The Latest Advances in the Use of Nanoparticles in Endodontics
Abstract
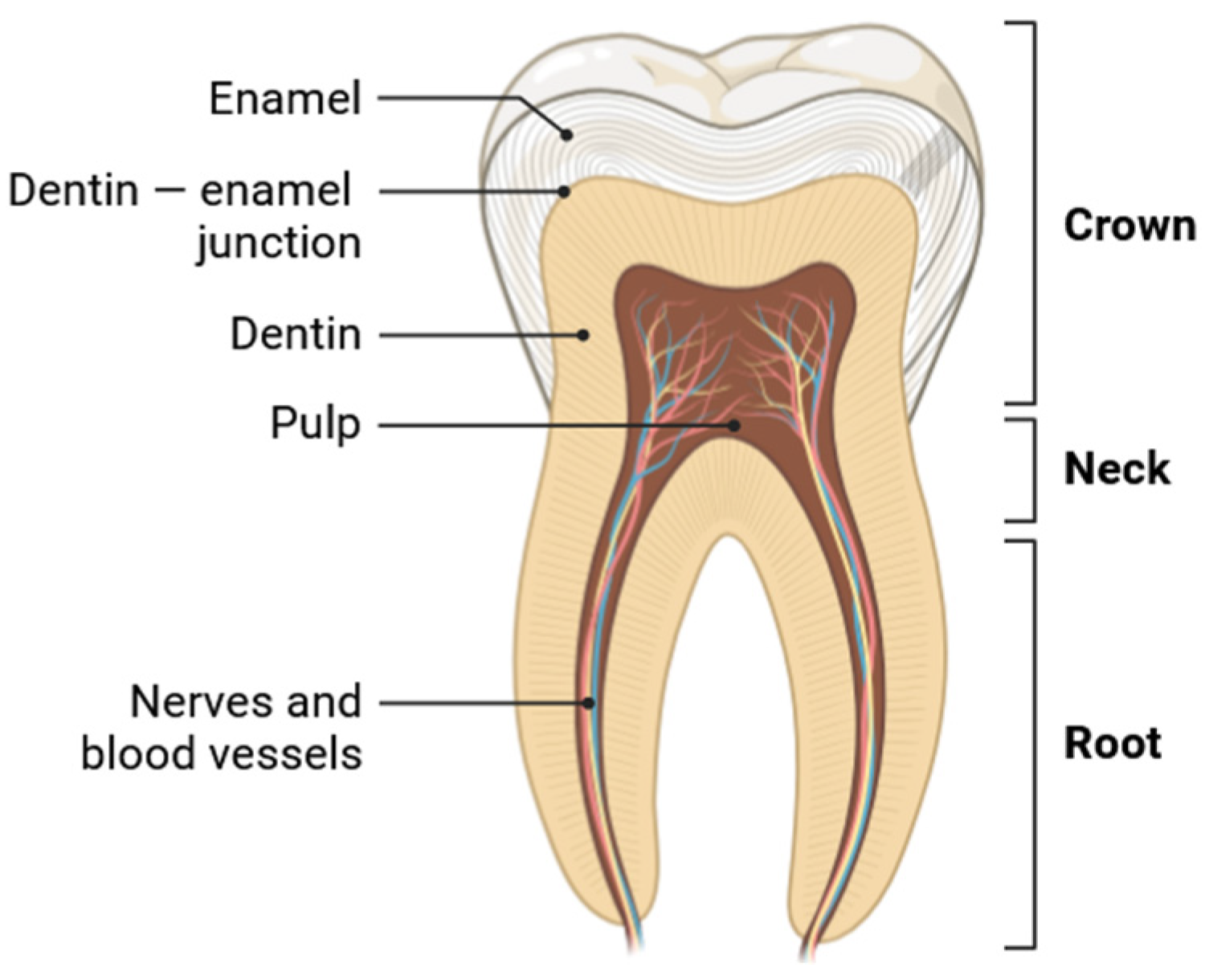
:1. Introduction
2. Nanomaterials in Endodontics
2.1. Nanohydroxyapatite
2.2. Bioglass
2.3. Nanocompounds Based on Chitosan
2.4. Metallic Nanoparticles
2.4.1. Nanosilver
2.4.2. Metal Oxide Nanoparticles
2.5. Graphene Oxide Nanoplatelets
2.6. Nanodiamond
2.7. Regenerative Endodontic Treatment at Nanoscale
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The tooth: Its structure and properties. Dent. Clin. 2017, 61, 651–668. [Google Scholar]
- Mondelli, J.; Sene, F.; Ramos, R.P.; Benetti, A.R. Tooth structure and fracture strength of cavities. Braz. Dent. J. 2007, 18, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Abbott, P.V. Pulp, root canal, and periradicular conditions. In Endodontic Advances and Evidence-Based Clinical Guidelines; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 85–116. [Google Scholar] [CrossRef]
- Cabaña-Muñoz, M.E.; Pelaz Fernández, M.J.; Parmigiani-Cabaña, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics 2023, 15, 2109. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory Response Mechanisms of the Dentine–Pulp Complex and the Periapical Tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Bakhsh, A.; Moyes, D.; Proctor, G.; Mannocci, F.; Niazi, S.A. The impact of apical periodontitis, non-surgical root canal retreatment and periapical surgery on serum inflammatory biomarkers. Int. Endod. J. 2022, 55, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Aggarwal, A.; Pandey, V.; Bansal, N. Regenerative Endodontics-Potential Approaches in Revitalizing the Tooth Pulp—A Review Article. J. Adv. Med. Dent. Sci. Res. 2019, 7, 27–32. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Liu, B.; Tulu, U.S.; Helms, J.A. Wnt-responsive odontoblasts secrete new dentin after superficial tooth injury. J. Dent. Res. 2018, 97, 1047–1054. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Paju, S. Dentin-Pulp and Periodontal Anatomy and Physiology. In Essential Endodontology: Prevention and Treatment of Apical Periodontitis; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 11–58. [Google Scholar] [CrossRef]
- Shah, D.; Lynd, T.; Ho, D.; Chen, J.; Vines, J.; Jung, H.-D.; Kim, J.-H.; Zhang, P.; Wu, H.; Jun, H.-W.; et al. Pulp–Dentin Tissue Healing Response: A Discussion of Current Biomedical Approaches. J. Clin. Med. 2020, 9, 434. [Google Scholar] [CrossRef]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of Root Canal Treatments Using a New Calcium Silicate Root Canal Sealer: A Non-Randomized Clinical Trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef]
- Karamifar, K.; Tondari, A.; Saghiri, M.A. Endodontic periapical lesion: An overview on the etiology, diagnosis and current treatment modalities. Eur. Endod. J. 2020, 5, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Kaval, M.E.; Güneri, P.; Çalışkan, M.K. Regenerative endodontic treatment of perforated internal root resorption: A case report. Int. Endod. J. 2018, 51, 128–137. [Google Scholar] [CrossRef]
- Metlerska, J.; Fagogeni, I.; Nowicka, A. Efficacy of autologous platelet concentrates in regenerative endodontic treatment: A systematic review of human studies. J. Endod. 2019, 45, 20–30. [Google Scholar] [CrossRef]
- Dąbrowski, W.; Puchalska, W.; Ziemlewski, A.; Ordyniec-Kwaśnica, I. Guided Endodontics as a Personalized Tool Complicated Clinical Cases. Int. J. Environ. Res. Public Health 2022, 19, 9958. [Google Scholar] [CrossRef] [PubMed]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Barot, T.; Rawtani, D.; Kulkarni, P. Nanotechnology-based materials as emerging trends for dental applications. Rev. Adv. Mater. Sci. 2021, 60, 173–189. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional fillers for dental resin composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Schmalz, G.; Galler, K.M. Biocompatibility of biomaterials–Lessons learned and considerations for the design of novel materials. Dent. Mater. 2017, 33, 382–393. [Google Scholar] [CrossRef]
- Besinis, A.; De Peralta, T.; Tredwin, C.J.; Handy, R.D. Review of nanomaterials in dentistry: Interactions with the oral microenvironment, clinical applications, hazards, and benefits. ACS Nano 2015, 9, 2255–2289. [Google Scholar] [CrossRef]
- Cuppini, M.; Zatta, K.C.; Mestieri, L.B.; Grecca, F.S.; Leitune, V.C.B.; Guterres, S.S.; Collares, F.M. Antimicrobial and anti-inflammatory drug-delivery systems at endodontic reparative material: Synthesis and characterization. Dent. Mater. 2019, 35, 457–467. [Google Scholar] [CrossRef]
- Samiei, M.; Farjami, A.; Dizaj, S.M.; Lotfipour, F. Nanoparticles for antimicrobial purposes in Endodontics: A systematic review of in vitro studies. Mater. Sci. Eng. C 2016, 58, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.T.; De Andrade, F.B.; De Vasconcelos, L.R.S.M.; Midena, R.Z.; Pereira, T.C.; Kuga, M.C.; Bernardineli, N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2019, 51, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Bhandi, S.; Mehta, D.; Mashyakhy, M.; Chohan, H.; Testarelli, L.; Thomas, J.; Dhillon, H.; Raj, A.T.; Madapusi Balaji, T.; Varadarajan, S.; et al. Antimicrobial Efficacy of Silver Nanoparticles as Root Canal Irrigant’s: A Systematic Review. J. Clin. Med. 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Abusrewil, S.; Alshanta, O.A.; Albashaireh, K.; Alqahtani, S.; Nile, C.J.; Scott, J.A.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: What’s new in 2020? Crit. Rev. Microbiol. 2020, 46, 194–212. [Google Scholar] [CrossRef]
