The Spread of Invasive and Poisonous Plants: A Lesson from Alkaloids
Abstract
:1. The Biosynthesis of Plant Bio-Molecules in a Changing Environment
2. Alkaloids from Invasive Plants: A Successful Strategy for Environmental Adaptation but a Risk for Food Safety
3. Biosynthesis and Health Implications of Tropane Alkaloids
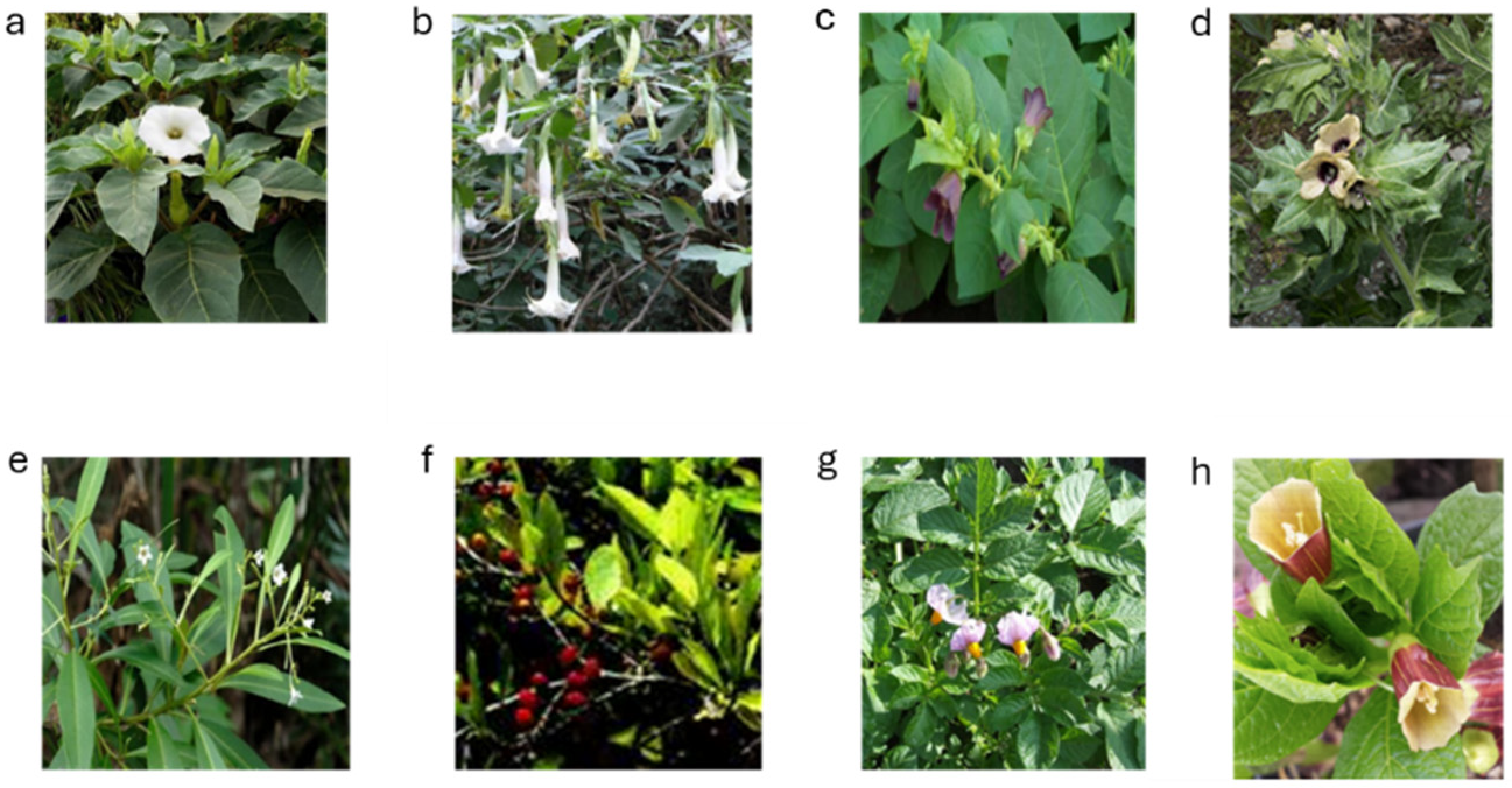
3.1. Tropane Alkaloid-Producing Plants
3.2. Biosynthesis of Tropane Alkaloids
3.3. Effect on Humans and Mechanism of Action
4. Biosynthesis and Health Implications of Pyrrolizidine Alkaloids
4.1. Pyrrolizidine Alkaloid-Producing Plants
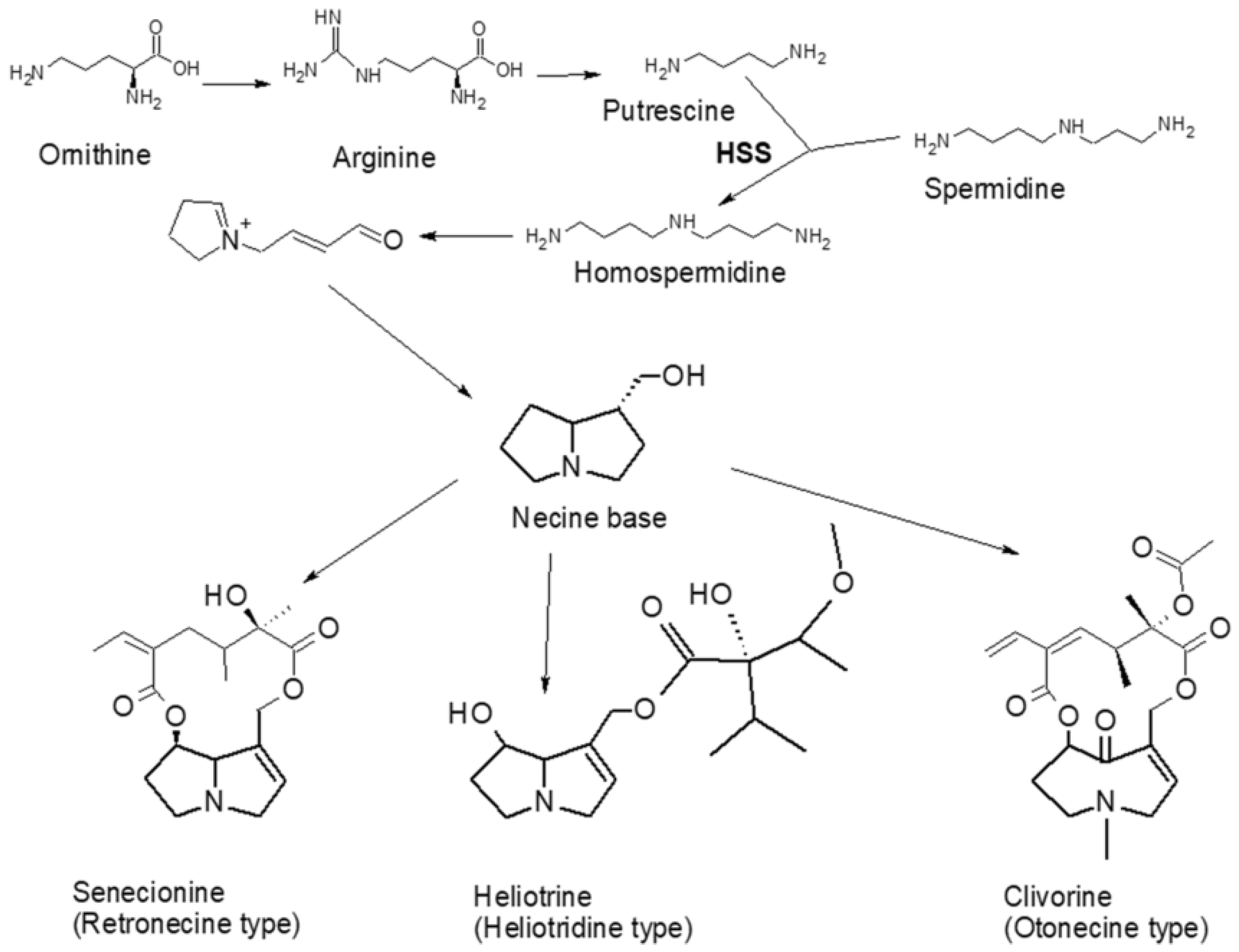
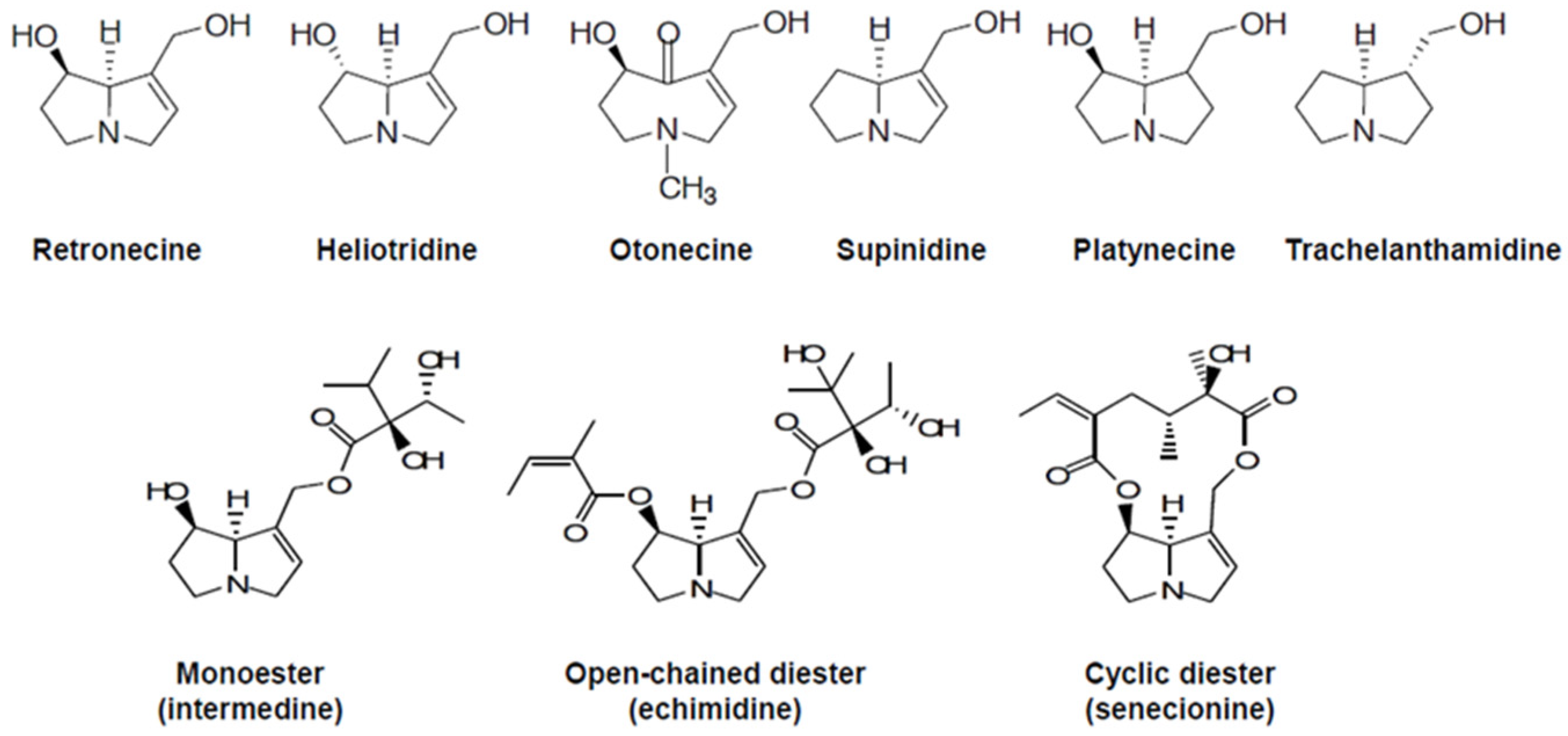
4.2. Biosynthesis of Pyrrolizidine Alkaloids
4.3. Effect on Humans and Mechanism of Action
5. Food and Feed Contaminations of TAs and PAs
6. Molecular Markers for Detecting Invasive Plant Contaminants in Food Crops
7. Analytical Methods for Detection
8. Perspectives to Mitigate Invasiveness and Food and Feed Contamination
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.Q.; Dudareva, N. Plant Specialized Metabolism. Curr. Biol. 2023, 33, R473–R478. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Xuan, T.D.; Khanh, T.D.; Tran, H.D.; Trung, N.T. Allelochemicals and Signaling Chemicals in Plants. Molecules 2019, 24, 2737. [Google Scholar] [CrossRef]
- Gaillard, M.D.P.; Glauser, G.; Robert, C.A.M.; Turlings, T.C.J. Fine-Tuning the ‘Plant Domestication-Reduced Defense’ Hypothesis: Specialist vs Generalist Herbivores. New Phytol. 2018, 217, 355–366. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy1[OPEN]. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Daehler, C.C. Performance Comparisons of Co-Occurring Native and Alien Invasive Plants: Implications for Conservation and Restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Wei, H.; Huang, M.; Quan, G.; Zhang, J.; Liu, Z.; Ma, R. Turn Bane into a Boon: Application of Invasive Plant Species to Remedy Soil Cadmium Contamination. Chemosphere 2018, 210, 1013–1020. [Google Scholar] [CrossRef]
- Yin, W.; Zhou, L.; Yang, K.; Fang, J.; Biere, A.; Callaway, R.M.; Wu, M.; Yu, H.; Shi, Y.; Ding, J. Rapid Evolutionary Trade-Offs between Resistance to Herbivory and Tolerance to Abiotic Stress in an Invasive Plant. Ecol. Lett. 2023, 26, 942–954. [Google Scholar] [CrossRef]
- Yahyazadeh, M.; Jerz, G.; Winterhalter, P.; Selmar, D. The Complexity of Sound Quantification of Specialized Metabolite Biosynthesis: The Stress Related Impact on the Alkaloid Content of Catharanthus Roseus. Phytochemistry 2021, 187, 112774. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Zhou, L.; Yin, W.; Ding, J. Trade-Offs between Chemical Resistance to Herbivory and Responses to Abiotic Stresses in Invasive Plants. J. Plant Ecol. 2024, 17, rtae007. [Google Scholar] [CrossRef]
