Aromatic Herbs of the Lamiaceae Family as Functional Ingredients in Wheat Tortilla
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tortillas
2.2. Water Activity Determination
2.3. Water Content Determination
2.4. Texture Analysis
2.5. Organoleptic Evaluation of Tortillas
2.6. Color Determination
- ΔE* < 1: color differences are not obvious to the human eye.
- 1 < ΔE* < 3: the human eye can detect subtle color differences depending on the specific hue.
- ΔE* > 3: color differences are obvious to the human eye.
2.7. Lyophilization of Tortillas
2.8. Preparation of Extracts from Freeze-Dried Tortillas
2.9. Aromatic Plant Leaf Extraction
2.10. Determination of Total Phenolic Content
2.11. Determination of Total Antioxidant Capacity
2.12. UHPLC-DAD Analysis of Phenolic Compounds in Plant Leaf Extracts
2.13. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristic and Organoleptic Assessment of Tortillas
3.2. Color Determination
3.3. Phenolic Content and Antioxidant Properties of Aromatic Plants and Tortillas
3.4. Profile and Concentrations of Phenolic Bioactive Compounds in Herbs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vouillamoz, J.F.; Christ, B. Thymus vulgaris L.: Thyme. In Medicinal, Aromatic and Stimulant Plants. Handbook of Plant Breeding; Novak, J., Blüthner, W.D., Eds.; Springer: Cham, Switzerland, 2020; Volume 12, pp. 547–557. [Google Scholar]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef] [PubMed]
- Ramos da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; de Jesus Pereira Franco, C.; Oliveira dos Anjos, T.; Cascaes, M.M.; Almeida da Costa, W.; de Aguiar Andrade, E.H.; Santana de Oliveira, M. Lamiaceae essential oils, phytochemical profile, antioxidant, and biological activities. Evid. Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M.M. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef]
