Determination of Diffusion Coefficients of Bisphenol A (BPA) in Polyethylene Terephthalate (PET) to Estimate Migration of BPA from Recycled PET into Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Materials
2.2. Sample Preparation for the Determination of Bisphenol A in PET
2.3. Migration Kinetics of Bisphenol A from PET into Food Simulants
2.4. Quantification of Bisphenol A in Dichloromethane Extracts and Food Simulants
3. Results
3.1. Concentations of Bisphenol A in Recyclate Containing PET Bottles in Europe
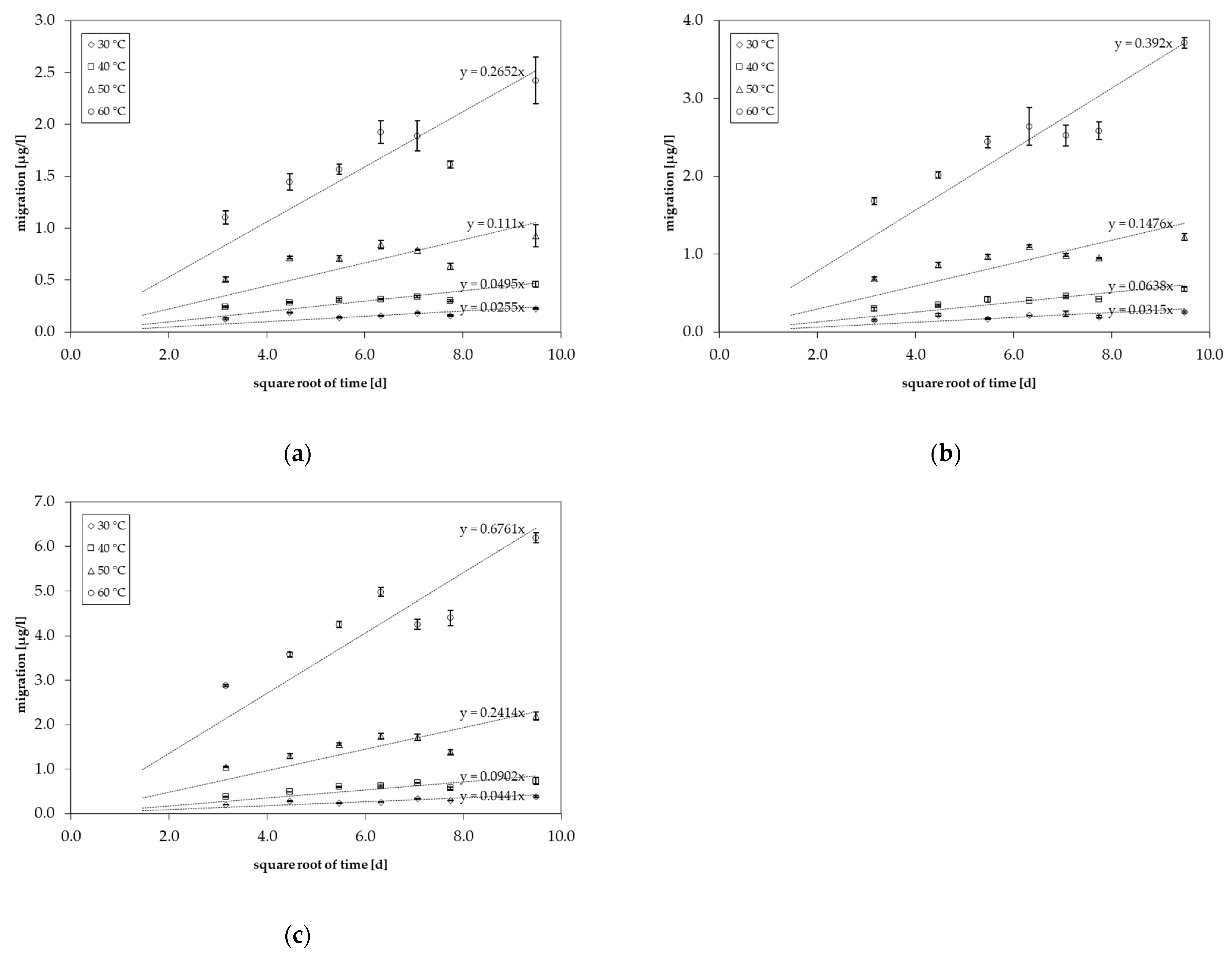
3.2. Migration Kinetics into Food Simulants
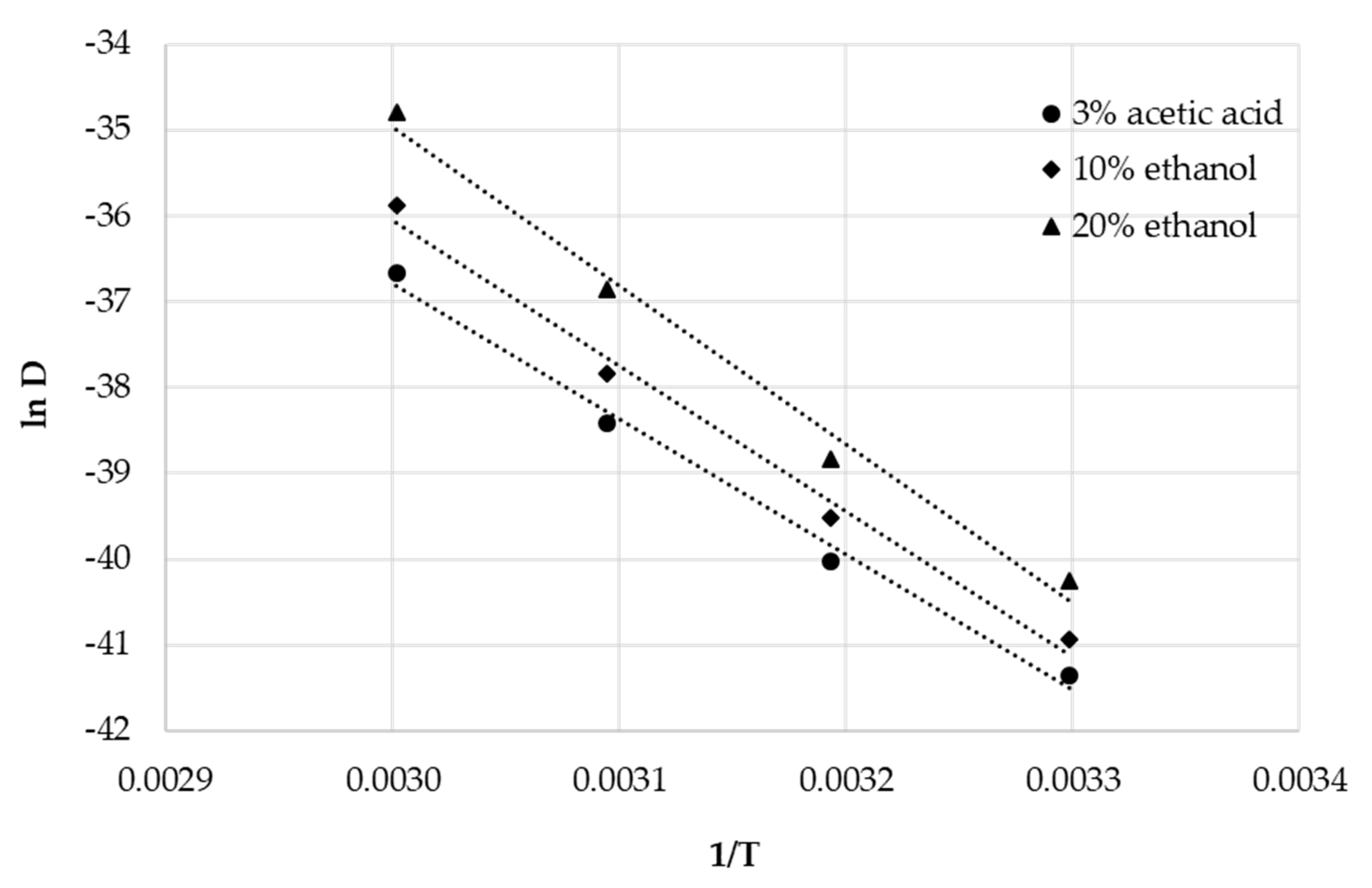
3.3. Diffusion Coefficients and Activation Energies of Diffusion
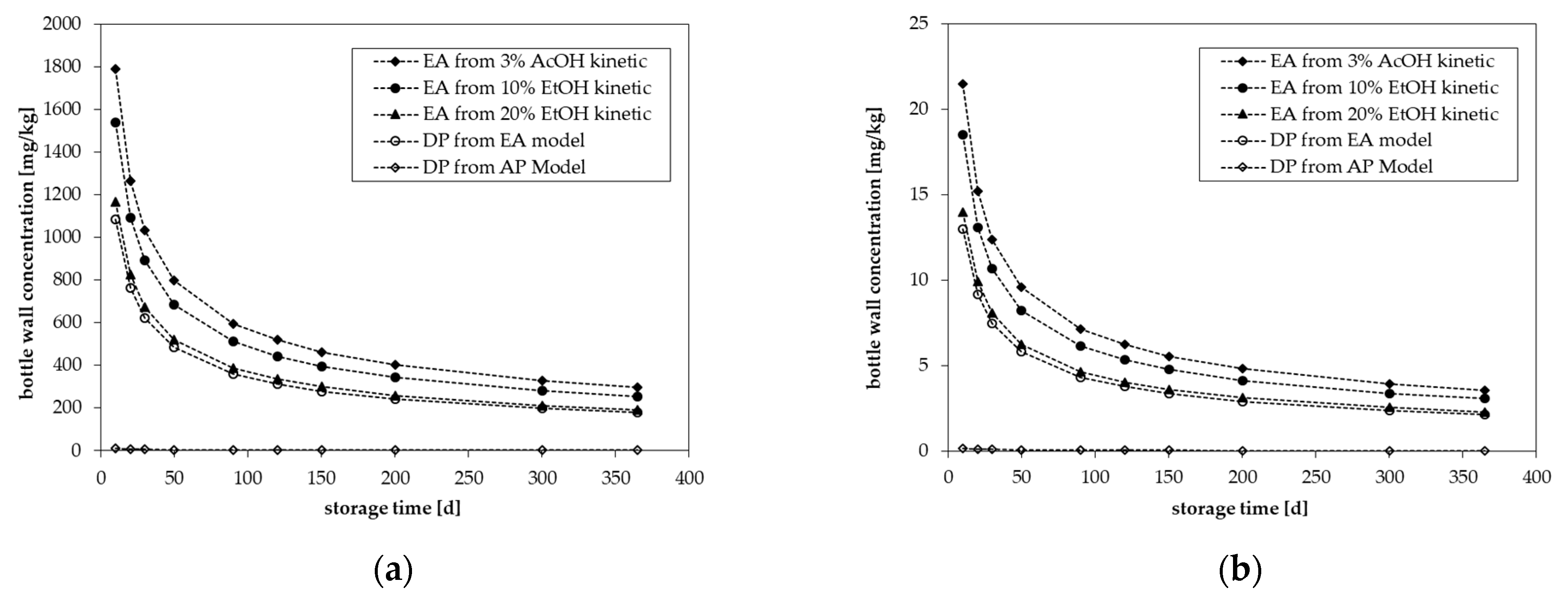
3.4. Migration Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriacos, D. Polycarbonates. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; Chapter 17; pp. 457–485. ISBN 978-0-323-35824-8. [Google Scholar] [CrossRef]
- Kadri, Z.; Mechnou, I.; Zyade, S. Migration of bisphenol a from epoxy coating to foodstuffs. Mater. Today Proc. 2021, 45, 7584–7587. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. A probabilistic approach to model bisphenol A (BPA) migration from packaging to meat products. Sci. Total Environ. 2023, 854, 158815. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-L.; Popovi, S.; Dabeka, R.W. Trends of bisphenol A occurrence in canned food products from 2008–2020. Food Addit. Contam. Part A 2023, 40, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Sadrabad, E.K.; Hashemi, S.A.; Nadjarzadeh, A.; Askari, E.; Mohajeri, F.A.; Ramroudi, F. Bisphenol A release from food and beverage containers—A review. Food Sci. Nutr. 2023, 11, 3718–3728. [Google Scholar] [CrossRef]
- Holmes, R.; Ma, J.; Andra, S.S.; Wang, H.-S. Effect of common consumer washing methods on bisphenol A release in tritan drinking bottles. Chemosphere 2021, 277, 130355. [Google Scholar] [CrossRef] [PubMed]
- Kaykhaii, M.; Yavari, E.; Sargazi, G.; Ebrahimi, A.K. Highly sensitive determination of bisphenol A in bottled water samples by HPLC after its extraction by a novel Th-MOF Pipette-Tip Micro-SPE. J. Chromatogr. Sci. 2020, 58, 373–382. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Dreolin, N.; Aznar, M.; Moret, S.; Nerin, C. Development and validation of a LC–MS/MS method for the analysis of bisphenol A in polyethylene terephthalate. Food Chem. 2019, 274, 246–253. [Google Scholar] [CrossRef]
