3D X-ray Tomography Analysis of Mg–Si–Zn Alloys for Biomedical Applications: Elucidating the Morphology of the MgZn Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Casting of Mg Alloys
2.2. Microstructural and Compositional Analyses
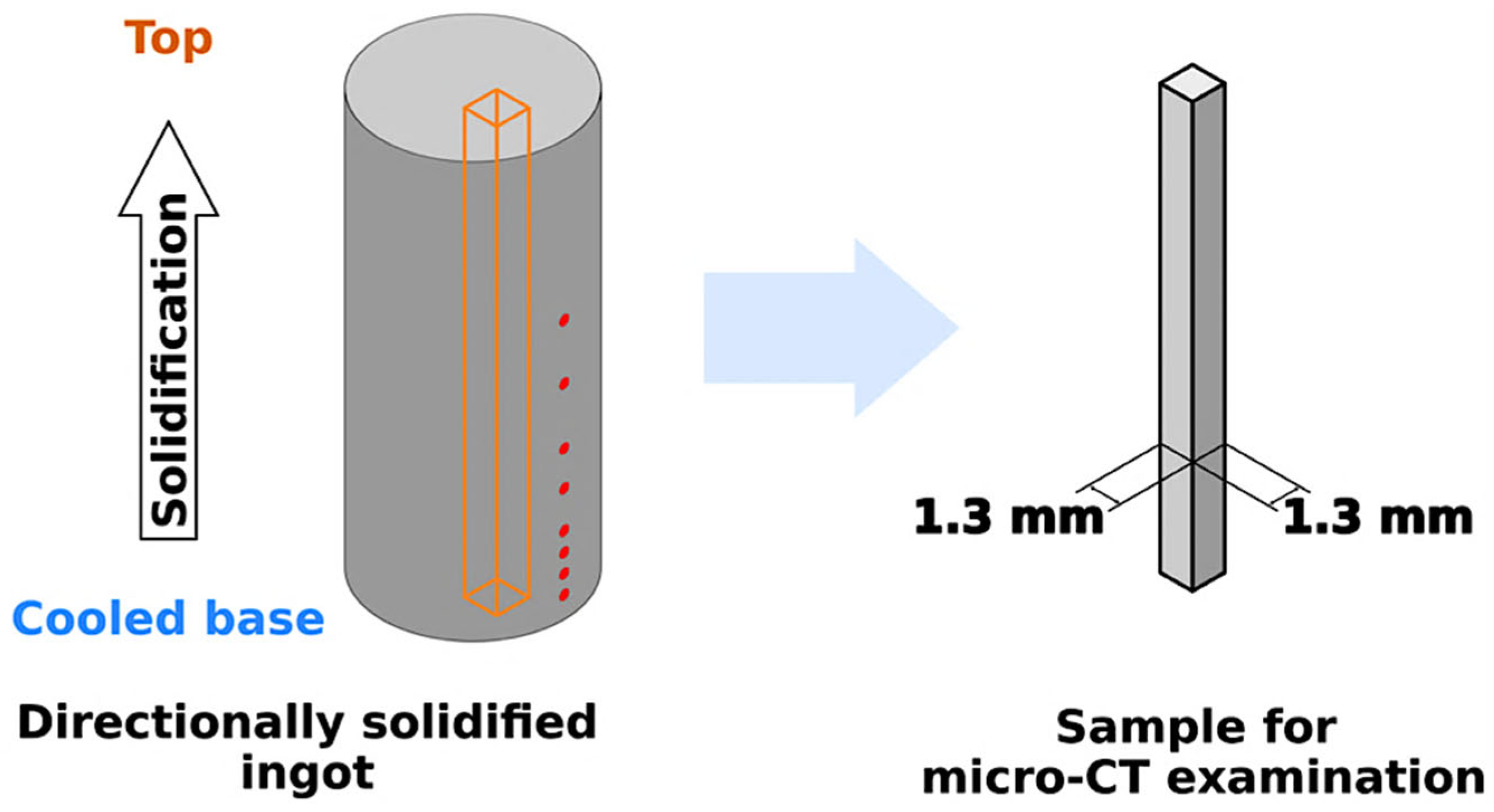
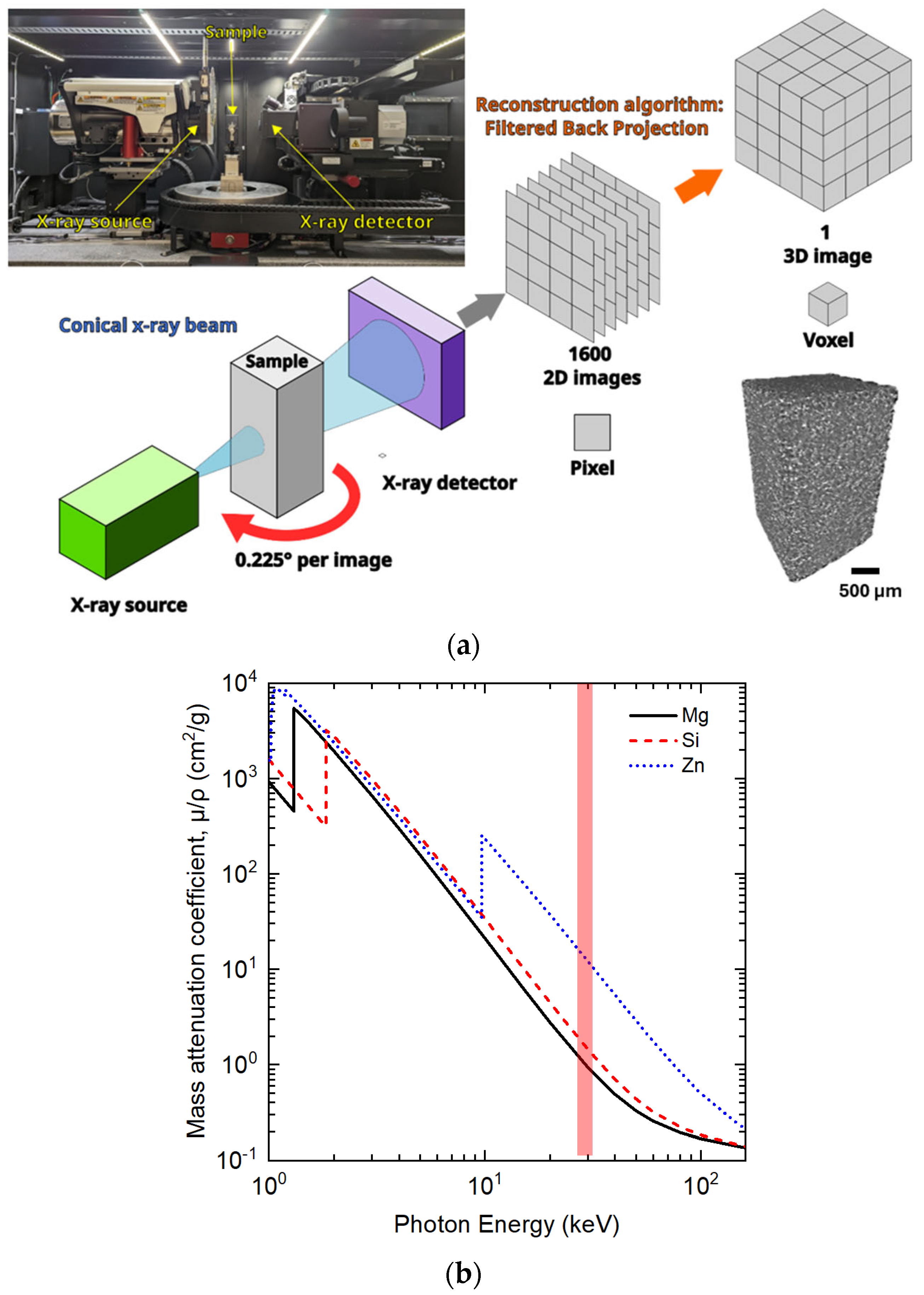
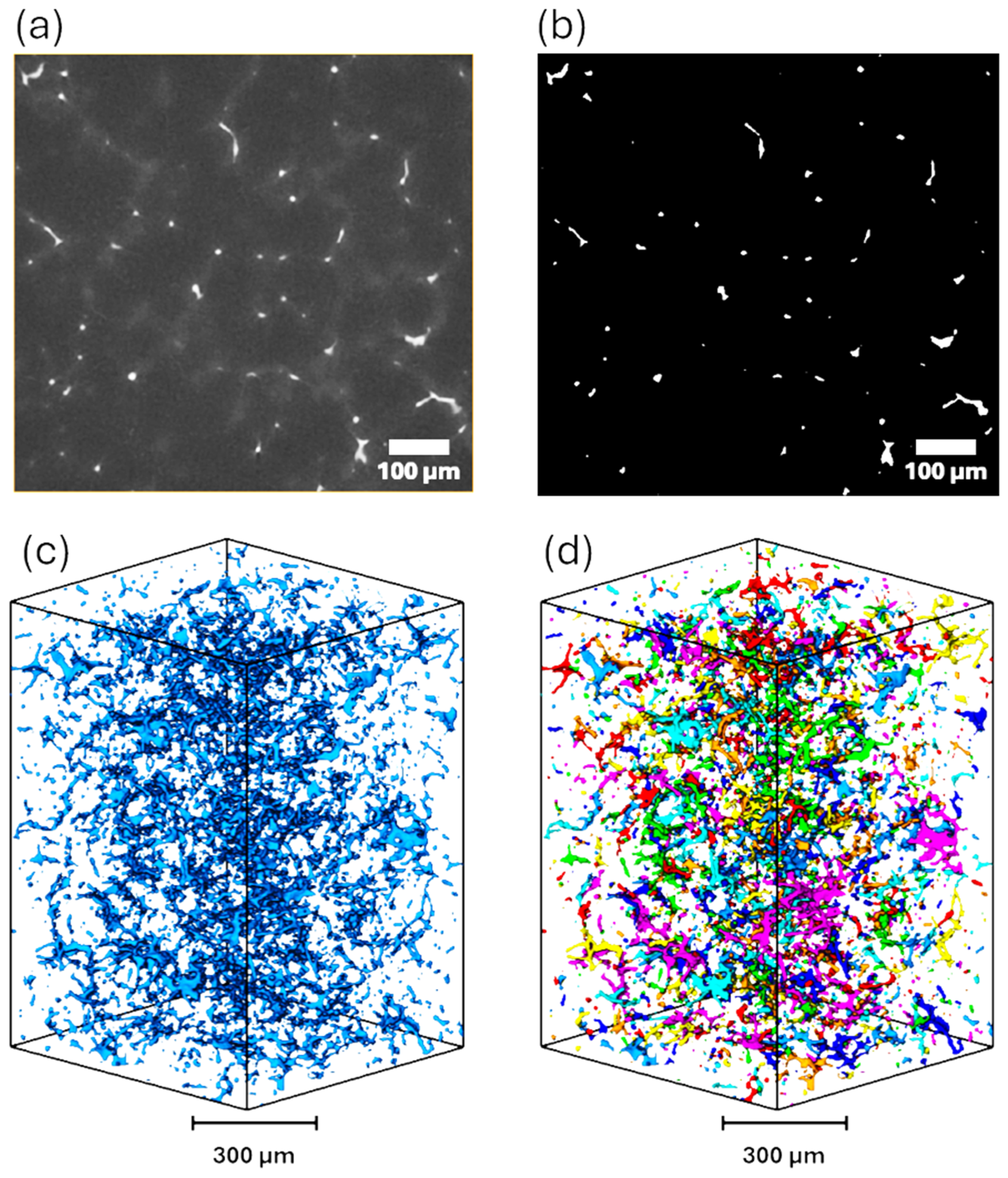
2.3. 3D Microstructural Analyses via X-ray Computed Tomography
3. Results and Discussion
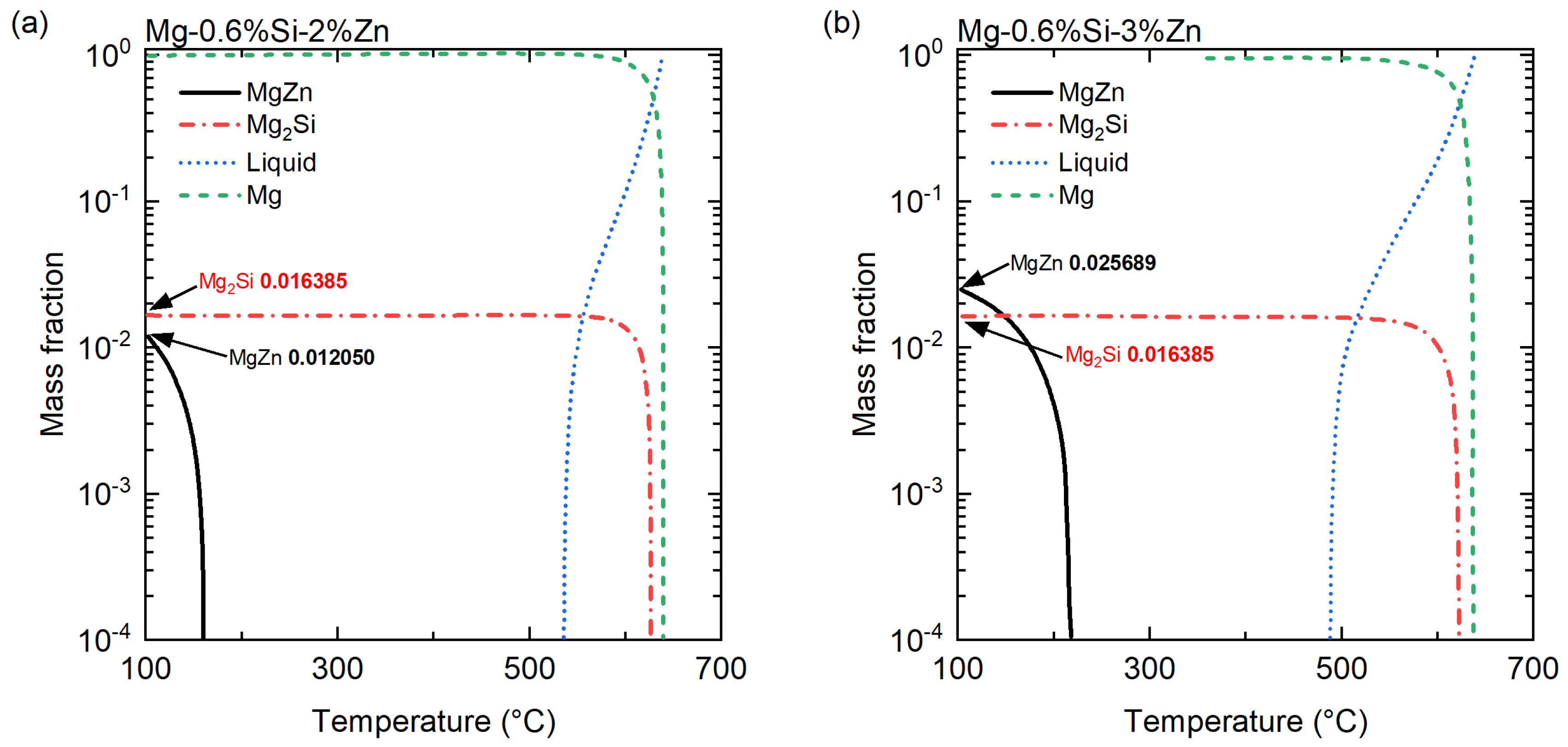