- Al-Bakhsh, B.A.J.; Shafiei, F.; Hashemian, A.; Shekofteh, K.; Bolhari, B.; Behroozibakhsh, M. In-vitro bioactivity evaluation and physical properties of an epoxy-based dental sealer reinforced with synthesized fluorine-substituted hydroxyapatite, hydroxyapatite and bioactive glass nanofillers. Bioact. Mater. 2019, 4, 322–333. [Google Scholar] [CrossRef]
- Chandak, P.G.; Chandak, M.G.; Relan, K.N.; Chandak, M.; Rathi, C.; Patel, A. Nanoparticles in endodontics—A review. J. Evol. Med. Dent. Sci. 2021, 10, 976–983. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Ziental, D.; Czarczynska-Goslinska, B.; Michalak, M.; Wysocki, M.; Güzel, E.; Sobotta, L. Nanotechnology for Dentistry: Prospects and Applications. Nanomaterials 2023, 13, 2130. [Google Scholar] [CrossRef]
- Elfakhri, F.; Alkahtani, R.; Li, C.; Khaliq, J. Influence of filler characteristics on the performance of dental composites: A comprehensive review. Ceram. Int. 2022, 48, 27280–27294. [Google Scholar] [CrossRef]
- Wong, J.; Zou, T.; Lee, A.H.C.; Zhang, C. The potential translational applications of nanoparticles in endodontics. Int. J. Nanomed. 2021, 16, 2087–2106. [Google Scholar] [CrossRef]
- Capuano, N.; Amato, A.; Dell’Annunziata, F.; Giordano, F.; Folliero, V.; Di Spirito, F.; More, P.R.; De Filippis, A.; Martina, S.; Amato, M.; et al. Nanoparticles and Their Antibacterial Application in Endodontics. Antibiotics 2023, 12, 1690. [Google Scholar] [CrossRef]
- Afkhami, F.; Forghan, P.; Gutmann, J.L.; Kishen, A. Silver Nanoparticles and Their Therapeutic Applications in Endodontics: A Narrative Review. Pharmaceutics 2023, 15, 715. [Google Scholar] [CrossRef]
- Liu, F.; Hong, T.; Xie, J.; Zhan, X.; Wang, Y. Application of Reactive Oxygen Species-Based Nanomaterials in Dentistry: A Review. Crystals 2021, 11, 266. [Google Scholar] [CrossRef]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic Nanoparticles—Friends or Foes in the Battle against Antibiotic-Resistant Bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Clift, F.J.M.T.C. Artificial methods for the remineralization of hydroxyapatite in enamel. Mater. Today Chem. 2021, 21, 100498. [Google Scholar] [CrossRef]
- Shellis, R.P.; Barbour, M.E.; Parker, D.M.; Addy, M.; Lussi, A. Effects of calcium and phosphate on dissolution of enamel, dentin and hydroxyapatite in citric acid. Swiss Dent. J. SSO–Sci. Clin. Top. 2023, 133, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–-Past, present, and future in bone regeneration. Bone Tissue Regen. Insights 2016, 7, BTRI-S36138. [Google Scholar] [CrossRef]
- Cheng, H.; Chabok, R.; Guan, X.; Chawla, A.; Li, Y.; Khademhosseini, A.; Jang, H.L. Synergistic interplay between the two major bone minerals, hydroxyapatite and whitlockite nanoparticles, for osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 2018, 69, 342–351. [Google Scholar] [CrossRef]
- Irfan, M.; Irfan, M. Overview of hydroxyapatite; composition, structure, synthesis methods and its biomedical uses. Biomed. Lett. 2020, 6, 17–22. [Google Scholar]
- Emtiazi, G.; Shapoorabadi, F.A.; Mirbagheri, M. Chemical and Biological Synthesis of HydroxyApatite: Advantage and Application. Int. J. Microbiol. Curr. Res. 2019, 1, 20–22. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. Hydroxylapatite and Related Minerals in Bone and Dental Tissues: Structural, Spectroscopic and Mechanical Properties from a Computational Perspective. Biomolecules 2021, 11, 728. [Google Scholar] [CrossRef]
- Heshmatpour, F.; Lashteneshaee, S.H.; Samadipour, M. Study of in vitro bioactivity of nano hydroxyapatite composites doped by various cations. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2063–2068. [Google Scholar] [CrossRef]
- Alagarsamy, K.; Vishwakarma, V.; Kaliaraj, G.S.; Viswanathan, K.; Chavali, M. Implant application of bioactive nano-hydroxyapatite powders—A comparative study. Mater. Res. Express 2018, 5, 015405. [Google Scholar] [CrossRef]
- Yoshida, S.; Sugii, H.; Itoyama, T.; Kadowaki, M.; Hasegawa, D.; Tomokiyo, A.; Maeda, H. Development of a novel direct dental pulp-capping material using 4-META/MMA-TBB resin with nano hydroxyapatite. Mater. Sci. Eng. C 2021, 130, 112426. [Google Scholar] [CrossRef] [PubMed]
- Haghgoo, R.; Asgary, S.; Abbas, F.M.; Hedeshi, R.M. Nano-Hydroxyapatite and Calcium-Enriched Mixture for Pulp Capping of Sound Primary Teeth: A Randomized Clinical Trial. Iran. Endod. J. 2015, 10, 107–111. [Google Scholar]
- Kato, C.; Suzuki, M.; Shinkai, K.; Katoh, Y. Histopathological and immunohistochemical study on the effects of a direct pulp capping experimentally developed adhesive resin system containing reparative dentin-promoting agents. Dent. Mater. J. 2011, 30, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Suzuki, M.; Kato, C.; Shinkai, K.; Ogawa, M.; Yamauchi, J. Observation of calcium phosphate powder mixed with an adhesive monomer experimentally developed for direct pulp capping and as a bonding agent. Dent. Mater. J. 2011, 29, 15–24. [Google Scholar] [CrossRef]
- Okamoto, H.; Arai, K.; Matsune, K.; Hirukawa, S.; Matsunaga, S.; Kiba, H.; Maeda, T. The usefulness of new hydroxyapatite as a pulp capping agent in rat molars. Int. J. Oral. Health Sci. 2006, 5, 50–56. [Google Scholar] [CrossRef]