- Heywood, V.H. Plant Conservation in the Anthropocene–Challenges and Future Prospects. Plant Divers. 2017, 39, 314–330. [Google Scholar] [CrossRef]
- Munné-Bosch, S. Achieving the Impossible: Prevention and Eradication of Invasive Plants in Mediterranean-Type Ecosystems. Trends Plant Sci. 2024, 29, 437–446. [Google Scholar] [CrossRef]
- Amirkia, V.; Heinrich, M. Alkaloids as Drug Leads—A Predictive Structural and Biodiversity-Based Analysis. Phytochem. Lett. 2014, 10, xlviii–liii. [Google Scholar] [CrossRef]
- Leete, E.; Marion, L.; Spenser, I.D. Biogenesis of Hyoscyamine. Nature 1954, 174, 650–651. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yukimune, Y.; Yamada, Y. Putrescine and Putrescine N-Methyltransferase in the Biosynthesis of Tropane Alkaloids in Cultured Roots of Hyoscyamus Albus-I. Biochemical Studies. Planta 1989, 178, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Biastoff, S.; Brandt, W.; Dräger, B. Putrescine N-Methyltransferase-The Start for Alkaloids. Phytochemistry 2009, 70, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Docimo, T.; Reichelt, M.; Schneider, B.; Kai, M.; Kunert, G.; Gershenzon, J.; D’Auria, J.C. The First Step in the Biosynthesis of Cocaine in Erythroxylum Coca: The Characterization of Arginine and Ornithine Decarboxylases. Plant Mol. Biol. 2012, 78, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Jirschitzka, J.; Dolke, F.; D’Auria, J.C. Increasing the Pace of New Discoveries in Tropane Alkaloid Biosynthesis, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 68, ISBN 9780124080614. [Google Scholar]
- Chavez, B.G.; Srinivasan, P.; Glockzin, K.; Kim, N.; Montero Estrada, O.; Jirschitzka, J.; Rowden, G.; Shao, J.; Meinhardt, L.; Smolke, C.D.; et al. Elucidation of Tropane Alkaloid Biosynthesis in Erythroxylum Coca Using a Microbial Pathway Discovery Platform. Proc. Natl. Acad. Sci. USA 2022, 119, e2215372119. [Google Scholar] [CrossRef]
- Chavez, B.G.; Leite Dias, S.; D’Auria, J.C. The Evolution of Tropane Alkaloids: Coca Does It Differently. Curr. Opin. Plant Biol. 2024, 81, 102606. [Google Scholar] [CrossRef]
- Zhang, F.; Qiu, F.; Zeng, J.; Xu, Z.; Tang, Y.; Zhao, T.; Gou, Y.; Su, F.; Wang, S.; Sun, X.; et al. Revealing Evolution of Tropane Alkaloid Biosynthesis by Analyzing Two Genomes in the Solanaceae Family. Nat. Commun. 2023, 14, 1446. [Google Scholar] [CrossRef]