- Carović-StanKo, K.; PeteK, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Chroho, M.; Rouphael, Y.; Petropoulos, S.A.; Bouissane, L. Carvacrol and Thymol Content Affects the Antioxidant and Antibacterial Activity of Origanum compactum and Thymus zygis Essential Oils. Antibiotics 2024, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, D.; Cabo, M.L.; Rodríguez-Herrera, J.J. Antimicrobial activity of essential oils against Staphylococcus aureus biofilms. Food Sci. Technol. Int. 2015, 21, 559–570. [Google Scholar] [CrossRef]
- Šegvić Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007, 44, 36–42. [Google Scholar] [CrossRef]
- Garza-González, J.N.; Vargas-Villarreal, J.; Verde-Star, M.J.; Rivas-Morales, C.; Oranday-Cárdenas, A.; Hernandez-García, M.E.; De La Garza-Salinas, L.; González-Salazar, F. Antiprotozoal activity of a Thymus vulgaris methanol extract and its fractions. Health 2017, 9, 1081–1094. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Shaffie, N.M.; Omer, E.A. The protection of Thymus vulgaris leaves alcoholic extract against hepatotoxicity of alcohol in rats. Asian Pac. J. Trop. Med. 2017, 10, 361–371. [Google Scholar] [CrossRef]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crop Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Shahar, B.; Indira, A.; Santosh, O.; Dolma, N.; Chongtham, N. Nutritional composition, antioxidant activity and characterization of bioactive compounds from Thymus serpyllum L.: An underexploited wild aromatic plant. Meas. Food 2023, 10, 100092. [Google Scholar] [CrossRef]
- Stahl-Biskup, E.; Holthuijzen, J. Essential oil and glycosidically bound volatiles of lemonscented thyme, Thymus × citriodorus (Pers.) Schreb. Flavour. Fragr. J. 1995, 10, 225–229. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Cavaleiro, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Thymus × citriodorus: An Emerging Aromatic and Medicinal Hybrid Plant with Relevant Bioactive Potential. Rev. Bras. Farm. 2023, 33, 1089–1109. [Google Scholar] [CrossRef]
