Study of the Effects of Plasma Pretreatment on the Microstructure of Peanuts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Instruments and Equipment
2.3. Experimental Procedure
2.3.1. Plasma Pretreatment of Peanuts
2.3.2. Observation of Peanut Surface Microstructure under a Scanning Electron Microscope
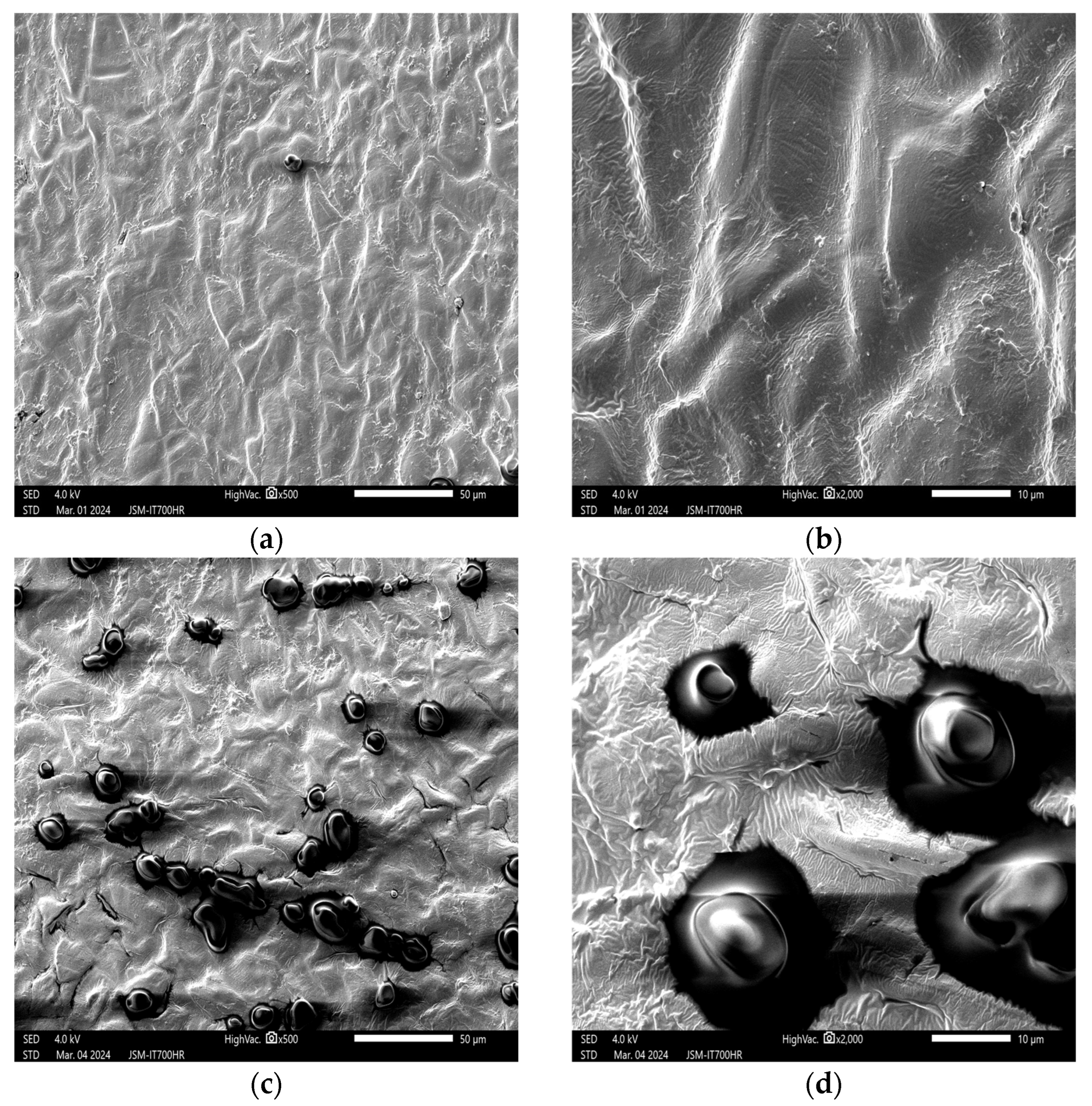
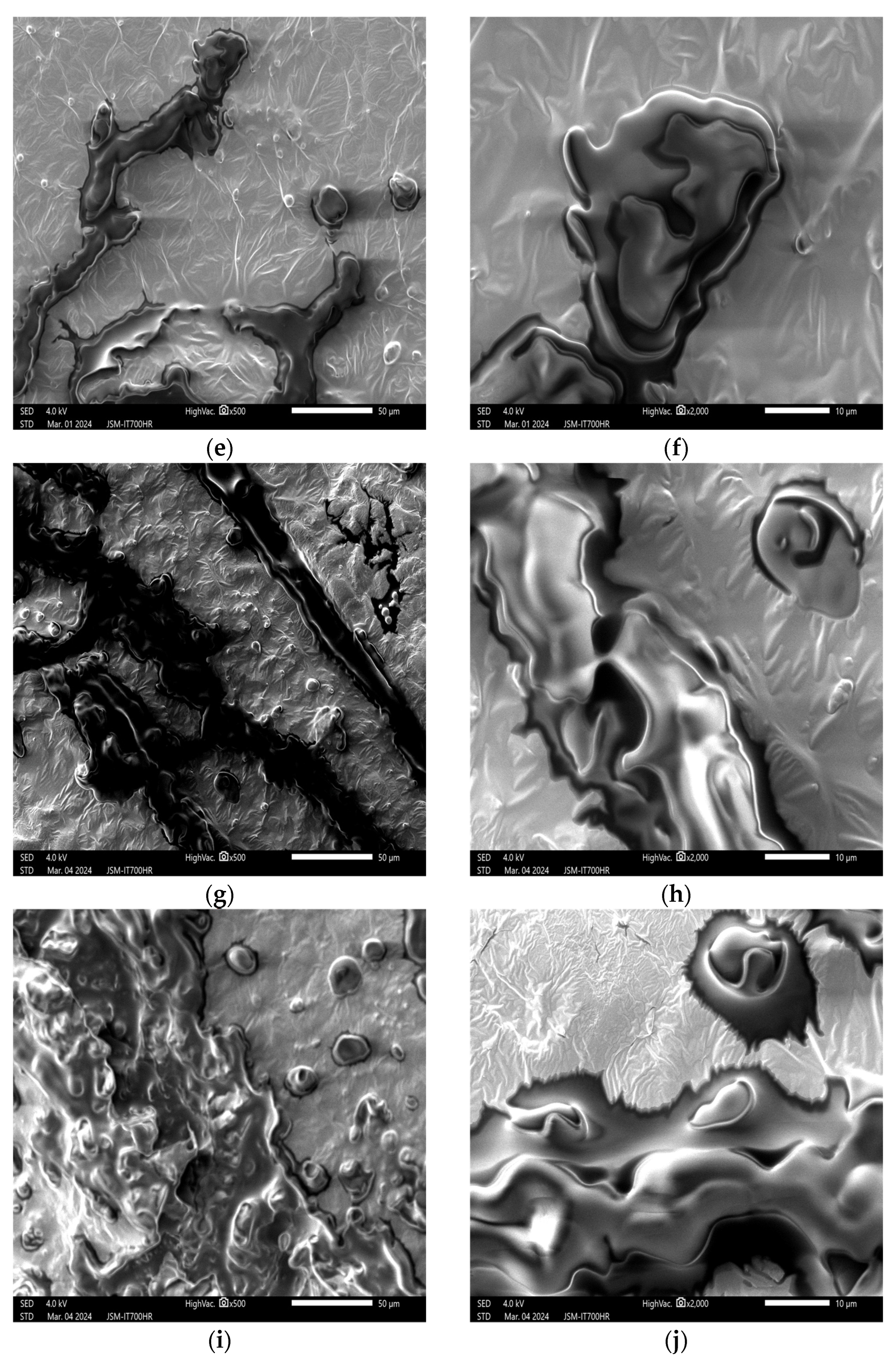
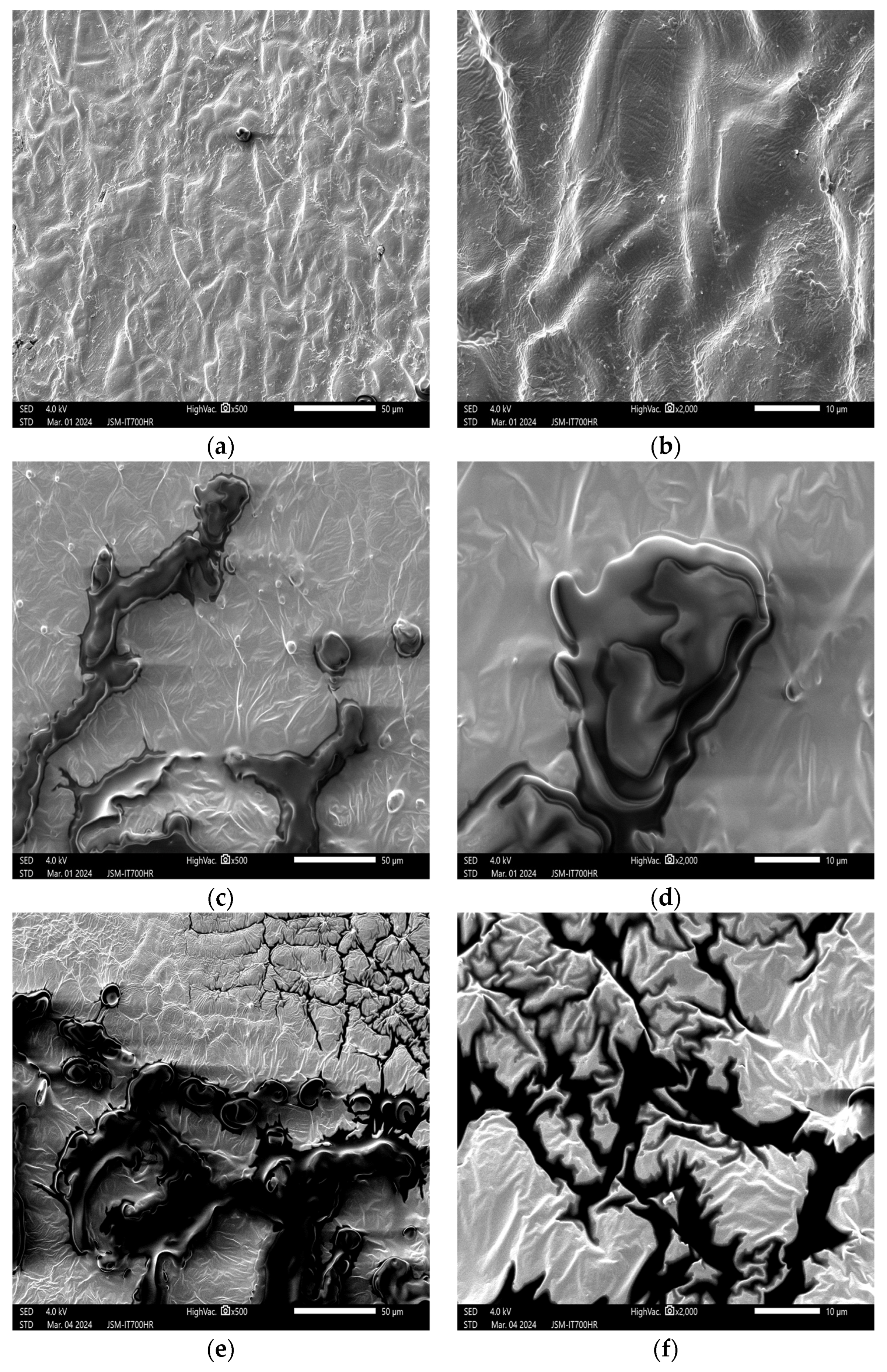
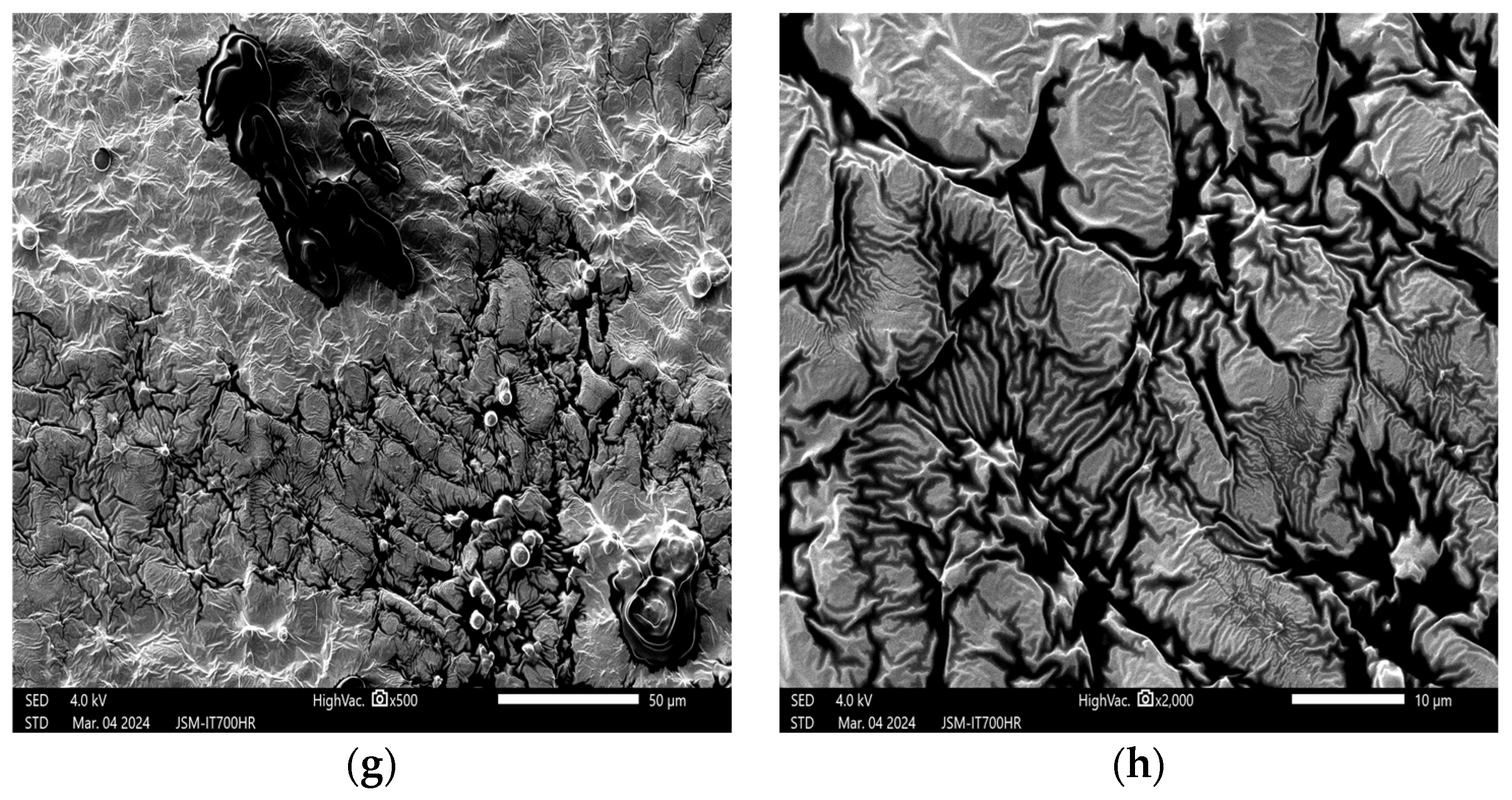
3. Results and Discussion
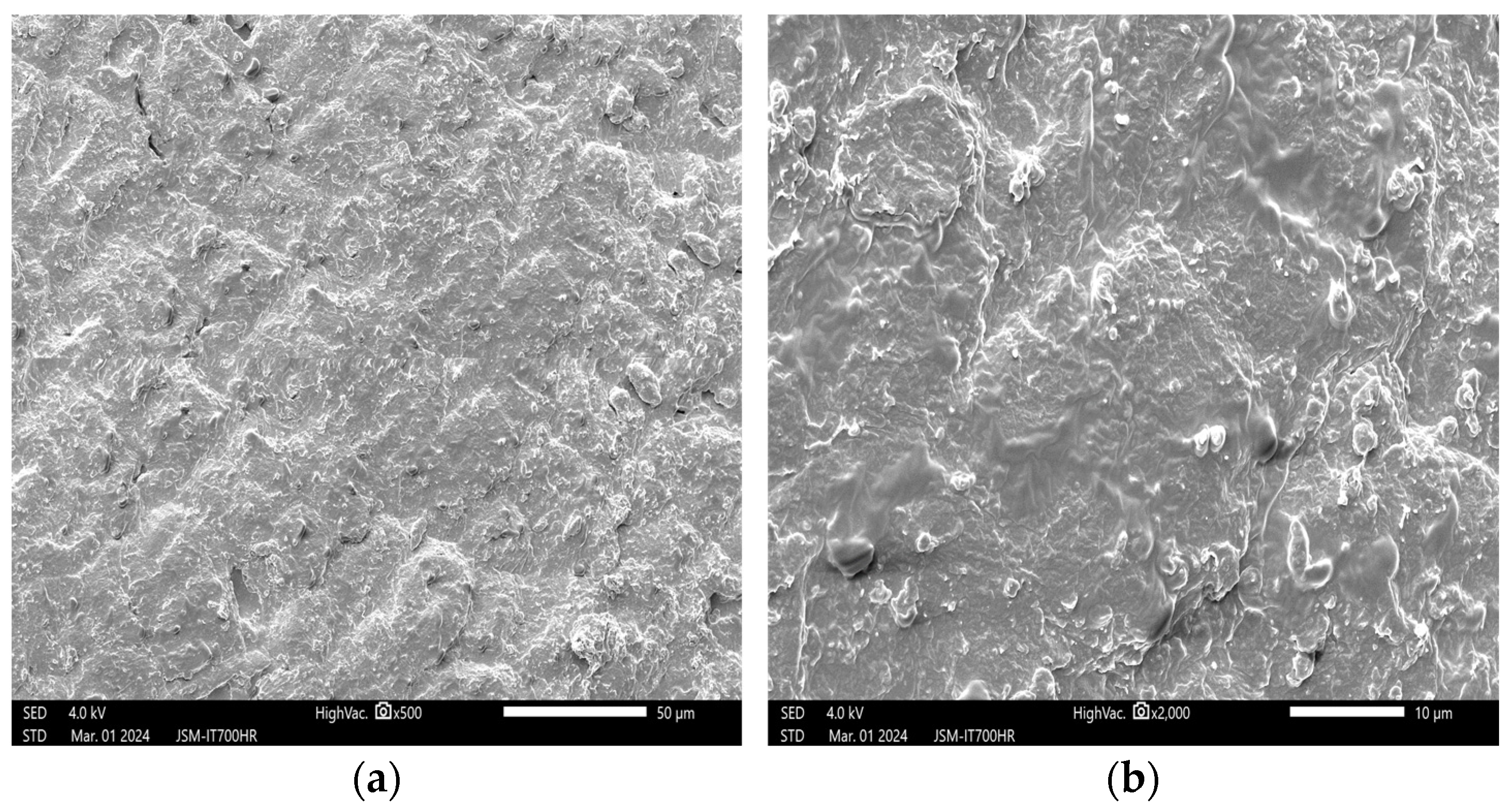
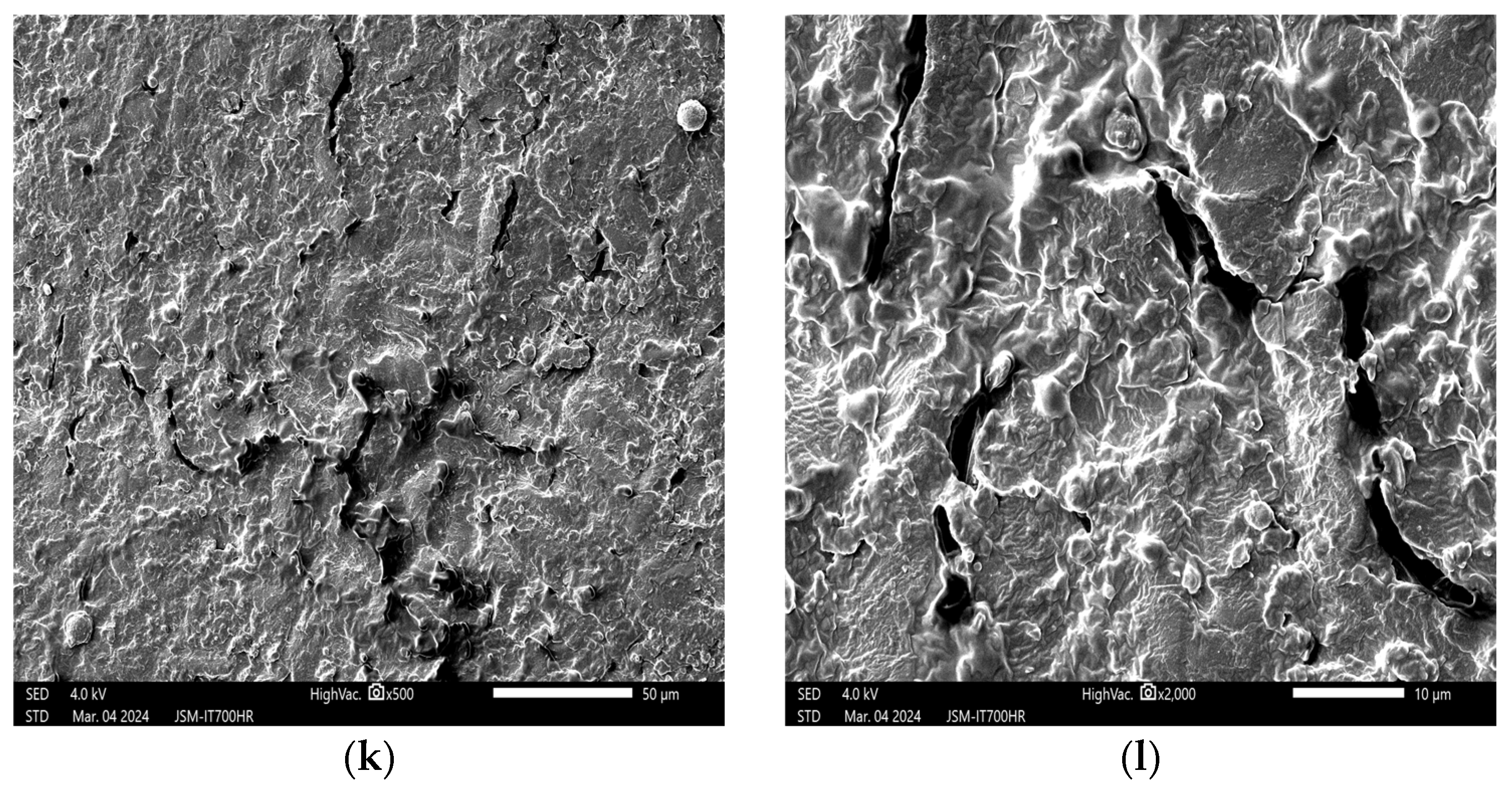
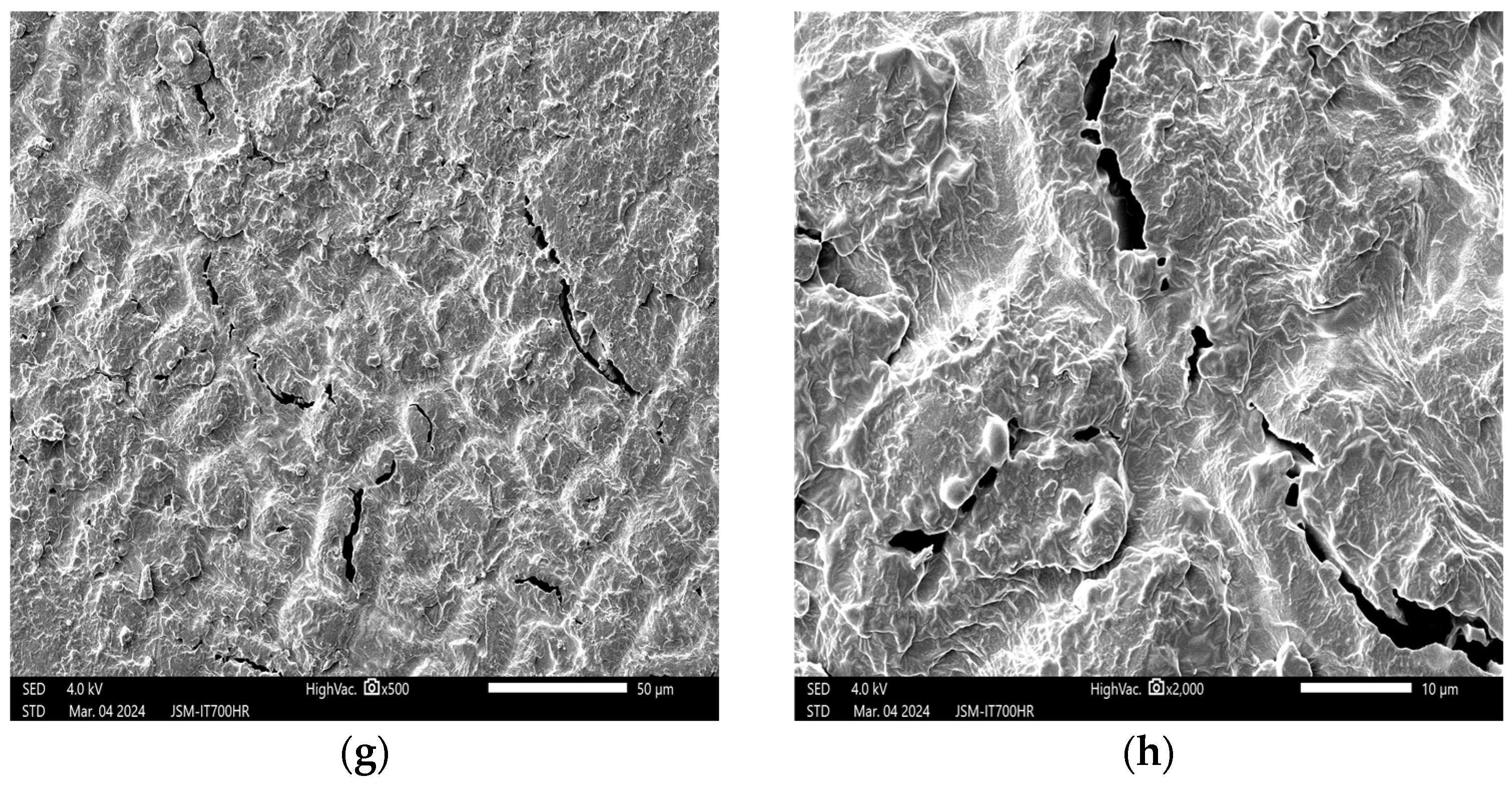
3.1. Effects of Plasma on the Structure of Peanut Seed Coat
3.1.1. Impact of Plasma Treatment Power on the Structure of Peanut Seed Coat
3.1.2. Impact of Plasma Treatment Duration on the Structure of Peanut Seed Coat
3.1.3. Impact of Plasma Treatment Gas Type on the Structure of Peanut Seed Coat
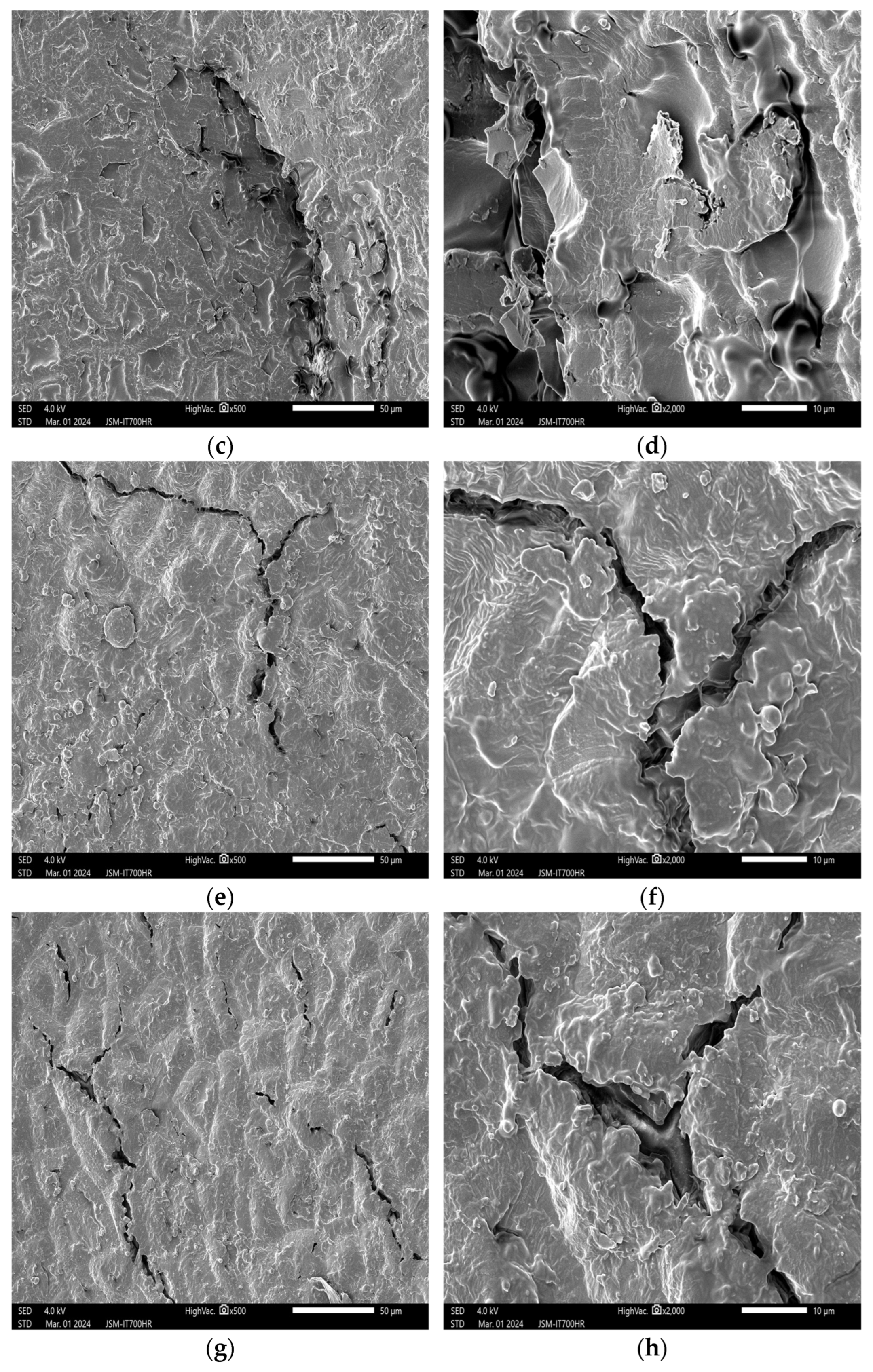
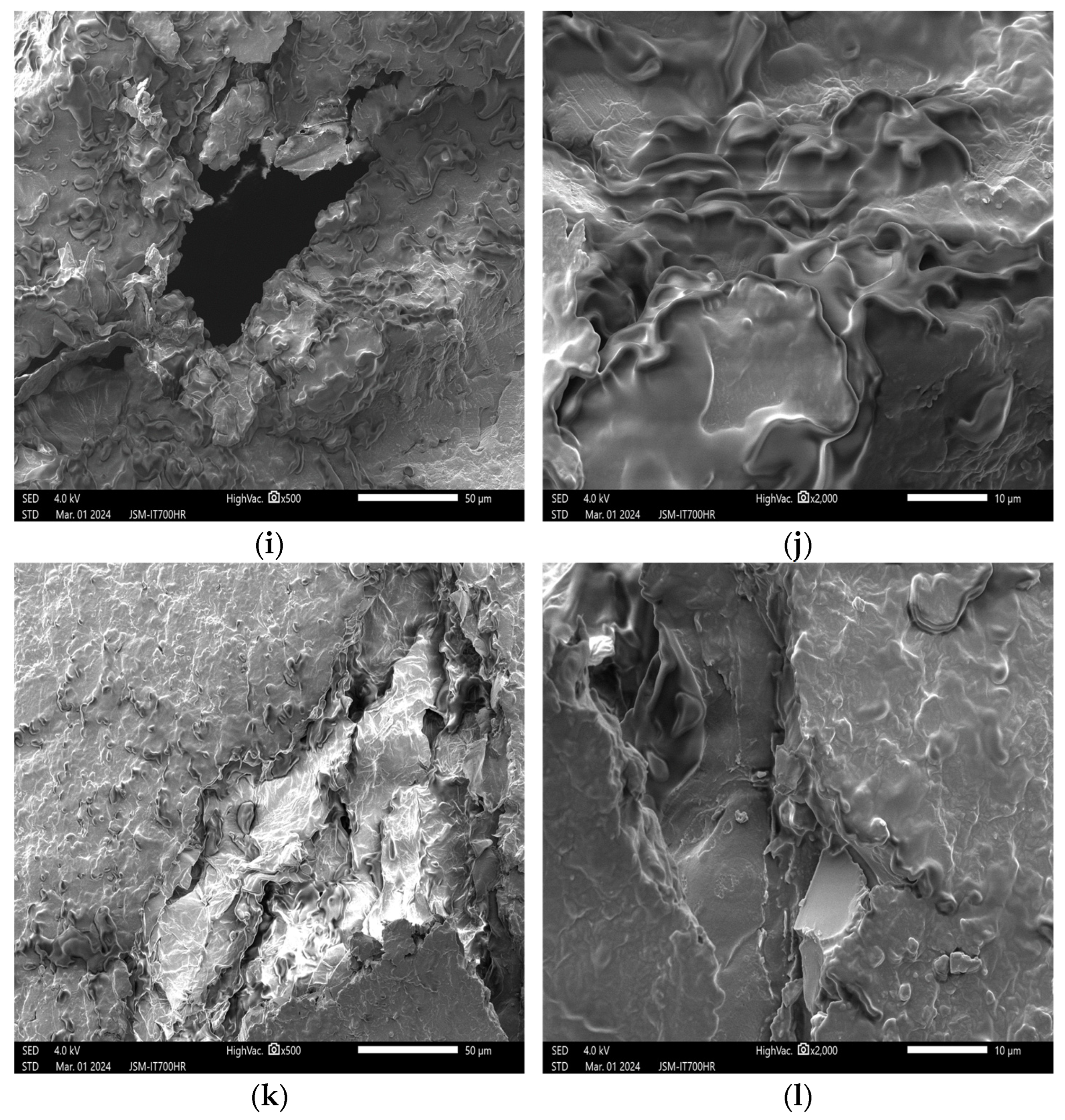
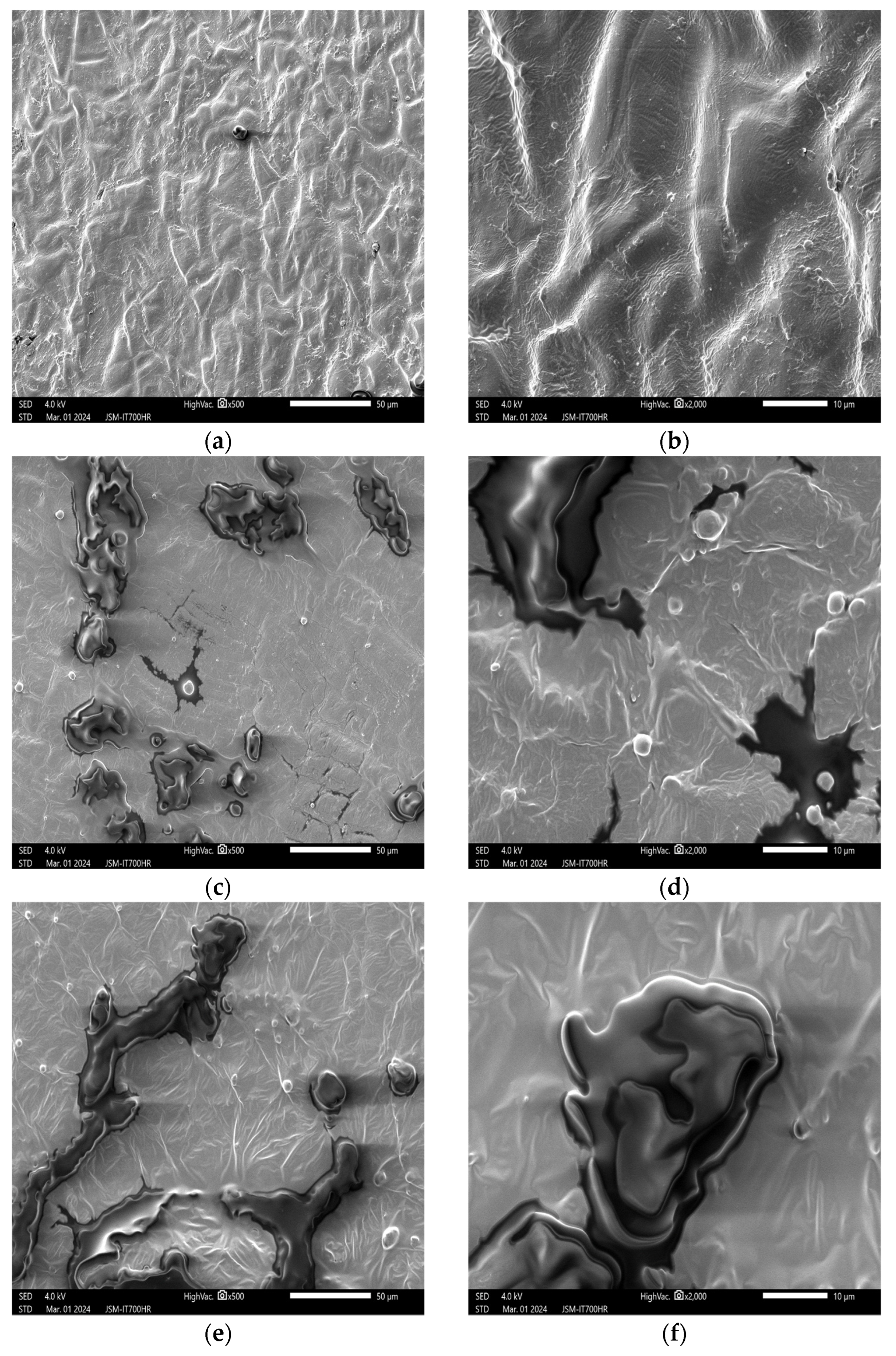
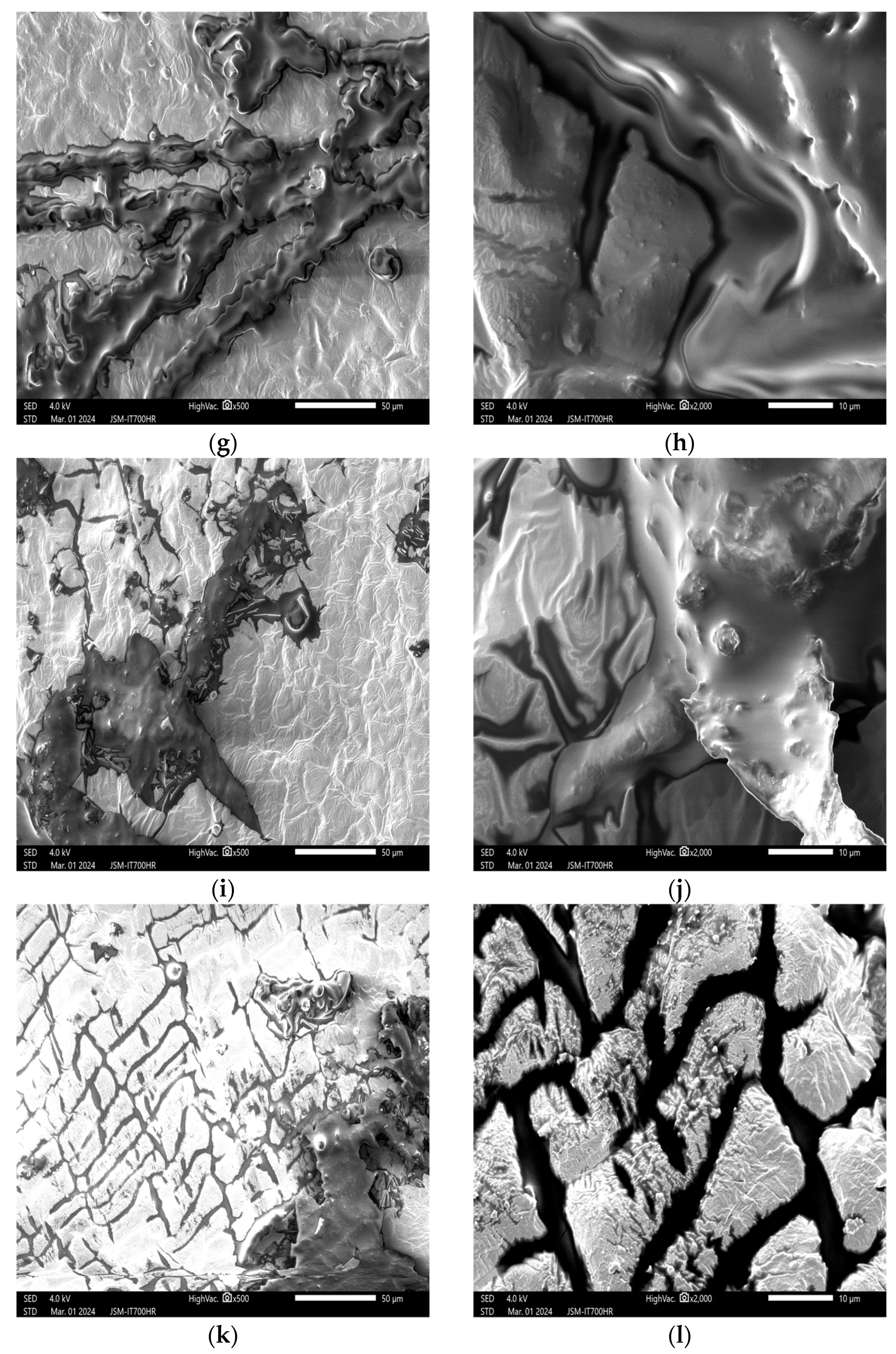
3.2. Impact of Plasma Treatment on the Appearance and Structure of Peanut Embryos
3.2.1. Impact of Plasma Treatment Power on the Appearance and Structure of Embryos
3.2.2. Impact of Plasma Treatment Duration on the Appearance and Structure of Embryos
3.2.3. Impact of Plasma Treatment Gas Type on the Appearance and Structure of Embryos
