Chaves Thermal Spring Water Impact on Skin Health: Potential Cosmetic Application
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Chaves Thermal Water Characterization
2.2. Evaluation of the Antioxidant Capacity of CHAVES Thermal Spring Water
2.3. Skin Enzyme Inhibition
2.3.1. Elastase Inhibition Assay
2.3.2. Collagenase Inhibition Assay
2.3.3. Tyrosinase Inhibition Assay
2.4. Cell Culture Assays
2.4.1. Cytotoxicity Assay
2.4.2. Quantification of Pro-Collagen 1 α1 and Fibronectin
2.4.3. Exposure to Urban Particulate Matter
2.5. Population of the Study of Skin Microbiota
2.6. Measurement of Skin Biometric Parameters
2.7. Collection of Skin Microbiota
2.8. DNA Extraction and Quantitative Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Characterization of Chaves Thermal Water
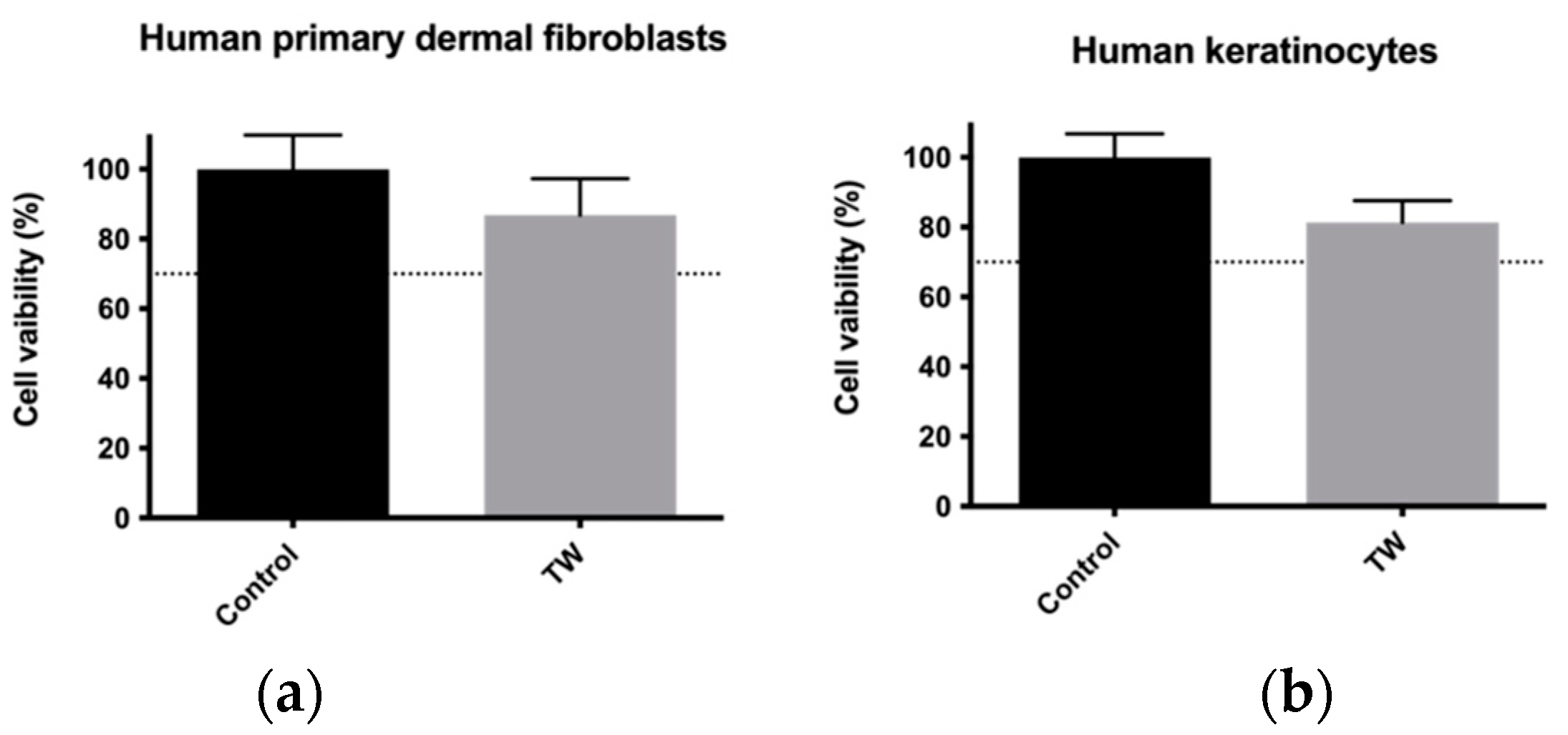
3.2. Cytotoxicity Assessment
3.3. Antioxidant and Antiaging Enzyme Activities
3.4. Cosmetic and Skincare Properties
3.4.1. Collagen and Fibronectin Production
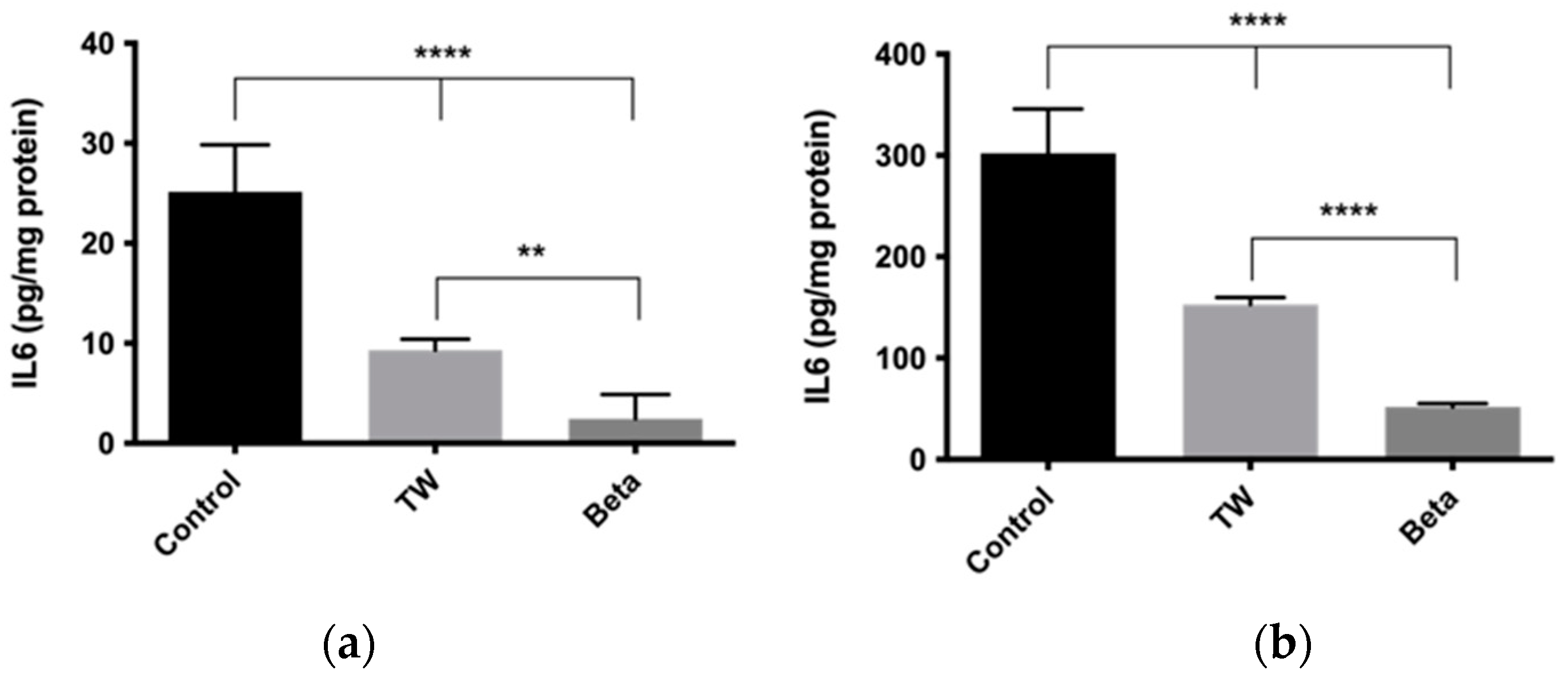
3.4.2. Cellular Exposure to Urban Particulate Matter
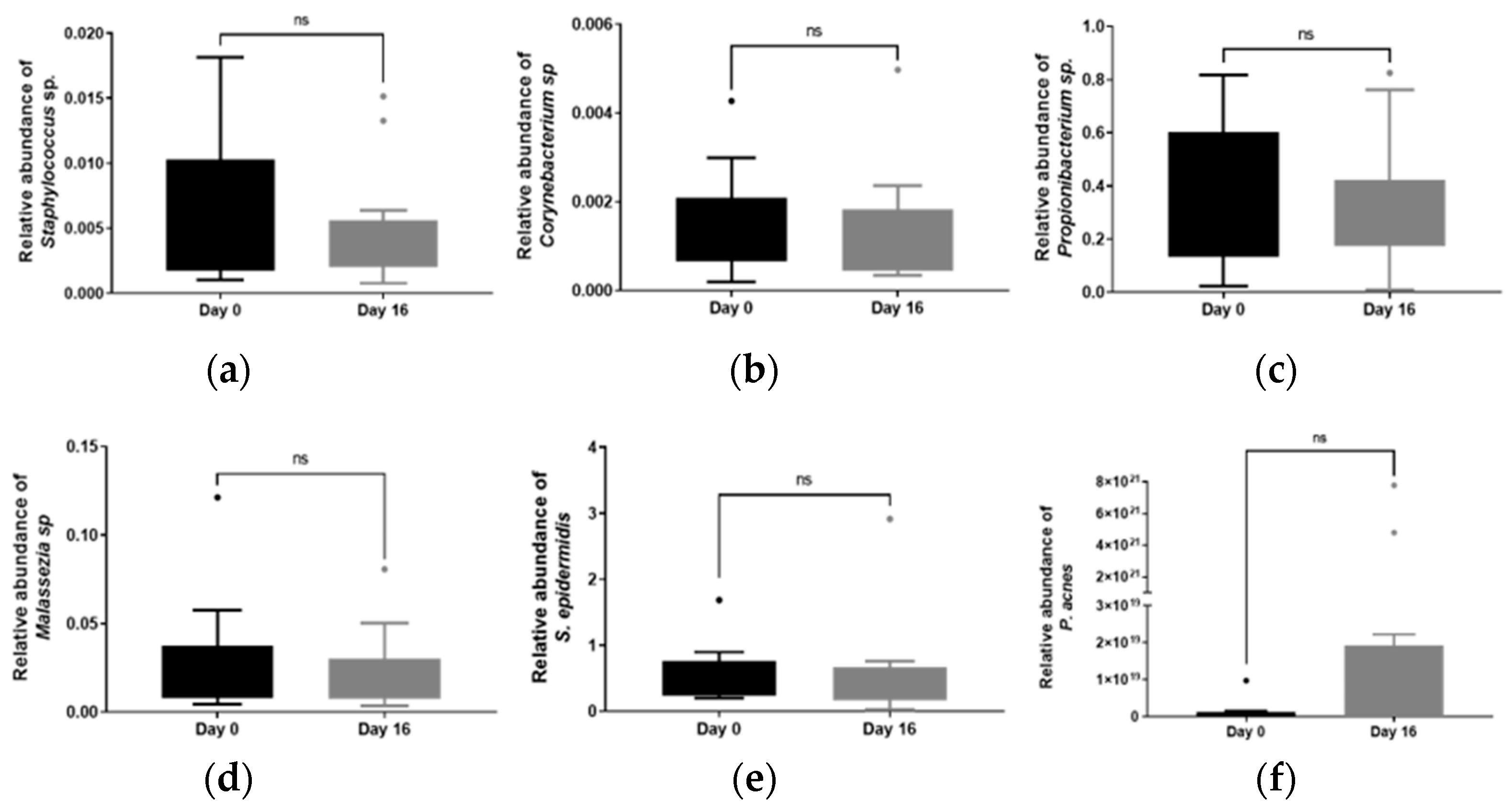
3.5. Evaluation of Skin Parameters and Skin Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghersetich, I.; Freedman, D.; Lotti, T. Balneology Today; Wiley Online Library: Hoboken, NJ, USA, 2000; pp. 346–348. [Google Scholar]
- Komatina, M. Medical Geology: Effects of Geological Environments on Human Health; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Huang, A.; Seité, S.; Adar, T. The use of balneotherapy in dermatology. Clin. Dermatol. 2018, 36, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, D.; Garrigue, E. Eau thermale d’Avène et dermatite atopique: Avène’s thermal water and atopic dermatitis. In Annales de Dermatologie et de Vénéréologie; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Geat, D.; Giovannini, M.; Barlocco, E.G.; Pertile, R.; Farina, S.; Pace, M.; Filippeschi, C.; Girolomoni, G.; Cristofolini, M.; Baldo, E. Characteristics associated with clinical response to Comano thermal spring water balneotherapy in pediatric patients with atopic dermatitis. Ital. J. Pediatr. 2021, 47, 91. [Google Scholar] [CrossRef] [PubMed]