- Holmes, H.L. Chapter VI The Chemistry of the Tropane Alkaloids. Alkaloids Chem. Physiol. 1950, 1, 271–374. [Google Scholar] [CrossRef]
- Griffin, W.J.; Lin, G.D. Chemotaxonomy and Geographical Distribution of Tropane Alkaloids. Phytochemistry 2000, 53, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. [Google Scholar] [CrossRef] [PubMed]
- Dräger, B. Analysis of Tropane and Related Alkaloids. J. Chromatogr. A 2002, 978, 1–35. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Tain, T.; Yu, J.-Y.; Li, J.; Xu, B.; Chen, J.; D’Auria, J.C.; Huang, J.-P.; Huang, S.-X. Genomic and Structural Basis for Evolution of Tropane Alkaloid Biosynthesis. Proc. Natl. Acad. Sci. USA 2023, 120, e2302448120. [Google Scholar] [CrossRef] [PubMed]
- Kohnen-Johannsen, K.L.; Kayser, O. Tropane Alkaloids: Chemistry, Pharmacology, Biosynthesis and Production. Molecules 2019, 24, 796. [Google Scholar] [CrossRef] [PubMed]
- Mccullough, K.J. Second Supplements to the 2nd Edition of Rodd’s Chemistry of Carbon Compounds; Sainsbury, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume IV, pp. 509–554. [Google Scholar]
- Maurya, V.K.; Kumar, S.; Kabir, R.; Shrivastava, G.; Shanker, K.; Nayak, D.; Khurana, A.; Manchanda, R.K.; Gadugu, S.; Kar, S.K.; et al. Dark Classics in Chemical Neuroscience: An Evidence-Based Systematic Review of Belladonna. ACS Chem. Neurosci. 2020, 11, 3937–3954. [Google Scholar] [CrossRef]
- Dräger, B. Chemistry and Biology of Calystegines. Nat. Prod. Rep. 2004, 21, 211–223. [Google Scholar] [CrossRef]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. Plant Toxins 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Casado, N.; Gañán, J.; Morante-Zarcero, S.; Sierra, I. Recent Food Alerts and Analytical Advances Related to the Contamination of Tropane and Pyrrolizidine Alkaloids in Food. Front. Chem. Biol. 2024, 3, 1360027. [Google Scholar] [CrossRef]
- González-Gómez, L.; Morante-Zarcero, S.; Pérez-Quintanilla, D.; Sierra, I. Occurrence and Chemistry of Tropane Alkaloids in Foods, with a Focus on Sample Analysis Methods: A Review on Recent Trends and Technological Advances. Foods 2022, 11, 407. [Google Scholar] [CrossRef]
- Guerra-Doce, E.; Rihuete-Herrada, C.; Micó, R.; Risch, R.; Lull, V.; Niemeyer, H.M. Direct Evidence of the Use of Multiple Drugs in Bronze Age Menorca (Western Mediterranean) from Human Hair Analysis. Sci. Rep. 2023, 13, 4782. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Sharma, N.; An, S.S.A. Beauty of the Beast: Anticholinergic Tropane Alkaloids in Therapeutics. Nat. Prod. Bioprospect. 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E. Hallucinogenic Plants; Golden Press: New York, NY, USA, 1976. [Google Scholar]
