Innovative Approaches for Minimizing Disinfection Byproducts (DBPs) in Water Treatment: Challenges and Trends
Abstract
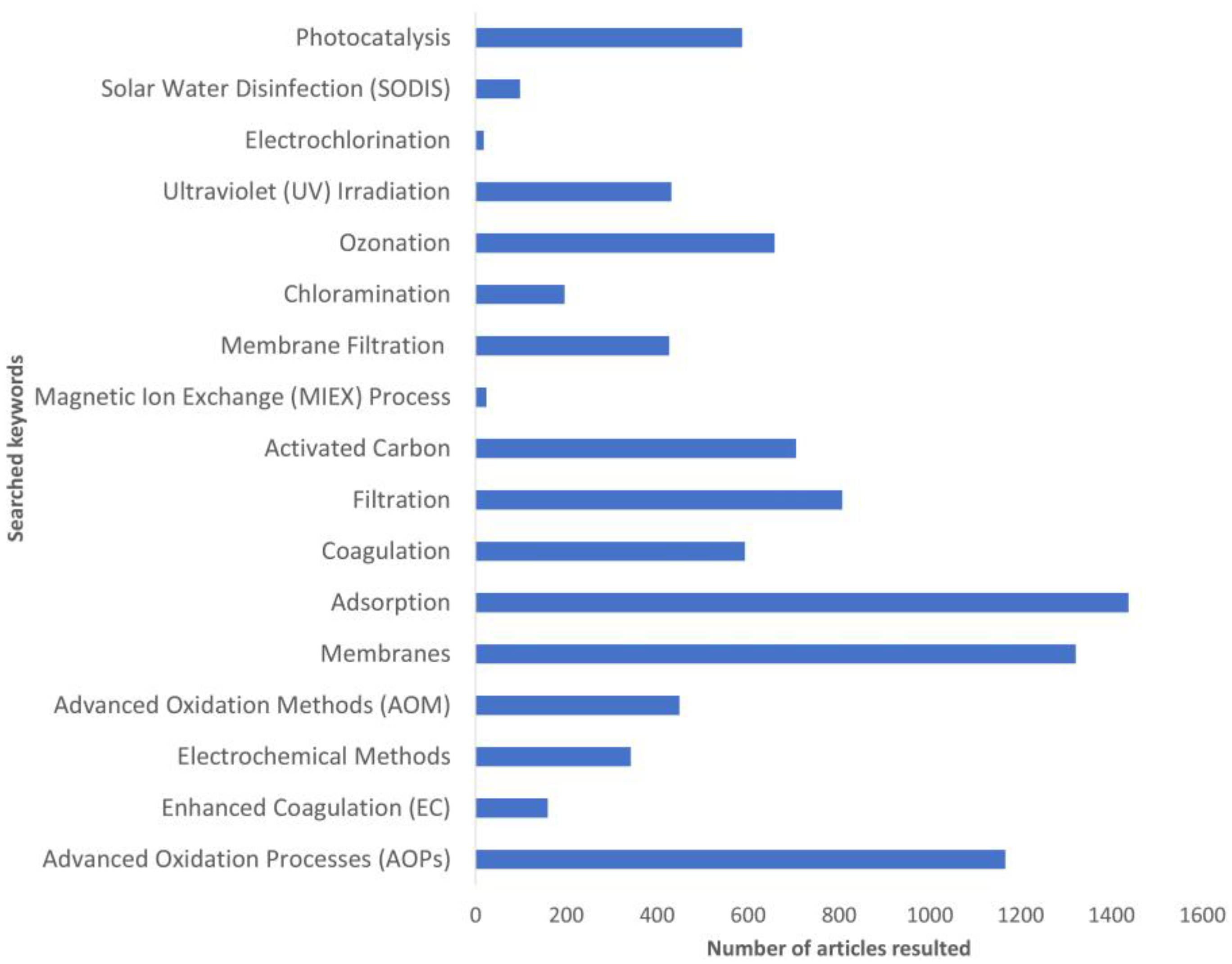
:1. Introduction
2. Methodology
3. DBPs
3.1. DBPs Categories
3.2. Health Effects of DBPs
3.3. DBPs Precursors—Natural Organic Matter (NOM)
- Coagulation and flocculation: This help to agglomerate small particles into larger, more easily settleable flocs.
- Sedimentation: The flocculated particles settle from the water during this sedimentation stage (Figure 2).
- Bed filtration: The water then undergoes filtration through sand (Figure 3), gravel, and other materials, effectively removing suspended solids and turbidity.
- Disinfection: Finally, the water is disinfected, commonly by using chlorine, chloramines, ozone, or chlorine dioxide, before being distributed through the supply network.