- Kulisch, Á.; Mándó, Z.; Sándor, E.; Lengyel, Z.; Illés, A.; Kósa, J.; Árvai, K.; Lakatos, P.; Tóbiás, B.; Papp, M.; et al. Evaluation of the effects of Lake Hévíz sulfur thermal water on skin microbiome in plaque psoriasis: An open label, pilot study. Int. J. Biometeorol. 2023, 67, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Khalilzadeh, S.; Shirbeigi, L.; Naghizadeh, A.; Mehriardestani, M.; Shamohammadi, S.; Tabarrai, M. Use of mineral waters in the treatment of psoriasis: Perspectives of Persian and conventional medicine. Dermatol. Ther. 2019, 32, e12969. [Google Scholar] [CrossRef] [PubMed]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The role of thermal water in chronic skin diseases management: A review of the literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Antunes, J.D.M.; Daher, D.V.; Giaretta, V.M.D.A.; Ferrari, M.F.M.; Posso, M.B.S. Hydrotherapy and crenotherapy in the treatment of pain: Integrative review. BrJP 2019, 2, 187–198. [Google Scholar] [CrossRef]
- Meier, B.P.; Dillard, A.J.; Osorio, E.; Lappas, C.M. A behavioral confirmation and reduction of the natural versus synthetic drug bias. Med. Decis. Mak. 2019, 39, 360–370. [Google Scholar] [CrossRef]
- Hwa, C.; Bauer, E.A.; Cohen, D.E. Skin biology. Dermatol. Ther. 2011, 24, 464–470. [Google Scholar] [CrossRef]
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five functional aspects of the epidermal barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Oliveira, A.L.S.; Pedrosa, S.S.; Pintado, M.; Pinto-Ribeiro, I.; Madureira, A.R. Skin microbiota and the cosmetic industry. Microb. Ecol. 2023, 86, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Hüls, A. Air pollution and skin aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef]
- Rembiesa, J.; Ruzgas, T.; Engblom, J.; Holefors, A. The impact of pollution on skin and proper efficacy testing for anti-pollution claims. Cosmetics 2018, 5, 4. [Google Scholar] [CrossRef]
- Braga, P.C.; Ceci, C.; Marabini, L.; Nappi, G. The antioxidant activity of sulphurous thermal water protects against oxidative DNA damage: A comet assay investigation. Drug Res. 2013, 63, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Sambataro, G.; Sasso, M.D.; Culici, M.; Alfieri, M.; Nappi, G. Antioxidant effect of sulphurous thermal water on human neutrophil bursts: Chemiluminescence evaluation. Respiration 2008, 75, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Prandelli, C.; Parola, C.; Buizza, L.; Delbarba, A.; Marziano, M.; Salvi, V.; Zacchi, V.; Memo, M.; Sozzani, S.; Calza, S.; et al. Sulphurous thermal water increases the release of the anti-inflammatory cytokine IL-10 and modulates antioxidant enzyme activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 633–646. [Google Scholar] [CrossRef]