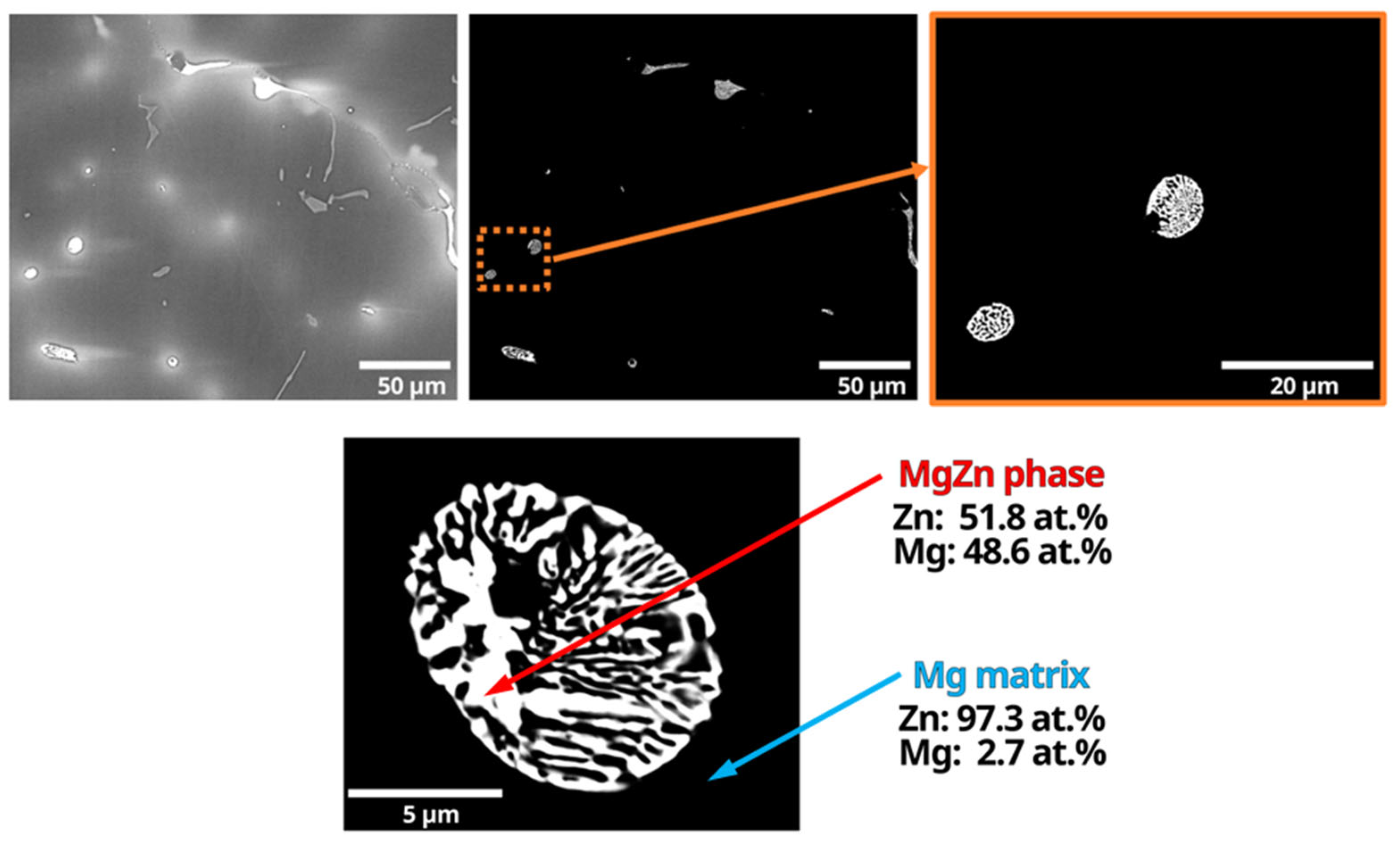
3.1. Compositional Analyses
3.2. Microstructural Analyses Using X-ray Computed Tomography (XCT)
3.2.1. Impact of Composition
3.2.2. Impact of Cooling Rate
4. Summary and Conclusions
- (1)
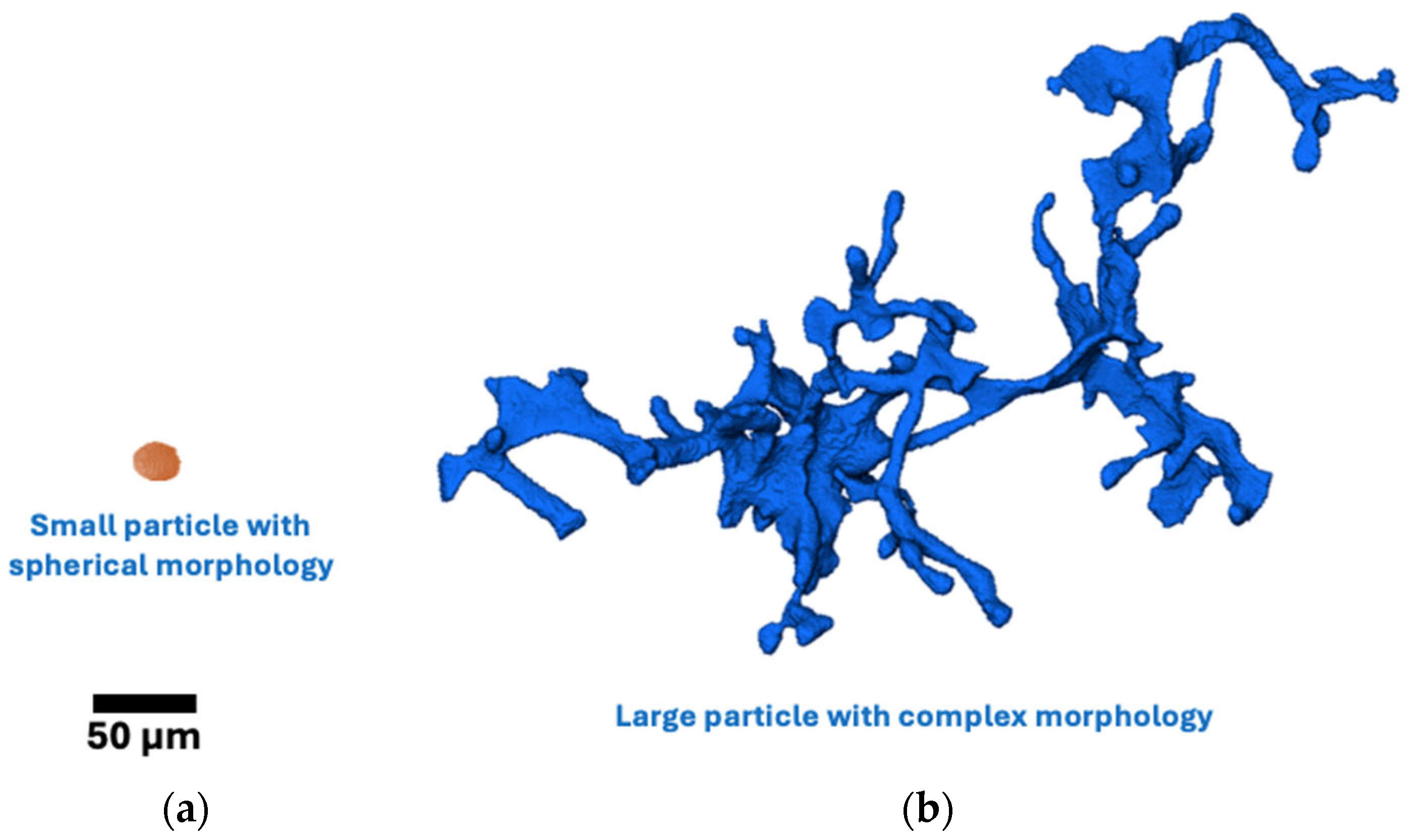
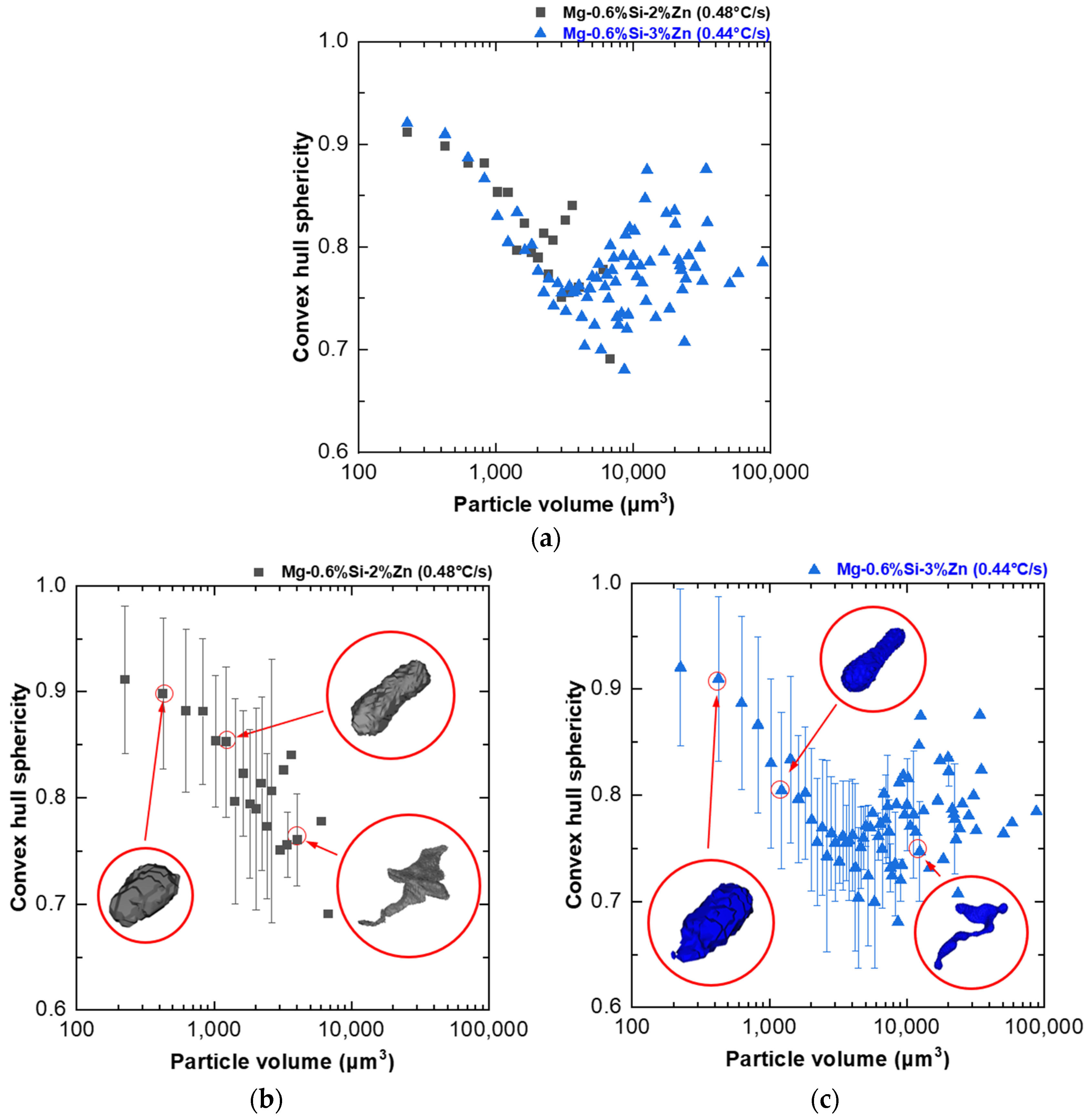
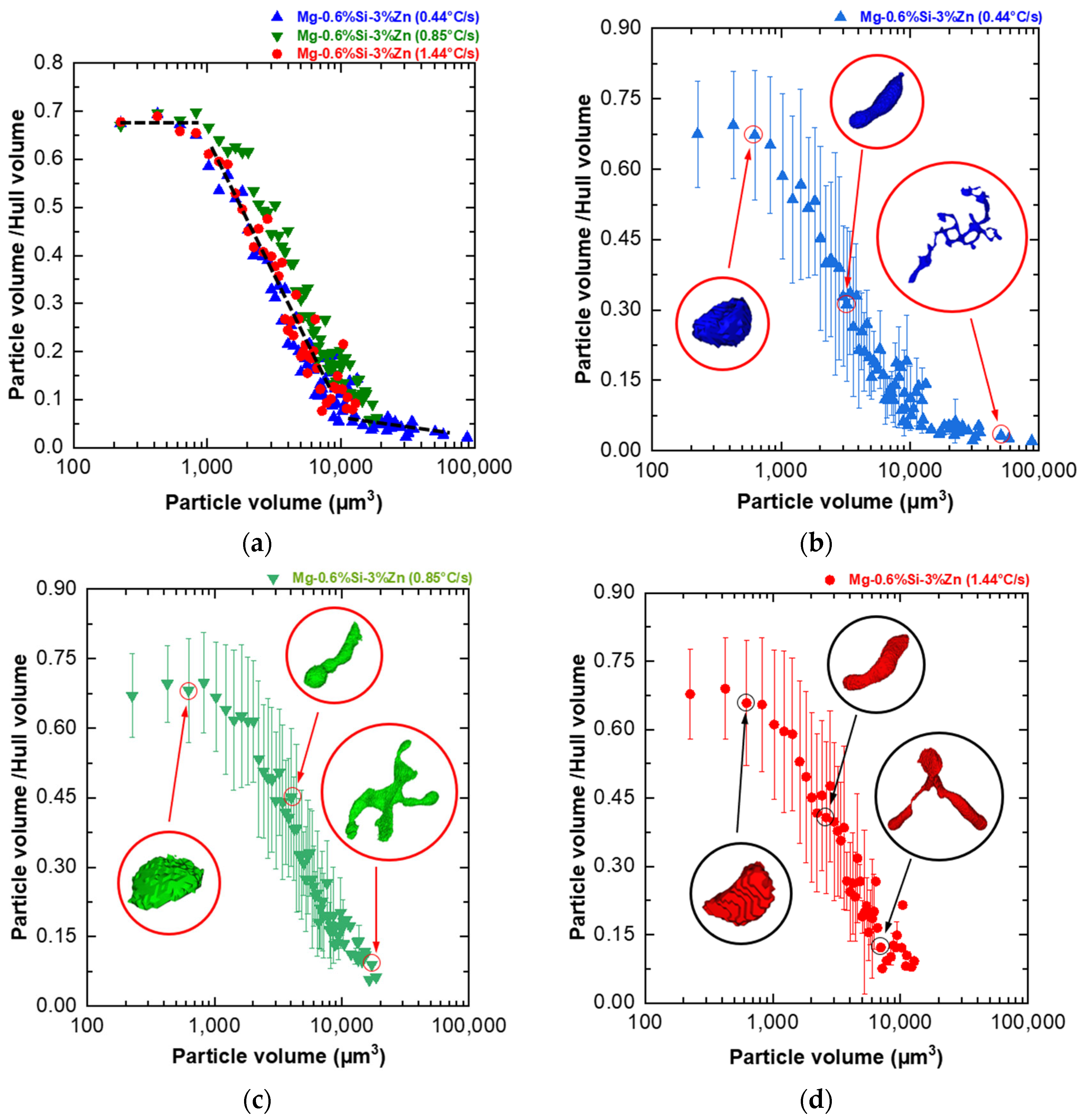
- The results demonstrate a tendency for the formation of a greater number of large particles with