Abstract
Sample preparation is a key step in the analytical procedure. This step is a time- and labor-consuming process, and often it is also expensive, with costs being influenced by the consumption of materials and reagents. Additionally, the toxicity of the reagents, waste generation, and energy consumption affect the environment and the safety of the analyst. New trends in sample preparation are focused on the development of miniaturized methods that are consistent with the principles of green sample preparation and contribute to environmental sustainability. The results of a comprehensive assessment of ten methods of preparing water samples for the determination of UV filters using gas chromatography are presented. Three assessment tools were used for this purpose: AGREEprep (the analytical greenness metric for sample preparation), BAGI (the blue applicability grade index), and the RGB 12 algorithm (red–green–blue model). All the differences and similarities between the three aforementioned metrics are discussed in this manuscript. The results of the evaluation of the most frequently used microextraction methods show their ecological friendliness, effectiveness, and practicality. The results of this assessment will allow researchers to identify the strengths and weaknesses of the given methods and select those that meet their requirements.
1. Introduction
UV filters are a group of chemicals commonly used in a wide range of cosmetic products to protect the skin from the harmful effects of UV radiation [1,2]. Organic UV filters have a highly lipophilic character, and most of them are classified as water-resistant and therefore tend to accumulate in the fatty tissues of living organisms [3,4]. They are considered emerging contaminants since they easily enter the natural environment, where they accumulate, causing harmful effects on flora and fauna despite being present at the ng/L level. Therefore, developing sensitive and selective analytical methods for their environmental monitoring is of high interest [5]. As exemplified by the Web of Science database, the results for the combination keywords “UV filters” and “environmental water” showed a growing trend in the amount of research (from 2002 to 2023) on the contamination of the waters by UV filters (Figure 1).
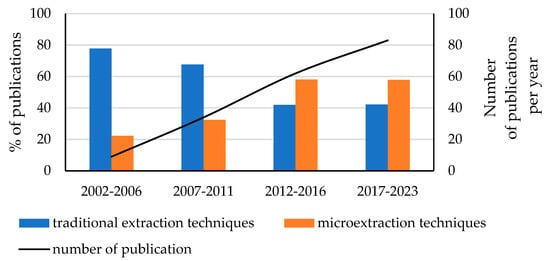
Figure 1.
Evolution of the number of publications (%) concerning the determination of UV filters in environmental water samples (2002–2023) by using traditional extraction techniques and microextraction techniques (188 articles on the determination of UV filters in water samples).
Based on the results obtained from the database, an increasing number of applied microextraction techniques compared to conventional techniques can be observed. The most applied classic extraction methods include solid-phase extraction (SPE), liquid–liquid extraction (LLE), fabric phase sorptive extraction (FPSE), Quick, Easy, Cheap, Effective, Rugged, and Safe Extraction (QuEChERS), and magnetic nanoparticles dispersive solid-phase extraction (MNPs-based dSPE). Microextraction techniques include solid-phase microextraction (SPME), stir bar sorptive extraction (SBSE), dispersive solid-phase extraction (dSPE), microextraction by packed sorbent (MEPS), bar adsorptive microextraction (BAµE), stir bar sorptive dispersive microextraction (SBSDME), single-drop microextraction (SDME), in situ suspended aggregate microextraction (iSAME), hollow-fibre liquid-phase microextraction (HFLPME), dispersive liquid–liquid microextraction (DLLME), ultrasounds-assisted dispersive liquid–liquid microextraction (USA-DLLME), vortex-assisted dispersive liquid–liquid microextraction (VA-DLLME), and ultrasounds-assisted emulsification microextraction (USAE-ME). Based on the literature review, it is concluded that the most popular microextraction techniques for determining UV filters are the following: SPME (~24%); DLLME, with various variants (~24%); SBSE (~16%); MSPE (~5%); and others (~16%). For instrumental techniques, the most common choices for the detection and quantification of the compounds studied in water samples remain gas chromatography and liquid chromatography, coupled with mass spectrometry [5,6,7,8].
Selecting the most appropriate analytical method for determining UV filters in water samples, among many developed methods, is not an easy task. When choosing a method, validation criteria should be taken into account, i.e., accuracy, precision, sensitivity, and selectivity, as well as economic and practical factors, i.e., costs, time, and ease of use. Moreover, special attention is currently being paid to the ecological aspect of the analytical method. According to the principles of Green Analytical Chemistry (GAC), methods should be used that do not pose a threat to human health and the environment.