- Zlotos, D.P.; Bender, W.; Holzgrabe, U. Muscarinic Receptor Agonists and Antagonists. Expert Opin. Ther. Pat. 1999, 9, 1029–1053. [Google Scholar] [CrossRef]
- Reas, H.W.; Tsai, T.H. The Antagonism by Atropine of the Response of the Nictitating Membrane to Sympathetic Nerve Stimulation. J. Pharmacol. Exp. Ther. 1966, 152, 186–196. [Google Scholar] [PubMed]
- Ebert, U.; Siepmann, M.; Oertel, R.; Wesnes, K.A.; Kirch, W. Pharmacokinetics and Pharmacodynamics of Scopolamine after Subcutaneous Administration. J. Clin. Pharmacol. 1998, 38, 720–726. [Google Scholar] [CrossRef]
- Honkavaara, P.; Pyykkö, I. Effects of Atropine and Scopolamine on Bradycardia and Emetic Symptoms in Otoplasty. Laryngoscope 1999, 109, 108–112. [Google Scholar] [CrossRef]
- Kim, N.; Estrada, O.; Chavez, B.; Stewart, C.; D’Auria, J.C. Tropane and Granatane Alkaloid Biosynthesis: A Systematic Analysis. Molecules 2016, 21, 1510. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Smolke, C.D. Engineering a Microbial Biosynthesis Platform for de Novo Production of Tropane Alkaloids. Nat. Commun. 2019, 10, 3634. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; de Nijs, M.; Castellari, M.; Hortos, M.; MacDonald, S.; Crews, C.; Hajslova, J.; Stranska, M. Occurrence of Tropane Alkaloids in Food. EFSA Support. Publ. 2016, 13, 1140E. [Google Scholar] [CrossRef]
- Lamp, J.; Knappstein, K.; Walte, H.G.; Krause, T.; Steinberg, P.; Schwake-Anduschus, C. Transfer of Tropane Alkaloids (Atropine and Scopolamine) into the Milk of Subclinically Exposed Dairy Cows. Food Control 2021, 126, 108056. [Google Scholar] [CrossRef]
- Spina, S.P.; Taddei, A. Teenagers with Jimson Weed (Datura Stramonium ) Poisoning. J. Emerg. Med. 2007, 9, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Binaglia, M.; Baert, K.; Schutte, M.; Serafimova, R. Overview of Available Toxicity Data for Calystegines. EFSA J. 2019, 17, e05574. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Pyrrolizidine Alkaloids in Food and Feed. EFSA J. 2011, 9, 2406. [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Duebecke, A.; Mulder, P.P.J.; Cuneah, O.; Barel, S. Pyrrolizidine and Tropane Alkaloids in Teas and the Herbal Teas Peppermint, Rooibos and Chamomile in the Israeli Market. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 2058–2067. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Homospermidine Synthase, the First Pathway-Specific Enzyme of Pyrrolizidine Alkaloid Biosynthesis, Evolved from Deoxyhypusine Synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef]