4. DBP Minimization Approaches
4.1. DBP Precursors Removal
4.2. Water Treatment Methods Modifications
- Eliminating prechlorination or relocating the chlorination point: by avoiding chlorination at the initial stages of water treatment or changing the location where chlorine is introduced, the formation of THMs and HAAs can be reduced.
- Implementing enhanced coagulation practices: enhanced coagulation techniques help to remove organic precursors before chlorination, thereby reducing the formation of DBPs.
- Optimizing chlorine dosing using disinfection benchmarking: water treatment plants can ensure effective disinfection while minimizing DBP formation by closely monitoring chlorine dosage and comparing it with established benchmarks.
- Transitioning to chloramines for secondary disinfection: chloramines, compounds formed by combining chlorine with ammonia, are less reactive than free chlorine and can help decrease the formation of THMs and HAAs.
- Exploring alternative DBP minimization strategies: this includes investigating novel treatment methods or combinations of treatments aimed at reducing the formation of DBPs during water chlorination.
4.2.1. Eliminating Prechlorination
4.2.2. Switching to Chloramines for Secondary Disinfection
4.3. Coagulation
4.4. Filtration
4.5. Advanced Water Treatment Technologies
- Enhanced coagulation;
- Activated carbon;
- Membrane filtration;
- Magnetic ion exchange (MIEX) process.
4.5.1. Enhanced Coagulation
4.5.2. Activated Carbon
4.5.3. Membrane Filtration
4.5.4. Membrane Nanofiltration
4.5.5. Magnetic Ion Exchange (MIEX) Process
4.6. Advanced Oxidation Processes (AOPs)
4.7. Alternative to Chlorine Disinfectants
4.7.1. Chloramination
4.7.2. Ozonation
4.7.3. Ultraviolet (UV) Irradiation
4.7.4. Electrochlorination
4.7.5. Solar Water Disinfection (SODIS)
4.7.6. Photocatalysis
5. Machine Learning Approaches
6. Recent Research Trends
7. Conclusions and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, T.; Karimi-Maleh, H.; Dragoi, E.N.; Puri, P.; Zhang, D.; Zhang, Z. Traditional Methods and Biosensors for Detecting Disinfection By-Products in Water: A Review. Environ. Res. 2023, 237, 116935. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-B.; Lin, Y.-L.; Xu, B.; Huang, H.; Zhang, T.-Y.; Tian, F.-X.; Gao, N.-Y. Degradation of Chlortoluron during UV Irradiation and UV/Chlorine Processes and Formation of Disinfection by-Products in Sequential Chlorination. Chem. Eng. J. 2016, 283, 412–419. [Google Scholar] [CrossRef]
- Han, J.; Zhai, H.; Zhang, X.; Liu, J.; Sharma, V.K. Effects of Ozone Dose on Brominated DBPs in Subsequent Chlor(Am)Ination: A Comprehensive Study of Aliphatic, Alicyclic and Aromatic DBPs. Water Res. 2024, 250, 121039. [Google Scholar] [CrossRef]
- Tian, F.-X.; Xu, B.; Lin, Y.-L.; Hu, C.-Y.; Zhang, T.-Y.; Xia, S.-J.; Chu, W.-H.; Gao, N.-Y. Chlor(Am)Ination of Iopamidol: Kinetics, Pathways and Disinfection by-Products Formation. Chemosphere 2017, 184, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Golfinopoulos, S.; Nikolaou, A. The Screening of Disinfection By-Products in Large and Small Water Systems in Greece. Desalination Water Treat. 2017, 58, 80–85. [Google Scholar] [CrossRef]
- Mazhar, M.A.; Khan, N.A.; Ahmed, S.; Khan, A.H.; Hussain, A.; Rahisuddin; Changani, F.; Yousefi, M.; Ahmadi, S.; Vambol, V. Chlorination Disinfection By-Products in Municipal Drinking Water—A Review. J. Clean. Prod. 2020, 273, 123159. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, W.; Yao, D.; Yin, D. Control of Aliphatic Halogenated DBP Precursors with Multiple Drinking Water Treatment Processes: Formation Potential and Integrated Toxicity. J. Environ. Sci. 2017, 58, 322–330. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Sun, Z.; Dong, H.; Yu, J.; Qiang, Z. Removal of Disinfection By-Product Precursors in Drinking Water Treatment Processes: Is Fluorescence Parallel Factor Analysis a Promising Indicator? J. Hazard. Mater. 2021, 418, 126298. [Google Scholar] [CrossRef]
- Lei, X.; Xie, Z.; Sun, Y.; Qiu, J.; Yang, X. Recent Progress in Identification of Water Disinfection Byproducts and Opportunities for Future Research. Environ. Pollut. 2023, 337, 122601. [Google Scholar] [CrossRef]