- EU Commission. Commission directive 2002/72/EC of 6 August 2002 relating to plastic materials and articles intended to come into contact with foodstuffs. Off. J. Eur. Communities L 2002, 220, 18–58. [Google Scholar]
- EU Commission. Commission Directive 2004/19/EC of 1 March 2004 amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with foodstuffs. Off. J. L 2004, 71, 8–21. [Google Scholar]
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to 2,2-bis(4-hydroxyphenyl)propane (Bisphenol A); Question number EFSA-Q-2005-100. EFSA J. 2006, 5, 428. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Statement on the ANSES reports on Bisphenol, A. EFSA J. 2011, 9, 2475. [Google Scholar] [CrossRef]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- EU Commission. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union L 2018, 41, 6–12. [Google Scholar]
- EU Commission. Commission Directive (EU) 2020/2184 of the European Parliament and of the council of 16 December 2020 on the quality of water intended for human consumption (recast). Off. J. Eur. Union L 2020, 435, 1–62. [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids). Scientific Opinion on the re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, 6857. [Google Scholar] [CrossRef]
- European Medicines Agency: Report on Divergent Opinion between EFSA and EMA on Bisphenol-A_Final Adopted EMA/150385/2023; European Medicines Agency: Amsterdam, The Netherlands, 2023.
- Leist, M.; Buettner, A.; Diel, P.; Eisenbrand, G.; Epe, B.; Först, P.; Grune, T.; Haller, D.; Heinz, V.; Hellwig, M.; et al. Controversy on health-based guidance values for bisphenol A—The need of criteria for studies that serve as a basis for risk assessment. Arch. Toxicol. 2024, 98, 1967–1973. [Google Scholar] [CrossRef]
- Bisphenol A: BfR Proposes Health Based Guidance Value, Current Exposure Data Are Needed for a Full Risk Assessment: BfR Opinion No 018/2023 issued 19 April 2023; Bundesinstitut für Risikobewertung: Berlin, Germany, 2023. [CrossRef]
- The Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment (COT), 28 June 2024. Available online: https://cot.food.gov.uk/Position%20paper%20on%20bisphenol%20A (accessed on 25 August 2024).