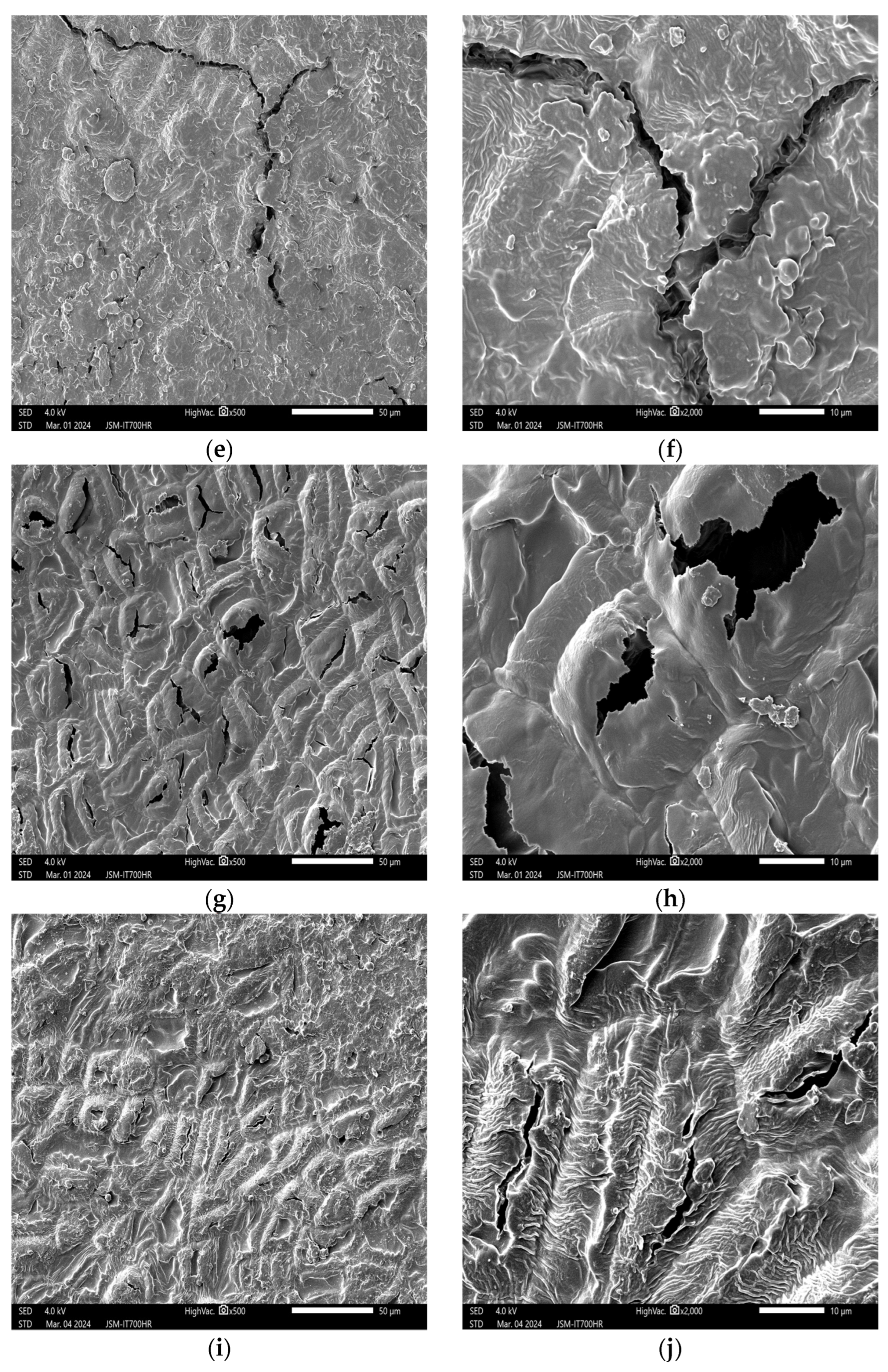
3.3. Effects of Plasma Treatment on the Internal Structure of Peanuts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.H.; Ding, T. Understanding the impact of nonthermal plasma on food constituents and microstructure—A review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, Z.; Xing, C.; Chen, H.J.; Zhang, J.H.; Yan, W.J. Degradation of deoxynivalenol in wheat by double dielectric barrier discharge cold plasma: Identification and pathway of degradation products. J. Sci. Food Agr. 2023, 103, 2347–2356. [Google Scholar] [CrossRef]
- Chaple, S.; Sarangapani, C.; Jones, J.; Carey, E.; Causeret, L.; Genson, A.; Duffy, B.; Bourke, P. Effect of atmospheric cold plasma on the functional properties of whole wheat (Triticum aestivum L.) grain and wheat flour. Innov. Food Sci. Emerg. 2020, 66, 102529. [Google Scholar] [CrossRef]
- Sainz-García, E.; Alba-Elías, F. Advances in the Application of Cold Plasma Technology in Foods. Foods 2023, 12, 1388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y. Research on the Effect of Cold Plasma Treatment on Peanut Quality Enhancement. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2022. [Google Scholar] [CrossRef]