- Kiba, W.; Imazato, S.; Takahashi, Y.; Yoshioka, S.; Ebisu, S.; Nakano, T. Efficacy of polyphasic calcium phosphates as a direct pulp capping material. J. Dent. 2010, 38, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; He, G.; Tan, Z.; Gong, P.; Li, X.Y.; Chen, Z.Q.; Zhu, Z.L. Biocompatibility of nano-TiO2/HA bioceramic coating for nerve regeneration around dental implants. Key Eng. Mater. 2007, 330, 1393–1396. [Google Scholar] [CrossRef]
- Mietto, B.S.; Jhelum, P.; Schulz, K.; David, S. Schwann cells provide iron to axonal mitochondria and its role in nerve regeneration. J. Neurosci. 2021, 41, 7300–7313. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Liang, M.Z.; Chen, L. Current progress of mitochondrial transplantation that promotes neuronal regeneration. Transl. Neurodegener. 2019, 8, 17. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef]
- D’Elía, N.L.; Mathieu, C.; Hoemann, C.D.; Laiuppa, J.A.; Santillán, G.E.; Messina, P.V. Bone-repair properties of biodegradable hydroxyapatite nano-rod superstructures. Nanoscale 2015, 7, 18751–18762. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. Part B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Roberts, H.W.; Kirkpatrick, T.C.; Bergeron, B.E. Thermal analysis and stability of commercially available endodontic obturation materials. Clin. Oral. Investig. 2017, 21, 2589–2602. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Abdulkareem, B.; Nwannenna, C.S. Synthesis techniques, characterization and mechanical properties of natural derived hydroxyapatite scaffolds for bone implants: A review. SN Appl. Sci. 2021, 3, 822. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Jouda, N.S.; Essa, A.F. Preparation and study of the structural, physical and mechanical properties of hydroxyapatite nanocomposite. Mater. Today Proc. 2021, 47, 5999–6005. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Ruan, D.; Xu, H.; Wang, F.; Lei, W.; Xia, M. Efficient removal of heavy metal ions by diethylenetriaminepenta (methylene phosphonic) acid-doped hydroxyapatite. Sci. Total Environ. 2022, 849, 157557. [Google Scholar] [CrossRef] [PubMed]
- Sinulingga, K.; Sirait, M.; Siregar, N.; Abdullah, H. Synthesis and characterizations of natural limestone-derived nano-hydroxyapatite (HAp): A comparison study of different metals doped HAps on antibacterial activity. RSC Adv. 2021, 11, 15896–15904. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Cao, X.; Zhang, Z.; Liu, Y. Effect of doping cation on the adsorption properties of hydroxyapatite to uranium. J. Solid State Chem. 2023, 317, 123687. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Olender, E.; Malina, D.; Tyliszczak, B. Effect of calcination parameters on behavior of bone hydroxyapatite in artificial saliva and its biosafety. Mater. Chem. Phys. 2018, 206, 158–165. [Google Scholar] [CrossRef]
- Al-Quraini, A.A.; Al-Aodah, A.F.; Al-Qadhi, A.A.M.; Ahmad, A.M.M. Incorporation of Hydroxyapatite and Calcium Triphosphate in the Epoxy Resin-Based Sealer. Eur. Dent. Res. Biomater. J. 2021, 2, 047–051. [Google Scholar] [CrossRef]
- Mulyawati, E.; Soesatyo, M.H.; Sunarintyas, S.; Handajani, J. Apical sealing ability of calcite-synthesized hydroxyapatite as a filler of epoxy resin-based root canal sealer. Contemp. Clin. Dent. 2020, 11, 136–140. [Google Scholar] [CrossRef]
- del Carpio-Perochena, A.; Nicholson, E.; Singh, C.V.; Camilleri, J.; Kishen, A. Impact of dentin conditioning and sealer modification with chitosan-hydroxyapatite nanocomplexes on the antibacterial and mechanical characteristics of root dentin. J. Endod. 2022, 48, 1319–1326. [Google Scholar] [CrossRef]
- Landzberg, G.; Hussein, H.; Kishen, A. A novel self-mineralizing antibacterial tissue repair varnish to condition root-end dentin in endodontic microsurgery. J. Endod. 2021, 47, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Zhang, X.; Kishen, A. Impact of dentin substrate modification with chitosan-hydroxyapatite precursor nanocomplexes on sealer penetration and tensile strength. J. Endod. 2019, 45, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Sodhi, R.N.; Kishen, A. Interfacial characterization of dentin conditioned with chitosan hydroxyapatite precursor nanocomplexes using time-of-flight secondary ion mass spectrometry. J. Endod. 2019, 45, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Jones, J.R. Bioactive glasses: Frontiers and challenges. Front. Bioeng. Biotechnol. 2015, 3, 194. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Mohammed, H.; Al-Hadeethi, Y.; Bakry, A.S.; Umar, A.; Hussein, M.A.; Abbassy, M.A.; Vaidya, K.G.; Al Berakdar, G.; Mkawi, E.M.; et al. Silica-Based Bioactive Glasses and Their Applications in Hard Tissue Regeneration: A Review. Pharmaceuticals 2021, 14, 75. [Google Scholar] [CrossRef]
- Simila, H.O.; Boccaccini, A.R. Sol-gel bioactive glass containing biomaterials for restorative dentistry: A review. Dent. Mater. 2022, 38, 725–747. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Koc, B.; Blunn, G.; Bartolo, P. Novel 3D Bioglass Scaffolds for Bone Tissue Regeneration. Polymers 2022, 14, 445. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Young, G.; Jones, J.R.; Rankin, S. Bioglass/carbonate apatite/collagen composite scaffold dissolution products promote human osteoblast differentiation. Mater. Sci. Eng. C 2021, 118, 111393. [Google Scholar] [CrossRef]