- Draft Regulation on the Use of Bisphenol A (BPA) and Other Bisphenols and Their Derivatives with Harmonised Classification for Specific Hazardous Properties in Certain Materials and Articles Intended to Come into Contact with Food, Amending Regulation (EU) No 10/2011 and Repealing Regulation (EU) 2018/213, 5 June 2024. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=PI_COM%3AAres%282024%29988745 (accessed on 25 August 2024).
- EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF). Scientific Opinion on the criteria to be used for safety evaluation of a mechanical recycling process to produce recycled PET intended to be used for manufacture of materials and articles in contact with food. EFSA J. 2011, 9, 2184. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA J. 2019, 17, 5708. [Google Scholar] [CrossRef]
- Kroes, R.; Kleiner, J.; Renwick, A. The threshold of toxicological concern concept in risk assessment. Toxicol. Sci. 2005, 86, 226–230. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Recycling of post-consumer packaging materials into new food packaging applications—Critical review of the European approach and future perspectives. Sustainability 2022, 14, 824. [Google Scholar] [CrossRef]
- Franz, R.; Gmeiner, M.; Gruner, A.; Kemmer, D.; Welle, F. Diffusion behaviour of the acetaldehyde scavenger 2-aminobenzamide in polyethylene terephthalate for beverage bottles. Food Addit. Contam. Part A 2016, 33, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Welle, F.; Franz, R. Migration of antimony from PET bottles into beverages: Determination of the activation energy of diffusion and migration modelling compared to literature data. Food Addit. Contam. Part A 2011, 28, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Stärker, C.; Welle, F. Migration of bisphenol A from can coatings into beverages at the end of shelf life compared to regulated test conditions. Beverages 2019, 5, 3. [Google Scholar] [CrossRef]
- Gehring, C.; Welle, F. Migration testing of polyethylene terephthalate: Comparison of regulated test conditions with migration into real food at the end of shelf life. Packag. Technol. Sci. 2018, 31, 771–780. [Google Scholar] [CrossRef]
- Gehring, C.; Welle, F. Migration of acetaldehyde scavengers from PET bottles. Ref. Modul. Food Sci. 2017, 1–6. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Migration measurement and modelling from poly(ethylene terephthalate) (PET) into softdrinks and fruit juices in comparison with food simulants. Food Addit. Contam. Part A 2008, 25, 1033–1046. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union L 2011, 12, 1–89.
- Begley, T.; Castle, L.; Feigenbaum, A.; Franz, R.; Hinrichs, K.; Lickly, T.; Mercea, P.; Milana, M.; O’Brian, A.; Rebre, S.; et al. Evaluation of migration models that might be used in support of regulations for food-contact plastics. Food Addit. Contam. 2005, 22, 73–90. [Google Scholar] [CrossRef]
- Practical Guidelines on the Application of Migration Modelling for the Estimation of Specific Migration, EU Report 27529 EN. 2015; ISBN 978-92-79-52790-6. Available online: https://op.europa.eu/de/publication-detail/-/publication/1b79bc61-97f6-11e5-983e-01aa75ed71a1 (accessed on 19 February 2024).
- Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 1845–1851. [Google Scholar] [CrossRef]
- Ewender, J.; Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate)—Validation data. Pack. Techn. Sci. 2022, 35, 405–413. [Google Scholar] [CrossRef]
- Nunez, S.S.; Ortuno, N.; Fernandez-Duran, S.; Molto, J.; Conesa, J.A. Analysis and removal of bisphenols in recycled plastics using polyethylene glycol. Sci. Rep. 2024, 14, 12824. [Google Scholar] [CrossRef]
- Nguyen, P.-M.; Berrard, C.; Daoud, N.; Saillard, P.; Peyroux, J.; Vitrac, O. Assessment of chemical risks and circular economy implications of recycled PET in food packaging with functional barriers. Resour. Environ. Sustain. 2024, 17, 100163. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Zheng, J.-L.; Ren, J.-H.; Luo, J.; Cui, X.-Y.; Ma, L.Q. Effects of storage temperature and duration on release of antimony and bisphenol a from polyethylene terephthalate drinking water bottles of China. Environ. Pollut. 2014, 192, 113–120. [Google Scholar] [CrossRef]
- Guart, A.; Bono-Blay, F.; Borrell, A.; Lacorte, S. Migration of plasticizers phthalates, bisphenol A and alkylphenols from plastic containers and evaluation of risk. Food Addit. Contam. Part A 2011, 28, 676–685. [Google Scholar] [CrossRef]
- Amiridou, D.; Voutsa, D. Alkyphenols and phthalates in bottled waters. J. Hazard. Mater. 2011, 185, 281–286. [Google Scholar] [CrossRef]
- Ginter-Kramarczyk, D.; Zembrzuska, J.; Kruszelnicka, I.; Zajac-Woznialis, A.; Cislak, M. Influence of temperature on the quantity of Bisphenol A in bottled drinking water. Int. J. Environ. Res. Public Health 2022, 19, 5710. [Google Scholar] [CrossRef] [PubMed]
- Baz, L.; Alharbi, A.; Al-Zahrani, M.; Alkhabbaz, S.; Alsousou, R.; Aljawadri, H. The effect of different storage conditions on the levels of bisphenol A in bottled drinking water in Jeddah City, Saudi Arabia. Adv. Public Health 2023, 2023, 8278428. [Google Scholar] [CrossRef]
- Marsolea, A.C.; Chiriac, F.L.; Orbeci, C.; Bobirica, L.; Bobirica, C. Migration and leaching behaviour of bisphenol A from polyethylene terephthalate water bottles under different storage conditions. Int. J. Food Sci. Technol. 2023, 58, 5609–5615. [Google Scholar] [CrossRef]
- Khanniria, E.; Bayanatia, M.; Koushkia, M.R.; Ferdosia, R.; Sohrabvandia, S.; Esmaeilia, S.; Akbarib, M.E.; Forouharc, P. Migration of bisphenol A and several phthalate acid contaminants into bottled drinking water: Influence of storage conditions and their health risks. Int. J. Environ. Anal. Chem. 2023. preview. [Google Scholar] [CrossRef]
| Concentration [mg/kg] | ||||||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2021 | 2022 | 2023 | 2019–2023 | |
| maximum | 5.28 | 4.68 | 20.0 | 56.6 | 18.4 | 56.6 |