GAC is an aspect of green analytical chemistry that was introduced in the late 1990s. It is a concept that is based on twelve principles related to the environment, health, and safety [9]. GAC takes into account, among other things, the use of safe solvents/reagents, the generation of toxic waste, and the safety of the analysts. In 2022, López-Lorente et al. [10] proposed ten principles of Green Sample Preparation (GSP) that aim to develop greener analytical procedures. In GSP, the GAC principles have been extended to include the use of solvents/reagents from renewable sources and reusable and/or recyclable materials. Additionally, GSP takes into account sample throughput, miniaturization, and the automation of the method. However, in 2021, Nowak et al. [11] introduced a new concept of sustainable development in analytical chemistry, the so-called White Analytical Chemistry (WAC), which is an extension of GAC. The authors of WAC proposed the WAC principles as an alternative to the 12 GAC principles but including not only green aspects (such as the toxicity of reagents, the number and amount of reagents and waste, energy, and other media, as well as other direct impacts). WAC also takes into account criteria such as analytical efficiency (scope of application, limits of quantification and detection, precision, and accuracy) and practical/economic criteria (cost-efficiency, time-efficiency, requirements, and operational simplicity). The compliance of analytical methods with the GAC, GSP, and WAC principles is a basic requirement in the development of sustainable analytical methods.
Over the last few years, various metric tools have been introduced to assess the environmental performance of analytical methods, including the National Environmental Method Index (NEMI) [12], the Analytical Eco-Scale [13], the Green Analytical Procedure Index (GAPI) [14], the Analytical Greenness Calculator (AGREE) [15], the RGB 12 algorithm [11], the Analytical Method Greenness Score (AMGS) [16], the Blue Applicability Grade Index (BAGI) [17], the Complementary Green Analytical Procedure Index (ComplexGAPI) [18], and the Analytical Greenness Metric for Sample Preparation (AGREEprep) [19]. All of these tools graphically and/or numerically reflect the compliance of a given analytical method with the GAC principles.
The main aim of this work was to assess the ecological and practical aspects of the water sample preparation step for the determination of UV filters by GC-MS using the AGREEprep, BAGI, and RGB 12 tools. These are the newest tools that are most often chosen for the evaluation of analytical procedures due to their versatility, usefulness, and simplicity of use.
Using these three metrics simultaneously will provide comprehensive information about the strengths and weaknesses of the analytical procedures used. At the same time, the presented correlations between the used metrics can be a guide for the analyst when deciding on the selection of a metric tool.
The assessment of analytical methods is necessary to understand their impact on the environment and can be helpful for analytical chemists when choosing a method for determining cosmetic ingredients and other compounds in water samples.
2. Materials and Methods
2.1. AGREEprep
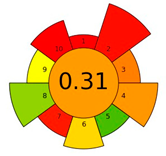
AGREEprep is a new analytical “greenness” metric that was published by Wojnowski et al. [19] in 2022. The free version of the software can be obtained from https://mostwiedzy.pl/AGREE (accessed on 3 May 2024) [20]. AGREEprep is based on ten steps of assessment that correspond to the ten principles of GSP. In order to assess the “greenness” of an analytical method, AGREEprep is based on ten individual steps which are presented in Table 1. Each criterion is scored from 0 to 1, with the extremes representing the worst and best performance, respectively. Moreover, each criterion has a default weight taken into account in the overall score, but the assessors can change this value at their discretion if there are valid reasons to do so [20]. However, in our work, we did not change the value of the criteria because we considered the default weights assigned to the criteria to be correct.

Table 1.
Description of the criteria and graphical presentation of results for AGREEprep, WAC, and BAGI metrics.
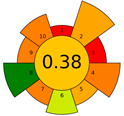
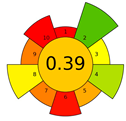
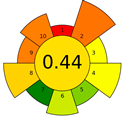
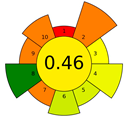
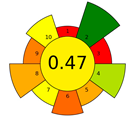
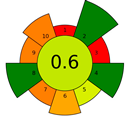
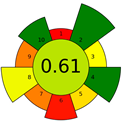
The result of the AGREEprep assessment is a colorful round pictogram that maps the degree of compliance of evaluated criteria within the rules of GAC. The color of the circle inside the pictogram and the overall score within it indicate the overall environmental performance of the sample preparation in a given analytical method. The overall score can range from 0 to 1, with a score of 0 being the worst result and 1 being the best result, taking into account the scores from all criteria or the lack of a sample-preparation step. On the outer part of the circle, there are ten parts, corresponding to the ten criteria. Each part may have a different length, depending on the weight assigned to a given criterion. However, the color of a given part indicates its score—a highest criterion score is indicated in green, and a lowest criterion score is indicated in red. A result between 0 and 1 is represented by a color gradient between red and green, e.g., yellow and orange in different shades, ac-cording to the value assigned by the AGREEprep calculator.
