Improved Functions of Fermented Coffee by Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Coffee Extract-Resistant LAB Strains
2.2. Acidity Change of the Fermented Coffee Extract with Selected LAB Strains Applied
2.3. Change in the Cell Mass of the Fermented Coffee Extract with Selected LAB Strains
2.4. Sample Preparation for Bioactivity Assays
2.5. Measurement of the Antimicrobial Activity of the Fermented Coffee Extract
2.6. Measurement of the Antioxidant Activity of the Fermented Coffee Extract
2.7. Measurement of the Caffeine Content of the Fermented Coffee Extract
2.8. Measurement of the Chlorogenic Acid Content of the Fermented Coffee Extract
2.9. Statistic Analysis
3. Results
3.1. Selection of Coffee Extract-Resistant LAB Strains
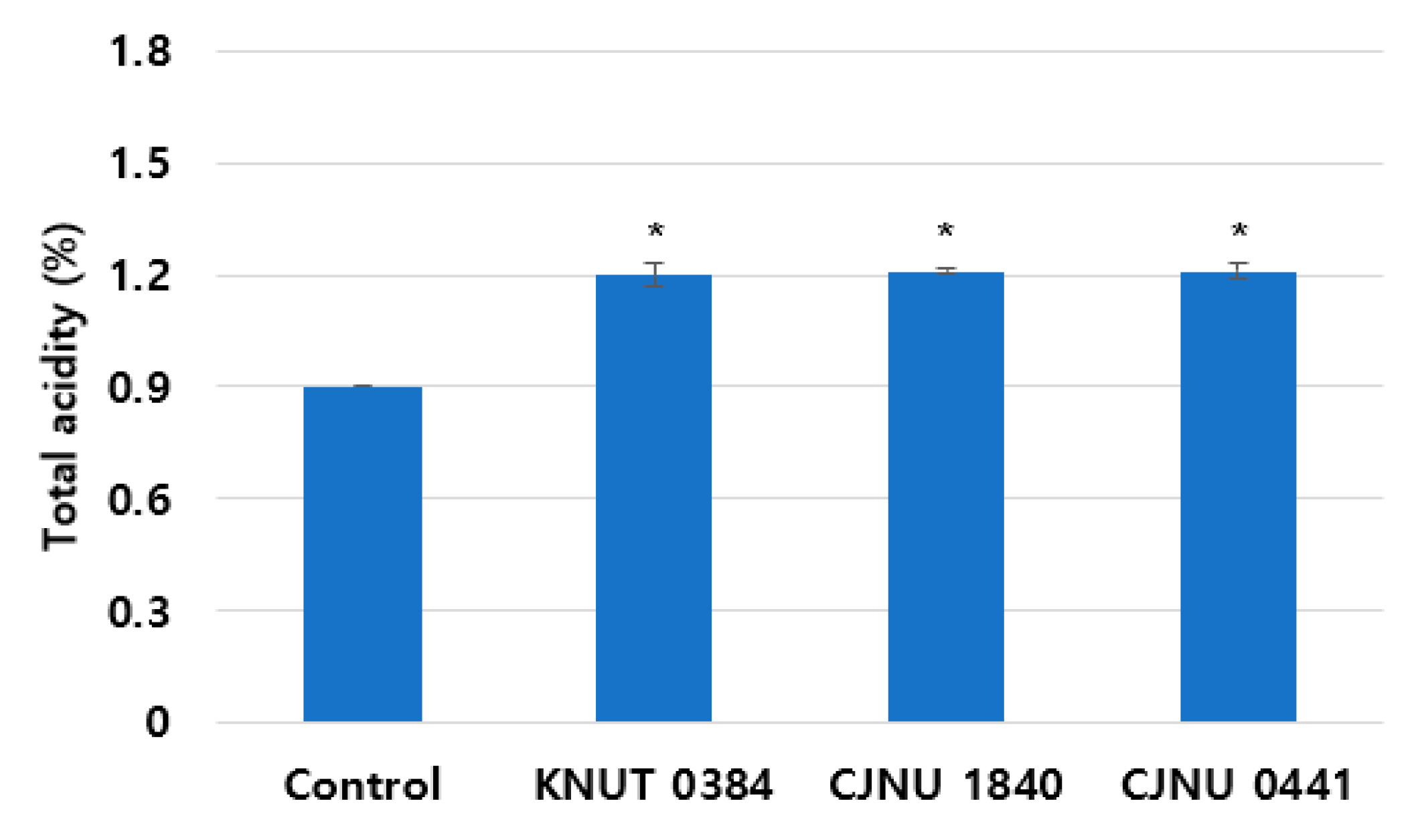
3.2. Acidity Change of the Fermented Coffee Extract
3.3. Increased Cell Mass of the Fermented Coffee Extract
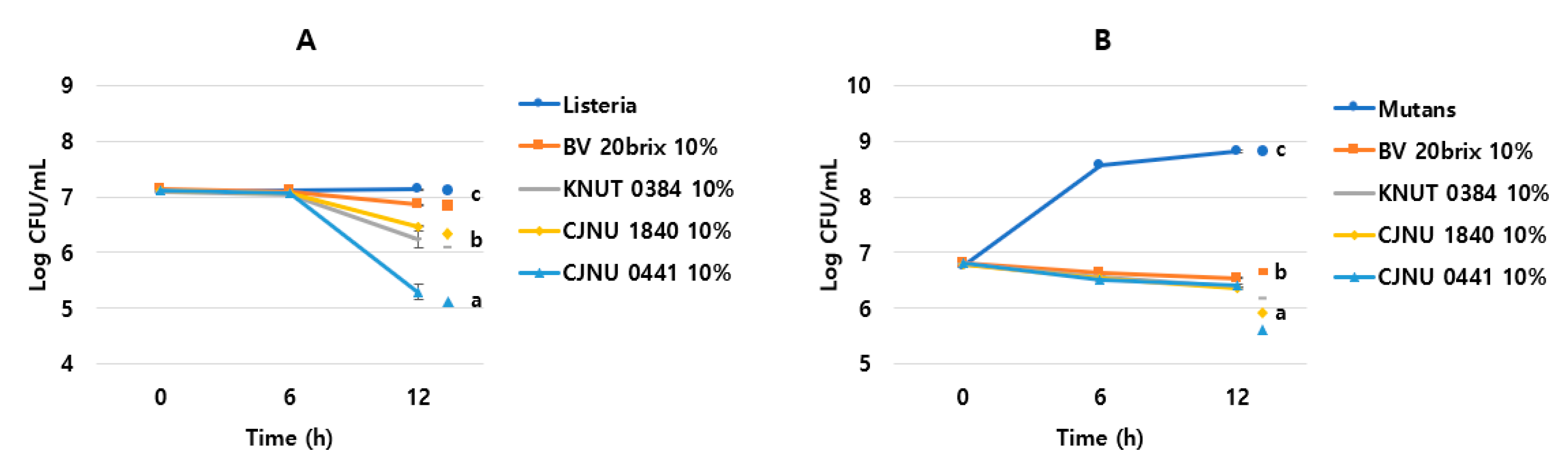
3.4. Increased Antimicrobial Activity of the Fermented Coffee Extract
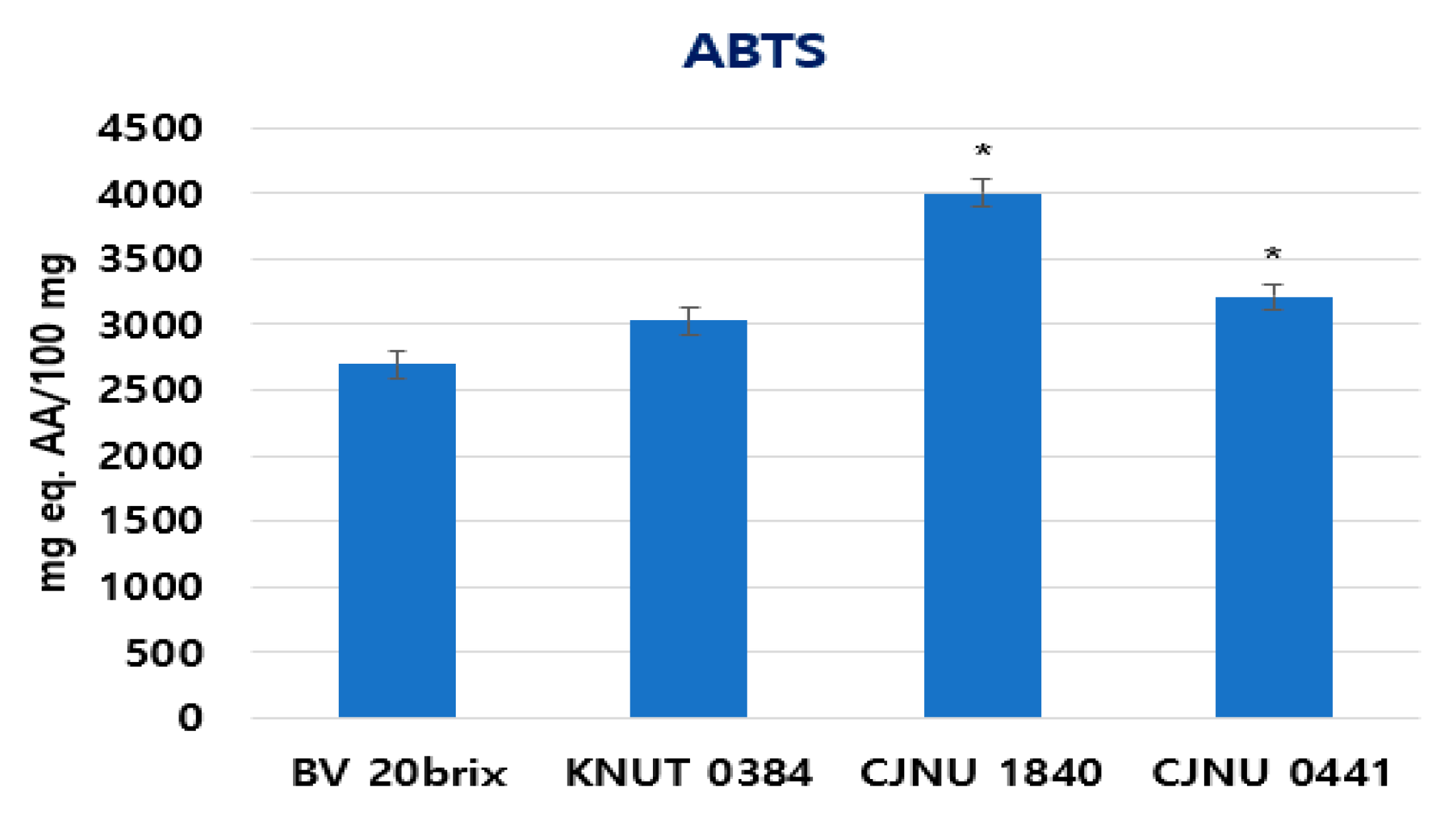
3.5. Increased Anti-Oxidative Activity of the Fermented Coffee Extract
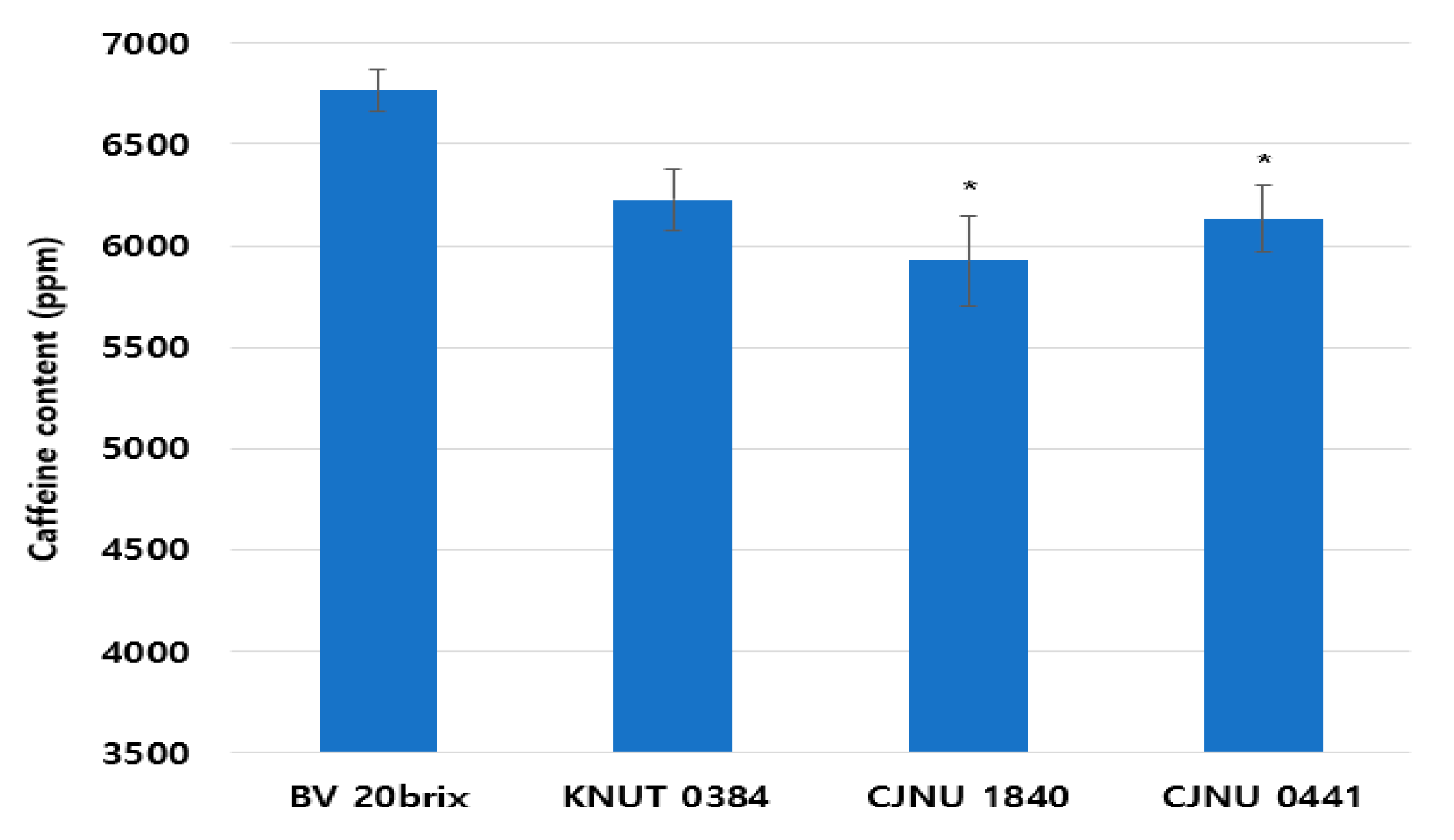
3.6. Decreased Caffeine Content of the Fermented Coffee Extract
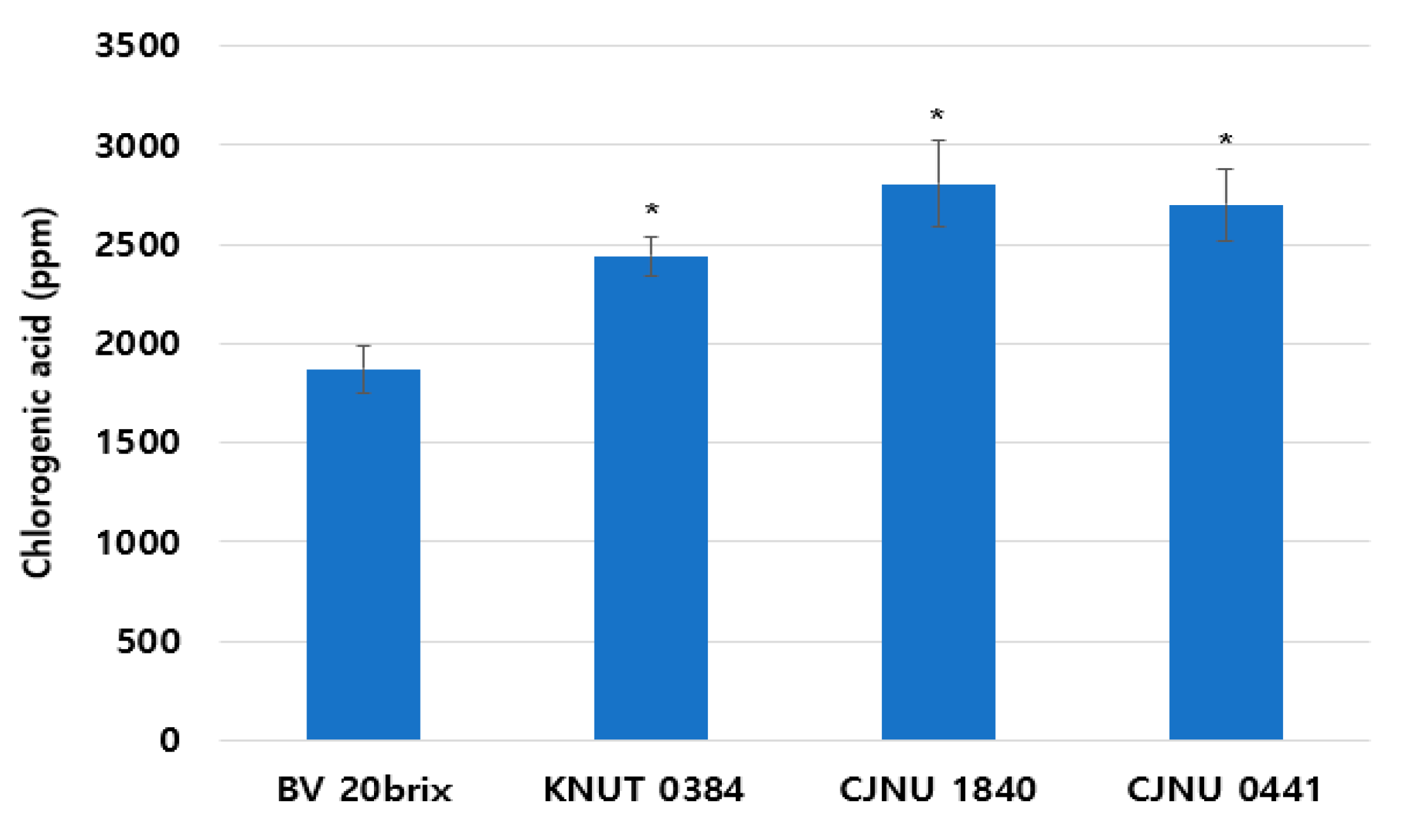
3.7. Increased Chlorogenic Acid Content in the Fermented Coffee Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of Coffee Brewing Methods on the Chromatographic and Spectroscopic Profiles, Antioxidant and Sensory Properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial Activity of Coffee Extracts and Selected Coffee Chemical Compounds against Enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Bae, J.