complex interconnected morphologies as the cooling rate decreased and Zn content increased. Cooling rate and Zn content very slightly influence the particle morphology distribution of MgZn eutectic particles within the analyzed parameters;
- (2)
- SEM and EDS analysis also revealed that these MgZn particles consist of the eutectic microconstituent Mg-MgZn, rather than solely MgZn as previously reported in the literature. This discovery offers a new perspective on the composition of MgZn particles in these alloys;
- (3)
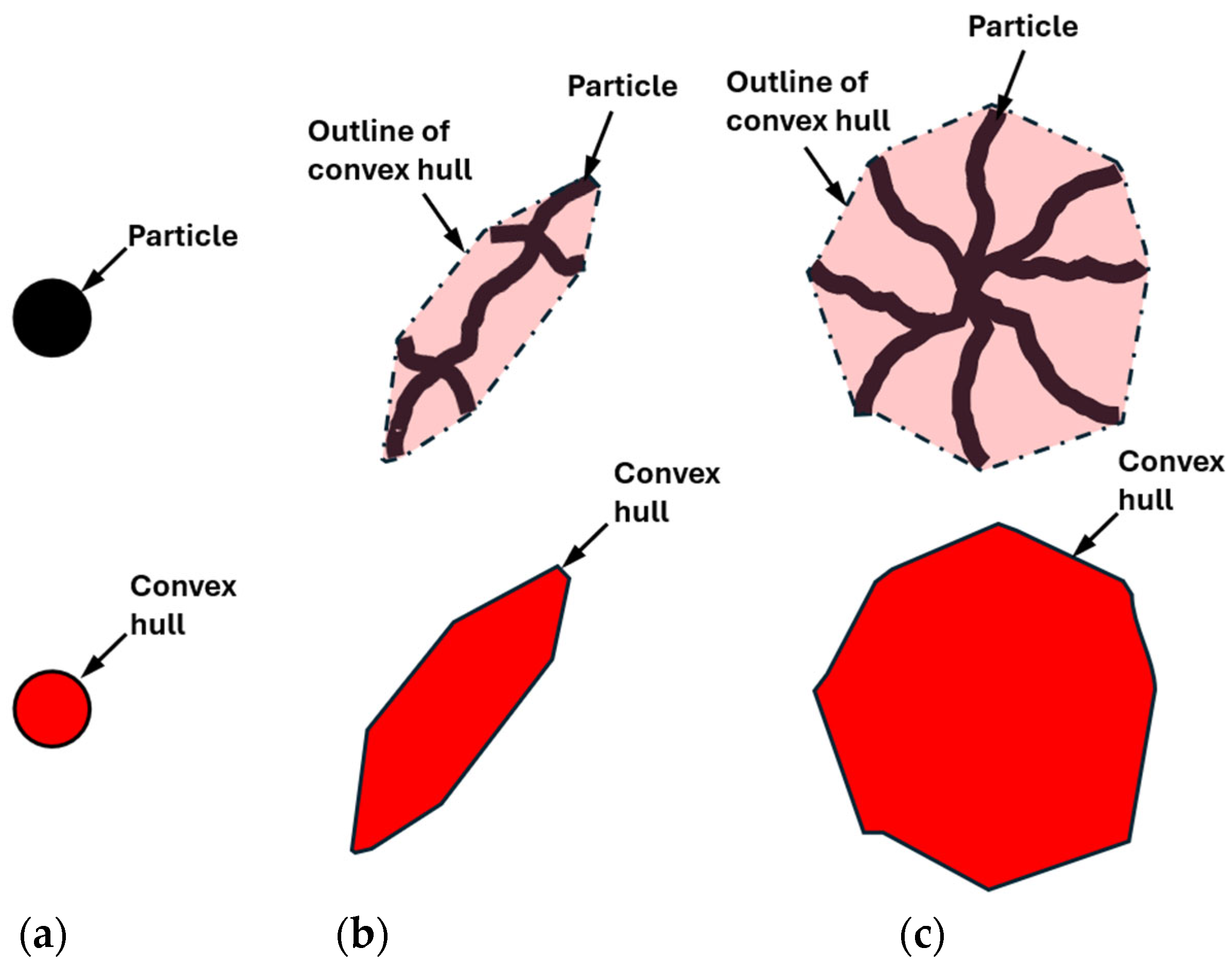
- A novel particle-to-convex hull ratio (p) was used to quantify the morphology of the different MgZn eutectic particles captured. Smaller particles were found to have simpler morphologies (high p value), while larger particles were found to have more complex morphologies (low p values). Furthermore, it was found that the drop in the p value for the alloys studied in this paper always occurred in the 1000–10,000 µm3 volume range;