- Gavahian, M.; Sarangapani, C.; Misra, N.N. Cold plasma for mitigating agrochemical and pesticide residue in food and water: Similarities with ozone and ultraviolet technologies. Food Res. Int. 2021, 141, 110138. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, R.; Jaspin, S.; Bhavadharini, B.; Pare, A.; Pandiselvam, R.; Mahendran, R. Chlorpyrifos pesticide reduction in soybean using cold plasma and ozone treatments. Lwt 2022, 159, 113193. [Google Scholar] [CrossRef]
- Cong, L.; Huang, M.; Zhang, J.; Yan, W.J. Effect of dielectric barrier discharge plasma on the degradation of malathion and chlorpyrifos on lettuce. J. Sci. Food Agr. 2021, 101, 424–432. [Google Scholar] [CrossRef]
- Mir, S.A.; Siddiqui, M.W.; Dar, B.N.; Shah, M.A.; Wani, M.H.; Roohinejad, S.; Annor, G.A.; Mallikarjunan, K.; Chin, C.F.; Ali, A. Promising applications of cold plasma for microbial safety, chemical decontamination and quality enhancement in fruits. J. Appl. Microbiol. 2020, 129, 474–485. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shen, M.; Hou, J.F.; Shao, H.L.; Dong, Y.H.; Jiang, J.F. Improving seed germination and peanut yields by cold plasma treatment. Plasma Sci. Technol. 2016, 18, 1027. [Google Scholar] [CrossRef]
- Terebun, P.; Kwiatkowski, M.; Starek, A.; Reuter, S.; Mok, Y.; Pawłat, J. Impact of short time atmospheric plasma treatment on onion seeds. Plasma Chem. Plasma Process. 2021, 41, 559–571. [Google Scholar] [CrossRef]
- Prakash, S.D.; Siliveru, K.; Zheng, Y. Emerging applications of cold plasma technology in cereal grains and products. Trends Food Sci. Technol. 2023, 141, 104177. [Google Scholar] [CrossRef]