- Tacheau, C.; Weisgerber, F.; Fagot, D.; Bastien, P.; Verdier, M.P.; Liboutet, M.; Sore, G.; Bernard, B.A. Vichy Thermal Spring Water (VTSW), a cosmetic ingredient of potential interest in the frame of skin ageing exposome: An in vitro study. Int. J. Cosmet. Sci. 2018, 40, 377–387. [Google Scholar] [CrossRef]
- Rasmont, V.; Valois, A.; Gueniche, A.; Sore, G.; Kerob, D.; Nielsen, M.; Berardesca, E. Vichy volcanic mineralizing water has unique properties to strengthen the skin barrier and skin defenses against exposome aggressions. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 5–15. [Google Scholar] [CrossRef]
- Joly, F.; Gardille, C.; Barbieux, E.; Lefeuvre, L. eneficial effect of a thermal spring water on the skin barrier recovery after injury: Evidence for claudin-6 expression in human skin. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 273–276. [Google Scholar]
- Mias, C.; Maret, A.; Gontier, E.; Carrasco, C.; Satge, C.; Bessou-Touya, S.; Coubetergues, H.; Bennett-Kennett, R.; Dauskardt, R.H.; Duplan, H. Protective properties of Avène Thermal Spring Water on biomechanical, ultrastructural and clinical parameters of human skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 15–20. [Google Scholar] [CrossRef]
- Zeichner, J.; Seite, S. From probiotic to prebiotic using thermal spring water. J. Drugs Dermatol. 2018, 17, 657–662. [Google Scholar]
- Tamás, B.; Gabriella, K.; Kristóf; Anett, I.; Pál, K.J.; Bálint, T.; Péter, L.; Márton, P.; Katalin, N. The Effects of Lakitelek Thermal Water and Tap Water on Skin Microbiome, a Randomized Control Pilot Study. Life 2023, 13, 746. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- Aires-Barros, L.; Marques, J.M.; Graça, R.C. Elemental and isotopic geochemistry in the hydrothermal area of Chaves, Vila Pouca de Aguiar (northern Portugal). Environ. Geol. 1995, 25, 232–238. [Google Scholar] [CrossRef]
- Vaz, M.; Fernandes, P.O.; Ferreira, F.A.; Alves, M.J.; Costa, V.; Nunes, A. The importance-satisfaction matrix as a strategic tool for Termas de Chaves thermal spa priority improvements. J. Tour. Sustain. Well-Being 2023, 11, 52–65. [Google Scholar]