- (4)
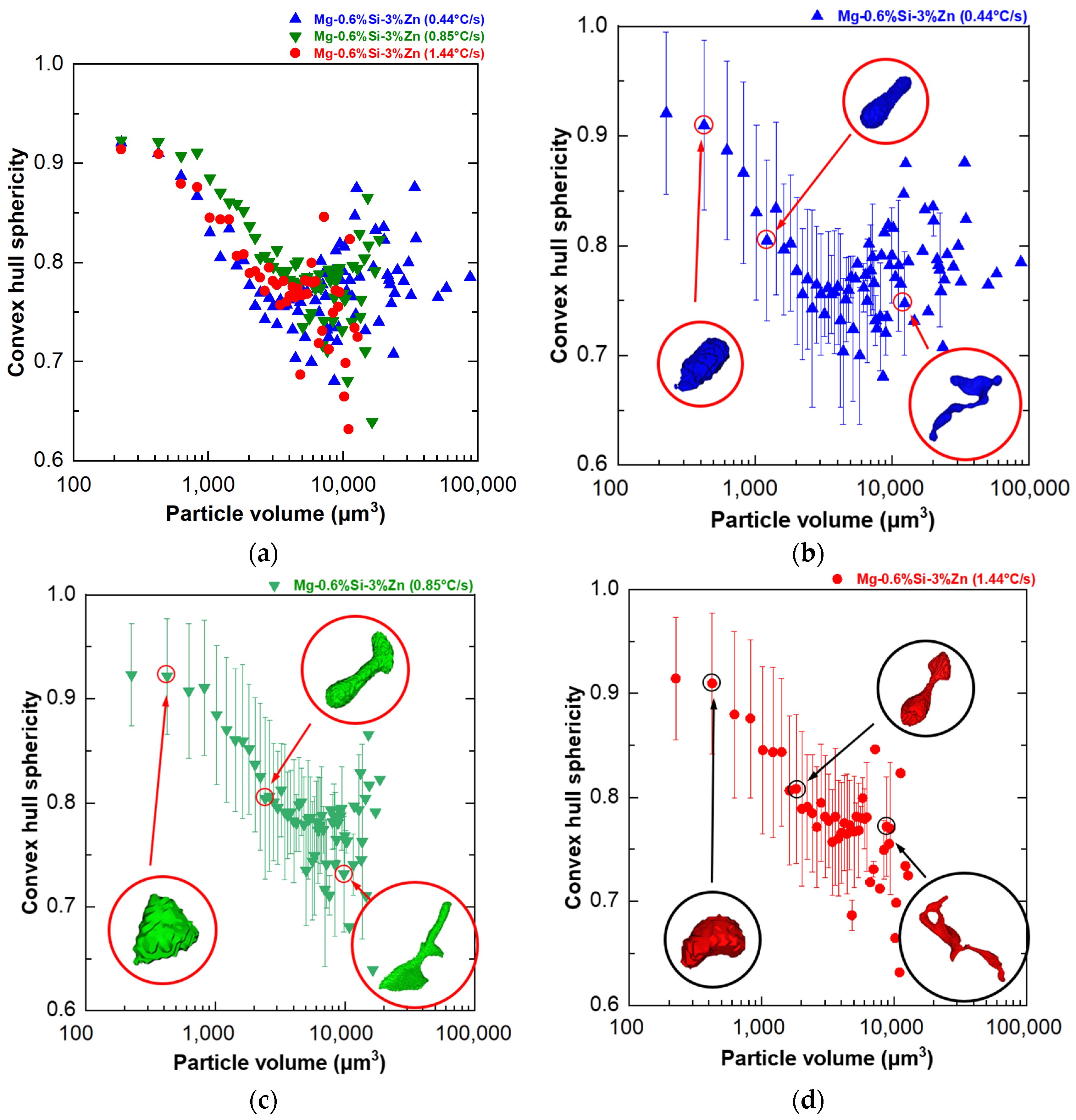
- In addition to p, the sphericity of the convex hull(s) of the particles was also quantified to understand the overall shape anisotropy of the network of MgZn particles in the microstructure. A trend of decrease in the sphericity of the convex hull of particles(s) was observed in the 1000–10,000 µm3 particle volume range with an increase (decrease in anisotropy) observed for particles larger than 10,000 µm3;
- (5)
- The branching behavior of MgZn particles and their spatial distribution during the early stages of particle formation demonstrated that increasing the Zn content and maintaining low cooling rates facilitate branching. This branching behavior initiates the interconnected network observed in the literature through particle interconnection, underscoring the critical role of particle morphology in network formation.