- Wilkesmann, S.; Fellenberg, J.; Nawaz, Q.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Primary osteoblasts, osteoblast precursor cells or osteoblast-like cell lines: Which human cell types are (most) suitable for characterizing 45S5-bioactive glass? J. Biomed. Mater. Res. 2020, 108, 663–674. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Shahin, K.; Mirström, M.M.; Li, D. Cellulose-reinforced bioglass composite as flexible bioactive bandage to enhance bone healing. Ceram. Int. 2021, 47, 416–423. [Google Scholar] [CrossRef]
- Kido, H.W.; Gabbai-Armelin, P.R.; Magri, A.M.P.; Fernandes, K.R.; Cruz, M.A.; Santana, A.F.; Rennó, A.C.M. Bioglass/collagen scaffolds combined with bone marrow stromal cells on bone healing in an experimental model in cranial defects in rats. J. Biomater. Appl. 2023, 37, 1632–1644. [Google Scholar] [CrossRef]
- Sonatkar, J.; Kandasubramanian, B. Bioactive glass with biocompatible polymers for bone applications. Eur. Polym. J. 2021, 160, 110801. [Google Scholar] [CrossRef]
- Mahato, A.; De, M.; Bhattacharjee, P.; Kumar, V.; Mukherjee, P.; Singh, G.; Kundu, B.; Balla, V.K.; Nandi, S.K. Role of calcium phosphate and bioactive glass coating on in vivo bone healing of new Mg–Zn–Ca implant. J. Mater. Sci. Mater. Med. 2021, 32, 55. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, M.J.; Chlanda, A.; Oberbek, P.; Heljak, M.; Czarnecka, K.; Janeta, M.; John, Ł. Binary bioactive glass composite scaffolds for bone tissue engineering—Structure and mechanical properties in micro and nano scale. A preliminary study. Micron 2019, 119, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, D.; Li, H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact. Mater. 2021, 6, 823–835. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, H.; Bu, Z.; Liu, Z.; Lv, F.; Pan, M.; Cheng, L. Small extracellular vesicles released from bioglass/hydrogel scaffold promote vascularized bone regeneration by transferring miR-23a-3p. Int. J. Nanomed. 2022, 17, 6201. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, F.; Sun, L.; Tang, F.; Gao, W.; Xie, W.; Chen, X. Bioactive glass activates VEGF paracrine signaling of cardiomyocytes to promote cardiac angiogenesis. Mater. Sci. Eng. C 2021, 124, 112077. [Google Scholar] [CrossRef]
- Tian, T.; Hu, Q.; Shi, M.; Liu, C.; Wang, G.; Chen, X. The synergetic effect of hierarchical pores and micro-nano bioactive glass on promoting osteogenesis and angiogenesis in vitro. J. Mech. Behav. Biomed. Mater. 2023, 146, 106093. [Google Scholar] [CrossRef]
- Corral Nunez, C.; Altamirano Gaete, D.; Maureira, M.; Martin, J.; Covarrubias, C. Nanoparticles of Bioactive Glass Enhance Biodentine Bioactivity on Dental Pulp Stem Cells. Materials 2021, 14, 2684. [Google Scholar] [CrossRef]
- Taddei, P.; Di Foggia, M.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Guttapercha Improves In Vitro Bioactivity and Dentin Remineralization Ability of a Bioglass Containing Polydimethylsiloxane-Based Root Canal Sealer. Molecules 2023, 28, 7088. [Google Scholar] [CrossRef]
- Pedano, M.S.; Li, X.; Yoshihara, K.; Landuyt, K.V.; Van Meerbeek, B. Cytotoxicity and Bioactivity of Dental Pulp-Capping Agents towards Human Tooth-Pulp Cells: A Systematic Review of In-Vitro Studies and Meta-Analysis of Randomized and Controlled Clinical Trials. Materials 2020, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Liu, S.; Zhu, L.; Liang, Q.; Chen, X.; Dong, Y. Evaluation of pulp response to novel bioactive glass pulp capping materials. J. Endod. 2017, 43, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive Glass Applications in Dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Niu, W.; Lei, B.; Boccaccini, A.R. Immunomodulatory bioactive glasses for tissue regeneration. Acta Biomater. 2021, 133, 168–186. [Google Scholar] [CrossRef]
- Hanada, K.; Morotomi, T.; Washio, A.; Yada, N.; Matsuo, K.; Teshima, H.; Kitamura, C. In vitro and in vivo effects of a novel bioactive glass-based cement used as a direct pulp capping agent. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 107, 161–168. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, J.; Liu, L.; Hou, G.; Yu, Y.; Yuan, C.; Wang, X. New gutta percha composite with high thermal conductivity and low shear viscosity contributed by the bridging fillers containing ZnO and CNTs. Compos. B Eng. 2019, 173, 106903. [Google Scholar] [CrossRef]
- Alhashimi, R.A.; Mannocci, F.; Sauro, S. Bioactivity, cytocompatibility and thermal properties of experimental Bioglass-reinforced composites as potential root-canal filling materials. J. Mech. Behav. Biomed. Mater. 2017, 69, 355–361. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Martinelli, J.R.; Bauer, J.; Haapasalo, M.; Shen, Y.; Bradaschia-Correa, V.; Gavini, G. Micropush-out dentine bond strength of a new gutta-percha and niobium phosphate glass composite. Int. Endod. J. 2015, 48, 451–459. [Google Scholar] [CrossRef]
- Jafari, N.; Habashi, M.S.; Hashemi, A.; Shirazi, R.; Tanideh, N.; Tamadon, A. Application of bioactive glasses in various dental fields. Biomater. Res. 2022, 26, 31. [Google Scholar] [CrossRef]