- Clesceri, L.S. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DA, USA, 1998; Volume 9. [Google Scholar]
- ISO 6222:1999; Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium. International Organization for Standardization: Geneva, Switzerland, 1999.
- Gonçalves, B.; Falco, V.; Moutinho-Pereira, J.; Bacelar, E.; Peixoto, F.; Correia, C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. J. Agric. Food Chem. 2009, 57, 265–273. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices; Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Carvalho, M.J.; Pedrosa, S.S.; Mendes, A.; Azevedo-Silva, J.; Fernandes, J.; Pintado, M.; Oliveira, A.L.S.; Madureira, A.R. Anti-Aging Potential of a Novel Ingredient Derived from Sugarcane Straw Extract (SSE). Int. J. Mol. Sci. 2023, 25, 21. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.J.; Pinto-Ribeiro, I.; Castro, C.; Pedrosa, S.S.; Oliveira, A.L.; Pintado, M.; Madureira, A.R. Impact of a novel sugarcane straw extract-based ingredient on skin microbiota via a new preclinical in vitro model. Microbe 2023, 1, 100017. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.-M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Seite, S. Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example. Clin. Cosmet. Investig. Dermatol. 2013, 6, 23–28. [Google Scholar] [CrossRef]
- Merial-Kieny, C.; Castex-Rizzi, N.; Selas, B.; Mery, S.; Guerrero, D. Avène Thermal Spring Water: An active component with specific properties. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 2–5. [Google Scholar] [CrossRef]
- Ferreira, M.O.; Costa, P.C.; Bahia, M.F. Effect of São Pedro do Sul thermal water on skin irritation. Int. J. Cosmet. Sci. 2010, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Madeira, A.; Marto, J.; Graça, A.; Pinto, P.; Ribeiro, H. Monfortinho thermal water-based creams: Effects on skin hydration, psoriasis, and eczema in adults. Cosmetics 2019, 6, 56. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards optimal ph of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Nasermoaddeli, A.; Kagamimori, S. Balneotherapy in medicine: A review. Environ. Health Prev. Med. 2005, 10, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Ribet, V.; Alvarez-Georges, S.; Sibaud, V.; Guerrero, D.; Schmitt, A.; Redoulès, D. Modulation of Interleukin-8 and staphylococcal flora by Avène hydrotherapy in patients suffering from chronic inflammatory dermatoses. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 19–23. [Google Scholar] [CrossRef]