- Jurevičiūtė, R.; Ložienė, K.; Bruno, M.; Maggio, A.; Rosselli, S. Composition of essential oil of lemon thyme (Thymus × citriodorus) at different hydrodistillation times. Nat. Prod. Res. 2018, 33, 80–88. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Zinno, P.; Guantario, B.; Lombardi, G.; Ranald, G.; Finamore, A.; Allegra, S.; Mammano, M.M.; Fascella, G.; Raffo, A.; Roselli, M. Chemical Composition and Biological Activities of Essential Oils from Origanum vulgare Genotypes Belonging to the Carvacrol and Thymol Chemotypes. Plants 2023, 12, 1344. [Google Scholar] [CrossRef]
- Gonçalves Cattelan, M.; Bonatto Machado de Castilhos, M.; Juliana Pinsetta Sales, P.; Leite Hoffmann, F. Antibacterial activity of oregano essential oil against foodborne pathogens. Nutr. Food Sci. 2013, 43, 169–174. [Google Scholar] [CrossRef]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef]
- Duan, W.Y.; Zhu, X.M.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Wei, S.; Ma, P.A.; Hu, Y.S. Antifungal effects of carvacrol, the main volatile compound in Origanum vulgare L. essential oil, against Aspergillus flavus in postharvest wheat. Int. J. Food Microbiol. 2024, 410, 110514. [Google Scholar] [CrossRef]
- Bounar, R.; Krimat, S.; Boureghda, H.; Dob, T. Chemical analyses, antioxidant and antifungal effects of oregano and thyme essential oils alone or in combination against selected Fusarium species. Int. Food Res. J. 2020, 27, 66–77. [Google Scholar]
- Zhao, Y.; Yang, Y.H.; Ye, M.; Wang, K.B.; Fan, L.M.; Su, F.W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Bhat, V.; Sharma, S.M.; Shetty, V.; Shastry, C.S.; Rao, C.V.; Shenoy, S.; Saha, S.; Balaji, S. Characterization of Herbal Antifungal Agent, Origanum vulgare against Oral Candida spp. Isolated from Patients with Candida-Associated Denture Stomatitis: An In Vitro Study. Contemp. Clin. Dent. 2018, 9, S3–S10. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic Acid and Carnosol, Two Major Antioxidants of Rosemary, Act through Different Mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Romo Vaquero, M.; Yáñez-Gascón, M.J.; García Villalba, R.; Larrosa, M.; Fromentin, E.; Ibarra, A.; Roller, M.; Tomás-Barberán, F.; Espín de Gea, J.C.; García-Conesa, M.T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE 2012, 7, e39773. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Manilal, A.; Sabu, K.R.; Woldemariam, M.; Aklilu, A.; Biresaw, G.; Yohanes, T.; Seid, M.; Merdekios, B. Antibacterial Activity of Rosmarinus officinalis against Multidrug-Resistant Clinical Isolates and Meat-Borne Pathogens. Evid. Based Complement. Alternat. Med. 2021, 1, 6677420. [Google Scholar] [CrossRef]
- Saraiva, C.; Silva, A.C.; García-Díez, J.; Cenci-Goga, B.; Grispoldi, L.; Silva, A.F.; Almeida, J.M. Antimicrobial activity of Myrtus communis L. and Rosmarinus officinalis L. essential oils against Listeria monocytogenes in cheese. Foods 2021, 10, 1106. [Google Scholar] [CrossRef]
- Da Silva Bomfim, N.; Kohiyama, C.Y.; Nakasugi, L.P.; Nerilo, S.B.; Mossini, S.A.G.; Romoli, J.C.Z.; Graton Mikcha, J.M.; Abreu Filho, B.A.; Machinski, M.J. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A 2020, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.; Chalchat, J.C. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kačániová, M. In Vitro Antimicrobial Activity of Lavender, Mint, and Rosemary Essential Oils and the Effect of Their Vapours on Growth of Penicillium spp. in a Bread Model System. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Katsantonis, D.Ν.; Chatzopoulou, P.; Irakli, M.; Papageorgiou, M. Antifungal Activity of Aromatic Plants of the Lamiaceae Family in Bread. Foods 2020, 9, 1642. [Google Scholar] [CrossRef]
- Kulbat-Warycha, K.; Nawrocka, J.; Kozłowska, L.; Żyżelewicz, D. Effect of Light Conditions, Trichoderma Fungi and Food Polymers on Growth and Profile of Biologically Active Compounds in Thymus vulgaris and Thymus serpyllum. Int. J. Mol. Sci. 2024, 25, 4846. [Google Scholar] [CrossRef]