- Tafvizi, H.; Chowdhury, S.; Husain, T. Low Cost Activated Carbon for Removal of NOM and DBPs: Optimization and Comparison. Water 2021, 13, 2244. [Google Scholar] [CrossRef]
- Badawy, M.I.; Gad-Allah, T.A.; Ali, M.E.M.; Yoon, Y. Minimization of the Formation of Disinfection By-Products. Chemosphere 2012, 89, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Liu, M.; Yu, H.; Zou, D. Pressure–Driven Membrane Filtration Technology for Terminal Control of Organic DBPs: A Review. Sci. Total Environ. 2023, 904, 166751. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wei, D.-B.; Wei, J.; Hu, H.-Y. Screening and Estimating of Toxicity Formation with Photobacterium Bioassay during Chlorine Disinfection of Wastewater. J. Hazard. Mater. 2007, 141, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, H.; Wang, Y.; Qiang, Z.; Yang, M. Disinfection By-Product (DBP) Research in China: Are We on the Track? J. Environ. Sci. 2021, 110, 99–110. [Google Scholar] [CrossRef]
- Gui, X.; Zhang, H.; Ji, B.; Ma, J.; Xu, M.; Li, Y.; Yan, M. Study on the Control of Dichloroacetonitrile Generation by Two-Point Influent Activated Carbon-Quartz Sand Biofilter. Membranes 2022, 12, 137. [Google Scholar] [CrossRef]
- Richardson, S.; Ternes, T.; Van, D. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef]
- EC (European Community) Council. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance); European Community: Maastricht, The Netherlands, 2020; pp. 1–62. [Google Scholar]
- DeMarini, D.M. A Review on the 40th Anniversary of the First Regulation of Drinking Water Disinfection By-Products. Environ. Mol. Mutagen. 2020, 61, 588–601. [Google Scholar] [CrossRef]
- Nawrocki, J.; Andrzejewski, P. Nitrosamines and Water. J. Hazard. Mater. 2011, 189, 1–18. [Google Scholar] [CrossRef]
- Riyadh, A.; Peleato, N.M. Natural Organic Matter Character in Drinking Water Distribution Systems: A Review of Impacts on Water Quality and Characterization Techniques. Water 2024, 16, 446. [Google Scholar] [CrossRef]
- Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment. Water 2021, 13, 3073. [Google Scholar] [CrossRef]
- Mayer, B.K.; Daugherty, E.; Abbaszadegan, M. Disinfection Byproduct Formation Resulting from Settled, Filtered, and Finished Water Treated by Titanium Dioxide Photocatalysis. Chemosphere 2014, 117, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pulicharla, R.; Proulx, F.; Behmel, S.; Sérodes, J.-B.; Rodriguez, M.J. Trends in Ozonation Disinfection By-Products—Occurrence, Analysis and Toxicity of Carboxylic Acids. Water 2020, 12, 756. [Google Scholar] [CrossRef]
- Karwowska, B.; Sperczyńska, E. Organic Matter and Heavy Metal Ions Removal from Surface Water in Processes of Oxidation with Ozone, UV Irradiation, Coagulation and Adsorption. Water 2022, 14, 3763. [Google Scholar] [CrossRef]
- Poleneni, S.R.; Inniss, E.; Shi, H.; Yang, J.; Hua, B.; Clamp, J. Enhanced Flocculation Using Drinking Water Treatment Plant Sedimentation Residual Solids. Water 2019, 11, 1821. [Google Scholar] [CrossRef]
- Gerrity, D.; Mayer, B.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. A Comparison of Pilot-Scale Photocatalysis and Enhanced Coagulation for Disinfection Byproduct Mitigation. Water Res. 2009, 43, 1597–1610. [Google Scholar] [CrossRef]
- Sriboonnak, S.; Induvesa, P.; Wattanachira, S.; Rakruam, P.; Siyasukh, A.; Pumas, C.; Wongrueng, A.; Khan, E. Trihalomethanes in Water Supply System and Water Distribution Networks. Int. J. Environ. Res. Public Health 2021, 18, 9066. [Google Scholar] [CrossRef]
- Jiang, J.; Han, J.; Zhang, X. Nonhalogenated Aromatic DBPs in Drinking Water Chlorination: A Gap between NOM and Halogenated Aromatic DBPs. Environ. Sci. Technol. 2020, 54, 1646–1656. [Google Scholar] [CrossRef]
- Latif, S.; Alim, M.A.; Rahman, A.; Haque, M.M. A Review on Chlorination of Harvested Rainwater. Water 2023, 15, 2816. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Martínez-Menchón, M.; Vela, N.; Navarro, S. Removal Assessment of Disinfection By-Products (DBPs) from Drinking Water Supplies by Solar Heterogeneous Photocatalysis: A Case Study of Trihalomethanes (THMs). J. Environ. Manag. 2022, 321, 115936. [Google Scholar] [CrossRef]
