Hormonal Balance and Cardiovascular Health: Exploring the Interconnection between Menopause, Body Composition, and Thyroid Function in a Cohort of Hypertensive Women
Abstract
:1. Background
2. Material and Methods
2.1. Participants and Study Design
2.2. Study Procedures
2.3. Statistical Analysis
3. Results
3.1. Comparison of Cardiovascular and Body Composition Parameters between Menopausal Weight Gain and Menopausal Weight Maintenance Groups
3.2. Correlations between Blood Pressure Parameters and Body Composition
3.3. Correlations between Cardiovascular and Cardiometabolic Risk Indices and Blood Pressure and Body Composition Parameters
3.4. Correlation between Endocrine-Metabolic Setting and Blood Pressure and Body Composition
4. Discussion
Potential Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarcin, O.; Abanonu, G.B.; Yazic, D.; Tarcin, O. Association of metabolic syndrome parameters with TT3 and FT3/FT4 ratio in obese Turkish population. Metab. Syndr. Relat. Disord. 2012, 10, 137–142. [Google Scholar] [CrossRef]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Boscaro, A.; Martin, V.; Vazeille, E.; Barnich, N.; Chassaing, B.; Boisseau, N. Effect of Concurrent Training on Body Composition and Gut Microbiota in Postmenopausal Women with Overweight or Obesity. Med. Sci. Sports Exerc. 2022, 54, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, J.; Pedersen, A.T.; Green, C.J.; Harder-Lauridsen, N.M.; Solomon, T.P.; Thomsen, C.; Juul, A.; Pedersen, M.; Pedersen, J.T.; Mortensen, O.H.; et al. Menopause is associated with decreased whole body fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1227–E1236. [Google Scholar] [CrossRef]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P.; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Acevedo, C.; Chinnakotla, B.; Padilla, J.; Martinez-Lemus, L.A.; Gozal, D. Obesity and cardiovascular disease in women. Int. J. Obes. 2020, 44, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Du, F.M.; Kuang, H.Y.; Duan, B.H.; Liu, D.N.; Yu, X.Y. Effects of thyroid hormone and depression on common components of central obesity. J. Int. Med. Res. 2019, 47, 3040–3049. [Google Scholar] [CrossRef]
- Bakker, S.J.; ter Maaten, J.C.; Popp-Snijders, C.; Slaets, J.P.; Heine, R.J.; Gans, R.O. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J. Clin. Endocrinol. Metab. 2001, 86, 1206–1211. [Google Scholar] [CrossRef]
- De Pergola, G.; Ciampolillo, A.; Paolotti, S.; Trerotoli, P.; Giorgino, R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin. Endocrinol. 2007, 67, 265–269. [Google Scholar] [CrossRef]
- Yang, Q.; Wan, Y.H.; Hu, S.; Cao, Y.H. Associations between the levels of thyroid hormones and abdominal obesity in euthyroid post-menopausal women. Endokrynol. Pol. 2020, 71, 299–305. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; Volume 252, Available online: https://iris.who.int/handle/10665/42330 (accessed on 17 May 2024).