- de Araújo Bezerra, J.; Lamarão, C.V.; Sanches, E.A.; Rodrigues, S.; Fernandes, F.; Ramos, G.; Esmerino, E.A.; Cruz, A.; Campelo, P. Cold plasma as a pre-treatment for processing improvement in food: A review. Food Res. Int. 2023, 167, 112663. [Google Scholar] [CrossRef]
- Loureiro, A.C.; Souza, F.C.A.; Sanches, E.A.; Bezerra, J.A.; Lamarão, C.V.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Cold plasma technique as a pretreatment for drying fruits: Evaluation of the excitation frequency on drying process and bioactive compounds. Food Res. Int. 2021, 147, 110462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, C.; Zou, F.; Sun, Y.; Shang, N.; Wu, W. Impacts of cold plasma technology on sensory, nutritional and safety quality of food: A review. Foods 2022, 11, 2818. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, G.; Admassu, S.; Berhanu, T.; Tučeková, Z.; Krumpolec, R.; Černák, M. Optimization and influence of multi-hollow surface dielectric barrier discharge plasma operating conditions on the physical quality of peanut. Eur. Phys. J. D 2019, 73, 1–16. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Liang, Q.; Ma, Z.; Han, F.; Xu, Y.; Jin, Y.; Wu, W. Low temperature plasma pretreatment enhances hot-air drying kinetics of corn kernels. J. Food Process. Eng. 2019, 42, e13195. [Google Scholar] [CrossRef]