-H.; Park, J.-H.; Im, S.-S.; Song, D.-K. Coffee and Health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.statista.com/statistics/292595/global-coffee-consumption (accessed on 27 June 2023).
- Song, I.S.; Han, K.; Ryu, J.J.; Choi, Y.J.; Park, J.B. Coffee Intake as a Risk Indicator for Tooth Loss in Korean Adults. Sci. Rep. 2018, 8, 2392. [Google Scholar] [CrossRef]
- Samoggia, A.; Riedel, B. Consumers’ Perceptions of Coffee Health Benefits and Motives for Coffee Consumption and Purchasing. Nutrients 2019, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, M.; Stanek, N.; Barabosz, K.; Jaszkiewicz, A.; Kulesza, R.; Matejuk, R.; Andrzejewski, D.; Biłos, Ł.; Porada, A. Comparison of Chemical Compounds and Their Influence on the Taste of Coffee Depending on Green Beans Storage Conditions. Sci. Rep. 2022, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef]
- Wierzejska, R. Can Coffee Consumption Lower the Risk of Alzheimer’s Disease and Parkinson’s Disease? A Literature Review. Arch. Med. Sci. 2017, 13, 507–514. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Kałuzna-Czaplińska, J.; Rosiak, A.; Chrzescijanska, E. Antioxidant Properties of Green Coffee Extract. Forests 2020, 11, 557. [Google Scholar] [CrossRef]
- Lee, I.-C.; Lee, J.-S.; Lee, J.-H.; Kim, Y.; So, W.-Y. Anti-Oxidative and Anti-Inflammatory Activity of Kenya Grade AA Green Coffee Bean Extracts. Iran. J. Public Health 2019, 48, 2025. [Google Scholar] [CrossRef]