2.2. WAC
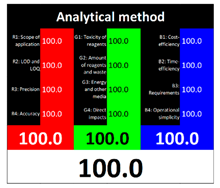
White analytical chemistry (WAC) is a concept of sustainable development in analytical chemistry, which is an extension of green analytical chemistry. WAC was designed and developed by Nowak et al. [11] in 2021, and it is a concept that encourages the harmony and integration of analytical, ecological, and practical characteristics, while aiming for the sustainability of analytical methods. In the WAC concept, the RGB (red, green, blue) model [21] is used to evaluate the analytical method. Just as the color white is created by mixing red, green, and blue light, the analytical method becomes white, and thus complete, when it achieves each primary color.
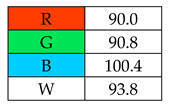
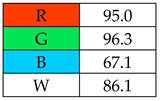
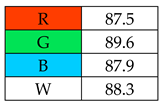
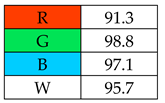
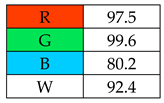
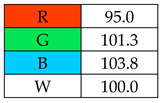
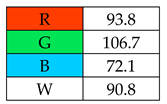
To evaluate the methods using the RGB 12 algorithm, which is the second version of the RGB model adapted to the 12 criteria of WAC, the available Excel template spreadsheet is used (access in Supplementary data in Nowak et al.’s work [11]), where specially prepared tables of red, green, and blue colors can be found. The template was designed to be able to evaluate and compare 10 methods simultaneously. The tables should be completed by assigning each criterion a point value ranging from 0 to 100. A value of 0 means the worst result, and 100 means that the method is well suited to the planned application. It is also possible to award more than 100 points for outstanding criteria in the evaluation of an analytical method. After completing the form, the assessment results are automatically calculated and presented in tabular form. The compliance of the method with a given WAC criterion is presented both numerically and visually by saturating a given value with color (a criterion value of 0 corresponds to black; 100 or more points correspond to full-color saturation). The value of arithmetic means for the red, green, and blue criteria are expressed individually as R (%), G (%), and B (%), while the overall result (whiteness—%) is given in the table and figure (Table 1).
2.3. BAGI
The blue applicability grade index (BAGI) is a new analytical “blueness” metric tool for evaluating the practicality of an analytical method, which was published by Manousi et al. [17] in 2023. The free version of the software can be obtained from https://mostwiedzy.pl/pl/justyna-plotka-wasylka,647762-1/BAGI (accessed on 3 February 2024). The blue color in the BAGI metric is inspired by the RGB model, and it may be considered complementary to the existing green metrics tools. In order to assess the applicability of an analytical method, BAGI takes into account the criteria shown in Table 1.
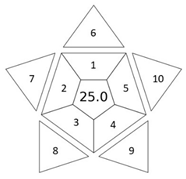
The overall result of assessing the method using BAGI is an asteroid pictogram with a number in the center. The hue of the scale of the pictogram reflects the compliance of the method with the designated criteria. There are four colors in the BAGI: dark blue for high compliance, blue for medium compliance, light blue for low compliance, and white for no compliance. The number in the center of the pictogram indicates the overall score for the analytical method, which is a number ranging from 25 to 100. A point value of 100 is assigned to a method with excellent performance, and a value of 25 indicates the worst performance of the method in terms of applicability. A method whose BAGI score is at least 60 points is considered practical. In the pictogram, criteria 1–5, located in its inner part, correspond to the stage of analytical determination or sample preparation stage. However, criteria 6–10, placed in the outer part, correspond to both mentioned stages. The result field takes on a shade that is the average shade of all criteria taken into account in BAGI.
3. Results and Discussion
3.1. Greenness, Blueness, and Whiteness Evaluation
The paper presents an assessment of the environmental impact and analytical suitability of ten methods of preparing water samples for the determination of UV filters described in the literature. One of the methods, solid phase extraction (SPE), is a classic extraction method, while the other nine are commonly used microextraction techniques. Table 2 presents assessed analytical methods and their literature sources. A brief description of the assessed sample preparation methods for analysis is presented in Supporting Information (Table S1).

Table 2.
Results from the evaluation of methods for preparing water samples for the analysis of UV filters obtained using the AGREEprep, BAGI, and WAC metrics.
All selected sample preparation procedures use gas chromatography and mass detection (GC-MS) for analysis. This made it possible to evaluate only the sample preparation step without taking into account the time, costs, and energy needed to perform the chromatographic analysis. In the case of methods using thermal desorption of analytes from the sorbent in the chromatograph dispenser, i.e., SBSE and SPME, the desorption step was included in the total assessment (time and energy consumption). It was also assumed that one sample was analyzed, and the time and costs incurred for its optimization were not taken into account when assessing the method. Using the example of determining UV filters in water samples, the environmental friendliness and functionality of commonly used microextraction methods were assessed using three tools, i.e., AGREEprep, BAGI, and RGB 12. AGREEprep assesses in detail the environmental impact of the use of a given analytical procedure, BAGI assesses its usefulness, while RGB 12 covers its comprehensive assessment ecological performance, analytical performance, and its economic and practical aspects.