- Suquet, J.; Godo-Pla, L.; Valentí, M.; Verdaguer, M.; Martin, M.J.; Poch, M.; Monclús, H. Development of an Environmental Decision Support System for Enhanced Coagulation in Drinking Water Production. Water 2020, 12, 2115. [Google Scholar] [CrossRef]
- Siddique, M.S.; Lu, H.; Xiong, X.; Fareed, H.; Graham, N.; Yu, W. Exploring Impacts of Water-Extractable Organic Matter on Pre-Ozonation Followed by Nanofiltration Process: Insights from pH Variations on DBPs Formation. Sci. Total Environ. 2023, 876, 162695. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Sanchez-Huerta, C.; Ali, Z.; Wang, Y.; Fortunato, L.; Pinnau, I. Evaluation of Nanofiltration and Reverse Osmosis Membranes for Efficient Rejection of Organic Micropollutants. J. Membr. Sci. 2024, 693, 122357. [Google Scholar] [CrossRef]
- Ranthom, N.; Khongnakorn, W.; Jutaporn, P. MIEX Resin and Enhanced Coagulation Treatment of High-Bromide Natural Water: Chlorine Reactivity and DBP Precursors Removal. J. Environ. Chem. Eng. 2023, 11, 111497. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kümmerer, K. Transformation Products of Pharmaceuticals in Surface Waters and Wastewater Formed during Photolysis and Advanced Oxidation Processes—Degradation, Elucidation of Byproducts and Assessment of Their Biological Potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef]
- Lofrano, G.; Ubaldi, F.; Albarano, L.; Carotenuto, M.; Vaiano, V.; Valeriani, F.; Libralato, G.; Gianfranceschi, G.; Fratoddi, I.; Meric, S.; et al. Antimicrobial Effectiveness of Innovative Photocatalysts: A Review. Nanomaterials 2022, 12, 2831. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Chung Lan Mow, M.C.; Liao, X.; Wei, X.; Ma, G.; Wang, X.; Yu, H. Chloramine Disinfection of Levofloxacin and Sulfaphenazole: Unraveling Novel Disinfection Byproducts and Elucidating Formation Mechanisms for an Enhanced Understanding of Water Treatment. Molecules 2024, 29, 396. [Google Scholar] [CrossRef]
- Wünsch, R.; Hettich, T.; Prahtel, M.; Thomann, M.; Wintgens, T.; Gunten, U. von Tradeoff between Micropollutant Abatement and Bromate Formation during Ozonation of Concentrates from Nanofiltration and Reverse Osmosis Processes. Water Res. 2022, 221, 118785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hao, C.; Zhang, M.; Lan, B. Enhanced Adsorption of Bromoform onto Microplastic Polyethylene Terephthalate Exposed to Ozonation and Chlorination. Molecules 2023, 28, 259. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ying, Z.; Ma, M.; Huo, M.; Yang, W. Degradation of Micropollutants by UV–Chlorine Treatment in Reclaimed Water: pH Effects, Formation of Disinfectant Byproducts, and Toxicity Assay. Water 2019, 11, 2639. [Google Scholar] [CrossRef]
- Atrashkevich, A.; Alum, A.; Stirling, R.; Abbaszadegan, M.; Garcia-Segura, S. Approaching Easy Water Disinfection for All: Can in Situ Electrochlorination Outperform Conventional Chlorination under Realistic Conditions? Water Res. 2024, 250, 121014. [Google Scholar] [CrossRef]
- Ballesteros, M.; Brindley, C.; Sánchez-Pérez, J.A.; Fernández-Ibañez, P. Worldwide Research Trends on Solar-Driven Water Disinfection. Int. J. Environ. Res. Public Health 2021, 18, 9396. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Zhu, Y.; Gao, B.; Li, Q. Progress on Mechanism and Efficacy of Heterogeneous Photocatalysis Coupled Oxidant Activation as an Advanced Oxidation Process for Water Decontamination. Water Res. 2024, 251, 121119. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Ghosal, P.S. A Comprehensive Appraisal on Status and Management of Remediation of DBPs by TiO2 Based-Photocatalysts: Insights of Technology, Performance and Energy Efficiency. J. Environ. Manag. 2023, 328, 117011. [Google Scholar] [CrossRef] [PubMed]
- Asteris, P.G.; Alexakis, D.E.; Tsoukalas, M.Z.; Gamvroula, D.E.; Guney, D. Machine Learning Approach for Rapid Estimation of Five-Day Biochemical Oxygen Demand in Wastewater. Water 2023, 15, 103. [Google Scholar] [CrossRef]