- Nuhu, A.A. Bioactive Micronutrients in Coffee: Recent Analytical Approaches for Characterization and Quantification. ISRN Nutr. 2014, 2014, 384230. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional Properties of Coffee and Coffee By-Products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.Á.; Leake, D.S.; Ames, J.M. In Vitro Antioxidant Activity of Coffee Compounds and Their Metabolites. J. Agric. Food Chem. 2007, 55, 6962–6969. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Grześkowiak, T. Analytical Methods Applied for the Characterization and the Determination of Bioactive Compounds in Coffee. Eur. Food Res. Technol. 2015, 240, 19–31. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, D.S.; Kim, H.S.; Park, T.; Kim, J.K.; Kim, S.W.; Lee, B.H.; Lee, H.I.; Kim, I.; Jeong, Y.; et al. Bioactivity of Fermented Green Coffee Bean Extract Containing High Chlorogenic Acid and Surfactin. J. Med. Food 2019, 22, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Le Gall, G.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16-O-Methylcafestol Is Present in Ground Roast Arabica Coffees: Implications for Authenticity Testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef]
- de Mendes, G.A.; de Oliveira, M.A.L.; Rodarte, M.P.; dos Anjos, V.d.C.; Bell, M.J.V. Determination of Arabica and Robusta Species in Blends of Roasted Coffee by Mid Infrared Spectroscopy in Association with Mixture Design. Food Chem. Adv. 2024, 4, 100592. [Google Scholar] [CrossRef]
