Novel Aromatic Estolide Esters from Biobased Resources by a Green Synthetic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Synthesis of Ricinoleic-Based Oligoesters
2.3. Enzymatic Synthesis of the Aromatic Oligoesters
2.4. Structural Analysis of the Reaction Products
2.5. Viscosity Measurements
2.6. DSC Analysis
2.7. Biodegradation Studies
3. Results and Discussion
3.1. Synthesis of Ricinoleic Acid by Saponification of Castor Oil
- Color: intense brown-yellow;
- Melting point: 5.5–6 °C;
- Boiling point: 246 °C;
- Density at 20 °C: 940 kg/m3;
- Acid index: 186.34 mgKOH/g;
- Iodine index: 85.23 gI2/100 g;
- Refractive index at 20 °C: 1.4694.
3.2. Enzymatic Synthesis of Oligo (Ricinoleate) Aromatic Esters
3.3. Structural Characterization of the Estolide Ester Products
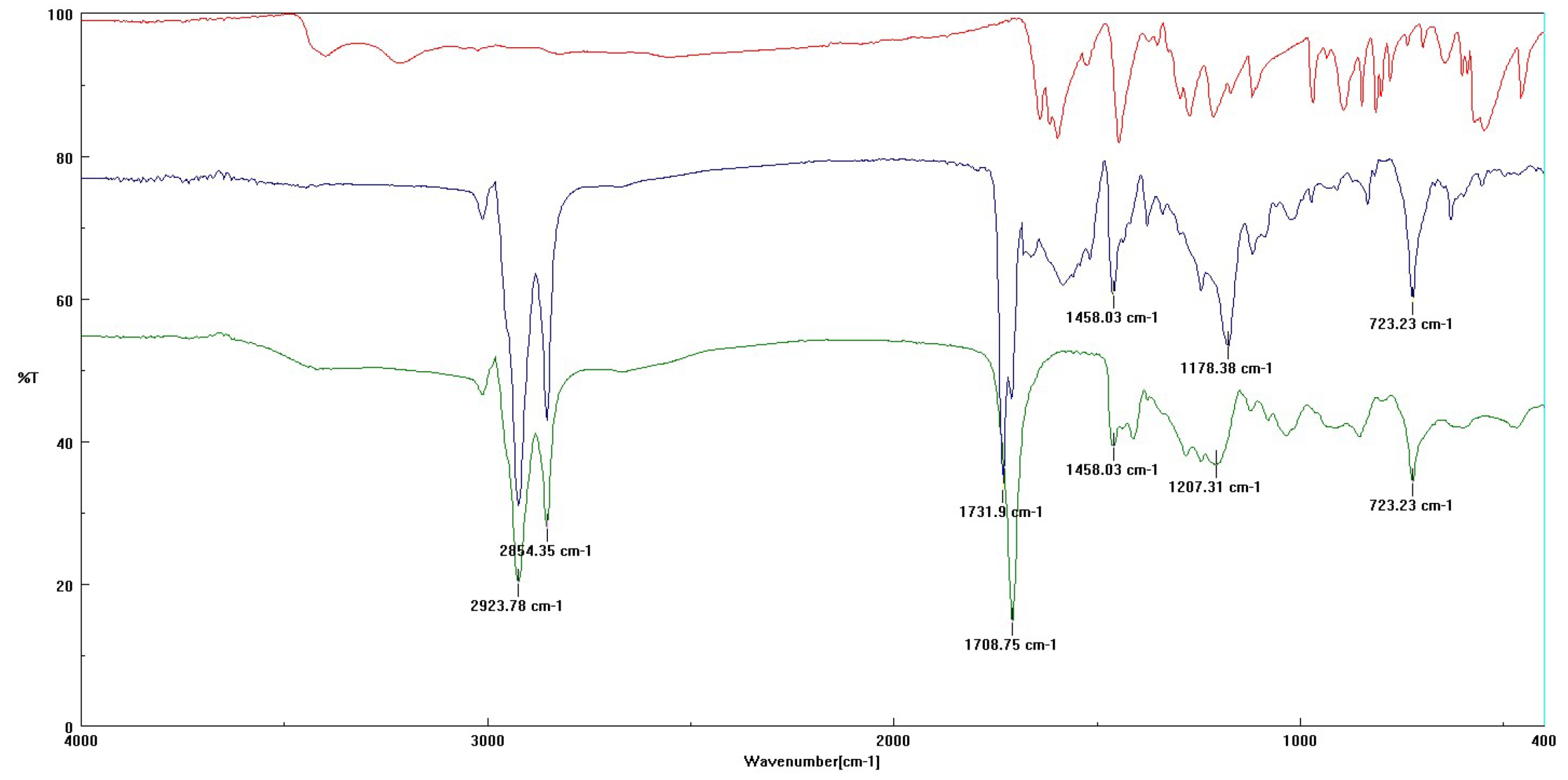
3.3.1. FT-IR Analysis
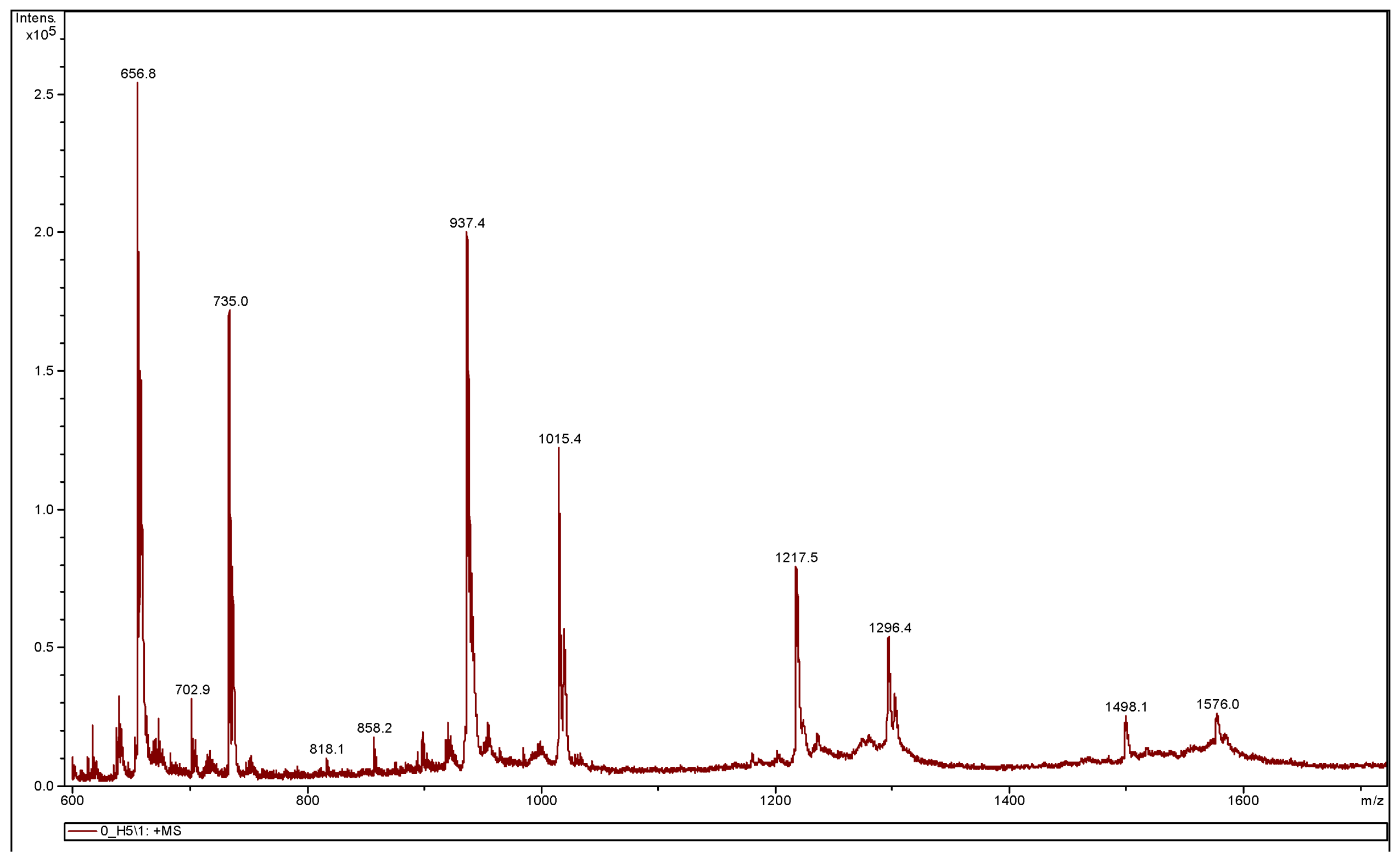
3.3.2. MALDI-TOF MS Analysis
3.3.3. Characterization of the Estolide Ester Products by NMR Spectroscopy
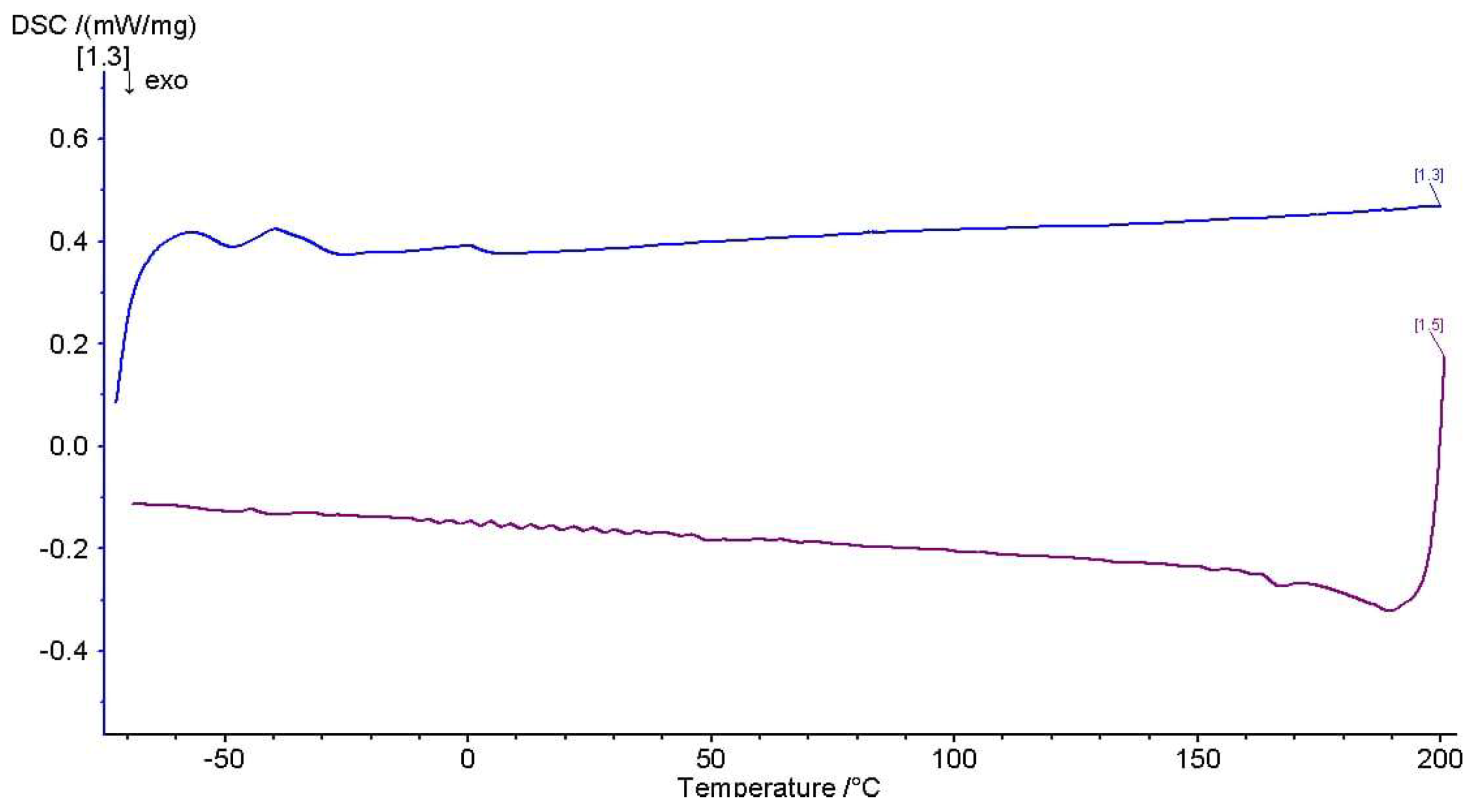
3.3.4. Thermal Analysis by Differential Scanning Calorimetry (DSC)
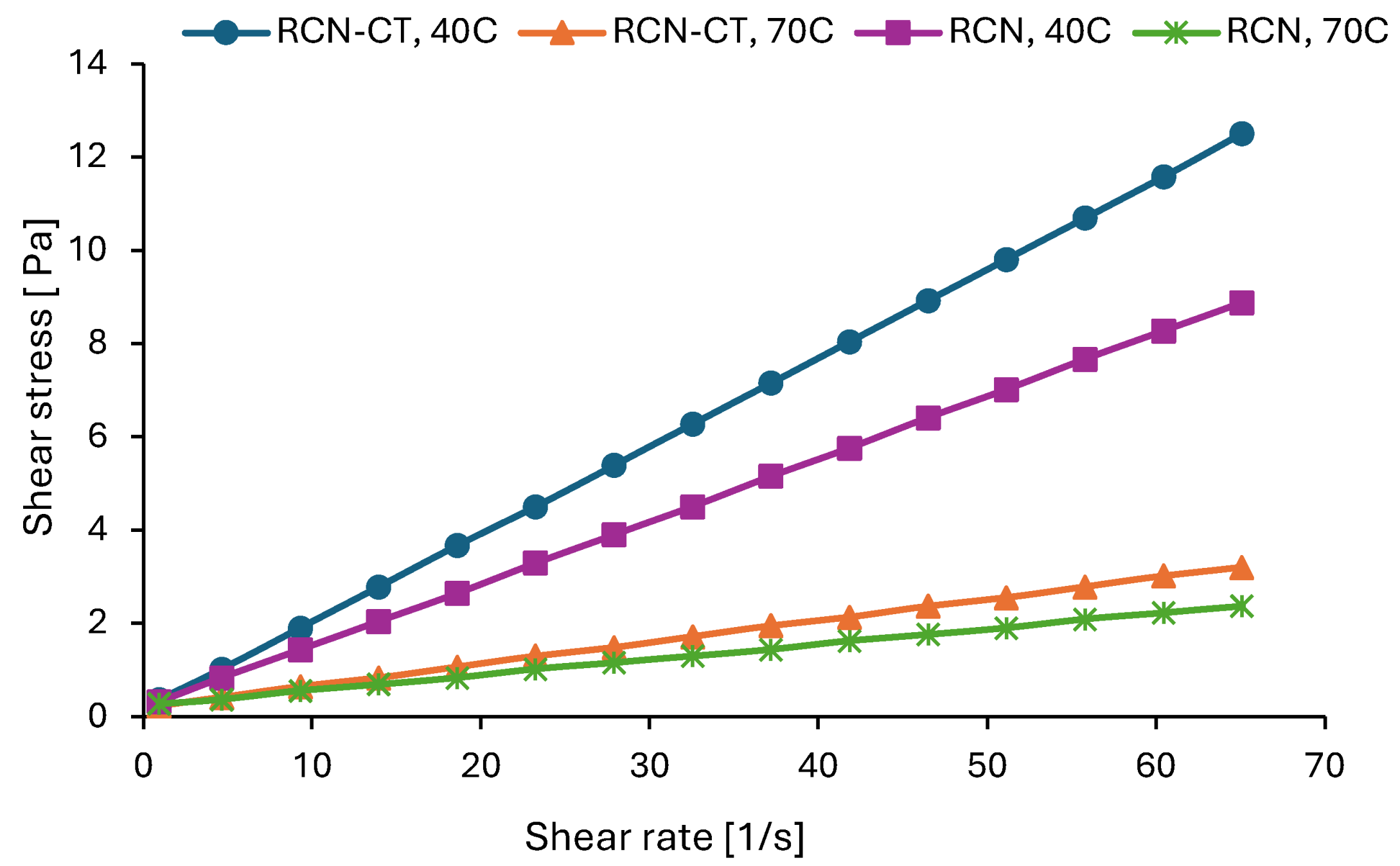
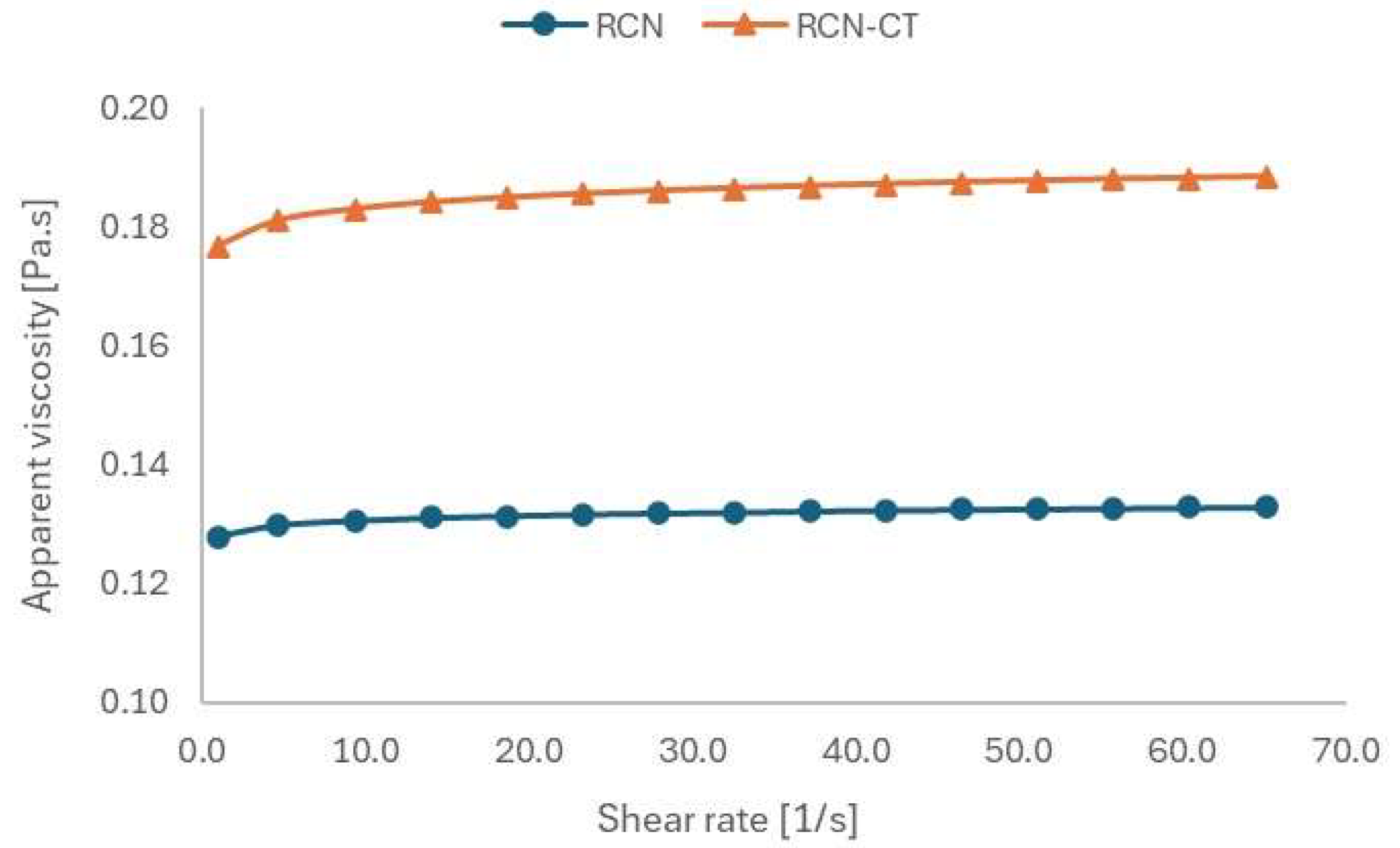
3.3.5. Rheological Characterization
3.4. Biodegradability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty acids and their derivatives as renewable platform molecules for the chemical industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Tschan, M.J.L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of biodegradable polymers from renewable resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Fraile, J.M.; García, J.I.; Herrerías, C.I.; Pires, E. Synthetic Transformations for the valorization of fatty acid derivatives. Synthesis 2017, 49, 1444–1460. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M.; Carvalho, A.J.F.; Trovatti, E. Progress of polymers from renewable resources: Furans, vegetable oils, and polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef] [PubMed]