- Pérez-Beltrán, C.H.; Pérez-Caballero, G.; Andrade, J.; Cuadros-Rodríguez, L.; Jiménez-Carvelo, A. Non-Targeted Spatially Offset Raman Spectroscopy-Based Vanguard Analytical Method to Authenticate Spirits: White Tequilas as a Case Study. Microchem. J. 2022, 183, 108126. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Wu, J.; Li, Y.; Han, J.; Song, Y.; Cao, W.; Zhou, X.-T. Developing and Validating a Clinlabomics-Based Machine-Learning Model for Early Detection of Retinal Detachment in Patients with High Myopia. J. Transl. Med. 2024, 22, 405. [Google Scholar] [CrossRef]
- Othman, S.; Mavani, N.R.; Hussain, M.A.; Rahman, N.A.; Ali, J.M. Artificial Intelligence-Based Techniques for Adulteration and Defect Detections in Food and Agricultural Industry: A Review. J. Agric. Food Res. 2023, 12, 100590. [Google Scholar] [CrossRef]
- Xu, Z.; Pan, Z.; Wang, Y.; Zhang, Y.; Leng, P. Research on a Machine Learning-Based Adaptive and Efficient Screening Model for Psychological Symptoms of Community Correctional Prisoners. Sci. Rep. 2024, 14, 9890. [Google Scholar] [CrossRef]
- Xia, L.; Han, Q.; Shang, L.; Wang, Y.; Li, X.; Zhang, J.; Yang, T.; Liu, J.; Liu, L. Quality Assessment and Prediction of Municipal Drinking Water Using Water Quality Index and Artificial Neural Network: A Case Study of Wuhan, Central China, from 2013 to 2019. Sci. Total Environ. 2022, 844, 157096. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mclellan, P.J. Models for Predicting Disinfection Byproduct (DBP) Formation in Drinking Waters: A Chronological Review. Sci. Total Environ. 2009, 407, 4189–4206. [Google Scholar] [CrossRef]
- Shahi, N.K.; Maeng, M.; Dockko, S. Models for Predicting Carbonaceous Disinfection By-Products Formation in Drinking Water Treatment Plants: A Case Study of South Korea. Environ. Sci. Pollut. Res. 2020, 27, 24594–24603. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, Z.; Guo, A.; Shen, L.; Sun, H.; Liang, Y.; Wu, F.; Lin, H. Radial Basis Function Artificial Neural Network (RBF ANN) as Well as the Hybrid Method of RBF ANN and Grey Relational Analysis Able to Well Predict Trihalomethanes Levels in Tap Water. J. Hydrol. 2020, 591, 125574. [Google Scholar] [CrossRef]
- Hu, G.; Mian, H.R.; Mohammadiun, S.; Rodriguez, M.J.; Hewage, K.; Sadiq, R. Appraisal of Machine Learning Techniques for Predicting Emerging Disinfection Byproducts in Small Water Distribution Networks. J. Hazard. Mater. 2023, 446, 130633. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, B.; Xu, X.; Huo, R.; Zhou, T.; Dong, X.; Ye, C.; Li, T.; Xie, L.; Pang, W. The Insightful Water Quality Analysis and Predictive Model Establishment via Machine Learning in Dual-Source Drinking Water Distribution System. Environ. Res. 2024, 250, 118474. [Google Scholar] [CrossRef]
- Peng, F.; Lu, Y.; Wang, Y.; Yang, L.; Yang, Z.; Li, H. Predicting the Formation of Disinfection By-Products Using Multiple Linear and Machine Learning Regression. J. Environ. Chem. Eng. 2023, 11, 110612. [Google Scholar] [CrossRef]
- Godo-Pla, L.; Emiliano, P.; Poch, M.; Valero, F.; Monclús, H. Benchmarking Empirical Models for THMs Formation in Drinking Water Systems: An Application for Decision Support in Barcelona, Spain. Sci. Total Environ. 2021, 763, 144197. [Google Scholar] [CrossRef]
- Kulkarni, P.; Chellam, S. Disinfection By-Product Formation Following Chlorination of Drinking Water: Artificial Neural Network Models and Changes in Speciation with Treatment. Sci. Total Environ. 2010, 408, 4202–4210. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Ansheng, Z.; Hong, H.; Liang, Y.; Sun, H.; Lin, H.; Chen, J. Regression Models Evaluating THMs, HAAs and HANs Formation upon Chloramination of Source Water Collected from Yangtze River Delta Region, China. Ecotoxicol. Environ. Saf. 2018, 160, 249–256. [Google Scholar] [CrossRef]
- Wang, P.; Ding, S.; Xiao, R.; An, G.; Fang, C.; Chu, W. Enhanced Coagulation for Mitigation of Disinfection By-Product Precursors: A Review. Adv. Colloid Interface Sci. 2021, 296, 102518. [Google Scholar] [CrossRef]