- Langi, T.M.; Paat, F.J.; Kusuma, S.D.A.; Oessoe, Y.Y.E.; Liwu, S.L.; Mamuaja, C.F.; Latumakulita, L.A.; Tooy, D.; Rumambi, D.P.; Pinatik, H.F.; et al. The effect of arabica and robusta coffee blends on caffeine content, acidity and organoleptic properties of instant coffee. J. Agric. 2023, 2, 183–192. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Antioxidant Activity, Total Polyphenol, Flavonoid and Tannin Contents of Fermented Green Coffee Beans with Selected Yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- de Pereira, G.V.M.; Soccol, V.T.; Soccol, C.R. Current State of Research on Cocoa and Coffee Fermentations. Curr. Opin. Food Sci. 2016, 7, 50–57. [Google Scholar] [CrossRef]
- Girma, B.; Sualeh, A. A Review of Coffee Processing Methods and Their Influence on Aroma. Int. J. Food Eng. Technol. 2022, 6, 7–16. [Google Scholar] [CrossRef]
- de Carvalho Neto, D.P.; de Melo Pereira, G.V.; Finco, A.M.O.; Letti, L.A.J.; da Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient Coffee Beans Mucilage Layer Removal Using Lactic Acid Fermentation in a Stirred-Tank Bioreactor: Kinetic, Metabolic and Sensorial Studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Sánchez-Riaño, A.M.; Vega-Oliveros, C.; Ladino-Garzón, W.L.; Orozco-Blanco, D.A.; Bahamón-Monje, A.F.; Gutiérrez-Guzmán, N.; Amorocho-Cruz, C.M. Effects of Cherries Sanitization Methods and Fermentation Times on Quality Parameters of Coffee Beans. Heliyon 2024, 10, e33508. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Kokawa, M.; Suzuki, T.; Khan, A.R.; Dong, W.; Nguyen, M.Q.; Kitamura, Y. Refermentation with Yeast and Lactic Acid Bacteria Isolates: A Strategy to Improve the Flavor of Green Coffee Beans. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef]