- Bodart, M.; de Peñarada, R.; Deneyer, A.; Flamant, G. Photometry and colorimetry characterization of materials in daylighting evaluation tools. Build. Env. 2008, 43, 2046–2058. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Viticult 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The Influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, D. Bioactive Properties of Extracts from Plectranthus barbatus (Coleus forskohlii) Roots Received Using Various Extraction Methods. Molecules 2022, 27, 8986. [Google Scholar] [CrossRef]
- Labuza, T.P. The Effect of Water Activity on Reaction Kinetics of Food Deterioration. Food Technol. 1980, 34, 36–41. [Google Scholar]
- López-Malo, A.; Alzamora, S.M. Water Activity and Microorganism Control: Past and Future. In Water Stress in Biological, Chemical, Pharmaceutical and Food Systems; Gutiérrez-López, G., Alamilla-Beltrán, L., del Pilar Buera, M., Welti-Chanes, J., Parada-Arias, E., Barbosa-Cánovas, G., Eds.; Food Engineering Series; Springer: New York, NY, USA, 2015; pp. 245–262. [Google Scholar]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Villanueva, R.L.; Marlett, J.A. Soft wheat flour functionality in tortillas as evaluated by subjective and objective tests. Cereal Chem. 1991, 68, 478–481. [Google Scholar]
- Wang, Y.; Flores, R.A.; Huang, L. Effects of flour particle size on the quality attributes of restructured whole wheat products. Cereal Chem. 2004, 81, 258–263. [Google Scholar]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Influence of fibre from different cereals on the rheological characteristics of wheat flour dough and on biscuit quality. Food Chem. 2007, 100, 1365–1370. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Papageorgiou, M. Aromatic plants of Lamiaceae family in a traditional bread recipe: Effects on quality and phytochemical content. J. Food Biochem. 2019, 43.11, e13020. [Google Scholar] [CrossRef]
- Mashau, M.E.; Mabodze, T.; Tshiakhatho, O.J.; Silungwe, H.; Ramashia, S.E. Evaluation of the content of polyphenols, antioxidant activity and physicochemical properties of tortillas added with Bambara groundnut flour. Molecules 2020, 25, 3035. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H. Chemical and biological changes of polyphenols caused by food thermal processing. Front. Nutr. 2022, 9, 948894. [Google Scholar] [CrossRef]