- Li, G.H.; Niu, L.N.; Zhang, W.; Olsen, M.; De-Deus, G.; Eid, A.A.; Tay, F.R. Ability of new obturation materials to improve the seal of the root canal system: A review. Acta Biomater. 2014, 10, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Aggarwal, H.; Tikku, A.P.; Singh, A.; Bains, R.; Mishra, S. Comparative evaluation of sealing ability of gutta percha and resilon as root canal filling materials-a systematic review. J. Oral Biol. Craniofacial Res. 2022, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.R.; Carvalho, C.; Paulo, S.; Martinho, J.P.; Coelho, A.S.; Paula, A.B.; Marto, C.M.; Carrilho, E.; Botelho, M.F.; Abrantes, A.M.; et al. Apical Sealing Ability of Two Calcium Silicate-Based Sealers Using a Radioactive Isotope Method: An In Vitro Apexification Model. Materials 2021, 14, 6456. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Gorustovich, A. Bioactive glasses as delivery systems for antimicrobial agents. J. Appl. Microbiol. 2017, 122, 1424–1437. [Google Scholar] [CrossRef]
- Zahid, S.; Shah, A.T.; Jamal, A.; Chaudhry, A.A.; Khan, A.S.; Khan, A.F. Biological behavior of bioactive glasses and their composites. RSC Adv. 2016, 6, 70197–70214. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Ren, B.; Li, X.; Wang, L.; Zhou, H.; Xu, H.H. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 5509. [Google Scholar] [CrossRef]
- Kannan, K.P.; Gunasekaran, V.; Sreenivasan, P.; Sathishkumar, P. Recent updates and feasibility of nanodrugs in the prevention and eradication of dental biofilm and its associated pathogens—A review. J. Dent. 2024, 143, 104888. [Google Scholar] [CrossRef]
- Correia, B.L.; Gomes, A.T.P.C.; Noites, R.; Ferreira, J.M.F.; Duarte, A.S. New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials 2022, 12, 1577. [Google Scholar] [CrossRef]
- Özdemir, O.; Kopac, T. Cytotoxicity and biocompatibility of root canal sealers: A review on recent studies. J. Appl. Biomater. 2022, 20, 22808000221076325. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.-J.; Dev Patel, K.; Campbell Knowles, J.; Lee, J.-H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Belal, R.S.I.; Edanami, N.; Yoshiba, K.; Yoshiba, N.; Ohkura, N.; Takenaka, S.; Noiri, Y. Comparison of calcium and hydroxyl ion release ability and in vivo apatite-forming ability of three bioceramic-containing root canal sealers. Clin. Oral Investig. 2022, 26, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Dobrzynski, W.; Zawadzka-Knefel, A.; Janecki, M.; Kurek, K.; Lubojanski, A.; Szymonowicz, M.; Rybak, Z.; Wiglusz, R.J. Nanomaterials Application in Orthodontics. Nanomaterials 2021, 11, 337. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial–a review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Supotngarmkul, A.; Panichuttra, A.; Ratisoontorn, C.; Nawachinda, M.; Matangkasombut, O. Antibacterial property of chitosan against E. faecalis standard strain and clinical isolates. Dent. Mater. J. 2020, 39, 456–463. [Google Scholar] [CrossRef]
- Song, S.; Kim, Y.J.; Lee, J.H.; Lee, J.; Shin, J.; Kim, J. Antibacterial effect on Enterococcus Faecalis and physical properties of chitosan added calcium hydroxide canal filling material. J. Korean Acad. Pediatr. Dent. 2021, 48, 198–208. [Google Scholar] [CrossRef]
- Caballero-Flores, H.; Nabeshima, C.K.; Sarra, G.; Moreira, M.S.; Arana-Chavez, V.E.; Marques, M.M.; de Lima Machado, M.E. Development and characterization of a new chitosan-based scaffold associated with gelatin, microparticulate dentin and genipin for endodontic regeneration. Dent. Mater. 2021, 37, e414–e425. [Google Scholar] [CrossRef] [PubMed]
- Sanap, P.; Hegde, V.; Ghunawat, D.; Patil, M.; Nagaonkar, N.; Jagtap, V. Current applications of chitosan nanoparticles in dentistry: A review. Int. J. Appl. Dent. Sci. 2020, 6, 81–84. [Google Scholar] [CrossRef]
- Rane, S.; Pandit, V.; Gaikwad, A.; Shinde, M.; Chavan, S.; Jadhav, A. Comparative evaluation of Apical Microleakage of Bioceramic sealer versus Bioceramic sealer mixed with Chitosan nanoparticles–An In-Vitro Study. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 179–188. [Google Scholar] [CrossRef]
- Skoskiewicz-Malinowska, K.; Kaczmarek, U.; Malicka, B.; Walczak, K.; Zietek, M. Application of chitosan and propolis in endodontic treatment: A review. Mini Rev. Med. Chem. 2017, 17, 410–434. [Google Scholar] [CrossRef]
- Loyola-Rodríguez, J.P.; Torres-Méndez, F.; Espinosa-Cristobal, L.F.; García-Cortes, J.O.; Loyola-Leyva, A.; González, F.J.; Contreras-Palma, G. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Functional Mater. 2019, 17, 2280800019851771. [Google Scholar] [CrossRef] [PubMed]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef]
- Kanathila, H.; Pangi, A.M.; Patil, S.; Shirlal, S.; Jaiswal, R. An Insight in to Various Metallic Oxide Nanoparticles as Antimicrobials and Their Applications in Dentistry. J. Evol. Med. Dent. Sci. 2021, 10, 2803–2809. [Google Scholar] [CrossRef]
- Nizami, M.Z.I.; Xu, V.W.; Yin, I.X.; Yu, O.Y.; Chu, C.-H. Metal and Metal Oxide Nanoparticles in Caries Prevention: A Review. Nanomaterials 2021, 11, 3446. [Google Scholar] [CrossRef]
- Bertani, R.; Bartolozzi, A.; Pontefisso, A.; Quaresimin, M.; Zappalorto, M. Improving the Antimicrobial and Mechanical Properties of Epoxy Resins via Nanomodification: An Overview. Molecules 2021, 26, 5426. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Mohamed, D.S.; Abd El-Baky, R.M.; Sandle, T.; Mandour, S.A.; Ahmed, E.F. Antimicrobial Activity of Silver-Treated Bacteria against other Multi-Drug Resistant Pathogens in Their Environment. Antibiotics 2020, 9, 181. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, C.; Wang, X.; Liu, D. Release strategies of silver ions from materials for bacterial killing. ACS Appl. Bio Mater. 2021, 4, 3985–3999. [Google Scholar] [CrossRef]