- Su, L.W.; Cheng, Y.H.; Hsiao, F.S.H.; Han, J.C.; Yu, Y.H. Optimization of Mixed Solid-State Fermentation of Soybean Meal by Lactobacillus Species and Clostridium Butyricum. Pol. J. Microbiol. 2018, 67, 297–305. [Google Scholar] [CrossRef] [PubMed]
- de Pereira, G.V.M.; de Carvalho Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of Lactic Acid Bacteria to Improve the Fermentation and Quality of Coffee during On-Farm Processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- Zhao, N.; Kokawa, M.; Amini, R.K.; Dong, W.; Kitamura, Y. Isolation of Yeast and LAB from Dry Coffee Pulp and Monitoring of Organic Acids in Inoculated Green Beans. Foods 2023, 12, 2622. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Coote, N.; Kirsop, B.H. Factors responsible for the decrease in ph during beer fermentations. J. Inst. Brew. 1976, 82, 149–153. [Google Scholar] [CrossRef]
- Silva, L.C.F.; Pereira, P.V.R.; da Cruz, M.A.D.; Costa, G.X.R.; Rocha, R.A.R.; Bertarini, P.L.L.; do Amaral, L.R.; Gomes, M.S.; Santos, L.D. Enhancing Sensory Quality of Coffee: The Impact of Fermentation Techniques on Coffea Arabica Cv. Catiguá MG2. Foods 2024, 13, 653. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Das, U.; Panda, S.K.; Saranraj, P. Microorganisms in Fermentation. In Essentials in Fermentation Technology; Springer Nature: Cham, Switzerland, 2019; pp. 1–39. [Google Scholar]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial Effect of Phenolic Compounds from Different Wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Rochín-Medina, J.J.; Ramírez, K.; Rangel-Peraza, J.G.; Bustos-Terrones, Y.A. Increase of Content and Bioactivity of Total Phenolic Compounds from Spent Coffee Grounds through Solid State Fermentation by Bacillus Clausii. J. Food Sci. Technol. 