- Castex-Rizzi, N.; Charveron, M.; Merial-Kieny, C. Inhibition of TNF-alpha induced-adhesion molecules by Avène Thermal Spring Water in human endothelial cells. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 6–11. [Google Scholar] [CrossRef]
- Nunes, F.; Rodrigues, M.; Ribeiro, M.P.; Ugazio, E.; Cavalli, R.; Abollino, O.; Coutinho, P.; Araujo, A.R.T.S. Incorporation of Cró thermal water in a dermocosmetic formulation: Cytotoxicity effects, characterization and stability studies and efficacy evaluation. Int. J. Cosmet. Sci. 2019, 41, 604–612. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Vaz, C.V.; Silva, A.; Correia, S.; Ferreira, R.; Breitenfeld, L.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R.; Pereira, C.; Cruz, M.T.; et al. In vitro evaluation of potential benefits of a silica-rich thermal water (Monfortinho Thermal Water) in hyperkeratotic skin conditions. Int. J. Biometeorol. 2020, 64, 1957–1968. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, G.; Corbella, M.; Jaber, O.; Marone, P.; Scevola, D.; Faga, A. Non-pathogenic microflora of a spring water with regenerative properties. Biomed. Rep. 2015, 3, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Portugal-Cohen, M.; Oron, M.; Merrik, E.; Ben-Amitai, D.; Yogev, H.; Zvulunov, A. A Dead Sea Water-Enriched Body Cream Improves Skin Severity Scores in Children with Atopic Dermatitis. J. Cosmet. Dermatol. Sci. Appl. 2011, 1, 99–106. [Google Scholar] [CrossRef]
- Joly, F.; Branka, J.-E.; Lefeuvre, L. Thermal Water from Uriage-les-Bains Exerts DNA Protection, Induction of Catalase Activity and Claudin-6 Expression on UV Irradiated Human Skin in Addition to Its Own Antioxidant Properties. J. Cosmet. Dermatol. Sci. Appl. 2014, 04, 99–106. [Google Scholar] [CrossRef]
- Knott, A.; Drenckhan, A.; Reuschlein, K.; Lucius, R.; Döring, O.; Böttger, M.; Stäb, F.; Wenck, H.; Gallinat, S. Corrigendum to Decreased fibroblast contractile activity and reduced fibronectin expression are involved in skin photoaging. J. Dermatol. Sci. 2010, 58, 232. [Google Scholar] [CrossRef]