- Nowacki, E.; Byerrum, R.U. Biosynthesis of Lupanine from Lysine and Other Labeled Compounds. Biochem. Biophys. Res. Commun. 1962, 7, 58–61. [Google Scholar] [CrossRef]
- Anke, S.; Niemüller, D.; Moll, S.; Hänsch, R.; Ober, D. Polyphyletic Origin of Pyrrolizidine Alkaloids within the Asteraceae. Evidence from Differential Tissue Expression of Homospermidine Synthase. Plant Physiol. 2004, 136, 4037–4047. [Google Scholar] [CrossRef]
- Tábuas, B.; Cruz Barros, S.; Diogo, C.; Cavaleiro, C.; Sanches Silva, A. Pyrrolizidine Alkaloids in Foods, Herbal Drugs, and Food Supplements: Chemistry, Metabolism, Toxicological Significance, Analytical Methods, Occurrence, and Challenges for Future. Toxins 2024, 16, 79. [Google Scholar] [CrossRef]
- Frölich, C.; Ober, D.; Hartmann, T. Tissue Distribution, Core Biosynthesis and Diversification of Pyrrolizidine Alkaloids of the Lycopsamine Type in Three Boraginaceae Species. Phytochemistry 2007, 68, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Picron, J.F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Analytical Strategies for the Determination of Pyrrolizidine Alkaloids in Plant Based Food and Examination of the Transfer Rate during the Infusion Process. Food Chem. 2018, 266, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ma, L.; He, X.; Cai, L.; Fu, P.P. 7-Glutathione Pyrrole Adduct: A Potential DNA Reactive Metabolite of Pyrrolizidine Alkaloids. Chem. Res. Toxicol. 2015, 28, 615–620. [Google Scholar] [CrossRef]
- Jank, B.; Rath, J. The Risk of Pyrrolizidine Alkaloids in Human Food and Animal Feed. Trends Plant Sci. 2017, 22, 191–193. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Worldwide Occurrence and Investigations of Contamination of Herbal Medicines by Tropane Alkaloids. Toxins 2017, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Montgomery, H.; Nugent, A.P.; Elliott, C.T. Tropane Alkaloid Contamination of Agricultural Commodities and Food Products in Relation to Consumer Health: Learnings from the 2019 Uganda Food Aid Outbreak. Compr. Rev. Food Sci. Food Saf. 2021, 20, 501–525. [Google Scholar] [CrossRef]
- Caprai, E.; Prizio, I.; Peloso, M.; Minkoumba Sonfack, G.; Bonan, S.; Benini, N.; Ghidini, S.; Varrà, M.O.; Zanardi, E.; Lanza, G.T.; et al. Case Reports of Tropane Alkaloid Contamination in Spinach from Italy and Its Potential Implications for Consumer Health. Food Control 2024, 160, 110334. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on Tropane Alkaloids in Food and Feed. EFSA J. 2013, 11, 3386. [CrossRef]