- Starowicz, M.; Lelujka, E.; Ciska, E.; Lamparski, G.; Sawicki, T.; Wronkowska, M. The Application of Lamiaceae Lindl. Promotes Aroma Compounds Formation, Sensory Properties, and Antioxidant Activity of Oat and Buckwheat-Based Cookies. Molecules 2020, 25, 5626. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U.; Dziki, D. Bread enriched with quinoa leaves–The influence of protein–phenolics interactions on the nutritional and antioxidant quality. Food Chem. 2014, 162, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Hou, G.G.; Sun, J.; Wan, X.; Dubat, A. Effect of green tea powder on the quality attributes and antioxidant activity of whole-wheat flour pan bread. LWT Food Sci. Technol. 2017, 79, 342–348. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.; Domingues, M.R.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus × citriodorus using a validated HPLC–UV and ESI–MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G. Polyphenolic and molecular variation in Thymus species using HPLC and SRAP analyses. Sci. Rep. 2021, 11, 5019. [Google Scholar] [CrossRef]
- Jafari Khorsand, G.; Morshedloo, M.R.; Mumivand, H.; Emami Bistgani, Z.; Maggi, F.; Khademi, A. Natural diversity in phenolic components and antioxidant properties of oregano (Origanum vulgare L.) accessions, grown under the same conditions. Sci. Rep. 2022, 12, 5813. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
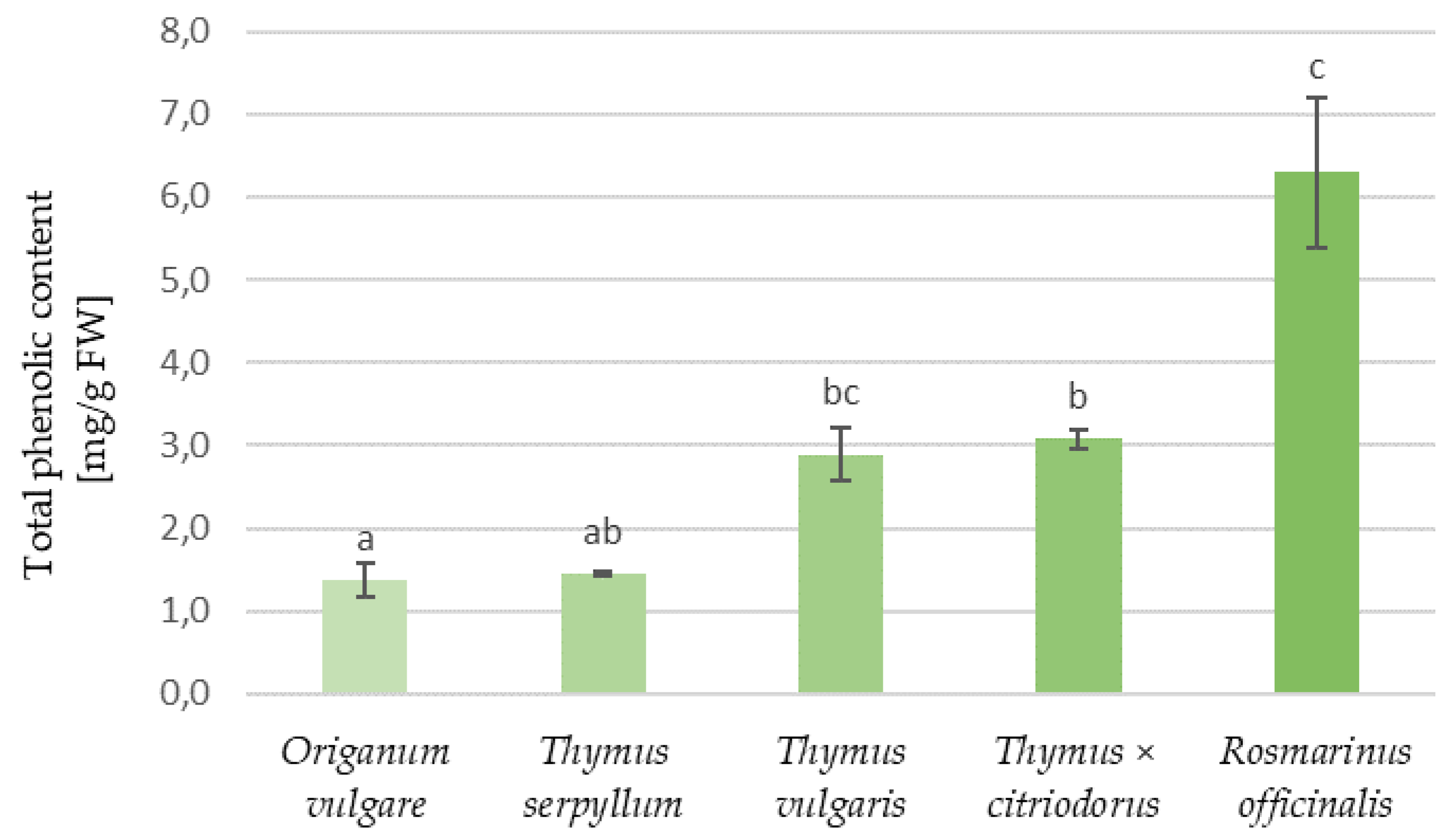

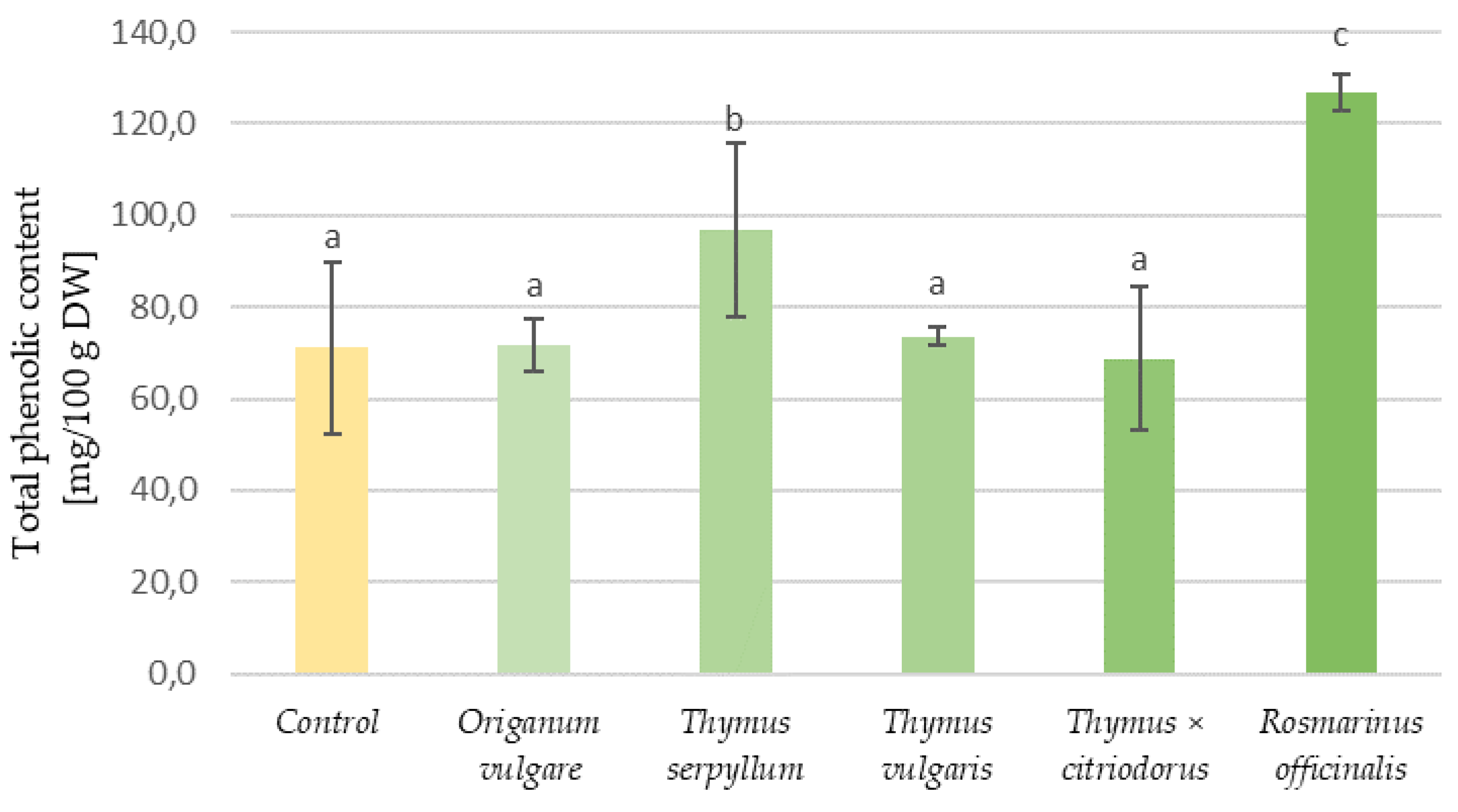
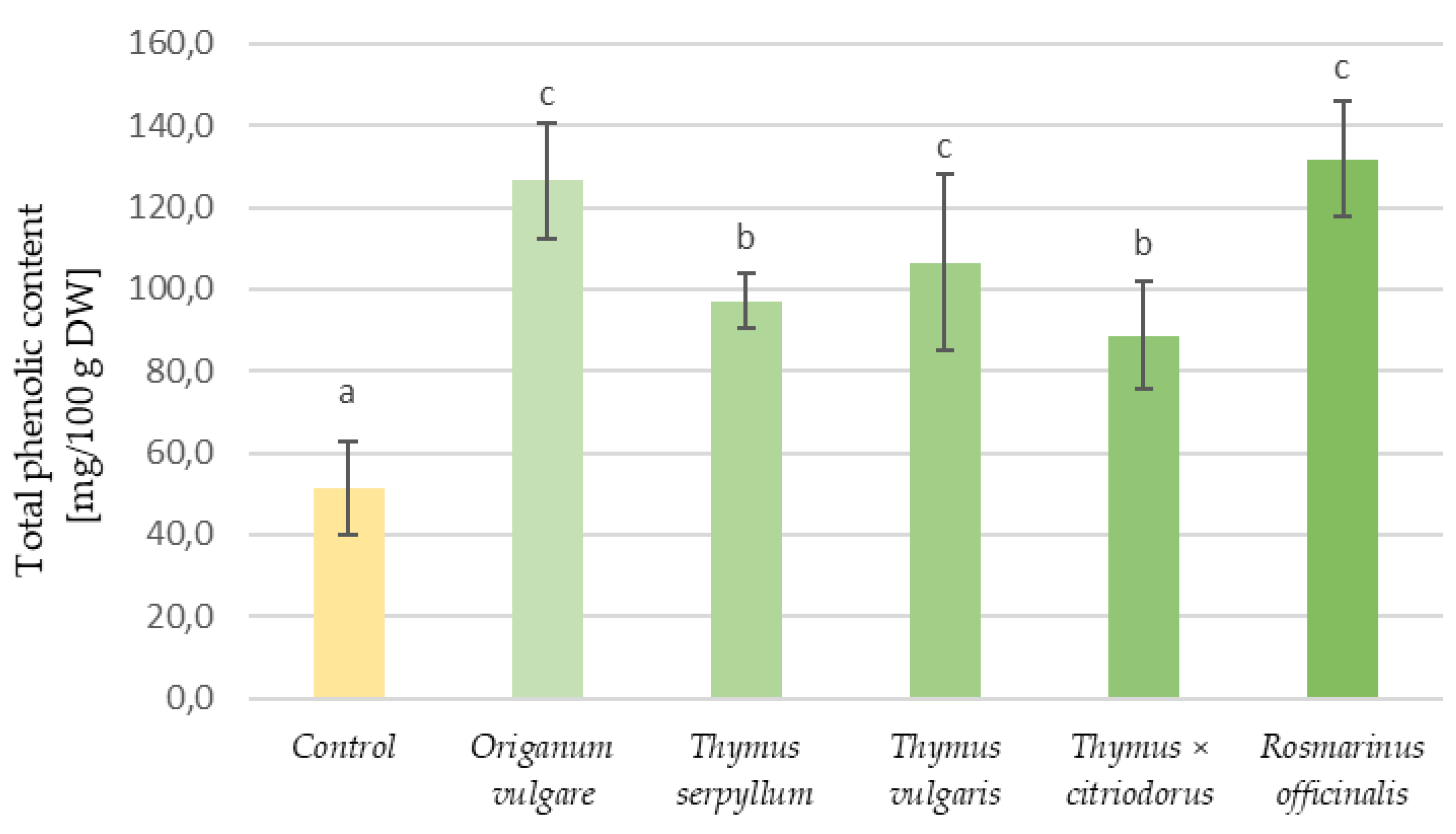
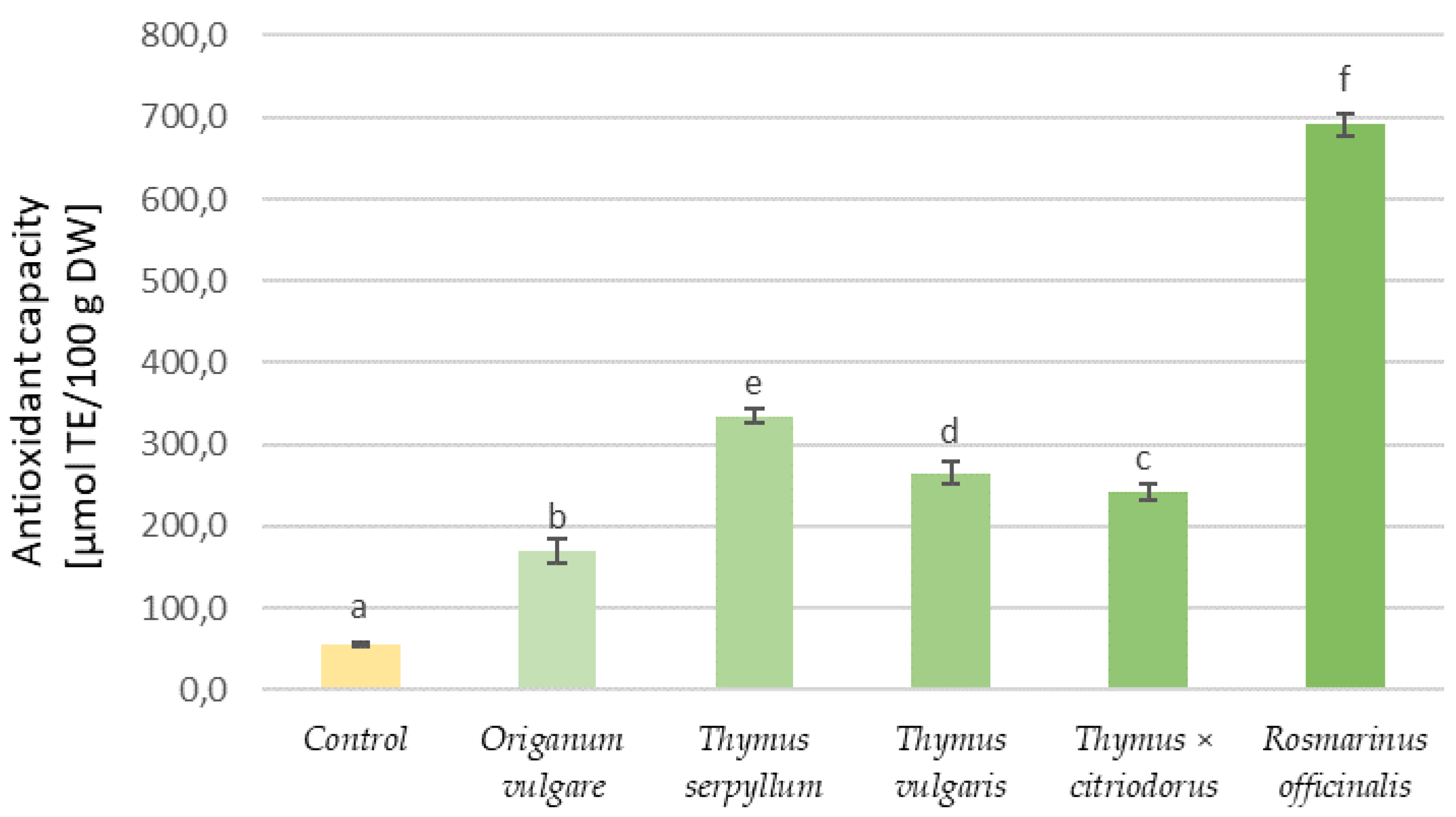

| Type of Tortilla | Samples | Water Activity | Water Content (%) | Hardness (kg) | Organoleptic Assessment (Points) |
|---|---|---|---|---|---|
| wheat tortilla | Control | 0.757 ± 0.027 b | 21.22 ± 1.58 ab | 8.34 ± 1.75 b | 3.3 ± 0.4 |
| Origanum vulgare | 0.794 ± 0.009 d | 20.14 ± 1.26 a | 11.12 ± 2.47 c | 3.5 ± 0.3 | |