- Yusuf, S.; Hawken, S.; Ôunpuu, S.; Bautista, L.; Franzosi, M.G.; Commerford, P.; Lang, C.C.; Rumboldt, Z.; Onen, C.L.; Lisheng, L.; et al. Obesity and the Risk of Myocardial Infarction in 27,000 Participants from 52 Countries: A Case-Control Study. Lancet 2005, 366, 1640–1649. [Google Scholar] [CrossRef]
- Tocci, G.; Biondi-Zoccai, G.; Forte, M.; Gallo, G.; Nardoianni, G.; Fiori, E.; D‘Ambrosio, L.; Di Pietro, R.; Stefanini, G.; Cannata, F.; et al. Effects of two-month treatment with a mixture of natural activators of autophagy on oxidative stress and arterial stiffness in patients with essential hypertension: A pilot study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2287–2293. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [PubMed]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A Preventable, Treatable, but Relapsing Disease. Nutrition 2020, 71, 1106–1115. [Google Scholar] [CrossRef]
- Liu, D.; Jiang, F.; Shan, Z.; Wang, B.; Wang, J.; Lai, Y.; Chen, Y.; Li, M.; Liu, H.; Li, C.; et al. A cross-sectional survey of relationship between serum TSH level and blood pressure. J. Hum. Hypertens. 2010, 24, 1348. [Google Scholar] [CrossRef]
- Eikås, J.G.; Gerdts, E.; Halland, H.; Midtbø, H.; Cramariuc, D.; Kringeland, E. Arterial Stiffness in Overweight and Obesity: Association with Sex, Age, and Blood Pressure. High. Blood Press. Cardiovasc. Prev. 2023, 30, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse Changes in Body Composition During the Menopausal Transition and Relation to Cardiovascular Risk: A Contemporary Review. Womens Health Rep. 2022, 3, 573–581. [Google Scholar] [CrossRef]
- Ko, S.H.; Jung, Y. Energy Metabolism Changes and Dysregulated Lipid Metabolism in Postmenopausal Women. Nutrients 2021, 13, 4556. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Lang, X.; Li, Y.; Zhang, D.; Zhang, Y.; Wu, N.; Zhang, Y. FT3/FT4 ratio is correlated with all-cause mortality, cardiovascular mortality, and cardiovascular disease risk: NHANES 2007-2012. Front. Endocrinol. 2022, 13, 964822. [Google Scholar] [CrossRef]