| minimum | 0.033 | 0.020 | 0.073 | 0.260 | 0.050 | 0.020 |
| mean value | 0.715 | 0.737 | 2.42 | 4.16 | 1.50 | 1.74 |
| median | 0.450 | 0.592 | 0.893 | 0.867 | 0.760 | 0.703 |
| mean rPET content | 71% | 76% | 98% | 100% | 100% | 90% |
| analyzed samples | 23 | 116 | 51 | 56 | 129 | 375 |
| Food Simulant | Storage Time [d] | Concentration [µg/L] | |||
|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | ||
| 3% acetic acid | 10 | 0.128 ± 0.010 | 0.242 ± 0.010 | 0.511 ± 0.022 | 1.11 ± 0.06 |
| 20 | 0.188 ± 0.005 | 0.287 ± 0.007 | 0.716 ± 0.012 | 1.45 ± 0.08 | |
| 30 | 0.141 ± 0.005 | 0.310 ± 0.012 | 0.710 ± 0.029 | 1.57 ± 0.05 | |
| 40 | 0.155 ± 0.003 | 0.316 ± 0.005 | 0.841 ± 0.039 | 1.93 ± 0.11 | |
| 50 | 0.182 ± 0.006 | 0.341 ± 0.012 | 0.790 ± 0.007 | 1.89 ± 0.15 | |
| 60 | 0.159 ± 0.004 | 0.301 ± 0.009 | 0.634 ± 0.029 | 1.61 ± 0.04 | |
| 90 | 0.225 ± 0.008 | 0.460 ± 0.026 | 0.926 ± 0.106 | 2.42 ± 0.22 | |
| 10% ethanol | 10 | 0.154 ± 0.014 | 0.296 ± 0.026 | 0.688 ± 0.024 | 1.69 ± 0.044 |
| 20 | 0.221 ± 0.016 | 0.349 ± 0.012 | 0.863 ± 0.033 | 2.02 ± 0.042 | |
| 30 | 0.172 ± 0.009 | 0.423 ± 0.035 | 0.969 ± 0.034 | 2.44 ± 0.07 | |
| 40 | 0.215 ± 0.002 | 0.409 ± 0.002 | 1.10 ± 0.01 | 2.64 ± 0.25 | |
| 50 | 0.235 ± 0.033 | 0.462 ± 0.009 | 0.990 ± 0.017 | 2.53 ± 0.13 | |
| 60 | 0.198 ± 0.013 | 0.424 ± 0.002 | 0.953 ± 0.009 | 2.59 ± 0.12 | |
| 90 | 0.260 ± 0.010 | 0.547 ± 0.029 | 1.22 ± 0.043 | 3.72 ± 0.070 | |
| 20% ethanol | 10 | 0.205 ± 0.005 | 0.383 ± 0.004 | 1.05 ± 0.02 | 2.88 ± 0.02 |
| 20 | 0.286 ± 0.009 | 0.491 ± 0.004 | 1.30 ± 0.06 | 3.58 ± 0.06 | |
| 30 | 0.244 ± 0.005 | 0.603 ± 0.013 | 1.57 ± 0.03 | 4.25 ± 0.07 | |
| 40 | 0.259 ± 0.004 | 0.626 ± 0.018 | 1.74 ± 0.06 | 4.98 ± 0.10 | |
| 50 | 0.336 ± 0.010 | 0.700 ± 0.007 | 1.72 ± 0.07 | 4.26 ± 0.12 | |
| 60 | 0.298 ± 0.012 | 0.576 ± 0.026 | 1.39 ± 0.04 | 4.40 ± 0.17 | |
| 90 | 0.386 ± 0.019 | 0.736 ± 0.078 | 2.19 ± 0.10 | 6.20 ± 0.11 | |
| Food Simulant | Temperature [°C] | Diffusion Coefficient DP [cm2/s] | Activation Energy EA [kJ/mol] | Pre-Exponential Factor D0 [cm2/s] | Correlation Coefficient r2 |
|---|---|---|---|---|---|
| 3% acetic acid | 30 | 1.10 × 10−18 | |||
| 40 | 4.14 × 10−18 | 131.2 | 3.87 × 104 | 0.9922 | |
| 50 | 2.08 × 10−17 | ||||
| 60 | 1.19 × 10−16 | ||||
| 10% ethanol | 30 | 1.67 × 10−18 | |||
| 40 | 6.87 × 10−18 | 140.9 | 2.63 × 106 | 0.9901 | |
| 50 | 3.68 × 10−17 | ||||
| 60 | 2.60 × 10−16 | ||||
| 20% ethanol | 30 | 3.29 × 10−18 | |||
| 40 | 1.37 × 10−17 | 153.6 | 7.71 × 108 | 0.9883 | |
| 50 | 9.84 × 10−17 | ||||
| 60 | 7.72 × 10−16 |
| Temperature [°C] | Diffusion Coefficient [cm2/s] |
|---|---|
| 25 | 8.45 × 10−15 |
| 30 | 1.65 × 10−14 |
| 40 | 5.84 × 10−14 |