- Ronda, J.C.; Lligadas, G.; Galià, M.; Cádiz, V. Vegetable oils as platform chemicals for polymer synthesis. Eur. J. Lipid Sci. Technol. 2011, 113, 46–58. [Google Scholar] [CrossRef]
- Kim, H.K.H.; Hou, C.T. Biodegradable photo-crosslinked thin polymer networks based on vegetable oil hydroxy fatty acid. J. Am. Oil Chem. Soc. 2010, 87, 1451–1459. [Google Scholar] [CrossRef]
- Metzger, J.O.; Bornscheuer, U. Lipids as renewable resources: Current state of chemical and biotechnological conversion and diversification. Appl. Microbiol. Biotechnol. 2006, 71, 13–22. [Google Scholar] [CrossRef]
- Kim, K.; Oh, D. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kim, H.R.; Hou, C.T.; Kang, S.C. Bioconverted products of essential fatty acids as potential antimicrobial agents. New Biotechnol. 2009, 26, 122–130. [Google Scholar] [CrossRef]
- Hou, C.T. Biotechnology for fats and oils: New oxygenated fatty acids. New Biotechnol. 2009, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A.; Carabin, I.G.; Griffiths, J.C. Toxicology and pharmacology of sodium ricinoleate. Food Chem. Toxicol. 2006, 44, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Biresaw, G.; Cermak, S.C.; Isbell, T.A.; Ngo, H.L.; Chen, L.; Durham, A.L. Fatty acid estolides: A review. J. Am. Oil Chem. Soc. 2020, 97, 231–241. [Google Scholar] [CrossRef]
- Ewing, T.A.; Blaauw, R.; Li, C.; Venkitasubramanian, P.; Hagberg, E.; van Haveren, J. Synthesis and Applications of Fatty Acid Estolides. In Sustainable Green Chemistry in Polymer Research. Volume 1: Biocatalysis and Biobased Materials; Cheng, H.N., Gross, R.A., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2023; Volume 1450, pp. 145–161. [Google Scholar] [CrossRef]