- Sun, H.X.; Ren, L.; Wang, H.X.; Yu, G.Q.; Shi, P.X. Comprehensive evaluation of appearance and nutritional quality of edible peanuts. Chin. J. Oil Crop Sci. 2023, 45, 907–915. [Google Scholar] [CrossRef]
- Devi, Y.; Thirumdas, R.; Sarangapani, C.; Deshmukh, R.R.; Annapure, U.S. Influence of cold plasma on fungal growth and aflatoxins production on groundnuts. Food Control. 2017, 77, 187–191. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.H.; Sun, D.W. Blocking and degradation of aflatoxins by cold plasma treatments: Applications and mechanisms. Trends Food Sci. Technol. 2021, 109, 647–661. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Xiao, J.; Xiong, X.; Zhang, Y.; Jiang, W. Effect of plasma treatment on the quality of peanut oil. Morn. Food Sci. Technol. 2016, 32, 203–208. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Yousefi, M.; Nematollahi, A.; Khorshidian, N. Effect of atmospheric cold plasma treatment on technological and nutrition functionality of protein in foods. Eur. Food Res. Technol. 2021, 247, 1579–1594. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, H.; Liu, X.L.; Xiang, Q.S.; Chen, Y.F.; Zhang, Y.Y. Effects of Plasma Treatment on Functional Properties and Structure of Peanut Protein. J. Chin. Cere Oils 2021, 36, 54–59+66. [Google Scholar]