- (6)
- Similar trends for p and s versus particle size were observed in particle morphology at different cooling rates and could be attributed to the similarity of dendritic structures within the cooling rate range of 0.44 °C/s to 1.44 °C/s, as indicated by the minimal variation in dendritic spacing.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018, 7, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Merson, D.; Brilevsky, A.; Myagkikh, P.; Tarkova, A.; Prokhorikhin, A.; Kretov, E.; Frolova, T.; Vinogradov, A. The Functional Properties of Mg–Zn–X Biodegradable Magnesium Alloys. Materials 2020, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.C.; Esdaille, C.J.; Ogueri, K.S.; Kan, H.-M.; Laurencin, S.J.; Nair, L.S.; Laurencin, C.T. The Mechanism of Metallosis After Total Hip Arthroplasty. Regen. Eng. Transl. Med. 2021, 7, 247–261. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant. Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Riaz, U.; Shabib, I.; Haider, W. The current trends of Mg alloys in biomedical applications-A review. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1970–1996. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Oryan, A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int. Wound J. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Burlingame, B. Editorial: What is a nutrient? J. Food Compos. Anal. 2001, 14, 1. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Vormann, J. Magnesium: Nutrition and metabolism. Mol. Asp. Med. 2003, 24, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Mabuchi, M.; Kubota, K.; Higashi, K. Tensile strength, ductility and fracture of magnesium-silicon alloys. J. Mater. Sci. 1996, 31, 1529–1535. [Google Scholar] [CrossRef]
- Hu, X.S.; Wu, K.; Zheng, M.Y.; Gan, W.M.; Wang, X.J. Low frequency damping capacities and mechanical properties of Mg–Si alloys. Mater. Sci. Eng. A 2007, 452–453, 374–379. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon. Nutr. Rev. 1975, 33, 257–261. [Google Scholar] [CrossRef]
- Henstock, J.R.; Canham, L.T.; Anderson, S.I. Silicon: The evolution of its use in biomaterials. Acta Biomater. 2015, 11, 17–26. [Google Scholar] [CrossRef]
- Carlisle, E.M. The nutritional essentiality of silicon. Nutr. Rev. 1982, 40, 193–198. [Google Scholar] [CrossRef]
- Nielsen, F.H. Update on the possible nutritional importance of silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef]
- Martin, K.R. Silicon: The health benefits of a metalloid. Met. Ions Life Sci. 2013, 13, 451–473. [Google Scholar] [CrossRef]
- Wang, W.; Gao, M.; Huang, Y.; Tan, L.; Yang, K.; Hort, N. Microstructures, Corrosion and Mechanical Properties of Mg–Si Alloys as Biodegradable Implant Materials; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kerstetter, J.E. Nutrition in bone health revisited: A story beyond calcium. J. Am. Coll. Nutr. 2000, 19, 715–737. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wei, X.; Yang, L.; Xu, J.; Song, C. Effect of Zn on the microstructure and mechanical properties of Mg–Si alloy. Mater. Sci. Eng. A 2010, 527, 3195–3199. [Google Scholar] [CrossRef]
- Zhang, B.P.; Wang, Y.; Geng, L. Research on Mg-Zn-Ca Alloy as Degradable Biomaterials—Physics and Chemistry; InTech: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Zhang, E.; Yang, L.; Xu, J.; Chen, H. Microstructure, mechanical properties and bio-corrosion properties of Mg-Si(-Ca, Zn) alloy for biomedical application. Acta Biomater. 2010, 6, 1756–1762. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Azghandi, S.H.M.; Weiss, M.; Arhatari, B.D.; Adrien, J.; Maire, E.; Barnett, M.R. A rationale for the influence of grain size on failure of magnesium alloy AZ31: An in situ X-ray microtomography study. Acta Mater. 2020, 200, 619–631. [Google Scholar] [CrossRef]
- Wang, Y.; Shuai, S.; Huang, C.; Jing, T.; Chen, C.; Hu, T.; Wang, J.; Ren, Z. Revealing the Diversity of Dendritic Morphology Evolution during Solidification of Magnesium Alloys using Synchrotron X-ray Imaging: A Review. Acta Metall. Sin. Engl. Lett. 2021, 35, 177–200. [Google Scholar] [CrossRef]
- Singh, S.S.; Schwartzstein, C.; Williams, J.J.; Xiao, X.; De Carlo, F.; Chawla, N. 3D microstructural characterization and mechanical properties of constituent particles in Al 7075 alloys using X-ray synchrotron tomography and nanoindentation. J. Alloys Compd. 2014, 602, 163–174. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, W.; Koe, B.; Connolley, T.; Irvine, S.; Allan, P.K.; Schlepütz, C.M.; Zhang, W.; Wang, F.; Eskin, D.G.; et al. 3D characterisation of the Fe-rich intermetallic phases in recycled Al alloys by synchrotron X-ray microtomography and skeletonisation. Scr. Mater. 2018, 146, 321–326. [Google Scholar] [CrossRef]
- Sarvesha, R.; Alam, W.; Gokhale, A.; Guruprasad, T.S.; Bhagavath, S.; Karagadde, S.; Jain, J.; Singh, S.S. Quantitative assessment of second phase particles characteristics and its role on the deformation response of a Mg-8Al-0.5Zn alloy. Mater. Sci. Eng. A 2019, 759, 368–379. [Google Scholar] [CrossRef]