- Salimon, J.; Nallathamby, N.; Salih, N.; Abdullah, B.M. Synthesis and physical properties of estolide ester using saturated fatty acid and ricinoleic acid. J. Anal. Methods Chem. 2011, 2011, 263624. [Google Scholar] [CrossRef]
- de Haro, J.C.; del Prado Garrido, M.; Pérez, A.; Carmona, M.; Rodríguez, J.F. Full conversion of oleic acid to estolides esters, biodiesel and choline carboxylates in three easy steps. J. Clean. Prod. 2018, 184, 579–585. [Google Scholar] [CrossRef]
- Arslan, A.; Rancke-Madsen, A.; Brask, J. Enzymatic synthesis of estolides from castor oil. Catalysts 2020, 10, 835. [Google Scholar] [CrossRef]
- Bodalo-Santoyo, A.; Bastida-Rodriguez, J.; Maximo-Martin, M.F.; Montiel-Morte, M.C.; Murcia-Almagro, M.D. Enzymatic biosynthesis of ricinoleic acid estolides. Biochem. Eng. J. 2005, 26, 155–158. [Google Scholar] [CrossRef]
- Martin-Arjol, I.; Isbell, T.A.; Manresa, A. Mono-estolide synthesis from trans-8-hydroxy-fatty acids by lipases in solvent-free media and their physical properties. J. Am. Oil Chem. Soc. 2015, 92, 1125–1141. [Google Scholar] [CrossRef]
- Todea, A.; Otten, L.G.; Frissen, A.E.; Arends, I.; Peter, F.; Boeriu, C.G. Selectivity of lipases for estolides synthesis. Pure Appl. Chem. 2015, 87, 51–58. [Google Scholar] [CrossRef]
- Auffray, P.; Pallas, S. Green Estolides Enzymatic Synthesis of Fatty Acids Polyesters & Estolides. Available online: https://cellar-c2.services.clever-cloud.com/s3.seqens.com/uploads/sites/8/2020/12/White-papers-estolidesv2-1.pdf (accessed on 17 June 2024).