- Perharič, L.; Juvan, K.A.; Stanovnik, L. Acute Effects of a Low-Dose Atropine/Scopolamine Mixture as a Food Contaminant in Human Volunteers. J. Appl. Toxicol. 2013, 33, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Perharič, L.; Koželj, G.; Družina, B.; Stanovnik, L. Risk Assessment of Buckwheat Flour Contaminated by Thorn-Apple (Datura stramonium L.) Alkaloids: A Case Study from Slovenia. Food Addit. Contam. Part A 2013, 30, 321–330. [Google Scholar] [CrossRef]
- Binaglia, M. Assessment of the Conclusions of the Joint FAO/WHO Expert Meeting on Tropane Alkaloids. EFSA J. 2022, 20, e07229. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157.
- Picron, J.F.; Herman, M.; Van Hoeck, E.; Goscinny, S. Monitoring of Pyrrolizidine Alkaloids in Beehive Products and Derivatives on the Belgian Market. Environ. Sci. Pollut. Res. 2020, 27, 5693–5708. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, P.; Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Pyrrolizidine Alkaloids and Beehive Products: A Review. Food Chem. 2021, 342, 128384. [Google Scholar] [CrossRef]
- Celano, R.; Piccinelli, A.L.; Campone, L.; Russo, M.; Rastrelli, L. Determination of Selected Pyrrolizidine Alkaloids in Honey by Dispersive Liquid-Liquid Microextraction and Ultrahigh-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 8689–8699. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Campone, L.; Rastrelli, L.; Piccinelli, A.L. Salting-out Assisted Liquid-Liquid Extraction for the Rapid and Simple Simultaneous Analysis of Pyrrolizidine Alkaloids and Related N-Oxides in Honey and Pollen. J. Food Compos. Anal. 2022, 108, 104457. [Google Scholar] [CrossRef]
- Mulder, P.P.J.; López, P.; Castelari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of Pyrrolizidine Alkaloids in Animal- and Plant-Derived Food: Results of a Survey across Europe. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Food, E.; Authority, S. Dietary Exposure Assessment to Pyrrolizidine Alkaloids in the European Population. EFSA J. 2016, 14, e04572. [Google Scholar] [CrossRef]
- Kast, C.; Dübecke, A.; Kilchenmann, V.; Bieri, K.; Böhlen, M.; Zoller, O.; Beckh, G.; Lüllmann, C. Analysis of Swiss Honeys for Pyrrolizidine Alkaloids. J. Apic. Res. 2014, 53, 75–83. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements. EFSA J. 2017, 15, e04908. [Google Scholar] [CrossRef]
- European Commission. Europe 2020 A European Strategy for Smart, Sustainable and Inclusive Growth; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- HMPC (Committee on Herbal Medicinal Products)-European Medicines Agency (EMA). Overview of Comments Received on the Second Draft Public Statement on the Use of Herbal Medicinal Products Containing Toxic Unsaturated Pyrrolizidine Alkaloids (PAs) (EMA/HMPC/893108/2011). 2014; 44.