3.1.1. AGREEprep Assessment
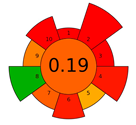
The use of AGREEprep allows the critical evaluation of each step of sample preparation from the GAC point of view. The results of the assessment performed using AGREEprep are presented in Table 2. As expected, the lowest score (0.19) was obtained by the SPE method, in which only one of the principles, energy use, was green (criterion 8). Classic extraction methods, including SPE, are characterized by high consumption of toxic solvents (criterion 2 uses safer solvents, and criterion 10 is safe for the operator), generate large amounts of waste (criterion 4), and are time-consuming (criterion 6). The SPE method, among the classical methods, is the most frequently used method for analyzing UV filters, which is the reason for presenting it in this work to compare it with microextraction methods.
However, the lowest AGREEprep score among the presented microextraction methods was given to the SBSE method, whose score was 0.3. Such a low assessment of the greenness of this method is due to the long time (criterion 6) of extraction (180 min) and desorption (15 min), as well as the energy (criterion 8) consumed during sample mixing, especially during the desorption step.
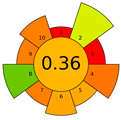
The USAEME method received a low AGREEprep score of 0.36. Only 100 µL of chloroform was used for extraction, which has a high score effect on the assessment of criterion 2. In this procedure, a large amount of sample was used (10 mL; criterion 5), to which 2 g of NaCl was added, together with the solvent. Due to the solvent contamination of the aqueous solution, all of the solution was treated as waste, which significantly lowered the score for criterion 4.
Sample preparation using the DLLME method takes very little time (~5 min), which results in a favorable result for criterion 6 (sample throughput). In this method, the sample is subjected to a short centrifugation (3 min), thanks to which the energy consumption is low, which is why criterion 8 has a green rating. However, the use of two hazardous solvents (1 mL acetone and 60 µL chlorobenzene) negatively affects criteria 2 and 10 (use safer solvents and safe for the operator), and it also results in a low AGREEprep score of 0.38.
The UV filters contain phenolic hydroxyl groups, which cause the low sensitivity of the GC analysis. The use of derivatization increases sensitivity and improves separation and shape peaks. Therefore, derivatization is often used in their analysis. Derivatization (on-fiber silylation) was used in the next assessed method—SPME. However, its use has a negative impact on the assessment of the ecological effectiveness of the method. The SPME with the derivatization method received a score of 0.39 in the AGREEprep evaluation. The reagents used for sample acidification and derivatization (MSTFA), and the time needed to perform derivatization, extraction, and desorption (~45 min) reduce the scores for criteria 6 and 10 (sample throughput and safety for the operator). Additionally, the energy consumed for mixing, sample heating, and thermal desorption reduces the score for criterion 8. For comparison, the SPME procedure (without derivatization) obtained the highest result of 0.61 among the assessed methods. A much smaller impact on the reduction of the greenness rating due to derivatization was demonstrated for other methods, i.e., DLLME (ultrasound-assisted) [32] and SBSE [33]. The AGREEprep rating for the DLLME method decreased from 0.38 to 0.3, and for SBSE from 0.3 to 0.28. In these methods, unlike SPME, derivatization is carried out simultaneously with extraction, which does not result in increased time and energy consumption.
The example of the MEPS method shows the differences in the AGREEprep assessment for the fully automated and manual MEPS method, which received scores of 0.44 and 0.46, respectively. Both methods have one green criterion: the manual MEPS scores green on criterion 8 (energy consumption), while the fully automated method scores green on criterion 7 (integration, automation). However, criterion 8 has a higher weight in the AGREEprep metric, which causes a difference in the evaluation of both methods. The rating of both MEPS techniques is not the highest, influenced by the fact that solvents are used for extraction (low criterion 2), and it additionally reduces the criteria related to waste generation and safety for the operator (criteria 4 and 10).
Dispersive micro- solid-phase extraction (DmSPE) is a solvent-free method, with a positive impact on criterion 2, which received the green status. However, the total score for this procedure is not high, and amounted to 0.47. The derivatization step of analytes reduces the overall evaluation of the method. After extraction, the analytes, together with the sorption bed, are placed in the injection port, where they are derivatized and then thermally desorbed. The use of a derivatization reagent (BSTFA) extends the sample preparation time (criterion 6—sample throughput) and reduces criteria 2 and 10 (use safer reagents and operator safety).