- Harry-O’Kuru, R.E. 4-Hydroxy-3-methoxycinnamate esters of milkweed oil: Synthesis and characterization. Lipids 2005, 40, 1179–1183. [Google Scholar] [CrossRef]
- Evans, K.O.; Harry-O’Kuru, R.E. Antioxidation behavior of milkweed oil 4-hydroxy-3-methoxycinnamate esters in phospholipid bilayers. J. Am. Oil Chem. Soc. 2013, 90, 1719–1727. [Google Scholar] [CrossRef]
- Yamamoto, A.; Nemoto, K.; Yoshida, M.; Tominaga, Y.; Imai, Y.; Ata, S.; Takenaka, Y.; Abe, H.; Sato, K. Improving thermal and mechanical properties of biomass-based polymers using structurally ordered polyesters from ricinoleic acid and 4-hydroxycinnamic acids. RSC Adv. 2020, 10, 36562–36570. [Google Scholar] [CrossRef] [PubMed]
- Bodalo, A.; Bastida, J.; Maximo, M.F.; Montiel, M.C.; Gomez, M.; Murcia, M.D. Production of ricinoleic acid estolide with free and immobilized lipase from Candida rugosa. Biochem. Eng. J. 2008, 39, 450–456. [Google Scholar] [CrossRef]
- Bitcan, I.; Pellis, A.; Petrovici, A.; Dreavă, D.M.; Păușescu, I.; Peter, F.; Nagy, L.; Gardossi, L.; Todea, A.; Kéki, S. One-pot green synthesis and characterization of novel furan-based oligoesters. Sustain. Chem. Pharm. 2023, 35, 101229. [Google Scholar] [CrossRef]
- Gordon, D.R.; Tancig, K.J.; Onderdonk, D.A.; Gantz, C.A. Assessing the invasive potential of biofuel species proposed for Florida and the United States using the Australian Weed Risk Assessment. Biomass Bioenergy 2011, 35, 74–79. [Google Scholar] [CrossRef]
- Greco-Duarte, J.; Collaço, A.C.A.; Costa, A.M.M.; Silva, L.O.; Da Silva, J.A.C.; Torres, A.G.; Fernandez-Lafuente, R.; Freire, D.M.G. Understanding the degree of estolide enzymatic polymerization and the effects on its lubricant properties. Fuel 2019, 245, 286–293. [Google Scholar] [CrossRef]