2018, 55, 915–923. [Google Scholar] [CrossRef]
- Santos da Silveira, J.; Durand, N.; Lacour, S.; Belleville, M.P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-State Fermentation as a Sustainable Method for Coffee Pulp Treatment and Production of an Extract Rich in Chlorogenic Acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Farah, A.; Lima, J.d.P. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Yosboonruang, A.; Ontawong, A.; Thapmamang, J.; Duangjai, A. Antibacterial Activity of Coffea Robusta Leaf Extract against Foodborne Pathogens. J. Microbiol. Biotechnol. 2022, 32, 1003–1010. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Jiménez-Monreal, A.M.; García-Jiménez, L.; Almela, L.; García-Diz, L.; Mariscal-Arcas, M.; Murcia, M.A. Assessment of Antimicrobial Activity of Coffee Brewed in Three Different Ways from Different Origins. Eur. Food Res. Technol. 2011, 233, 497–505. [Google Scholar] [CrossRef]
- Le, Y.J.; He, L.Y.; Li, S.; Xiong, C.J.; Lu, C.H.; Yang, X.Y. Chlorogenic Acid Exerts Antibacterial Effects by Affecting Lipid Metabolism and Scavenging ROS in Streptococcus Pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef] [PubMed]
- Hatiningsih, S.; Antara, N.S.; Gunam, I.B.W. Microbiological and Physicochemical Changes of Green Coffee (Coffea Arabica) Fermentation in Kintamani, Bangli, Bali. Sci. J. Food Technol. 2018, 5, 123–138. [Google Scholar]