- Cassol, G.S.; Shang, C.; Li, J.; Ling, L.; Yang, X.; Yin, R. Dosing Low-Level Ferrous Iron in Coagulation Enhances the Removal of Micropollutants, Chlorite and Chlorate during Advanced Water Treatment. J. Environ. Sci. 2022, 117, 119–128. [Google Scholar] [CrossRef]
- Diaz, M.; Daer, S.; Pype, M.L.; Keller, J.; Doederer, K.; Ledezma, P. Tri-Protonated Ferrate: A Novel Electro-Synthesised Pre-Oxidant/Coagulant for NOM and DBP Precursors Removal in Drinking Water Production. J. Water Process Eng. 2024, 57, 104570. [Google Scholar] [CrossRef]
- Lin, Q.; Dong, F.; Miao, Y.; Li, C.; Fei, W. Removal of Disinfection Byproducts and Their Precursors during Drinking Water Treatment Processes. Water Environ. Res. 2019, 92, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Jiang, J.; Yang, T.; Cao, Y. Oxidative Treatment of NOM by Selective Oxidants in Drinking Water Treatment and Its Impact on DBP Formation in Postchlorination. Sci. Total Environ. 2023, 858, 159908. [Google Scholar] [CrossRef]
- Yin, T.; Wu, Y.; Shi, P.; Li, A.; Xu, B.; Chu, W.; Pan, Y. Anion-Exchange Resin Adsorption Followed by Electrolysis: A New Disinfection Approach to Control Halogenated Disinfection Byproducts in Drinking Water. Water Res. 2019, 168, 115144. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent Advances in Carbon Nanomaterial-Based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Jiang, J.; Pu, J.; Takizawa, S.; Hou, L.; Yang, Y. Insights into Graphene Oxide/Ferrihydrite Adsorption as Pretreatment during Ultrafiltration: Membrane Fouling Mitigation and Disinfection by-Product Control. J. Hazard. Mater. 2022, 436, 129098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Maness, J.; Cuthbertson, A.; Kimura, S.; Liberatore, H.; Richardson, S.; Stanford, B.; Sun, M.; Knappe, D. Treating Water Containing Elevated Bromide and Iodide Levels with Granular Activated Carbon and Free Chlorine: Impacts on Disinfection Byproduct Formation and Calculated Toxicity. Environ. Sci. Water Res. Technol. 2020, 6, 3460–3475. [Google Scholar] [CrossRef]
- Soliman, A.M.; Elshorbagy, W.; Maraqa, M.A.; Al-Issai, L.M.; Sheikh, E.S.E.; Elhaty, I.A.; Ayesh, A.I.; Pál, T. In-Situ, Facile and Green Preparation of Nanoscale Silver Supported on Activated Carbon: Disinfection Properties and Removal of Inorganic DBPs from Drinking Water. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100621. [Google Scholar] [CrossRef]
- Lau, S.; Feng, Y.; Gu, A.; Russell, C.; Pope, G.; Mitch, W. Cytotoxicity Comparison between Drinking Water Treated by Chlorination with Postchloramination versus Granular Activated Carbon (GAC) with Postchlorination. Environ. Sci. Technol. 2023, 57, 13699–13709. [Google Scholar] [CrossRef]
- Siddique, M.S.; Song, Q.; Xiong, X.; Fareed, H.; Graham, N.; Yu, W. Hydrolyzed Polyacrylonitrile UF-Membrane for Surface and TAP Water Treatment: Influence on DBPs Formation and Removal. Chem. Eng. J. 2023, 471, 144314. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Dong, H.; Wang, Q.; Liu, S.; Wu, M.; Yu, J.; Qiang, Z. Removal of Carbonaceous and Nitrogenous Disinfection By-Product Precursors in Biological Activated Carbon Process of Drinking Water: Is Service Life a Pivotal Factor? Chem. Eng. J. 2023, 465, 142875. [Google Scholar] [CrossRef]
- Youngwilai, A.; Khan, E.; Phungsai, P.; Therdkiattikul, N.; Limpiyakorn, T.; Mhuantong, W.; Ratpukdi, T.; Supanchaiyamat, N.; Hunt, A.J.; Ngernyen, Y.; et al. Comparative Investigation of Known and Unknown Disinfection By-Product Precursor Removal and Microbial Community from Biological Biochar and Activated Carbon Filters. Water Res. 2024, 261, 121994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, Y.; Zhang, J.; Peng, X.; Xu, P.; Niu, Y.; Dong, B. Control of Chlorination Disinfection By-Products in Drinking Water by Combined Nanofiltration Process: A Case Study with Trihalomethanes and Haloacetic Acids. Chemosphere 2024, 358, 142121. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lou, Y.; Li, A.; Wei, Y.; Li, H.; Zhou, M.; Li, Y. Effects of Pre-Oxidation by Ozone, Permanganate and Ferrate on Generation and Toxicities of Disinfection Byproducts. Int. J. Environ. Sci. Technol. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Rougé, V.; von Gunten, U.; Allard, S. Efficiency of Pre-Oxidation of Natural Organic Matter for the Mitigation of Disinfection Byproducts: Electron Donating Capacity and UV Absorbance as Surrogate Parameters. Water Res. 2020, 187, 116418. [Google Scholar] [CrossRef]