- Ortega-Requena, S.; Serrano-Arnaldos, M.; Montiel, M.C.; Máximo, F.; Bastida, J.; Murcia, M.D. Biocatalytic synthesis of polymeric esters used as emulsifiers. Chem. Biochem. Eng. Q. 2019, 33, 79–86. [Google Scholar] [CrossRef]
- Cermak, S.C.; Brandon, K.B.; Isbell, T.A. Synthesis and physical properties of estolides from Lesquerella and castor fatty acid esters. Ind. Crops Prod. 2006, 23, 54–64. [Google Scholar] [CrossRef]
- Croitoru, R.; Fiţigău, F.; van den Broek, L.A.M.; Frissen, A.E.; Davidescu, C.M.; Boeriu, C.G.; Peter, F. Biocatalytic acylation of sugar alcohols by 3-(4-hydroxyphenyl)propionic acid. Process Biochem. 2012, 47, 1894–1902. [Google Scholar] [CrossRef]
- Buzatu, A.R.; Frissen, A.E.; van den Broek, L.A.M.; Todea, A.; Motoc, M.; Boeriu, C.G. Chemoenzymatic synthesis of new aromatic esters of mono- and oligosaccharides. Processes 2020, 8, 1638. [Google Scholar] [CrossRef]
- Naik, S.; Basu, A.; Saiki, R.; Madan, B.; Paul, P.; Chaterjee, R.; Brask, J.; Svendsen, A. Lipases for use in industrial biocatalysis: Specificity of selected structural groups of lipases. J. Mol. Catal. B Enzym. 2010, 65, 18–23. [Google Scholar] [CrossRef]
- Flourat, A.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.-H.; Allais, F. Accessing p-hydroxycinnamic acids: Chemical synthesis, biomass recovery or engineered microbial production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Hou, J.; Chao, N.; Gai, Y.; Jiang, S. Three steps in one pot: Biosynthesis of 4-hydroxycinnamyl alcohols using immobilized whole cells of two genetically engineered Escherichia coli strains. Microb. Cell Factories 2017, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Standard Practice for Calculating Viscosity Index. Available online: https://wiki.anton-paar.com/en/astm-d2270-viscosity-index-vi-from-40c-and-100c/ (accessed on 17 July 2024).