- Chávez-Andrade, G.M.; Tanomaru-Filho, M.; Bernardi, M.I.B.; de Toledo Leonardo, R.; Faria, G.; Guerreiro-Tanomaru, J.M. Antimicrobial and biofilm anti-adhesion activities of silver nanoparticles and farnesol against endodontic microorganisms for possible application in root canal treatment. Arch. Oral. Biol. 2019, 107, 3985–3999. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, H.O.; Buzoglu, H.D.; Calt, S.; Stabholz, A.; Steinberg, D. Effect of ethylenediaminetetraacetic acid and sodium hypochlorite irrigation on Enterococcus faecalis biofilm colonization in young and old human root canal dentin: In vitro study. J. Endod. 2010, 36, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Firouzmandi, M.; Mohaghegh, M.; Jafarpisheh, M. Effect of silver diamine fluoride on the bond durability of normal and carious dentin. J. Clin. Exp. Dent. 2020, 12, e468. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Bapat, P.; Alexander, A.; Kesharwani, P. Recent advances of gold nanoparticles as biomaterial in dentistry. Int. J. Pharm. 2020, 586, 119596. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.; Tajuddin, N.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Altunbek, M.; Baysal, A.; Çulha, M. Influence of surface properties of zinc oxide nanoparticles on their cytotoxicity. Colloids Surf. B Biointerfaces 2019, 121, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gharpure, S.; Ankamwar, B. Synthesis and antimicrobial properties of zinc oxide nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 5977–5996. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, X.; Quan, Y.; Tian, Y.; Chen, J.; Li, L. Visible light-induced photocatalytic and antibacterial adhesion properties of superhydrophilic TiO2 nanoparticles. Sci. Rep. 2024, 14, 7940. [Google Scholar] [CrossRef]
- Khater, M.S.; Kulkarni, G.R.; Khater, S.S.; Gholap, H.; Patil, R. Study to elucidate effect of titanium dioxide nanoparticles on bacterial membrane potential and membrane permeability. Mater. Res. Express 2020, 7, 035005. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Patil, N.R.; Nataraj, S.K.; Sannaikar, M.S.; Inamdar, S.R. Synthesis and photoluminescence properties of titanium oxide (TiO2) nanoparticles: Effect of calcination temperature. Optik 2019, 194, 163070. [Google Scholar] [CrossRef]
- Pinto, D.; Bernardo, L.; Amaro, A.; Lopes, S. Mechanical properties of epoxy nanocomposites using titanium dioxide as reinforcement–a review. Constr. Build. Mater. 2015, 95, 506–524. [Google Scholar] [CrossRef]
- Alshahrani, F.A.; Gad, M.M.; Al-Thobity, A.M.; Akhtar, S.; Kashkari, A.; Alzoubi, F.; Yilmaz, B. Effect of treated zirconium dioxide nanoparticles on the flexural properties of autopolymerized resin for interim fixed restorations: An in vitro study. J. Prosthet. Dent. 2023, 130, 257–264. [Google Scholar] [CrossRef]
- Ergun, G.; Sahin, Z.; Ataol, A.S. The effects of adding various ratios of zirconium oxide nanoparticles to poly (methyl methacrylate) on physical and mechanical properties. J. Oral Sci. 2018, 60, 304–315. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, H.; Liu, H.; Zhu, M.; Zhu, X.X. Surface modification of ZrO2 nanoparticles and its effects on the properties of dental resin composites. ACS Appl. Bio Mater. 2020, 3, 5300–5309. [Google Scholar] [CrossRef]
- Alhavaz, A.; Rezaei Dastjerdi, M.; Ghasemi, A.; Ghasemi, A.; Alizadeh Sahraei, A. Effect of untreated zirconium oxide nanofiller on the flexural strength and surface hardness of autopolymerized interim fixed restoration resins. J. Esthet. Restor. Dent. 2017, 29, 264–269. [Google Scholar] [CrossRef]
- Chęcińska, K.; Chęciński, M.; Sikora, M.; Nowak, Z.; Karwan, S.; Chlubek, D. The Effect of Zirconium Dioxide (ZrO2) Nanoparticles Addition on the Mechanical Parameters of Polymethyl Methacrylate (PMMA): A Systematic Review and Meta-Analysis of Experimental Studies. Polymers 2022, 14, 1047. [Google Scholar] [CrossRef]
- Souza, J.C.; Silva, J.B.; Aladim, A.; Carvalho, O.; Nascimento, R.M.; Silva, F.S.; Henriques, B. Effect of zirconia and alumina fillers on the microstructure and mechanical strength of dental glass ionomer cements. Open Dent. J. 2016, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.R.; Karrar, N.; Abdulsahib, S.J.; Ibrahim, J.S.; Haneen, T.A. Clinical Application of Iron Oxide Nanoparticles in Dentistry A Review. IJDDT 2021, 11, 1501–1506. [Google Scholar]
- Araujo, H.C.; da Silva, A.C.G.; Paiao, L.I.; Magario, M.K.W.; Frasnelli, S.C.T.; Oliveira, S.H.P.; Monteiro, D.R. Antimicrobial, antibiofilm and cytotoxic effects of a colloidal nanocarrier composed by chitosan-coated iron oxide nanoparticles loaded with chlorhexidine. J. Dent. 2020, 101, 103453. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhang, F.; Wang, L.; Chen, B.; Reynolds, M.A.; Xu, H.H. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Sharifian, S.; Loghmani, A.; Nayyerain, S.; Javanbakht, S.; Daneii, P. Application of Magnesium Oxide Nanoparticles in Dentistry: A Literature Review. Eur. J. Dent. 2023, 12, 1–6. [Google Scholar] [CrossRef]
- Liang, K.; Wang, S.; Tao, S.; Xiao, S.; Zhou, H.; Wang, P.; Xu, H.H. Dental remineralization via poly (amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral Sci. 2019, 11, 15. [Google Scholar] [CrossRef]