- Allard, S.; Kristiana, I.; Andringa-Bate, C.; Joll, C.A. Alternative Application of Preformed Monochloramine as a Drinking Water Disinfectant for Redosing in Long Drinking Water Distribution System Servicing Remote Locations. Water Res. 2020, 185, 116083. [Google Scholar] [CrossRef]
- Bloodgood, M.A.; Chowdary, S.A.; Daiber, E.J.; Shi, H.; Granger, C.O.; Richardson, S.D. A Balancing Act: Optimizing Free Chlorine Contact Time to Minimize Iodo-DBPs, NDMA, and Regulated DBPs in Chloraminated Drinking Water. J. Environ. Sci. 2022, 117, 315–325. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Z.; Cui, F.; Liu, B. Comparative Study of UV/Chlorine and VUV/Chlorine as Ultrafiltration Membrane Pretreatment Techniques: Performance, Mechanisms and DBPs Formation. J. Hazard. Mater. 2023, 459, 132249. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Dong, L.; Rao, P.; Zhang, X.; Gao, N.; Yin, X. Mechanism, Kinetics and DBPs Formation of UV/NH2Cl Process on Sulfathiazole Removal in Aqueous Solution. Water Resour. Ind. 2023, 30, 100221. [Google Scholar] [CrossRef]
- Liu, H.; Lv, H.; Xu, H.; Rao, D.; Zhang, J.; Sun, B. Is Monochloramine Pre-Oxidation a Viable Strategy for Enhancing the Treatment Efficiency of Algae-Laden Water with Conventional Drinking Water Treatment Process? Chemosphere 2024, 352, 141312. [Google Scholar] [CrossRef]
- Wang, D.; Chen, X.; Luo, J.; Shi, P.; Zhou, Q.; Li, A.; Pan, Y. Comparison of Chlorine and Chlorine Dioxide Disinfection in Drinking Water: Evaluation of Disinfection Byproduct Formation under Equal Disinfection Efficiency. Water Res. 2024, 260, 121932. [Google Scholar] [CrossRef] [PubMed]
- Dowdell, K.S.; Olsen, K.; Paz, E.F.M.; Sun, A.; Keown, J.; Lahr, R.; Steglitz, B.; Busch, A.; LiPuma, J.J.; Olson, T.; et al. Investigating the Suitability of Online Flow Cytometry for Monitoring Full-Scale Drinking Water Ozone System Disinfection Effectiveness. Water Res. 2024, 257, 121702. [Google Scholar] [CrossRef]
- Rall, D.; Schweidtmann, A.M.; Aumeier, B.M.; Kamp, J.; Karwe, J.; Ostendorf, K.; Mitsos, A.; Wessling, M. Simultaneous Rational Design of Ion Separation Membranes and Processes. J. Membr. Sci. 2020, 600, 117860. [Google Scholar] [CrossRef]
- Krippl, M.; Dürauer, A.; Duerkop, M. Hybrid Modeling of Cross-Flow Filtration: Predicting the Flux Evolution and Duration of Ultrafiltration Processes. Sep. Purif. Technol. 2020, 248, 117064. [Google Scholar] [CrossRef]
- Dias, C.G.; Librantz, A.F.H.; dos Santos, F.C.R. Modeling and Simulation of an Intelligent System for Dosage Control of Post-Chlorination in Water Treatment Plants. Eng. Sanit. Ambient. 2020, 25, 323–332. [Google Scholar] [CrossRef]
- Yang, J.; Du, Q.; Ma, R.; Khan, A. Artificial Intelligence Simulation of Water Treatment Using a Novel Bimodal Micromesoporous Nanocomposite. J. Mol. Liq. 2021, 340, 117296. [Google Scholar] [CrossRef]
- Narges, S.; Ghorban, A.; Khotanlou, H.; Khazaei, M. Prediction of the Optimal Dosage of Coagulants in Water Treatment Plants through Developing Models Based on Artificial Neural Network Fuzzy Inference System (ANFIS). J. Environ. Health Sci. Eng. 2021, 19, 1543–1553. [Google Scholar] [CrossRef]
- Jayaweera, D.; Aziz, N. An Efficient Neural Network Model for Aiding the Coagulation Process of Water Treatment Plants. Environ. Dev. Sustain. 2022, 24, 1069–1085. [Google Scholar] [CrossRef]
- Hu, J.; Kim, C.; Halasz, P.; Kim, J.F.; Kim, J.; Szekely, G. Artificial Intelligence for Performance Prediction of Organic Solvent Nanofiltration Membranes. J. Membr. Sci. 2021, 619, 118513. [Google Scholar] [CrossRef]