- Viscosity Index of lubricants. Available online: https://www.klueber.com/us/en/products-service/tools-apps/viscosity-index-calculator/ (accessed on 17 July 2024).
- Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Grzybowski, Ł.; Czupryński, B. Glycerolysis of poly(lactic acid) as a way to extend the “life cycle” of this material. Polymers 2019, 11, 1963. [Google Scholar] [CrossRef] [PubMed]
- ISO Standard No. 17556:2019; Plastics—Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in Soil by Measuring the Oxygen Demand in a Respirometer or the Amount of Carbon Dioxide Evolved. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/74993.html#lifecycle (accessed on 17 July 2024).
- Zappaterra, F.; Renzi, M.; Piccardo, M.; Spennato, M.; Asaro, F.; Di Serio, M.; Vitiello, R.; Turco, R.; Todea, A.; Gardossi, L. Understanding marine biodegradation of bio-based oligoesters and plasticizers. Polymers 2023, 15, 1536. [Google Scholar] [CrossRef]
- Cunniff, P. (Ed.) Official Methods of Analysis of AOAC International, 16th ed.; Methods 920.213, 921.08, 972.28, 993.20; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ricinoleic-acid (accessed on 17 April 2023).
- Attia, N.K.; El-Mekkawi, S.A.; Elardy, O.A.; Abdelkader, E.A. Chemical and rheological assessment of produced biolubricants from different vegetable oils. Fuel 2020, 271, 117578. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry, 2nd ed.; Thermo Electron Karlsruhe: Karlsruhe, Germany, 2004; pp. 21–24. [Google Scholar]
- Soodoo, N.; Bouzidi, L.; Narine, S.S. Fundamental structure—Function relationships in vegetable oil-based lubricants: A critical review. Lubricants 2023, 11, 284. [Google Scholar] [CrossRef]
- Jenkins, S.; Martinez i Quer, A.; Fonseca, C.; Varrone, C. Microbial degradation of plastics: New plastic degraders, mixed cultures and engineering strategies. In Soil Microenvironment for Bioremediation and Polymer Production; Jamil, N., Kumar, P., Batool, R., Eds.; Scrivener Publishing: London, UK, 2020; pp. 215–238. [Google Scholar]

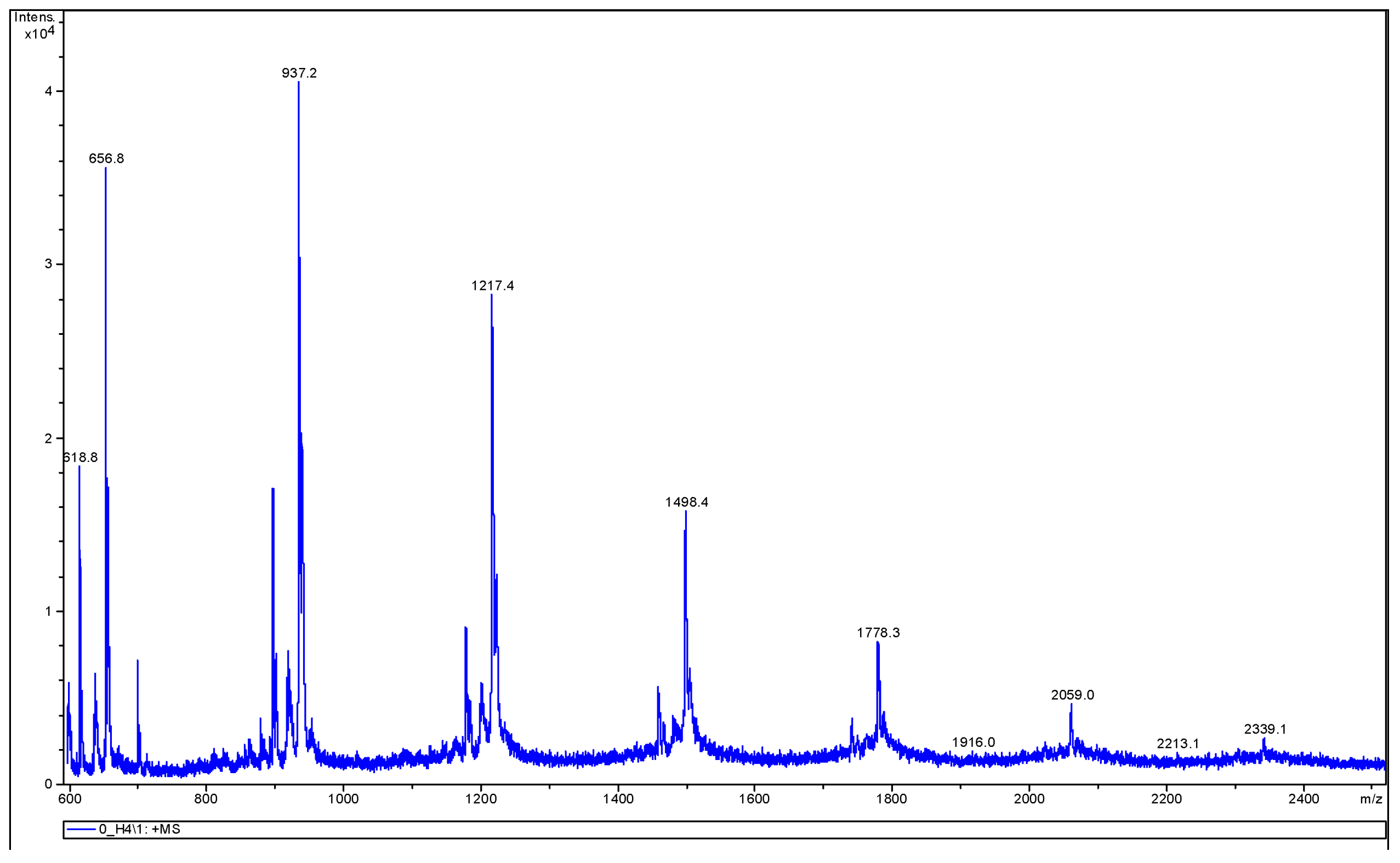
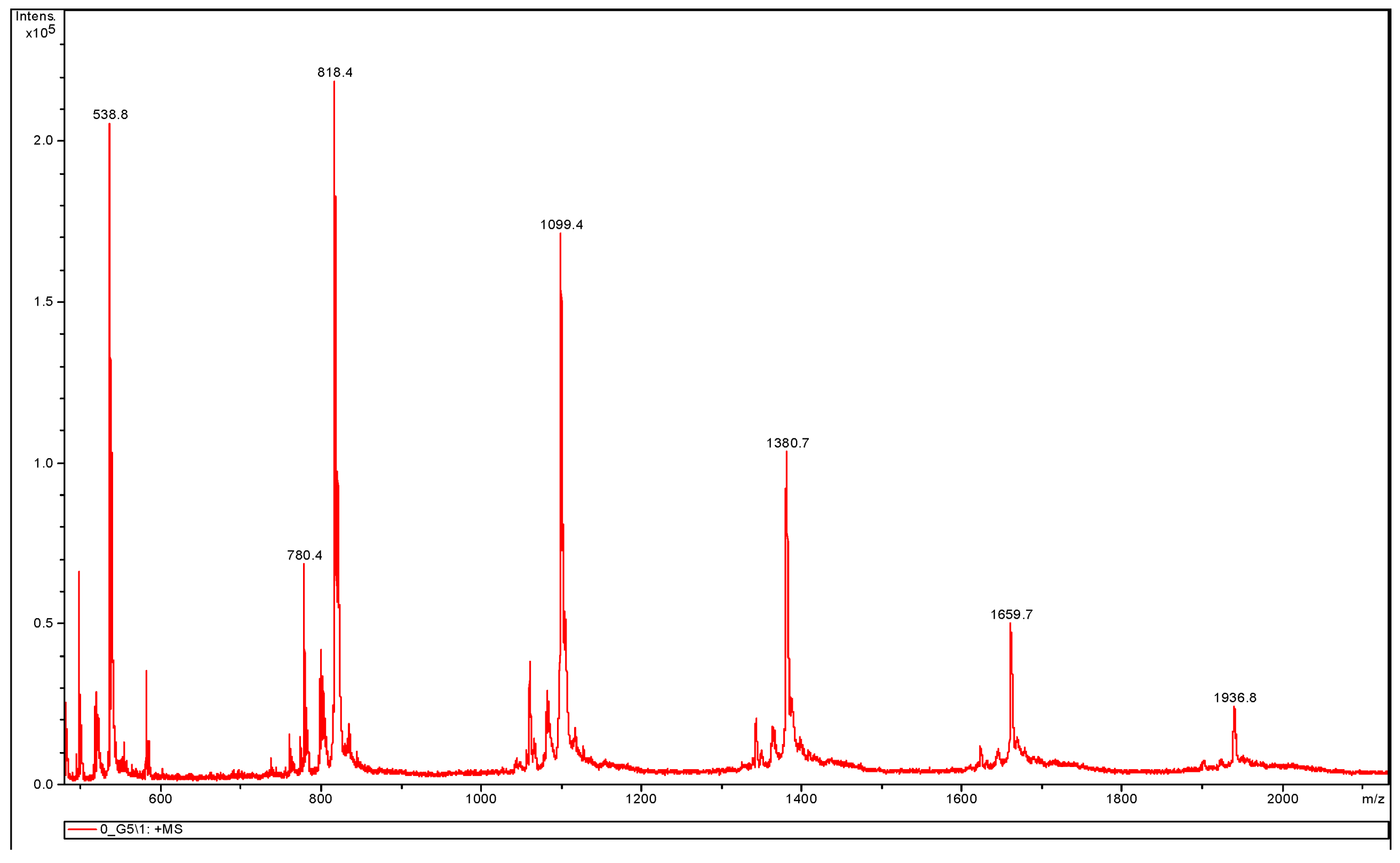
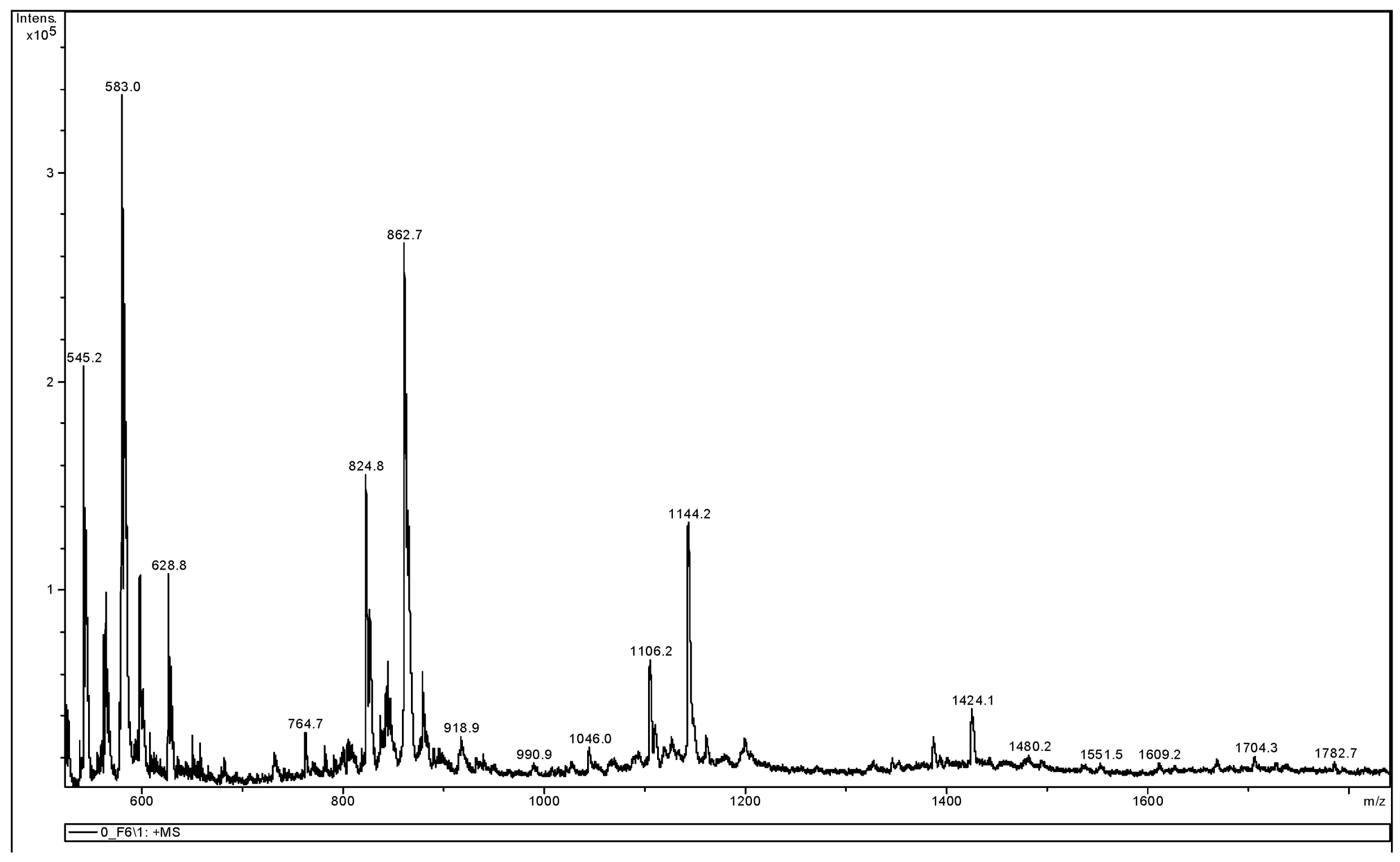


| Endcapping Compound | Mn [g/mole] | Mw [g/mole] | ĐM |
|---|---|---|---|
| - | 1104 | 1258 | 1.14 |
| Caffeic acid | 1003 | 1184 | 1.18 |
| Sinapic acid | 821 | 898 | 1.09 |
| Cinnamyl alcohol | 965 | 1035 | 1.07 |
| Sample | Kinematic Viscosity, cSt | Viscosity Index | |
|---|---|---|---|
| 40 °C | 100 °C | ||
| RCA | 142.5 | 10.6 | 29 |
| Oligo(ricinoleyl)-caffeate | 200.6 | 14.7 | 61 |