Only two microextraction methods achieved the green status, with scores of 0.6 and 0.61—SDME and DI-SPME, respectively. The SDME method obtained such a high rating compared to the previously discussed methods thanks to the use of a very small amount of solvent (3 µL of toluene) for extraction, which is injected into the injection port after extraction. This affects two green criteria—criterion 2 (use safer solvents) and criterion 4 (minimize waste). The favorable final assessment was also influenced by the use of only 2 mL of sample for analysis (criterion 5) and the consumption of a small amount of energy (criterion 8) due to magnetic stirring for 20 min. Compared to SDME, criterion 8 in the SPME method has a lower score due to the higher energy consumption during the thermal desorption step and the long operating time of the magnetic stirrer during the sorption step (45 min). However, because it is a solvent-free, waste-free method and no derivatization of analytes was used, three criteria obtained a green rating, with a result of 1 (criteria 2, 4, and 10—use safer reagents, minimize waste and operator safety), which resulted in the highest score (0.61) for the SPME method in terms of greenness.
3.1.2. WAC Assessment
The RGB 12 metric, compared to the AGREEprep and BAGI metrics, allows for a deeper analysis of the method in terms of its analytical performance and practical benefits. This metric allowed the authors to assign scores to individual categories according to their discretion (experience and needs). All data of the assessed procedures were compiled in the available Excel spreadsheet, and, taking into account the importance of each criterion, points were assigned to all parameters.
The scores obtained for each principle are presented in Table 2 and Figure 2, and the detailed elements of this assessment are presented in an Excel spreadsheet (Supporting Information).
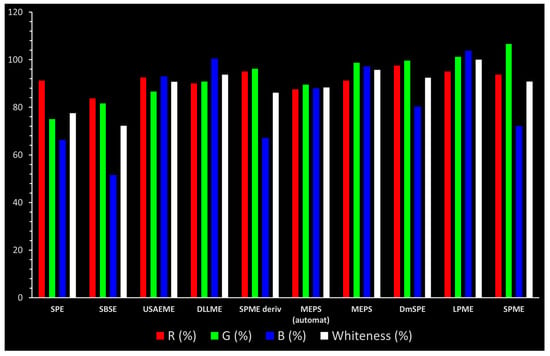
Figure 2.
Comparison of the main assessment outcomes from the RGB 12 tool. Values above 100 indicate additional capabilities exceeding current requirements.
RED Principles Rating (Analytical Performance)
The red category evaluates the method in terms of its applicability to its intended purpose. Precision, accuracy, limit of detection, and scope of application are assessed in this category. When determining the UV filters in the environmental samples, this category is of great importance due to the presence of these compounds at very low concentration levels and the wide range of UV filters used.
High redness results of 95% were obtained by the SPME (with derivatization) and SDME methods, while the DmSPE method was rated highest in this category, with a score of 97.5%. Of the assessed parameter components (R2), the lowest LOD (0.5–10 ng/L) was achieved by the SPME (on-fiber silylation) and DmSPE methods, earning them 100 points. These methods also showed high precision and accuracy, which contributed to the high final results in this category.
However, the LODs of the remaining methods were at a similar concentration level. The last points in this category were awarded to the SPE (70%) and SPME (75%) methods, for which the LOD was ~1–8 µg/L. However, it should be taken into account that the LOD was determined in laboratories using MS detectors operating at various parameters.
Green Principles Rating (green chemistry). The authors of this work entered the data of all assessed procedures into the Excel spreadsheet for the following categories: G1: toxicity of reagents, G2: amount of reagents and waste, G3: consumption of energy, and G4: direct impacts (safety). Based on these data, they assigned points from 0 to 120 to individual categories. It was decided to award 120 points to categories that showed 0 (reagents, waste, energy).
The greenest methods according to WAC are SPME, with a score of 106.7%, and SDME, with a score of 101.3%. The SPME method was awarded 120 points for the G1, G2, and G4 criteria. This is because only in this procedure no solvents and reagents were used. However, the SDME procedure requires the use of only 2 µL of solvent, which also contributes to the high rating of this method. Neither method generates waste and both are safe for the operator. The greenness rating of the remaining microextraction methods ranged from 81.7 to 99.6. These differences mainly resulted from the amount of energy used. As expected, SPE received the lowest greenness score (75), which is mainly due to the large amount of reagents used (~40 mL) and waste generated (30 mL). These parameters mean that for the G2 principle, SPE received only 20 points.
BLUE Principles Rating (Practical Side)
The assessment of practical and economic aspects includes the following categories: B1: cost-efficiency, B2: time-efficiency, B3: requirements, and B4: operational simplicity. In the blue principles, it was decided to distinguish procedures by awarding them 120 points for zero financial contribution to equipment and reagents in category B1, for methods whose sample preparation time is lower than 5 min in category B2, and for methods using less than 1 mL of sample in category B3.
Two of the assessed procedures, SDME and DLLME, received over 100 points: 103.8 and 100.4, respectively. The main advantages of these methods are their low costs and speed of implementation (B1 and B2). The MEPS (manually) and USAEME methods also have high scores, of over 90 points. However, the remaining methods (SBSE, SPME, SPE), due to the costs of purchasing accessories, automatic attachments (e.g., thermal desorption for SBSE), and derivatization reagents, received a low rating for category B1. Additionally, the total rating of these methods was lowered by the B3 category, which is influenced by the requirements for “advanced instruments and greater operator skills and experience”.