- Gao, S.; Ma, W.; Huang, S.; Lin, X.; Yu, M. Predictive Value of Free Triiodothyronine to Free Thyroxine Ratio in Euthyroid Patients With Myocardial Infarction With Nonobstructive Coronary Arteries. Front. Endocrinol. 2021, 12, 708216. [Google Scholar] [CrossRef] [PubMed]
- Melo E Silva, F.V.; Almonfrey, F.B.; Freitas, C.M.N.; Fonte, F.K.; Sepulvida, M.B.C.; Almada-Filho, C.M.; Cendoroglo, M.S.; Quadrado, E.B.; Amodeo, C.; Povoa, R.; et al. Association of Body Composition with Arterial Stiffness in Long-lived People. Arq. Bras. Cardiol. 2021, 117, 457–462. [Google Scholar] [PubMed]
- Cao, Q.; Fan, C.; Fei, Y.; Zhang, S.; Shen, T.; Meng, H. Waist-to-Height Ratio Is a Better Predictor of Hypertension in Women during Recovery from Anesthesia Compared to BMI, Waist-to-Hip Ratio, and Waist Circumference. Heliyon 2024, 10, e28297. [Google Scholar] [CrossRef] [PubMed]
- Ataee, Z.; Aghaee, A.; Sobhani, S.R.; Ebrahimi Miandehi, E.; Pirzadeh, P.; Alinezhad-Namaghi, M.; Eslami, S.; Sobhani, S. Evaluation of Arterial Stiffness and Its Relation to Innovative Anthropometric Indices in Persian Adults. Int. J. Hypertens. 2023, 2023, 2180923. [Google Scholar] [CrossRef]
- Therkelsen, K.E.; Pedley, A.; Speliotes, E.K.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arter. Thromb. Vasc. Biol. 2013, 33, 863–870. [Google Scholar] [CrossRef]
- Katsimardou, A.; Imprialos, K.; Stavropoulos, K.; Sachinidis, A.; Doumas, M.; Athyros, V. Hypertension in Metabolic Syndrome: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 12–18. [Google Scholar]
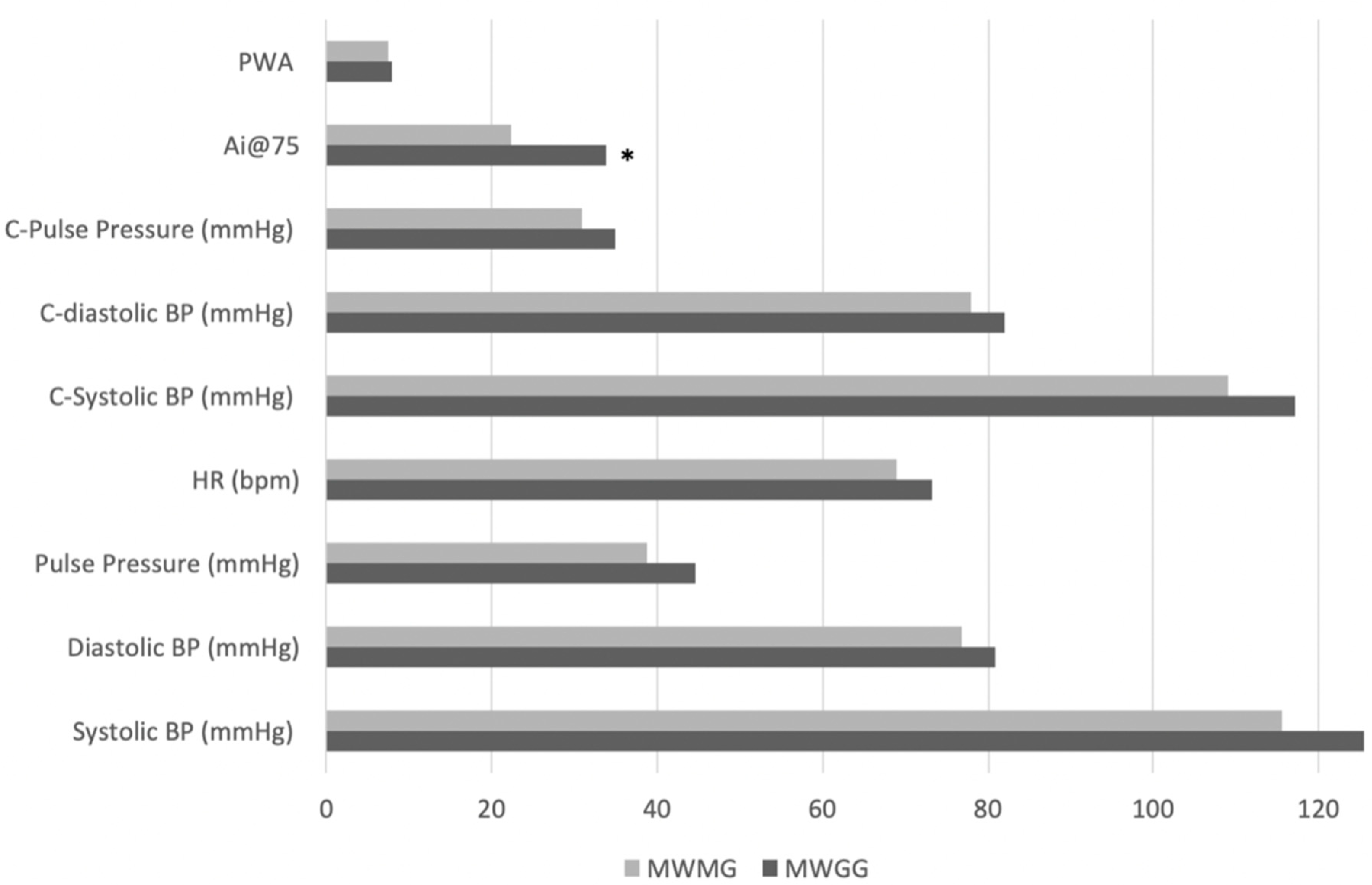

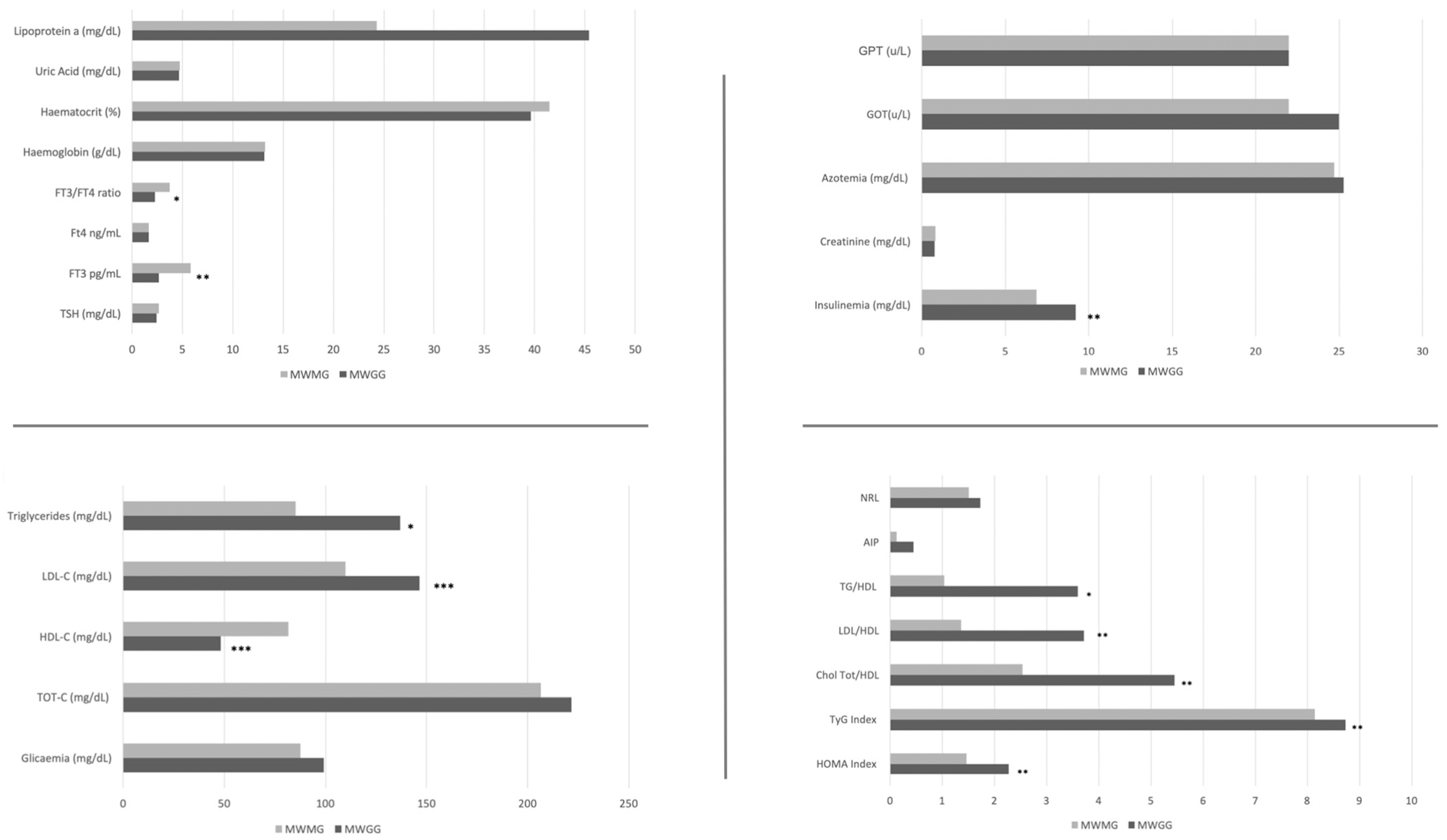
| MWGG (n = 12) | Max-Min | MWMG ( n = 8) | Max-Min | |
| Age (years) | ||||
| (Mean ± SD, range) | 56.08 ± 4.99 | 49–65 | 54.85 ± 5.75 | 46–60 |