- Sun, W.; He, J.; Zhang, Y.; He, R.; Zhang, X. Comprehensive functional evaluation of a novel collagen for the skin protection in human fibroblasts and keratinocytes. Biosci. Biotechnol. Biochem. 2023, 87, 724–735. [Google Scholar] [CrossRef]
- Mormile, I.; Tuccillo, F.; Della Casa, F.; D’aiuto, V.; Montuori, N.; De Rosa, M.; Napolitano, F.; de Paulis, A.; Rossi, F.W. The Benefits of Water from Nitrodi’s Spring: The In Vitro Studies Leading the Potential Clinical Applications. Int. J. Mol. Sci. 2023, 24, 13685. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Mühlberg, K.; Brenden, H.; Felsner, I.; Brynjolfsdottir, A.; Einarsson, S.; Krutmann, J. Bioactive molecules from the Blue Lagoon: In vitro and in vivo assessment of silica mud and microalgae extracts for their effects on skin barrier function and prevention of skin ageing. Exp. Dermatol. 2008, 17, 771–779. [Google Scholar] [CrossRef]
- de Araújo, L.A.; Addor, F.; Campos, P.M.B.G.M. Use of silicon for skin and hair care: An approach of chemical forms available and efficacy. An. Bras. de Dermatol. 2016, 91, 331–335. [Google Scholar] [CrossRef]
- Joly, F.; Charveron, M.; Aries, M.F.; Bidault, J.; Kahhak, L.; Beauvais, F.; Gall, Y. Effect of Avène Spring Water on the Activation of Rat Mast Cell by Substance P or Antigen. Ski. Pharmacol. Appl. Ski.Physiol. 1998, 11, 111–116. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Ski. Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.C.; Nikam, V.N.; Dandakeri, S.; Bhat, R.M. Transepidermal Water Loss in Psoriasis: A Case-control Study. Indian Dermatol. Online J. 2019, 10, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Henley, J.B.; Sarrazin, P.; Seité, S. Skin Microbiome in Patients With Psoriasis Before and After Balneotherapy at the Thermal Care Center of La Roche-Posay. J. Drugs Dermatol. 2015, 14, 1400–1405. [Google Scholar] [PubMed]
- Haftek, M.; Abdayem, R.; Guyonnet-Debersac, P. Skin Minerals: Key Roles of Inorganic Elements in Skin Physiological Functions. Int. J. Mol. Sci. 2022, 23, 6267. [Google Scholar] [CrossRef]
- Woolery-Lloyd, H.; Andriessen, A.; Day, D.; Gonzalez, N.; Green, L.; Grice, E.; Henry, M. Review of the microbiome in skin aging and the effect of a topical prebiotic containing thermal spring water. J. Cosmet. Dermatol. 2023, 22, 96–102. [Google Scholar] [CrossRef]