- Iqdiam, B.M.; Abuagela, M.O.; Boz, Z.; Marshall, S.M.; Goodrich-Schneider, R.; Sims, C.A.; Marshall, M.R.; MacIntosh, A.J.; Welt, B.A. Effects of atmospheric pressure plasma jet treatment on aflatoxin level, physiochemical quality, and sensory attributes of peanuts. J. Food Process. Preserv. 2019, 44, e14305. [Google Scholar] [CrossRef]
- Chen, Q. Research on the Changing Law of Drying Quality of Indica Rice Based on Cold Plasma Pretreatment. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2022. [Google Scholar] [CrossRef]
- Li, R.M.; Zhang, Z.J.; Yao, Q.; Wang, S.H.; Yin, J. Effects of cold plasma treatment on the appearance and structure of corn kernels. J. Chin. Cere Oils 2021, 36, 29–34. [Google Scholar]
- Li, S. Effects of low-temperature plasma pretreatment on drying kinetics and storage characteristics of corn kernels. Ph.D. Thesis, Jilin University, Changchun, China, 2020. [Google Scholar] [CrossRef]
- Wang, L.H.; Wei, Y.M.; Li, Z.X. Studies on the relationship between endosperm microstructure and quality of wheat grain. Acta Bot. Bori-Occid Sin. 1993, 150–153, 174–175. [Google Scholar]
- Ahmed, N.; Masood, A.; Siow, K.S.; Wee, M.M.R.; Auliya, R.Z.; Ho, W.K. Effect of H2O-based low-pressure plasma (LPP) treatment on the germination of bambara groundnut seeds. Agronomy 2021, 11, 338. [Google Scholar] [CrossRef]
- Wang, T.; Yu, S.H.L.; Xie, H.F.; Chen, M.N.; Wang, M.; Chen, N.; Pan, L.J.; Xu, J.; Feng, H.; Chi, X.Y. Effects of peanut seed coat changes on seed resistance to breakage. J. Peanut Sci. 2020, 49, 69–72. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, W.; Chen, F.S. Effect of ultrasonic treatment of peanut red coat on the extraction of peanut oil by water enzyme method and its quality. China Oils Fats 2024, 1–16. [Google Scholar] [CrossRef]
- Ahmed, N.; Siow, K.S.; Wee, M.F.M.R.; Patra, A. A study to examine the ageing behaviour of cold plasma-treated agricultural seeds. Sci. Rep. 2023, 13, 1675. [Google Scholar] [CrossRef]
- Li, B.; Peng, L.; Cao, Y.; Liu, S.; Zhu, Y.; Dou, J.; Yang, Z.; Zhou, C. Insights into Cold Plasma Treatment on the Cereal and Legume Proteins Modification: Principle, Mechanism, and Application. Foods 2024, 13, 1522. [Google Scholar] [CrossRef] [PubMed]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold plasma effects on enzyme activity in a model food system. Innov. Food Sci. Emerg. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Reineke, K.; Schlüter, O.; Schweiggert-Weisz, U.; Eisner, P. The effects of pulsed ultraviolet light, cold atmospheric pressure plasma, and gamma-irradiation on the immunoreactivity of soy protein isolate. Innov. Food Sci. Emerg. 2016, 38, 374–383. [Google Scholar] [CrossRef]
- Cao, X.Y.; Zhao, G.J.; Zhang, H.L.; Liu, X.G.; Wang, Q.; Liu, H.Z. Effect of frying process on quality and microstructure of peanuts with high oleic acid. J. Chin. Inst. Food Sci. Technol. 2022, 22, 200–206. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; He, X.Y.; Zhang, X.G.; Ma, X.L.; Yin, D.M. Microstructural analysis of oil bodies of peanut seeds at different developmental periods. J. Henan Agric. Univ. 2017, 51, 775–780. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kadam, D.; Annapure, U.S. Cold plasma: An alternative technology for the starch modification. Food Biophys. 2017, 12, 129–139. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Zhao, Y. Effect of storage conditions on the protein composition and structure of peanuts. ACS Omega 2022, 7, 21694–21700. [Google Scholar] [CrossRef]
- Yin, D.M.; Song, J.J.; Zhang, X.G.; Li, H.M.; Wang, Y.; Cui, D.Q. Microstructural analysis of peanut seeds at different developmental periods. J. Nuc. Agric. Sci. 2013, 27, 344–349. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yao, Q.; Li, X.; Yin, J.; Zhang, Z.; Zhou, X. Study of the Effects of Plasma Pretreatment on the Microstructure of Peanuts. Appl. Sci. 2024, 14, 7752. https://doi.org/10.3390/app14177752
Wang Y, Yao Q, Li X, Yin J, Zhang Z, Zhou X. Study of the Effects of Plasma Pretreatment on the Microstructure of Peanuts. Applied Sciences. 2024; 14(17):7752. https://doi.org/10.3390/app14177752
Chicago/Turabian StyleWang, Yingnan, Qu Yao, Xingjun Li, Jun Yin, Zhongjie Zhang, and Xianqing Zhou. 2024. "Study of the Effects of Plasma Pretreatment on the Microstructure of Peanuts" Applied Sciences 14, no. 17: 7752. https://doi.org/10.3390/app14177752