Whiteness
Whiteness is a summary assessment of the three components (red, green, and blue) and shows the overall usefulness of the procedure. This assessment tool enables the analyst to select a procedure that will meet the given expectations. The assessment results, together with knowledge of the matrix composition, amounts, properties, and expected concentrations of analytes, laboratory equipment, economic opportunities, and analyst skills will help to select the most appropriate procedure from among the highest-rated procedures.
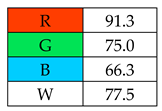
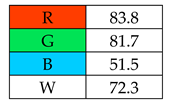
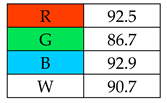
The highest whiteness rating (Table 2, Figure 2) of 100% was awarded to SDME, with only the red principle rated at less than 100 points. Assuming that the authors of this paper consider a result of more than 90% to indicate the whiteness of the method, six out of nine microextraction methods achieved satisfactory results. However, the lowest whiteness ratings were received by SBSE (72.3%) and SPE (77.5%), for which the blue principles had the greatest impact on the assessment.
3.1.3. BAGI Assessment
BAGI evaluates ten main attributes of an analytical procedure in terms of practicality. The results of the assessment performed using BAGI are presented in Table 2. Six of the assessed methods obtained a high score, higher than 60, indicating their practicality. According to this assessment, the highest score was 70 points, obtained by the DLLME and MEPS (automatic) methods, then MEPS (manually), USAEME, and SDME—65 points—and DmSPE—62.5 points.
The remaining methods, i.e., SPE, SPME, and SBSE, received scores in the range of 50–60 points. This assessment is influenced, among other things, by parameter 7 (reagents and materials), which gives a low score for the need to purchase “commercially available reagents and materials” such as SPE cartridges, SPME fibers, SBSE twisters, and derivatization reagents. Additionally, the SBSE and MEPS (fully automated) methods received 0 points for parameter 3 (analytical technique) for “instrumentation that is not commonly available in most labs”.
3.2. AGREEprep vs. GREEN Principle of WAC Assessment
It was verified whether the greenness assessment of the methods performed using the AGREEprep tool was consistent with the green principles of the WAC assessment. The methods assessed are presented in Table 2 in ascending order of score (from the AGREEprep assessment), and this order was in most cases confirmed after the assessment of the green principles of WAC. The only discrepancy was observed in the greenness assessment for the MEPS (fully automated) method, which obtained a higher result in the AGREEprep assessment. This higher score was related to criterion 7, where the automated method receives additional points in the AGREEprep assessment. However, in the RGB 12 algorithm, the automation of methods is assessed in the principle blue: 4 (operational simplicity). Despite this, a high degree of convergence was observed in the greenness assessments of both metrics used to evaluate the ten methods for preparing water samples for the analysis of UV filters using the GC-MS technique. This convergence is shown in Figure 3, which shows that the correlation of both assessments is high and amounts to 0.877.
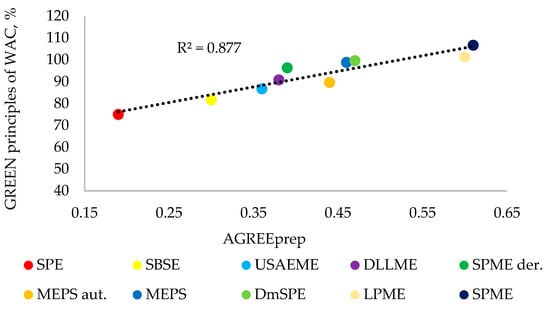
Figure 3.
Linear correlation between the AGREEprep and GREEN principles of WAC.
3.3. BAGI vs. BLUE Principle of WAC Assessment
The BAGI tool is used to assess the suitability of an analytical method. However, the RGB 12 algorithm includes the blue principle (practical side), which is one of the components of the total whiteness assessment of the method. The correlation between the results obtained using the BAGI and the blue principle tools is shown in Figure 4. Despite a moderate correlation (R2 ~ 0.7), there was some agreement between both assessments. The four highest-rated methods for suitability by both tools are DLLME, SDME, MEPS, and USAEME. These methods obtained results for BAGI > 60 points, and for the blue principles > 90%. However, the SPME, SBSE, and SPE methods were rated the lowest by both tools. As can be seen in Figure 4, a greater discrepancy in both assessments can be seen for two methods: SPE and MEPS (fully automated). In the case of the SPE method, the BAGI rating is low (50), which is influenced by principle 8 (preconcentration), which lowers the rating for methods that use additional concentration steps (solvent evaporation). However, in the case of the MEPS (fully automated) method, the blue rating in relation to BAGI is lowered by additional parameters assessed in the blue metric tool, i.e., B1 (total cost) and B4 (portability).