| Temperature, °C | R2 | |
|---|---|---|
| 40 | 0.999975 | |
| 55 | 0.999908 | |
| 70 | 0.99961 |
| Temperature, °C | R2 | |
|---|---|---|
| 40 | 0.999987 | |
| 55 | 0.999945 | |
| 70 | 0.99982 |
| ThOD a [mg/mg] | BOD5 [mg/mg] | Dt5 [%] | BOD10 [mg/mg] | Dt10 [%] | BOD21 [mg/mg] | Dt21 [%] |
|---|---|---|---|---|---|---|
| 2.156 | 0.276 | 13 | 0.432 | 20 | 0.652 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tămaș, A.; Bîtcan, I.; Nițu, S.; Paul, C.; Benea, I.C.; Rusu, G.I.; Perot, E.; Peter, F.; Todea, A. Novel Aromatic Estolide Esters from Biobased Resources by a Green Synthetic Approach. Appl. Sci. 2024, 14, 7832. https://doi.org/10.3390/app14177832
Tămaș A, Bîtcan I, Nițu S, Paul C, Benea IC, Rusu GI, Perot E, Peter F, Todea A. Novel Aromatic Estolide Esters from Biobased Resources by a Green Synthetic Approach. Applied Sciences. 2024; 14(17):7832. https://doi.org/10.3390/app14177832
Chicago/Turabian StyleTămaș, Andra, Ioan Bîtcan, Sabina Nițu, Cristina Paul, Ioana Cristina Benea, Gerlinde Iuliana Rusu, Elline Perot, Francisc Peter, and Anamaria Todea. 2024. "Novel Aromatic Estolide Esters from Biobased Resources by a Green Synthetic Approach" Applied Sciences 14, no. 17: 7832. https://doi.org/10.3390/app14177832