- Takhar, K.; Jindal, N.; Agarwal, R.; Rani, M.; Bansal, S. Comparative evaluation of the effect of different endodontic irrigation protocols on the microhardness of root canal dentin: An in vitro study. Dent. J. Adv. Stud. 2021, 9, 128–132. [Google Scholar] [CrossRef]
- Sistemática, D.; Gabriela, S.S.; Daniela, F.R.; Helia, B.T. Copper nanoparticles as potential antimicrobial agent in disinfecting root canals. A systematic review. Int. J. Odontostomat. 2016, 10, 547–554. [Google Scholar] [CrossRef]
- Xu, V.W.; Nizami, M.Z.I.; Yin, I.X.; Yu, O.Y.; Lung, C.Y.K.; Chu, C.H. Application of Copper Nanoparticles in Dentistry. Nanomaterials 2022, 12, 805. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Rygas, J.; Matys, J.; Wawrzyńska, M.; Szymonowicz, M.; Dobrzyński, M. The Use of Graphene Oxide in Orthodontics—A Systematic Review. J. Funct. Biomater. 2023, 14, 500. [Google Scholar] [CrossRef]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene Nanosheets to Improve Physico-Mechanical Properties of Bioactive Calcium Silicate Cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Trotta, A.; Camero, M.; Cordisco, M.; Dimuccio, M.M.; Corrente, M. Streptococcus mutans Associated with Endo-Periodontal Lesions in Intact Teeth. Appl. Sci. 2022, 12, 11837. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, S.V.; Limansubroto, A.N.; Yen, A.; Soundia, A.; Wang, C.-Y.; Shi, W.; Hong, C.; Tetradis, S.; Ho, D.; et al. Nanodiamond–gutta percha composite biomaterials for root canal therapy. ACS Nano 2015, 9, 11490–11501. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, J.; Rygas, J.; Homa, K.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. Antibacterial Activity of Endodontic Gutta-Percha—A Systematic Review. Appl. Sci. 2024, 14, 388. [Google Scholar] [CrossRef]
- Zhang, T.; Kalimuthu, S.; Rajasekar, V.; Xu, F.; Yiu, Y.C.; Hui, T.K.; Neelakantan, P.; Chu, Z. Biofilm inhibition in oral pathogens by nanodiamonds. Biomater. Sci. 2021, 9, 5127–5135. [Google Scholar] [CrossRef]
- Huang, C.-S.; Hsiao, C.-H.; Chang, Y.-C.; Chang, C.-H.; Yang, J.-C.; Gutmann, J.L.; Chang, H.-C.; Huang, H.-M.; Hsieh, S.-C. A Novel Endodontic Approach in Removing Smear Layer Using Nano and Submicron Diamonds with Intracanal Oscillation Irrigation. Nanomaterials 2023, 13, 1646. [Google Scholar] [CrossRef]
- Alghamdi, F.; Alsulaimani, M. Regenerative endodontic treatment: A systematic review of successful clinical cases. Dent. Med. Probl. 2021, 58, 555–567. [Google Scholar] [CrossRef]
- Murray, P.E. Review of guidance for the selection of regenerative endodontics, apexogenesis, apexification, pulpotomy, and other endodontic treatments for immature permanent teeth. Int. Endod. J. 2023, 56, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lee, H.W. Clinical potential of dental pulp stem cells in pulp regeneration: Current endodontic progress and future perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, T.W. Dental pulp stem cells: Advances to applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42. [Google Scholar] [CrossRef]
- Liu, H.; Lu, J.; Jiang, Q.; Haapasalo, M.; Qian, J.; Tay, F.R.; Shen, Y. Biomaterial scaffolds for clinical procedures in endodontic regeneration. Bioact. Mater. 2022, 12, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, S.; Cheayto, A.; Bassam, S.; Najar, M.; Berbéri, A.; Fayyad-Kazan, M. The effects of intracanal irrigants and medicaments on dental-derived stem cells fate in regenerative endodontics: An update. Stem Cell Rev. Rep. 2020, 16, 650–660. [Google Scholar] [CrossRef]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a new concept of regenerative endodontics based on mesenchymal stem cell-derived secretomes products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, L.; Li, J.; Ren, B. Nanofibrous scaffolds for regenerative endodontics treatment. Front. Bioeng. Biotechnol. 2022, 10, 1078453. [Google Scholar] [CrossRef]
- Cotti, E.; Ideo, F.; Pedrazzini, A.; Bardini, G.; Musu, D.; Kantarci, A. Proresolving mediators in endodontics: A systematic review. J. Endod. 2021, 47, 711–720. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Pang, L.; Pan, L.; Zhang, Q. Combination of resolvin E1 and lipoxin A4 promotes the resolution of pulpitis by inhibiting NF-κB activation through upregulating sirtuin 7 in dental pulp fibroblasts. Cell Prolif. 2022, 55, e13227. [Google Scholar] [CrossRef]
- Li, X.L.; Fan, W.; Fan, B. Dental pulp regeneration strategies: A review of status quo and recent advances. Bioact. Mater. 2024, 38, 258–275. [Google Scholar] [CrossRef]
- Seck, A.; Zein, N.; Nounsi, A.; Harmouch, E.; Vidal Varbanova, A.; Fernandez De Grado, G.; Fioretti, F. Nanomaterials for Endodontic Regeneration. In Stem Cells and Regenerative Medicine; IOS Press: Amsterdam, The Netherland, 2021; pp. 88–92. [Google Scholar]