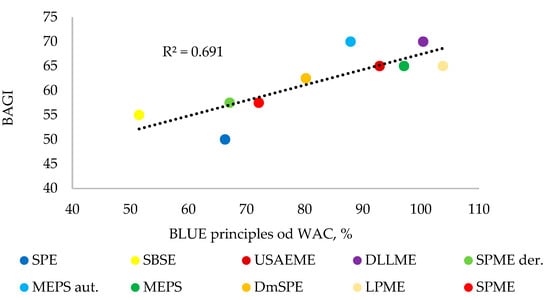
Figure 4.
Linear correlation between BAGI and blue principles of WAC.
3.4. Summary of Evaluation of Sample Preparation Methods
The data presented in the Introduction indicate that the most popular microextraction techniques for determining UV filters are SPME (~24%) and DLLME, with various variants (~24%). In the case of the SPME method, this result is very consistent with the AGREEprep evaluation, in which this technique obtained the highest result (0.61). It is a solvent-free and waste-free method, which makes it unrivalled in the “green” category. However, it can be noticed that the best evaluation results in all categories (green, blue, and white) were obtained by the SDME method. However, these highest ratings for the SDME method do not translate into the popularity of using this method in practice (~3%). This is probably mainly related to maintaining a stable solvent microdrop. However, another popular method used by analysts—DLLME—is easy to perform and cheap. Its frequent use coincides with the highest BAGI rating (70 points), indicating its practicality.
These observations confirm the fact that analysts, using their experience, accurately select the most beneficial techniques. These techniques are simple, cheap, fast, and solvent-free or use minimal amounts of solvents. They are also characterized by reliability, repeatability, and sensitivity. Additionally, what is important is that their use does not require the purchase of additional laboratory equipment. However, the evaluation tools used confirm and prove the selection of the most advantageous analytical technique, and they are used to evaluate newly developed procedures.
4. Conclusions
The results of the assessment of sample preparation procedures presented in this work, based on the example of determining UV filters in water samples, demonstrated the usefulness and effectiveness of all metric tools used. The WAC tool was found to evaluate the methods most comprehensively, as expected. The advantage of this tool is that it allows the analyst to independently assign points for individual principles according to individual problems and needs, although this task is time-consuming and difficult to perform. However, the AGREEprep and BAGI tools evaluate the method within a narrower scope (greenness and practicality), and their implementation is relatively simple and quick. As a result of the evaluations, AGREEprep and BAGI were highly consistent with the WAC tool. There is no doubt that the procedure assessment tools used in this work are useful and help the analyst decide which method to choose for use in the laboratory.
As shown by the evaluation carried out using three complementary tools, the SDME, SPME, and DLLME methods were rated the highest. Two methods, i.e., SPME and SDME, obtained the highest greenness results which was confirmed by the green principles of WAC (>100%). However, the best method in the practicality category is DLLME, which received 70 points in the BAGI assessment, and it was also confirmed by the blue principles of WAC (>100%).
The presented assessment results show that the use of expensive materials and devices and conducting additional steps for the procedure, e.g., derivatization, sonication, etc., negatively affect all assessment. The preferred and still-developing methods should be (if possible) simple, cheap, fast, and preferably solvent-free. However, when choosing a method, it is necessary to remember to maintain a balance between greenness, functionality, and usability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14177690/s1, Table S1: A brief description of the analytical methods assessed; Excel spreadsheet: WAC assessment.