- Wang, M.Y.; Williams, J.J.; Jiang, L.; De Carlo, F.; Jing, T.; Chawla, N. Three Dimensional (3D) Microstructural Characterization and Quantitative Analysis of Solidified Microstructures in Magnesium-Based Alloys. Metallogr. Microstruct. Anal. 2012, 1, 7–13. [Google Scholar] [CrossRef]
- Bandi, B.; Santillana, B.; Tiekink, W.; Koura, N.; Williams, M.; Srirangam, P. 2D automated SEM and 3D X-ray computed tomography study on inclusion analysis of steels. Ironmak. Steelmak. 2019, 47, 47–50. [Google Scholar] [CrossRef]
- Li, Z.; Limodin, N.; Tandjaoui, A.; Quaegebeur, P.; Balloy, D. Effects of duration of solution heat treatment on the evolution of 3D microstructure in AlSi7Cu3 alloy: A quantitative X-ray tomography study. Mater. Charact. 2021, 173, 110919. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Song, J.L.; Hu, W.X.; Li, J.C.; Xiong, S.M. Morphology transition of the primary silicon particles in a hypereutectic A390 alloy in high pressure die casting. Sci. Rep. 2017, 7, 14994. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Z.; Guo, Y.; Tao, D.; Li, J.; Wang, S. 3D morphology characterization of graphite and its effect on the thermal conductivity of vermicular graphite iron. Int. J. Mater. Res. 2019, 110, 591–599. [Google Scholar] [CrossRef]
- Díaz, F.V.; Peralta, M.E.; Fernandino, D.O. Study of Sphericity and Compactness Parameters in Spheroidal Graphite Iron Using X-ray Micro-computed Tomography and Image Processing. J. Nondestruct. Eval. 2021, 40, 11. [Google Scholar] [CrossRef]
- Subroto, T.; Mendis, C.; D’Elia, F.; Szakács, G.; Fife, J.; Hort, N.; Kainer, K.; Tolnai, D. 3D microstructural evolution on solidifying Mg–5Nd–5Zn alloy observed via in situ synchrotron tomography. Miner. Met. Mater. 2017, F8, 605–612. [Google Scholar] [CrossRef]
- de Gouveia, G.L.; Garcia, A.; Spinelli, J.E. Towards a morphological control of Mg2Si and superior tensile properties of high-Zn Mg-0.6Si (-Zn) alloys. Mater. Lett. 2021, 299, 130084. [Google Scholar] [CrossRef]
- Patnaik, S.; Ganju, E.; Yu, X.; Kang, M.; Park, J.; Kang, D.; Kamat, R.; Carsley, J.; Chawla, N. Advancing Sustainable Aluminum Alloy Development via Comprehensive 3D Morphological and Compositional Characterization of Fe-Rich Intermetallic Particles. Metall. Mater. Trans. A, 2024; in review. [Google Scholar]
- Ganju, E.; Kilic, M.; Prezzi, M.; Salgado, R.; Parab, N.; Chen, W.N. Effect of particle characteristics on the evolution of particle size, particle morphology, and fabric of sands loaded under uniaxial compression. Acta Geotech. 2021, 16, 3489–3516. [Google Scholar] [CrossRef]
- Stefanescu, D.M. Science and Engineering of Casting Solidification; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 3rd ed.; Trans Tech Publications Ltd.: Bäch, Switzerland, 1992. [Google Scholar]
- Kapinos, D.; Augustyn, B.; Szymanek, M. Methods of Introducing Alloying Elements into Liquid Magnesium. Metall. Foundry Eng. 2014, 40, 141. [Google Scholar] [CrossRef]
- Beucher, S.; Lantueoul, C. Use of watershed in contour detection. In Proceedings of the International Workshop of Image Detection: Real Time Edge and Motion Detection, Rennes, France, 17–21 September 1979. [Google Scholar]
- Chen, S.; Tu, J.; Hu, Q.; Xiong, X.; Wu, J.; Zou, J.; Zeng, X. Corrosion resistance and in vitro bioactivity of Si-containing coating prepared on a biodegradable Mg-Zn-Ca bulk metallic glass by micro-arc oxidation. J. Non-Cryst. Solids 2017, 456, 125–131. [Google Scholar] [CrossRef]
- Ben-Hamu, G.; Eliezer, D.; Shin, K.S. The role of Mg2Si on the corrosion behavior of wrought Mg–Zn–Mn alloy. Intermetallics 2008, 16, 860–867. [Google Scholar] [CrossRef]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H. The quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef]
- Wadell, H. Volume, Shape, and Roundness of Quartz Particles. J. Geol. 1935, 43, 250–280. [Google Scholar] [CrossRef]



| Sample ID | Si (wt.%) | Zn (wt.%) | Cooling Rate (°C/s) |
|---|---|---|---|
| Mg-0.6Si-2Zn-0.48 | 0.6 | 2 | 0.48 |
| Mg-0.6Si-3Zn-0.44 | 0.6 | 3 | 0.44 |
| Mg-0.6Si-3Zn-0.85 | 0.6 | 3 | 0.85 |
| Mg-0.6Si-3Zn-1.44 | 0.6 | 3 | 1.44 |
| Alloy | Mg | Si | Zn | Al | Ca | Fe | Cu |
|---|---|---|---|---|---|---|---|
| Mg-0.6Si-2Zn | 97.413 | 0.580 | 1.953 | 0.041 | 0.001 | 0.007 | 0.002 |
| Mg-0.6Si-3Zn | 96.304 | 0.654 | 2.976 | 0.053 | 0.001 | 0.008 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, G.L.d.; Ganju, E.; Moura, D.; Morankar, S.K.; Spinelli, J.E.; Chawla, N. 3D X-ray Tomography Analysis of Mg–Si–Zn Alloys for Biomedical Applications: Elucidating the Morphology of the MgZn Phase. Appl. Sci. 2024, 14, 8081. https://doi.org/10.3390/app14178081
Gouveia GLd, Ganju E, Moura D, Morankar SK, Spinelli JE, Chawla N. 3D X-ray Tomography Analysis of Mg–Si–Zn Alloys for Biomedical Applications: Elucidating the Morphology of the MgZn Phase. Applied Sciences. 2024; 14(17):8081. https://doi.org/10.3390/app14178081
Chicago/Turabian StyleGouveia, Guilherme Lisboa de, Eshan Ganju, Danusa Moura, Swapnil K. Morankar, José Eduardo Spinelli, and Nikhilesh Chawla. 2024. "3D X-ray Tomography Analysis of Mg–Si–Zn Alloys for Biomedical Applications: Elucidating the Morphology of the MgZn Phase" Applied Sciences 14, no. 17: 8081. https://doi.org/10.3390/app14178081