- Purwoko, T.; Suranto; Setyaningsih, R.; Marliyana, S.D. Caffeine Degradation by Food Microorganisms. Biodiversitas 2023, 24, 3495–3502. [Google Scholar] [CrossRef]
- Szlapinski, S.K.; Charrette, A.; Guthrie, N.; Hilmas, C.J. Paraxanthine Safety and Comparison to Caffeine. Front. Toxicol. 2023, 5, 1117729. [Google Scholar] [CrossRef]
| Time (min) | 0 | 8 | 30 | 35 | 40 | 50 | 60 |
|---|---|---|---|---|---|---|---|
| Solvent A | 100% | 100% | 0% | 0% | 100% | 100% | 100% |
| Solvent B | 0% | 0% | 100% | 100% | 0% | 0% | 0% |
| Fermentation | Cell Mass (g/L) | |
|---|---|---|
| Pediococcus pentosaceus KNUT 0384 | 0 h | 7.88 |
| 12 h | 8.78 | |
| Lacticaseibacillus paracasei CJNU 1840 | 0 h | 8.33 |
| 12 h | 10.86 | |
| Lactiplantibacillus plantarum CJNU 0441 | 0 h | 8.23 |
| 12 h | 10.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-G.; Abbas, A.; Moon, G.-S. Improved Functions of Fermented Coffee by Lactic Acid Bacteria. Appl. Sci. 2024, 14, 7596. https://doi.org/10.3390/app14177596
Kim S-G, Abbas A, Moon G-S. Improved Functions of Fermented Coffee by Lactic Acid Bacteria. Applied Sciences. 2024; 14(17):7596. https://doi.org/10.3390/app14177596
Chicago/Turabian StyleKim, Seon-Gyu, Aoun Abbas, and Gi-Seong Moon. 2024. "Improved Functions of Fermented Coffee by Lactic Acid Bacteria" Applied Sciences 14, no. 17: 7596. https://doi.org/10.3390/app14177596
APA StyleKim, S.-G., Abbas, A., & Moon, G.-S. (2024). Improved Functions of Fermented Coffee by Lactic Acid Bacteria. Applied Sciences, 14(17), 7596. https://doi.org/10.3390/app14177596