Author Contributions
Conceptualization, G.W. and I.N.; formal analysis, G.W.; methodology, G.W. and I.N.; writing—original draft preparation, G.W. and I.N.; writing—review and editing, G.W. and I.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Narloch, I.; Wejnerowska, G. An Overview of the Analytical Methods for the Determination of Organic Ultraviolet Filters in Cosmetic Products and Human Samples. Molecules 2021, 26, 4780. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hu, L.; Liu, J.; Tian, M.; Yang, L. Polyaniline-coated core-shell silica microspheres-based dispersive-solid phase extraction for detection of benzophenone-type UV filters in environmental water samples. Environ. Adv. 2021, 3, 10037. [Google Scholar] [CrossRef]
- Barón, E.; Gago-Ferrero, P.; Gorga, M.; Rudolph, I.; Mendoza, G.; Zapata, A.M.; Díaz-Cruz, S.; Barra, R.; Ocampo-Duque, W.; Páez, M.; et al. Occurrence of hydrophobic organic pollutants (BFRs and UV-filters) in sediments from South America. Chemosphere 2013, 92, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pozo, L.; del Carmen Gómez-Regalado, M.; Moscoso-Ruiz, I.; Zafra-Gómez, A. Analytical methods for the determination of endocrine disrupting chemicals in cosmetics and personal care products: A review. Talanta 2021, 234, 122642. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Organic UV filters in marine environments: An update of analytical methodologies, occurrence and distribution. Trends Environ. Anal. Chem. 2020, 25, e00079. [Google Scholar] [CrossRef]
- Chisvert, A.; Benedé, J.L.; Salvador, A. Current trends on the determination of organic UV filters in environmental water samples based on microextraction techniques—A review. Anal. Chim. Acta 2018, 1034, 22–38. [Google Scholar] [CrossRef]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. Advances in analytical methods and occurrence of organic UV-filters in the environment—A review. Sci. Total Environ. 2015, 526, 278–311. [Google Scholar] [CrossRef]
- Oubahmane, M.; Mihucz, V.G.; Vasanits, A. Recent trends in the determination of organic UV filters by gas chromatography-mass spectrometry in environmental samples. TrAC Trends Anal. Chem. 2023, 161, 116995. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green Analytical Methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huangh, Y.; et al. Correction: Making the move towards modernized greener separations: Introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereirac, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Advan. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method Evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef] [PubMed]
- Lambropoulou, D.A.; Giokas, D.L.; Sakkas, V.A.; Albanis, T.A.; Karayannis, M.I. Gas chromatographic determination of 2-hydroxy-4-methoxybenzophenone and octyldimethyl-p-aminobenzoic acid sunscreen agents in swimming pool and bathing waters by solid-phase microextraction. J. Chromatogr. A 2002, 967, 243–253. [Google Scholar] [CrossRef]
- Rodil, R.; Moeder, M. Development of a method for the determination of UV filters in water samples using stir bar sorptive extraction and thermal desorption–gas chromatography–mass spectrometry. J. Chromatogr. A 2008, 1179, 81–88. [Google Scholar] [CrossRef]
- Vila, M.; Lamas, J.P.; Garcia-Jares, C.; Dagnac, T.; Llompart, M. Ultrasound-assisted emulsification microextraction followed by gas chromatography–mass spectrometry and gas chromatography–tandem mass spectrometry for the analysis of UV filters in water. Microchem. J. 2016, 124, 530–539. [Google Scholar] [CrossRef]
- Negreira, N.; Rodríguez, I.; Rubí, E.; Cela, R. Dispersive liquid–liquid microextraction followed by gas chromatography–mass spectrometry for the rapid and sensitive determination of UV filters in environmental water samples. Anal. Bioanal. Chem. 2010, 398, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Negreira, N.; Rodríguez, I.; Ramil, M.; Rubí, E.; Cela, R. Sensitive determination of salicylate and benzophenone type UV filters in water samples using solid-phase microextraction, derivatization and gas chromatography tandem mass spectrometry. Anal. Chim. Acta 2009, 638, 36–44. [Google Scholar] [CrossRef]
- Moeder, M.; Schrader, S.; Winkler, U.; Rodil, R. At-line microextraction by packed sorbent-gas chromatography–mass spectrometry for the determination of UV filter and polycyclic musk compounds in water samples. J. Chromatogr. A 2010, 1217, 2925–2932. [Google Scholar] [CrossRef]
- Wejnerowska, G.; Narloch, I. Determination of Benzophenones in Water and Cosmetics Samples: A Comparison of Solid-Phase Extraction and Microextraction by Packed Sorbent Methods. Molecules 2021, 26, 6896. [Google Scholar] [CrossRef]
- Chung, W.H.; Tzing, S.H.; Ding, W.H. Optimization of dispersive micro solid-phase extraction for the rapid determination of benzophenone-type ultraviolet absorbers in aqueous samples. J. Chromatogr. A 2015, 1411, 17–22. [Google Scholar] [CrossRef]
- Okanouchi, N.; Honda, H.; Ito, R.; Kawaguchi, M.; Saito, K.; Nakazawa, H. Determination of Benzophenones in River-water Samples Using Drop-based Liquid Phase Microextraction Coupled with Gas Chromatography/Mass Spectrometry. Anal. Sci. 2008, 24, 627–630. [Google Scholar] [CrossRef]
- Yılmazcan, Ö.; Kanakaki, C.; Izgi, B.; Rosenberg, E. Fast determination of octinoxate and oxybenzone UV filters in swimming pool waters by gas chromatography/mass spectrometry after solid-phase microextraction. J. Sep. Sci. 2015, 38, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Chen, H.C.; Ding, W.H. Ultrasound-assisted dispersive liquid–liquid microextraction plus simultaneous silylation for rapid determination of salicylate and benzophenone-type ultraviolet filters in aqueous samples. J. Chromatogr. A 2013, 1302, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Ito, R.; Honda, H.; Endo, N.; Okanouchi, N.; Saito, K.; Saito, K.; Seto, Y.; Nakazawa, H. Simultaneous analysis of benzophenone sunscreen compounds in water sample by stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 2008, 1200, 260–263. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).