| 50 | 1.92 × 10−13 |
| 60 | 5.87 × 10−13 |
| Temperature [°C] | Diffusion Coefficient [cm2/s] | Activation Energy [kJ/mol] | Pre-Exponential Factor [cm2/s] |
|---|---|---|---|
| 25 | 1.09 × 10−18 | ||
| 30 | 3.33 × 10−18 | ||
| 40 | 2.72 × 10−17 | 165.8 | 1.22 × 1011 |
| 50 | 1.95 × 10−16 | ||
| 60 | 1.24 × 10−15 |
| Temperature | Storage Time [d] | Concentration [mg/kg] | ||||
|---|---|---|---|---|---|---|
| 3% Acetic Acid (EA exp) | 10% Ethanol (EA exp) | 20% Ethanol (EA exp) | DP Predicted EA Based Model | DP Predicted AP Model | ||
| 25 °C | 10 | 1792 | 1542 | 1167 | 1083 | 12.3 |
| 20 | 1267 | 1108 | 825 | 765 | 8.67 | |
| 30 | 1033 | 892 | 672 | 624 | 7.09 | |
| 50 | 800 | 686 | 521 | 487 | 5.52 | |
| 90 | 597 | 514 | 387 | 361 | 4.12 | |
| 120 | 519 | 444 | 336 | 314 | 3.57 | |
| 150 | 462 | 397 | 300 | 279 | 3.19 | |
| 200 | 402 | 344 | 260 | 226 | 2.76 | |
| 300 | 327 | 281 | 212 | 198 | 2.25 | |
| 365 | 297 | 255 | 192 | 179 | 2.04 | |
| 40 °C | 10 | 542 | 395 | 263 | 218 | 4.68 |
| 60 °C | 10 | 111 | 77.7 | 45.0 | 32.3 | 1.48 |
| Temperature | Storage Time [d] | Concentration [mg/kg] | ||||
|---|---|---|---|---|---|---|
| 3% Acetic Acid (EA exp) | 10% Ethanol (EA exp) | 20% Ethanol (EA exp) | DP Predicted EA Based Model | DP Predicted AP Model | ||
| 25 °C | 10 | 21.5 | 18.5 | 14.0 | 13.0 | 0.148 |
| 20 | 15.2 | 13.1 | 9.90 | 9.18 | 0.104 | |
| 30 | 12.4 | 10.7 | 8.07 | 7.49 | 0.0851 | |
| 50 | 9.60 | 8.23 | 6.25 | 5.84 | 0.0663 | |
| 90 | 7.16 | 6.17 | 4.65 | 4.33 | 0.0494 | |
| 120 | 6.23 | 5.33 | 4.03 | 3.77 | 0.0428 | |
| 150 | 5.54 | 4.77 | 3.60 | 3.35 | 0.0383 | |
| 200 | 4.82 | 4.13 | 3.12 | 2.91 | 0.0331 | |
| 300 | 3.93 | 3.37 | 2.54 | 2.38 | 0.0270 | |
| 365 | 3.57 | 3.06 | 2.30 | 2.15 | 0.0245 | |
| 40 °C | 10 | 6.50 | 4.74 | 3.16 | 2.62 | 0.0562 |
| 60 °C | 10 | 1.33 | 0.932 | 0.540 | 0.388 | 0.0178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juric, M.; Franz, R.; Welle, F. Determination of Diffusion Coefficients of Bisphenol A (BPA) in Polyethylene Terephthalate (PET) to Estimate Migration of BPA from Recycled PET into Foods. Appl. Sci. 2024, 14, 7704. https://doi.org/10.3390/app14177704
Juric M, Franz R, Welle F. Determination of Diffusion Coefficients of Bisphenol A (BPA) in Polyethylene Terephthalate (PET) to Estimate Migration of BPA from Recycled PET into Foods. Applied Sciences. 2024; 14(17):7704. https://doi.org/10.3390/app14177704
Chicago/Turabian StyleJuric, Mladen, Roland Franz, and Frank Welle. 2024. "Determination of Diffusion Coefficients of Bisphenol A (BPA) in Polyethylene Terephthalate (PET) to Estimate Migration of BPA from Recycled PET into Foods" Applied Sciences 14, no. 17: 7704. https://doi.org/10.3390/app14177704