- Manara, S.; Beghini, F.; Masetti, G.; Armanini, F.; Geat, D.; Galligioni, G.; Segata, N.; Farina, S.; Cristofolini, M. Thermal Therapy Modulation of the Psoriasis-Associated Skin and Gut Microbiome. Dermatol. Ther. 2023, 13, 2769–2783. [Google Scholar] [CrossRef]
- Instituto Nacional de Investigação Agrária e Veterinária (INIAV)Estudo do Microbismo Natural das Águas Minerais Naturais. 2018. Available online: https://hidrogenoma.dgeg.gov.pt/agua-mineral-natural/caldas-de-chaves (accessed on 27 May 2024).


| Chaves Thermal Spring Water | |
|---|---|
| Physicochemical characterization | |
| pH | 6.84 ± 0.01 |
| Conductivity (µS/cm) | 2532 ± 2.12 |
| Total dissolved solids (mg/L) | 1630 ± 10 |
| Minerals (mg/L) * | |
| Sodium (Na) | 576 ± 16.91 |
| Potassium (K) | 74.02 ± 1.02 |
| Silicon (Si) | 37.20 ± 0.34 |
| Calcium (Ca) | 26.03 ± 0.46 |
| Magnesium (Mg) | 7.62 ± 0.10 |
| Sulfur (S) | 4.89 ± 0.05 |
| Phosphor (P) | 4.02 ± 0.05 |
| Molybdenum (Mo) | 0.04 ± 0.001 |
| Manganese (Mn) | 0.03 ± 0.0004 |
| Cadmium (Cd) | 0.02 ± 0.0003 |
| Enzyme Relative Inhibition (%) | Antioxidant Activity (%) | ||||
|---|---|---|---|---|---|
| Elastase | MMP-1 | Tyrosinase | ABTS | DPPH | |
| Chaves thermal spring water | 14.22 ± 4.29 b | 0.70 ± 0.25 b | 0.005 ± 0.00 b | 9.09 ± 3.88 b | ND |
| Vitamin C (8 mg/mL) | 90.54 ±1.51 a | 96.26 ± 0.31 a | 99.26 ± 0.31 a | − | − |
| Vitamin C (0.075 mg/mL) | − | − | − | 91.94 ± 2.03 b | 86.56 ± 0.48 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Ribeiro, I.; Castro, C.; Rocha, P.E.; Carvalho, M.J.; Pintado, A.; Mendes, A.; Pedrosa, S.S.; Capeto, P.; Azevedo-Silva, J.; Oliveira, A.L.S.; et al. Chaves Thermal Spring Water Impact on Skin Health: Potential Cosmetic Application. Appl. Sci. 2024, 14, 7911. https://doi.org/10.3390/app14177911
Pinto-Ribeiro I, Castro C, Rocha PE, Carvalho MJ, Pintado A, Mendes A, Pedrosa SS, Capeto P, Azevedo-Silva J, Oliveira ALS, et al. Chaves Thermal Spring Water Impact on Skin Health: Potential Cosmetic Application. Applied Sciences. 2024; 14(17):7911. https://doi.org/10.3390/app14177911
Chicago/Turabian StylePinto-Ribeiro, Inês, Cláudia Castro, Pedro Emanuel Rocha, Maria João Carvalho, Ana Pintado, Adélia Mendes, Sílvia Santos Pedrosa, Paula Capeto, João Azevedo-Silva, Ana L. S. Oliveira, and et al. 2024. "Chaves Thermal Spring Water Impact on Skin Health: Potential Cosmetic Application" Applied Sciences 14, no. 17: 7911. https://doi.org/10.3390/app14177911