- Setshedi, K.; Mutingwende, N.; Ngqwala, N. The Use of Artificial Neural Networks to Predict the Physicochemical Characteristics of Water Quality in Three District Municipalities, Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 5248. [Google Scholar] [CrossRef]
- Mao, R.; Zhang, K.; Zhang, Q.; Xu, J.; Cen, C.; Pan, R.; Zhang, T. Joint Majorization of Waterworks and Secondary Chlorination Points Considering the Chloric Odor and Economic Investment in the DWDS Using Machine Learning and Optimization Algorithms. Water Res. 2022, 220, 118595. [Google Scholar] [CrossRef] [PubMed]




| Precursor Removal | Combined Use with | Efficiency Improvements | Direct Removal of DBPs |
|---|---|---|---|
| During prechlorination | Chemical oxidants | Solar photo-Fenton | Best results for brominated species |
| Before distribution network | Coagulation | TiO2 and ZnO semiconductors | |
| Membranes | |||
| Limitations | |||
| NOM shift to hydrophilic fractions → favors DBPs formation | |||
| Increased energy consumption → increased costs | |||
| Rechlorination needed when applied in the end of the process | |||
| DBPs Minimization | Techniques | Reference |
|---|---|---|
| Precursors and/or DBPs removal | ||
| Coagulation | Enhanced coagulation | [60] |
| Coagulation | Low-level ferrous iron | [61] |
| Coagulation | Tri-protonated ferrate as preoxidant/coagulant | [62] |
| Various techniques | Pre-ozonation, coagulation–sedimentation, sand filtration, and ozone combined with biological activated carbon (O3-BAC) | [63] |
| Various techniques | Adsorption, boiling, membrane filtration | [64] |
| Adsorption | Anion-exchange resin adsorption followed by electrolysis | [65] |
| Adsorption | Carbon nanomaterial-based adsorbents | [66] |
| Adsorption | Graphene oxide/ferrihydrite | [67] |
| Adsorption | Granular activated carbon | [68] |
| Adsorption | Nanoscale silver supported on activated carbon | [69] |
| Adsorption | GAC | [70] |
| Adsorption | Hydrolyzed polyacrylonitrile UF membrane | [71] |
| Adsorption | Biological activated carbon | [72] |
| Adsorption | Biological biochar and activated carbon filters | [73] |
| Adsorption | Nanofiltration | [74] |
| AOPs | Heterogenous photocatalysis followed by granular activated carbon | [68] |
| AOPs | Pre-oxidation by ozone, permanganate, and ferrate | [75] |
| AOPs | Pre-oxidation by ozone, permanganate, and ferrate | [76] |
| AOPs | Solar heterogeneous photocatalysis | [30] |
| AOPs | Pre-oxidation by ozone, chlorine dioxide, permanganate, and ferrate | [64] |
| Alternative disinfectants/modifying treatment | ||
| Preformed monochloramine | [77] | |
| Optimizing Cl2 contact time | [78] | |
| UV/chlorine and VUV/chlorine as ultrafiltration membrane pretreatment | [79] | |
| UV/NH2Cl | [80] | |
| Monochloramine | [81] | |
| O3, Fe(VI), Mn(VII), and ClO2 | [64] | |
| Cl2/ClO2 | [82] | |
| Ozone | [83] | |
| AI/machine learning | ||
| Membrane design | [84] | |
| Ultrafiltration processes | [85] | |
| Real-time monitoring of Cl2 dosage | [86] | |
| Adsorption on nanocomposite material | [87] | |
| Optimal coagulant dose by artificial neural network fuzzy inference system (ANFIS) | [88] | |
| Optimized coagulation process | [89] | |
| Nanofiltration membrane performance | [90] | |
| Prediction of physicochemical water quality characteristics | [91] | |
| Model chlorine, chloramine, and chlorine odor intensity | [92] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golfinopoulos, S.K.; Nikolaou, A.D.; Alexakis, D.E. Innovative Approaches for Minimizing Disinfection Byproducts (DBPs) in Water Treatment: Challenges and Trends. Appl. Sci. 2024, 14, 8153. https://doi.org/10.3390/app14188153
Golfinopoulos SK, Nikolaou AD, Alexakis DE. Innovative Approaches for Minimizing Disinfection Byproducts (DBPs) in Water Treatment: Challenges and Trends. Applied Sciences. 2024; 14(18):8153. https://doi.org/10.3390/app14188153
Chicago/Turabian StyleGolfinopoulos, Spyridon K., Anastasia D. Nikolaou, and Dimitrios E. Alexakis. 2024. "Innovative Approaches for Minimizing Disinfection Byproducts (DBPs) in Water Treatment: Challenges and Trends" Applied Sciences 14, no. 18: 8153. https://doi.org/10.3390/app14188153