- ESA Statement on Pyrrolizidine Alkaloids in Herbs and Spices. 2023, pp. 1–5. Available online: https://www.esa-spices.org/download/esa-position-statement.pdf (accessed on 4 September 2024).
- Vieira, M.B.; Faustino, M.V.; Lourenço, T.F.; Margarida Oliveira, M. DNA-Based Tools to Certify Authenticity of Rice Varieties—An Overview. Foods 2022, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Böhme, K.; Calo-Mata, P.; Barros-Velázquez, J.; Ortea, I. Review of Recent DNA-Based Methods for Main Food-Authentication Topics. J. Agric. Food Chem. 2019, 67, 3854–3864. [Google Scholar] [CrossRef]
- Lanubile, A.; Stagnati, L.; Marocco, A.; Busconi, M. DNA-Based Techniques to Check Quality and Authenticity of Food, Feed and Medicinal Products of Plant Origin: A Review. Trends Food Sci. Technol. 2024, 149, 104568. [Google Scholar] [CrossRef]
- Javanmardi, N.; Bagheri, A.; Moshtaghi, N.; Sharifi, A.; Kakhki, A.H. Identification of Safflower as a Fraud in Commercial Saffron Using RAPD/SCAR Marker. J. Cell Mol. Res. 2011, 3, 31–37. [Google Scholar]
- Marwal, A.; Sahu, A.K.; Gaur, R.K. Molecular Markers: Tool for Genetic Analysis; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780124160026. [Google Scholar]
- Scarano, D.; Rao, R. DNA Markers for Food Products Authentication. Diversity 2014, 6, 579–596. [Google Scholar] [CrossRef]
- Darling, J.A.; Blum, M.J. DNA-Based Methods for Monitoring Invasive Species: A Review and Prospectus. Biol. Invasions 2007, 9, 751–765. [Google Scholar] [CrossRef]
- Willocx, M.; Van der Beeten, I.; Asselman, P.; Delgat, L.; Baert, W.; Janssens, S.B.; Leliaert, F.; Picron, J.F.; Vanhee, C. Sorting out the Plants Responsible for a Contamination with Pyrrolizidine Alkaloids in Spice Seeds by Means of LC-MS/MS and DNA Barcoding: Proof of Principle with Cumin and Anise Spice Seeds. Food Chem. Mol. Sci. 2022, 4, 100070. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Xiong, C.; Zhao, B.; Chen, S. lin The Potential Power of Bar-HRM Technology in Herbal Medicine Identification. Front. Plant Sci. 2016, 7, 367. [Google Scholar] [CrossRef]
- Anthoons, B.; Lagiotis, G.; Drouzas, A.D.; de Boer, H.; Madesis, P. Barcoding High Resolution Melting (Bar-HRM) Enables the Discrimination between Toxic Plants and Edible Vegetables Prior to Consumption and after Digestion. J. Food Sci. 2022, 87, 4221–4232. [Google Scholar] [CrossRef] [PubMed]
- Bosmali, I.; Ordoudi, S.A.; Tsimidou, M.Z.; Madesis, P. Greek PDO Saffron Authentication Studies Using Species Specific Molecular Markers. Food Res. Int. 2017, 100, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesan, B.; Gerner-Smidt, P.; Allard, M.W.; Leuillet, S.; Winkler, A.; Xiao, Y.; Chaffron, S.; Van Der Vossen, J.; Tang, S.; Katase, M.; et al. The Use of next Generation Sequencing for Improving Food Safety: Translation into Practice. Food Microbiol. 2019, 79, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Casado, N.; Morante-Zarcero, S.; Sierra, I. The Concerning Food Safety Issue of Pyrrolizidine Alkaloids: An Overview. Trends Food Sci. Technol. 2022, 120, 123–139. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Serio, S.; Russo, M.; Rastrelli, L. An Analytical Platform for the Screening and Identification of Pyrrolizidine Alkaloids in Food Matrices with High Risk of Contamination. Food Chem. 2023, 406, 135058. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xu, J.; Liu, Y.; Xie, Y.; Xiong, A.; Wang, Z.; Yang, L. Mass Spectrometric Analysis Strategies for Pyrrolizidine Alkaloids. Food Chem. 2024, 445, 138748. [Google Scholar] [CrossRef]