| Menarche years (years) | ||||
| (Mean ± SD, range) | 11.58 ± 0.99 | 10–13 | 11.86 ± 1.06 | 10–13 |
| Menopause years (years) | ||||
| (Mean ± SD, range) | 51.33 ± 3.22 | 45–56 | 50.00 ± 3.06 | 44–54 |
| Years since menopause (years) | ||||
| (Mean ± SD, range) | 4.75 ± 2.89 | 2–9 | 4.86 ± 2.67 | 2–9 |
| Menopausal Weight Gain (Kg) | ||||
| (Mean ± SD, range) | 10.25 ± 2.92 | 2–13 | 2 ± 1 | 0–3 |
| MWGG (n = 12) | MWMG (n = 8) | |||
| Smoker (%) | 8.33 | 14.28 | ||
| Diabetes (%) | 8.33 | 14.28 | ||
| Dislipidemia (%) | 50.00 | 28.57 | ||
| Anti-hypertensive Drugs (%) | ||||
| 0 | 8.33 | 14.28 | ||
| 1 | 50.00 | 71.44 | ||
| 2 | 25.00 | 14.28 | ||
| 3 or more | 16.67 | 0 |
| Brachial Systolic BP | Brachial Diastolic BP | Heart Rate | Central Systolic BP | Central Diastolic BP | Ai@75 | PWA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Weight (Kg) | 0.18 | 0.48 | 0.08 | 0.75 | 0.12 | 0.61 | 0.15 | 0.54 | 0.08 | 0.72 | 0.49 | 0.03 | 0.08 | 0.75 |
| BMI (kg/m2) | 0.32 | 0.19 | 0.28 | 0.25 | 0.20 | 0.41 | 0.30 | 0.22 | 0.29 | 0.24 | 0.56 | 0.01 | 0.19 | 0.44 |
| WHR | 0.55 | 0.01 | 0.47 | 0.04 | −0.01 | 0.90 | 0.47 | 0.04 | 0.48 | 0.04 | 0.17 | 0.49 | 0.47 | 0.04 |
| Total FM (%) | 0.20 | 0.43 | 0.12 | 0.62 | 0.19 | 0.46 | 0.19 | 0.45 | 0.12 | 0.63 | 0.61 | 0.008 | 0.13 | 0.61 |
| Lean/Fat Mass | −0.19 | 0.44 | −0.09 | 0.74 | −0.13 | 0.61 | −0.19 | 0.46 | −0.08 | 0.75 | −0.63 | 0.006 | −0.16 | 0.53 |
| VAT (g) | 0.62 | 0.01 | 0.54 | 0.04 | 0.17 | 0.55 | 0.59 | 0.02 | 0.54 | 0.04 | 0.26 | 0.35 | 0.25 | 0.37 |
| IMAT (Kg) | 0.21 | 0.44 | 0.09 | 0.71 | 0.21 | 0.41 | 0.19 | 0.48 | 0.10 | 0.71 | 0.67 | 0.004 | 0.16 | 0.65 |
| ASMI | 0.19 | 0.47 | 0.14 | 0.60 | 0.20 | 0.45 | 0.12 | 0.65 | 0.16 | 0.54 | 0.01 | 0.95 | 0.17 | 0.53 |