- Ebrahimi, M.; Dissanayaka, W.L. Current Strategies in Pulp and Periodontal Regeneration Using Biodegradable Biomaterials. In Biodegradable Materials and Their Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 377–427. [Google Scholar]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The effect of silver, zinc oxide, and titanium dioxide nanoparticles used as final irrigation solutions on the fracture resistance of root-filled teeth. Clinical, Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, I.-R.; Kim, Y.; Kim, D.-H.; Park, S.-B.; Park, B.-S.; Bae, M.-K.; Kim, Y.-I. The Effect of Mesoporous Bioactive Glass Nanoparticles/Graphene Oxide Composites on the Differentiation and Mineralization of Human Dental Pulp Stem Cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, H.; Zhang, F.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Xu, H.H. Gold nanoparticles in injectable calcium phosphate cement enhance osteogenic differentiation of human dental pulp stem cells. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shurygina, I.A.; Shurygin, M.G. Nanoparticles in wound healing and regeneration. Met. Nanopart. Pharma 2017, 21–37. [Google Scholar] [CrossRef]
- Rajendran, N.K.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug. Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Lboutounne, H. Dental medicine nanosystems: Nanoparticles and their use in dentistry and oral health care. Int. J. Dent. Oral. Health 2017, 3, 145–157. [Google Scholar] [CrossRef]
- Vasiliu, S.; Racovita, S.; Gugoasa, I.A.; Lungan, M.-A.; Popa, M.; Desbrieres, J. The Benefits of Smart Nanoparticles in Dental Applications. Int. J. Mol. Sci. 2021, 22, 2585. [Google Scholar] [CrossRef]
- Montoya, C.; Roldan, L.; Yu, M.; Valliani, S.; Ta, C.; Yang, M.; Orrego, S. Smart dental materials for antimicrobial applications. Bioact. Mater. 2023, 24, 1–19. [Google Scholar] [CrossRef]
- Staniowski, T.; Zawadzka-Knefel, A.; Skośkiewicz-Malinowska, K. Therapeutic Potential of Dental Pulp Stem Cells According to Different Transplant Types. Molecules 2021, 26, 7423. [Google Scholar] [CrossRef]
- Özdemir, O.; Kopac, T. Recent Progress on the Applications of Nanomaterials and Nano-Characterization Techniques in Endodontics: A Review. Materials 2022, 15, 5109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moraes, G.; Zambom, C.; Siqueira, W.L. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef] [PubMed]
- Filip, D.G.; Surdu, V.-A.; Paduraru, A.V.; Andronescu, E. Current Development in Biomaterials—Hydroxyapatite and Bioglass for Applications in Biomedical Field: A Review. J. Funct. Biomater. 2022, 13, 248. [Google Scholar] [CrossRef] [PubMed]

| Application | Nanoparticles | Features/Properties |
|---|---|---|
| sealing agents | silver nanoparticles (AgNPs) | provide long-lasting antibacterial protection, reducing the risk of root canal reinfection |
| zinc oxide nanoparticles (ZnO-NPs) | exhibit antimicrobial properties, improve the mechanical and adhesive properties of sealants | |
| filling materials | nanohydroxyapatite (nHAp) | added to filling materials improves their mechanical and biological properties and supports the regeneration of tooth hard tissues |
| nanodiamonds | increase the mechanical strength of filling materials and improve their adhesive properties | |
| intrathecal drugs | carbon nanoparticles (e.g., fullerenes, carbon nanotubes) | used as drug carriers, enabling precise delivery of active substances to infection sites. |
| gold nanoparticles (AuNPs) | used for imaging, diagnostic and therapeutic purposes because of their optical and plasmonic properties, improving the effectiveness of intrathecal medications | |
| irrigation solutions | titanium oxide nanoparticles (TiO2-NPs) | in combination with UV radiation, TiO2-NPs generate reactive oxygen species that effectively disinfect root canals |
| silver nanoparticles (AgNPs) | adding to irrigation solutions increases their antimicrobial effectiveness, penetrate bacterial biofilm, used to disinfect root canals, effectively remove different strains of bacteria, including difficult-to-fight Gram-negative bacteria |
| Stem Cells | Role in RET |
|---|---|
| Dental Pulp Stem Cells (DPSCs) | capable of differentiating into many cell types, including odontoblasts, which are responsible for dentin formation |
| Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs) | differentiate into odontoblasts and other cell types that support pulp regeneration and differentiate into endothelial cells that contribute to the formation of blood vessels in the pulp |
| Immature Dental Pulp Stem Cells (IDPSs) | |
| Dental Follicle Stem Cells (DFSCs) | capable of differentiating into cells that can support the regeneration of periodontal tissues |
| Periodontal Ligament Stem Cells (PLSCs) | the potential to regenerate periodontal tissues, including root cement, periodontal ligament and alveolar bone |
| Stem Cells from Apical Papilla (SCAPs) | the ability to differentiate into odontoblasts, which form dentin, and into pulp-like cells that can contribute to the regeneration of vascular tissue |
| Supernumerary Tooth Stem Cells (SNTSCs) | research into their regenerative potential is still ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzejewska, Ż.A.; Rusztyn, B.; Łukaszuk, K.; Borys, J.; Borowska, M.; Antonowicz, B. The Latest Advances in the Use of Nanoparticles in Endodontics. Appl. Sci. 2024, 14, 7912. https://doi.org/10.3390/app14177912
Mierzejewska ŻA, Rusztyn B, Łukaszuk K, Borys J, Borowska M, Antonowicz B. The Latest Advances in the Use of Nanoparticles in Endodontics. Applied Sciences. 2024; 14(17):7912. https://doi.org/10.3390/app14177912
Chicago/Turabian StyleMierzejewska, Żaneta Anna, Bartłomiej Rusztyn, Kamila Łukaszuk, Jan Borys, Marta Borowska, and Bożena Antonowicz. 2024. "The Latest Advances in the Use of Nanoparticles in Endodontics" Applied Sciences 14, no. 17: 7912. https://doi.org/10.3390/app14177912