| Thymus serpyllum | 0.746 ± 0.004 a | 21.78 ± 1.12 b | 11.99 ± 2.19 c | 3.6 ± 0.3 | |
| Thymus vulgaris | 0.767 ± 0.023 c | 20.00 ± 1.36 a | 7.54 ± 1.31 b | 4.2 ± 0.2 | |
| Thymus × citriodorus | 0.735 ± 0.063 a | 21.61 ± 2.06 b | 11.17 ± 4.87 c | 4.3 ± 0,2 | |
| Rosmarinus officinalis | 0.774 ± 0.010 c | 21.42 ± 1.19 b | 8.53 ± 2.57 b | 4.3 ± 0.3 | |
| whole-grain tortilla | Control | 0.808 ± 0.015 e | 21.62 ± 0.98 b | 7.12 ± 1.20 b | 4.1 ± 0.2 |
| Origanum vulgare | 0.809 ± 0.008 e | 21.56 ± 1.58 b | 7.31 ± 2.65 b | 3.7 ± 0.2 | |
| Thymus serpyllum | 0.818 ± 0.009 f | 22.01 ± 1.12 b | 7.81 ± 1.06 b | 4.1 ± 0.1 | |
| Thymus vulgaris | 0.806 ± 0.002 e | 21.04 ± 1.13 ab | 5.58 ± 0.60 a | 3.8 ± 0.2 | |
| Thymus × citriodorus | 0.821 ± 0.023 f | 22.36 ± 2.15 b | 5.24 ± 0.24 a | 4.3 ± 0.1 | |
| Rosmarinus officinalis | 0.817 ± 0.004 ef | 21.54 ± 0.63 b | 7.27 ± 3.07 b | 4.6 ± 0.2 |
| Type of Tortilla | Herbs | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| wheat tortilla | Control | 74.61 ± 1.62 | −0.27 ± 0.11 | 18.68 ± 0.95 | 0.00 |
| Origanum vulgare | 74.22 ± 1,09 | −0.27 ± 0.10 | 15.05 ± 0.83 | 3.65 | |
| Thymus serpyllum | 76.57 ± 2.23 | −0.53 ± 0.21 | 13.94 ± 1.21 | 5.14 | |
| Thymus vulgaris | 72.62 ± 4.95 | −0.41 ± 0.33 | 18.29 ± 0.92 | 2.03 | |
| Thymus × citriodorus | 66.80 ± 1.14 | −0.62 ± 0.18 | 17.65 ± 0.19 | 7.89 | |
| Rosmarinus officinalis | 78.10 ± 1.61 | −0.94 ± 0.12 | 16.45 ± 0.97 | 4.20 | |
| whole-grain tortilla | Control | 64.60 ± 1.26 | 5.31 ± 0.29 | 15.03 ± 0.64 | 0.00 |
| Origanum vulgare | 61.64 ± 1.12 | 5.31 ± 0.25 | 15.39 ± 0.46 | 2.98 | |
| Thymus serpyllum | 65.64 ± 0.75 | 5.13 ± 0.15 | 15.85 ± 0.48 | 1.34 | |
| Thymus vulgaris | 57.07 ± 2.54 | 5.28 ± 1.36 | 15.39 ± 2.02 | 7.54 | |
| Thymus × citriodorus | 58.59 ± 1.98 | 5.80 ± 0.87 | 15.66 ± 0.71 | 6.06 | |
| Rosmarinus officinalis | 54.82 ± 0.77 | 7.26 ± 7.26 | 19.06 ± 0.16 | 10.76 |
| Phenolic Compound (mg/100 g FW) | RT (min) | Origanum vulgare | Thymus serpyllum | Thymus vulgaris | Thymus × citriodorus | Rosmarinus officinalis |