- Rizzo, S.; Celano, R.; Piccinelli, A.L.; Russo, M.; Rastrelli, L. Target Screening Method for the Quantitative Determination of 118 Pyrrolizidine Alkaloids in Food Supplements, Herbal Infusions, Honey and Teas by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Food Chem. 2023, 423, 136306. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Mao, H.; You, W.; Chen, J.; Xu, H.; Wu, J.; Gong, Y.; Guo, L.; Liu, T.; et al. Structural Annotation, Semi-Quantification and Toxicity Prediction of Pyrrolizidine Alkaloids from Functional Food: In Silico and Molecular Networking Strategy. Food Chem. Toxicol. 2023, 176, 113738. [Google Scholar] [CrossRef]
- Tsiokanos, E.; Tsafantakis, N.; Obé, H.; Beuerle, T.; Leti, M.; Fokialakis, N.; Grondin, A. Profiling of Pyrrolizidine Alkaloids Using a Retronecine-Based Untargeted Metabolomics Approach Coupled to the Quantitation of the Retronecine-Core in Medicinal Plants Using UHPLC-QTOF. J. Pharm. Biomed. Anal. 2023, 224, 115171. [Google Scholar] [CrossRef] [PubMed]
- Choudry, M.W.; Nawaz, P.; Jahan, N.; Riaz, R.; Ahmed, B.; Raza, M.H.; Fayyaz, Z.; Malik, K.; Afzal, S. RNA Based Gene Silencing Modalities to Control Insect and Fungal Plant Pests–Challenges and Future Prospects. Physiol. Mol. Plant Pathol. 2024, 130, 102241. [Google Scholar] [CrossRef]
- Hasebe, F.; Yuba, H.; Hashimoto, T.; Saito, K.; Funa, N.; Shoji, T. CRISPR/Cas9-Mediated Disruption of the PYRROLIDINE KETIDE SYNTHASE Gene Reduces the Accumulation of Tropane Alkaloids in Atropa Belladonna Hairy Roots. Biosci. Biotechnol. Biochem. 2021, 85, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Parks, H.M.; Cinelli, M.A.; Bedewitz, M.A.; Grabar, J.M.; Hurney, S.M.; Walker, K.D.; Jones, A.D.; Barry, C.S. Redirecting Tropane Alkaloid Metabolism Reveals Pyrrolidine Alkaloid Diversity in Atropa Belladonna. New Phytol. 2023, 237, 1810–1825. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Mehrotra, S.; Mishra, S. (Eds.) Tropane Alkaloids: Pathways, Potential and Biotechnological Applications; Springer: Singapore, 2021; ISBN 9789813345355. [Google Scholar]
- Son, N.; Chen, C.R.; Syu, C.H. Towards Artificial Intelligence Applications in Precision and Sustainable Agriculture. Agronomy 2024, 14, 239. [Google Scholar] [CrossRef]
- Mohidem, N.A.; Che’ya, N.N.; Juraimi, A.S.; Ilahi, W.F.F.; Roslim, M.H.M.; Sulaiman, N.; Saberioon, M.; Mohd Noor, N. How can unmanned aerial vehicles be used for detecting weeds in agricultural fields? Agriculture 2021, 11, 1004. [Google Scholar] [CrossRef]
- Istiak, M.A.; Syeed, M.M.M.; Hossain, M.S.; Uddin, M.F.; Hasan, M.; Khan, R.H.; Azad, N.S. Adoption of Unmanned Aerial Vehicle (UAV) Imagery in Agricultural Management: A Systematic Literature Review. Ecol. Inform. 2023, 78, 102305. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Alessandro, R.; Celano, R.; Piccinelli, A.L.; D’Amelia, V.; Docimo, T. The Spread of Invasive and Poisonous Plants: A Lesson from Alkaloids. Appl. Sci. 2024, 14, 8058. https://doi.org/10.3390/app14178058
D’Alessandro R, Celano R, Piccinelli AL, D’Amelia V, Docimo T. The Spread of Invasive and Poisonous Plants: A Lesson from Alkaloids. Applied Sciences. 2024; 14(17):8058. https://doi.org/10.3390/app14178058
Chicago/Turabian StyleD’Alessandro, Rosa, Rita Celano, Anna Lisa Piccinelli, Vincenzo D’Amelia, and Teresa Docimo. 2024. "The Spread of Invasive and Poisonous Plants: A Lesson from Alkaloids" Applied Sciences 14, no. 17: 8058. https://doi.org/10.3390/app14178058