| Correlations | HOMA Index | TyG Index | TG/hdl | Ctot/hdl | LDL/HDL | CV Risk: AIP | N/L Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| B-Systolic BP (mmHg) | 0.65 | 0.004 | 0.56 | 0.01 | 0.42 | 0.08 | 0.42 | 0.08 | 0.42 | 0.07 | 0.51 | 0.02 | 0.42 | 0.08 |
| B-Diastolic BP (mmHg) | 0.42 | 0.07 | 0.42 | 0.08 | 0.37 | 0.12 | 0.36 | 0.16 | 0.32 | 0.18 | 0.37 | 0.12 | 0.41 | 0.09 |
| HR (bpm) | 0.50 | 0.81 | 0.39 | 0.19 | 0.38 | 0.11 | 0.30 | 0.27 | 0.24 | 0.32 | 0.34 | 0.15 | 0.05 | 0.83 |
| C-Systolic BP (mmHg) | 0.57 | 0.01 | 0.52 | 0.02 | 0.39 | 0.10 | 0.41 | 0.08 | 0.41 | 0.08 | 0.48 | 0.04 | 0.40 | 0.06 |
| C-Diastolic BP (mmHg) | 0.43 | 0.07 | 0.43 | 0.07 | 0.37 | 0.12 | 0.36 | 0.13 | 0.33 | 0.17 | 0.37 | 0.12 | 0.40 | 0.09 |
| AI@75 | 0.46 | 0.05 | 0.21 | 0.39 | 0.25 | 0.30 | 0.23 | 0.35 | 0.23 | 0.35 | 0.27 | 0.26 | 0.28 | 0.24 |
| PWA | 0.49 | 0.04 | 0.18 | 0.47 | 0.08 | 0.73 | 0.15 | 0.56 | 0.18 | 0.45 | 0.18 | 0.45 | 0.54 | 0.01 |
| Weight (Kg) | 0.25 | 0.29 | 0.41 | 0.07 | 0.41 | 0.08 | 0.27 | 0.25 | 0.23 | 0.33 | 0.38 | 0.10 | 0.28 | 0.23 |
| BMI (Kg/m2) | 0.24 | 0.31 | 0.43 | 0.06 | 0.47 | 0.03 | 0.36 | 0.12 | 0.32 | 0.17 | 0.44 | 0.05 | 0.36 | 0.13 |
| WHR | 0.50 | 0.03 | 0.37 | 0.11 | 0.22 | 0.36 | 0.11 | 0.64 | 0.08 | 0.73 | 0.21 | 0.38 | 0.22 | 0.35 |
| Total FM (%) | 0.35 | 0.15 | 0.20 | 0.40 | 0.27 | 0.27 | 0.23 | 0.35 | 0.28 | 0.40 | 0.24 | 0.32 | 0.20 | 0.42 |
| Lean/Fat Mass | −0.35 | 0.14 | −0.16 | 0.51 | −0.28 | 0.26 | −0.26 | 0.29 | −0.24 | 0.32 | −0.24 | 0.32 | −0.17 | 0.48 |
| VAT (g) | 0.59 | 0.01 | 0.44 | 0.08 | 0.31 | 0.24 | 0.20 | 0.44 | 0.16 | 0.54 | 0.28 | 0.28 | 0.47 | 0.06 |
| IMAT (Kg) | 0.36 | 0.14 | 0.33 | 0.19 | 0.31 | 0.23 | 0.19 | 0.44 | 0.16 | 0.52 | 0.29 | 0.24 | 0.25 | 0.32 |
| ASMI | −0.16 | 0.51 | 0.32 | 0.19 | 0.21 | 0.40 | 0.10 | 0.67 | 0.10 | 0.70 | 0.24 | 0.35 | 0.19 | 0.46 |
| Correlations | Glicaemia | Insulinemia | TotColesterol | HDL | LDL | TG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| B-Systolic BP (mmHg) | 0.46 | 0.05 | 0.50 | 0.03 | 0.19 | 0.45 | −0.48 | 0.04 | 0.36 | 0.14 | 0.40 | 0.09 |