|---|---|---|---|---|---|---|
| Procyanidin B1 | 8.47 | nd | nd | 11.67 ± 0.51 b | 9.79 ± 0.96 a | 9.60 ± 0.52 a |
| Caffeic acid | 12.04 | 0.91 ± 0.02 a | 3.31 ± 0.14 b | 5.36 ± 0.04 c | 3.78 ± 0.31 b | 3.50 ± 0.34 b |
| Procyanidin B2 | 17.20 | 10.16 ± 1.13 b | 9.01 ± 0.58 a | 29.99 ± 1.67 d | 18.32 ± 0.21 c | nd |
| Epicatechin | 21.46 | nd | 6.24 ± 0.62 a | 8.12 ± 0.86 b | 7.92 ± 0.67 b | nd |
| Quercetin-3,7-di-O-glucoside | 26.00 | 4.10 ± 0.25 c | nd | 3.84 ± 0.91 b | 3.38 ± 0.03 a | nd |
| Epicatechin gallate | 27.72 | 22.35 ± 0.98 a | nd | 21.59 ± 1.37 a | nd | nd |
| Quercetin 3-O-galactoside | 28.41 | nd | 18.89 ± 1.12 b | 8.25 ± 0.47 a | 21.83 ± 2.23 c | 18.59 ± 1.25 b |
| Quercetin 3-O-glucoside | 29.50 | 4.02 ± 0.11 b | 5.34 ± 0.07 c | 5.80 ± 0.61 c | 1.93 ± 0.09 a | nd |
| Luteolin 7-O-glucoside (Cynaroside) | 31.69 | nd | 32.04 ± 1.08 b | 115.64 ± 8.25 d | 92.30 ± 6.21 c | 6.10 ± 0.17 a |
| Rosmarinic acid | 37.14 | nd | 42.70 ± 5.78 a | 125.30 ± 7.21 b | 186.38 ± 6.32 c | 416.07 ± 11.26 d |
| Quercetin | 37.83 | nd | 1.59 ± 0.03 a | 11.84 ± 1.01 c | 6.07 ± 0.72 b | 16.77 ± 1.01 d |
| Carnosic acid | 43.10 | nd | nd | nd | nd | 8.86 ± 0.49 |
| Naringenin | 45.65 | 7.40 ± 0.26 c | 5.25 ± 0.98 b | 2.99 ± 0.76 a | 5.35 ± 0.08 b | nd |
| Apigenin | 46.38 | 13.05 ± 1.05 c | nd | 4.97 ± 0.84 a | 7.00 ± 0.94 b | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbat-Warycha, K.; Stoińska, K.; Żyżelewicz, D. Aromatic Herbs of the Lamiaceae Family as Functional Ingredients in Wheat Tortilla. Appl. Sci. 2024, 14, 7584. https://doi.org/10.3390/app14177584
Kulbat-Warycha K, Stoińska K, Żyżelewicz D. Aromatic Herbs of the Lamiaceae Family as Functional Ingredients in Wheat Tortilla. Applied Sciences. 2024; 14(17):7584. https://doi.org/10.3390/app14177584
Chicago/Turabian StyleKulbat-Warycha, Kamila, Kinga Stoińska, and Dorota Żyżelewicz. 2024. "Aromatic Herbs of the Lamiaceae Family as Functional Ingredients in Wheat Tortilla" Applied Sciences 14, no. 17: 7584. https://doi.org/10.3390/app14177584






