.198 | 4.77 | BCC | FCC + HCP + IM [48] |
| Al3Si0.8Zn0.3Mg0.7Mn0.2 | 9.75 | −13.79 | 9.03 | 0.75 | 10.774 | 3.72 | IM | FCC + IM [48] |
| Al20Be20Fe10Si15Ti35 | 12.69 | −40.42 | 11.15 | 0.51 | 11.987 | 3.8 | IM | IM [49] |
| Al65Cr5Cu5Si15Mn5Ti5 | 9.68 | −20.22 | 7.7 | 0.58 | 8.023 | 3.95 | IM | IM [50] |
| AlCrFeMnTi0.25 | 12.71 | −11.74 | 6.27 | 1.77 | 6.283 | 5.88 | BCC | BCC + L21 [51] |
| Material | CALPHAD Results | Experimental [Reference] | |||
|---|---|---|---|---|---|
| 25 °C | 400 °C | 600 °C | 800 °C | ||
| CoCrFeMnNi | FCC + BCC + sigma | FCC | FCC | FCC | FCC [42] |
| CoCrFeNi | BCC + sigma + IM | FCC + BCC + sigma | FCC + sigma | FCC | FCC [43] |
| CoCrFeNiAl0.25 | BCC + sigma + IM | BCC + sigma + IM | FCC + sigma + IM | FCC | FCC [44] |
| CrFeMnCuCo | FCC + BCC + sigma+ IM | FCC + BCC + HCP + sigma | FCC + BCC | FCC + BCC | FCC [42] |
| WMoNbTa | BCC + IM | BCC | BCC | BCC | BCC [45] |
| WMoNbTaV | BCC + IM | BCC | BCC | BCC | BCC [45] |
| NbTaHfZrTi | HCP + BCC | BCC + HCP | BCC + HCP | BCC | BCC [46] |
| CrMnFe1.5Ni0.5Al0.5 | SIGMA + BCC + IM | SIGMA + BCC + IM | SIGMA + FCC + BCC + IM | BCCC + FFCC + IM | BCC + IM [47] |
| CrMnFe1.5Ni0.5Al1.2 | SIGMA + BCC + IM | BCC + SIGMA + IM | BCC + IM | BCC + IM | BCC [47] |
| Cr0.5Fe0.5NiAlCo0.5MnV | BCC + IM | BCC + IM | BCC + IM | BCC + FCC + IM | BCC [47] |
| CrNiCoMnV | FCC + BCC + IM | FCC + BCC | FCC + BCC | FCC + BCC | FCC + IM [42] |
| CrNiMnFeTi | FCC + IM | BCC + IM | BCC + IM | BCC + IM | BCC + FCC + IM [42] |
| Al3.4Cu0.5Si0.2Zn0.5Mg0.2 | FCC + HCP + IM | BCC + FCC + IM | LIQUID | LIQUID | FCC + HCP + IM [48] |
| Al3Si0.8Zn0.3Mg0.7Mn0.2 | FCC + IM | FCC + IM | IM | LIQUID | FCC + IM [48] |
| Al65Cr5Cu5Si15Mn5Ti5 | BCC + IM | FCC + BCC + IM | FCC + BCC | LIQUID | IM [50] |
| AlCrFeMnTi0.25 | BCC + FCC | BCC | BCC | BCC | BCC + L21 [51] |
| Composition of HEA Alloy | Melting-Casting | MA + SPS | AM | |||
|---|---|---|---|---|---|---|
| Phases | Mechanical Properties | Phases | Mechanical Properties | Phases | Mechanical Properties | |
| CoCrFeNiMn | FCC [84,85] | - | FCC [88] | Rc = 1987 MPa Vickers hardness = 646 HV | FCC + BCC [82,89,90] | Rt = 601 MPa |
| CoCrFeNiAl0.3 | FCC [91,92] | UTS = 528 MPa YTS = 257 MPa | FCC + BCC [78] | Rc = 1907 MPa Vickers hardness = 625 HV | FCC [93] | UTS = 896 MPa; YS = 730 MPa |
| CoCrFeNi | - | - | FCC + Cr7C3 [94] | Vickers hardness = 580 HV | FCC [95] | - |
| AlCoCrCuFeNi | FCC + BCC [96] | Hardness = 5.056 GPa Rc = 1.82 GPa | FCC + BCC [97] | Hardness = 8.13 GPa Elastic modulus = 172 GPa | BCC [98] | - |
| TiZrNbMo0.3V0.3 | BCC [99] | YS = 1312 MPa | - | - | FCC + BCC [100] | - |
| Ni1.5Co1.5CrFeTi0.5 | FCC [101] | YS = 896 MPa Rc = 1502 MPa Vickers hardness = 515 HV | FCC [102] | Vickers hardness = 442 HV Elastic modulus = 216 GPa Rt = 1384 MP | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramarin, S.; Badea, I.-C.; Mosinoiu, L.-F.; Mitrica, D.; Serban, B.-A.; Vitan, N.; Cursaru, L.-M.; Pogrebnjak, A. Structural Particularities, Prediction, and Synthesis Methods in High-Entropy Alloys. Appl. Sci. 2024, 14, 7576. https://doi.org/10.3390/app14177576
Caramarin S, Badea I-C, Mosinoiu L-F, Mitrica D, Serban B-A, Vitan N, Cursaru L-M, Pogrebnjak A. Structural Particularities, Prediction, and Synthesis Methods in High-Entropy Alloys. Applied Sciences. 2024; 14(17):7576. https://doi.org/10.3390/app14177576
Chicago/Turabian StyleCaramarin, Stefania, Ioana-Cristina Badea, Laurentiu-Florin Mosinoiu, Dumitru Mitrica, Beatrice-Adriana Serban, Nicoleta Vitan, Laura-Madalina Cursaru, and Alexander Pogrebnjak. 2024. "Structural Particularities, Prediction, and Synthesis Methods in High-Entropy Alloys" Applied Sciences 14, no. 17: 7576. https://doi.org/10.3390/app14177576