| B-Diastolic BP (mmHg) | 0.33 | 0.18 | 0.28 | 0.25 | 0.33 | 0.17 | −0.30 | 0.17 | 0.30 | 0.21 | 0.31 | 0.20 |
| HR (bpm) | 0.06 | 0.80 | 0.09 | 0.70 | 0.42 | 0.07 | −0.25 | 0.31 | 0.34 | 0.16 | 0.41 | 0.08 |
| C-Systolic BP (mmHg) | 0.44 | 0.06 | 0.42 | 0.08 | 0.16 | 0.52 | −0.47 | 0.04 | 0.32 | 0.19 | 0.35 | 0.16 |
| C-Diastolic BP (mmHg) | 0.33 | 0.17 | 0.29 | 0.23 | −0.34 | 0.16 | −0.30 | 0.21 | 0.31 | 0.20 | 0.32 | 0.18 |
| AI@75 | 0.14 | 0.56 | 0.56 | 0.01 | 0.10 | 0.67 | −0.35 | 0.15 | 0.09 | 0.70 | 0.20 | 0.40 |
| PWA | 0.31 | 0.20 | 0.43 | 0.69 | −0.21 | 0.40 | −0.26 | 0.30 | 0.01 | 0.93 | 0.02 | 0.92 |
| Weight (Kg) | 0.16 | 0.50 | 0.23 | 0.27 | 0.09 | 0.69 | −0.35 | 0.12 | 0.16 | 0.49 | 0.47 | 0.04 |
| BMI (Kg/m2) | 0.17 | 0.47 | 0.23 | 0.34 | 0.10 | 0.67 | −0.46 | 0.04 | 0.22 | 0.36 | 0.48 | 0.03 |
| WHR | 0.52 | 0.02 | 0.30 | 0.20 | −0.00 | 0.99 | −0.18 | 0.44 | 0.03 | 0.91 | 0.27 | 0.26 |
| Total FM (%) | 0.32 | 0.18 | 0.31 | 0.19 | −0.12 | 0.62 | −0.40 | 0.09 | 0.07 | 0.75 | 0.18 | 0.46 |
| Lean/Fat Mass | −0.32 | 0.18 | −0.33 | 0.18 | 0.18 | 0.47 | 0.44 | 0.06 | −0.06 | 0.79 | −0.14 | 0.57 |
| VAT (g) | 0.61 | 0.01 | 0.42 | 0.10 | 0.04 | 0.86 | −0.24 | 0.36 | 0.06 | 0.82 | 0.28 | 0.28 |
| IMAT (Kg) | 0.35 | 0.17 | 0.32 | 0.19 | −0.05 | 0.85 | −0.37 | 0.14 | 0.11 | 0.65 | 0.31 | 0.21 |
| ASMI | −0.08 | 0.76 | −0.24 | 0.34 | 0.35 | 0.16 | −0.05 | 0.83 | 0.27 | 0.27 | 0.43 | 0.08 |
| Correlations | TSH | FT3 | FT4 | FT3/FT4 Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | R | p | |
| B-Systolic BP (mmHg) | −0.07 | 0.77 | 0.11 | 0.65 | 0.09 | 0.72 | 0.13 | 0.62 |
| B-Diastolic BP (mmHg) | −0.11 | 0.67 | −0.04 | 0.88 | 0.21 | 0.40 | −0.01 | 0.96 |
| HR (bpm) | −0.21 | 0.41 | −0.38 | 0.12 | 0.37 | 0.14 | −0.50 | 0.03 |
| C-Systolic BP (mmHg) | −0.15 | 0.56 | 0.16 | 0.53 | 0.15 | 0.57 | 0.08 | 0.76 |
| C-Diastolic BP (mmHg) | −0.01 | 0.68 | −0.04 | 0.90 | 0.20 | 0.43 | 0.00 | 0.97 |
| AI@75 | 0.22 | 0.39 | −0.27 | 0.28 | −0.07 | 0.80 | −0.15 | 0.54 |
| PWA | 0.00 | 0.99 | 0.17 | 0.51 | −0.25 | 0.32 | 0.31 | 0.21 |
| Weight (Kg) | −0.16 | 0.52 | −0.41 | 0.08 | 0.30 | 0.22 | −0.58 | 0.01 |
| BMI (Kg/m2) | −0.40 | 0.09 | −0.43 | 0.07 | 0.33 | 0.17 | −0.59 | 0.009 |
| WHR | 0.02 | 0.91 | −0.05 | 0.83 | 0.16 | 0.51 | −0.10 | 0.68 |
| Total FM (%) | −0.04 | 0.87 | −0.45 | 0.06 | 0.23 | 0.37 | −0.63 | 0.006 |
| Lean/Fat Mass | −0.03 | 0.90 | 0.39 | 0.11 | −0.23 | 0.36 | 0.61 | 0.009 |
| VAT (g) | −0.10 | 0.70 | −0.17 | 0.53 | 0.31 | 0.25 | −0.32 | 0.23 |
| IMAT (Kg) | −0.08 | 0.74 | −0.49 | 0.05 | 0.22 | 0.41 | −0.68 | 0.003 |
| ASMI | −0.62 | 0.01 | −0.00 | 0.99 | −0.05 | 0.86 | 0.17 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pala, B.; Tocci, G.; Nardoianni, G.; Barbato, E.; Cianci, R.; Gualtieri, P.; Frank, G.; Di Renzo, L. Hormonal Balance and Cardiovascular Health: Exploring the Interconnection between Menopause, Body Composition, and Thyroid Function in a Cohort of Hypertensive Women. Appl. Sci. 2024, 14, 7772. https://doi.org/10.3390/app14177772
Pala B, Tocci G, Nardoianni G, Barbato E, Cianci R, Gualtieri P, Frank G, Di Renzo L. Hormonal Balance and Cardiovascular Health: Exploring the Interconnection between Menopause, Body Composition, and Thyroid Function in a Cohort of Hypertensive Women. Applied Sciences. 2024; 14(17):7772. https://doi.org/10.3390/app14177772
Chicago/Turabian StylePala, Barbara, Giuliano Tocci, Giulia Nardoianni, Emanuele Barbato, Rossella Cianci, Paola Gualtieri, Giulia Frank, and Laura Di Renzo. 2024. "Hormonal Balance and Cardiovascular Health: Exploring the Interconnection between Menopause, Body Composition, and Thyroid Function in a Cohort of Hypertensive Women" Applied Sciences 14, no. 17: 7772. https://doi.org/10.3390/app14177772







