Abstract
Human Immunodeficiency Virus (HIV) is a diploid, C-type enveloped retrovirus belonging to the Lentivirus genus, characterized by two positive-sense single-stranded RNA genomes, that transitioned from non-human primates to humans and has become globally widespread. In its advanced stages, HIV leads to Acquired Immune Deficiency Syndrome (AIDS), which severely weakens the immune system by depleting CD4+ helper T cells. Without treatment, HIV progressively impairs immune function, making the body susceptible to various opportunistic infections and complications, including cardiovascular, respiratory, and neurological issues, as well as secondary cancers. The envelope glycoprotein complex (Env), composed of gp120 and gp41 subunits derived from the precursor gp160, plays a central role in cycle entry. gp160, synthesized in the rough endoplasmic reticulum, undergoes glycosylation and proteolytic cleavage, forming a trimeric spike on the virion surface. These structural features, including the transmembrane domain (TMD), membrane-proximal external region (MPER), and cytoplasmic tail (CT), are critical for viral infectivity and immune evasion. Glycosylation and proteolytic processing, especially by furin, are essential for Env’s fusogenic activity and capacity to evade immune detection. The virus’s outer envelope glycoprotein, gp120, interacts with host cell CD4 receptors. This interaction, along with the involvement of coreceptors CXCR4 and CCR5, prompts the exposure of the gp41 fusogenic components, enabling the fusion of viral and host cell membranes. While this is the predominant pathway for viral entry, alternative mechanisms involving receptors such as C-type lectin and mannose receptors have been found. This review aims to provide an in-depth analysis of the structural features and functional roles of HIV entry proteins, particularly gp120 and gp41, in the viral entry process. By examining these proteins’ architecture, the review elucidates how their structural properties facilitate HIV invasion of host cells. It also explores the synthesis, trafficking, and structural characteristics of Env/gp160 proteins, highlighting the interactions between gp120, gp41, and the viral matrix. These contributions advance drug resistance management and vaccine development efforts.
1. Introduction
Human Immunodeficiency Virus (HIV) is a globally disseminated pathogen that originated from non-human primates and crossed into humans in the early 20th century [1]. In its advanced stages, HIV infection leads to the development of AIDS (Acquired Immune Deficiency Syndrome), which severely impairs the immune system by reducing the population of CD4+ (cluster of differentiation 4) helper T cells [2]. Untreated HIV infection progressively weakens the immune system, increasing the body’s vulnerability to a range of opportunistic infections and leading to potential complications, including cardiovascular, respiratory, and neurological symptoms, as well as the risk of developing secondary neoplastic diseases [3]. According to UNAIDS (United Nations Program on HIV and AIDS), in 2022, there were 39 million living with HIV. Of these, 1.3 million were newly infected, and there were 630,000 AIDS-related deaths [4]. In numerous countries, HIV infection has significantly impacted the economy, as the virus frequently affects individuals during their most economically productive years. The extensive repercussions on public health and overall well-being are immeasurable [1].
HIV is a diploid, C-type enveloped retrovirus belonging to the Lentivirus genus, characterized by two positive-sense single-stranded RNA genomes [5]. HIV-1, known for its high infectivity and role in driving the pandemic, along with HIV-2, is classified within the Retroviridae family. These viruses possess a unique trait that defies the central dogma of molecular biology. Specifically, upon infection, viral RNA genomes are initially reverse-transcribed into DNA. Subsequently, these DNA copies integrate into the host genome, establishing persistent infections. As long as the host cell remains viable, both viral genomic RNA (vRNA) and messenger RNA (mRNA) continue to be transcribed from the integrated viral genome [5,6]. Therefore, lentiviruses exhibit a broad spectrum of cellular tropism, enabling replication across various cell types. Beyond non-primate lentiviruses, such as those impacting fibroblasts and macrophages, HIV can infect a myriad of cell types, as evidenced by in vitro investigations, including but not limited to astrocytes, oligodendrocytes, microglia, in a lower proportion of neurons [7,8], bowel mucosal cells, B cells, CD8+ T cells, cervical cells, eosinophils, and others [9]. Conversely, in vivo, HIV predominantly targets and establishes reservoirs in CD4+ T lymphocytes, cells of the macrophage lineage, and dendritic cells [10,11,12,13]. While there is evidence of HIV involvement in trans-infection processes, the relevance of in vitro findings to in vivo infection for other cell types remains unclear.
HIV predominantly targets cells expressing CD4 receptors, including helper T cells, CD8+ T cells, eosinophils, macrophages, and neutrophils. The CD4 receptor typically engages major histocompatibility complex (MHC) molecules on the surfaces of adjacent cells under normal conditions [10,14]. Furthermore, the CD4 receptor is an endogenous ligand for MHC class II molecules, enhancing the interaction between antigen-presenting cells and CD4+ T lymphocytes. This interaction is stabilized by the engagement of protein kinase molecules, transmitting signals to the cytoplasmic domains of CD4 [15,16,17,18,19]. The outer envelope glycoprotein of HIV, gp120, engages with host cell CD4 receptors. Following this interaction, two host cell components, known as coreceptors, namely, CXCR4 (C-X-C Chemokine Receptor 4) and CCR5 (CC chemokine receptor 5), trigger the exposure of the gp41 fusogenic components by gp120. This exposure facilitates the fusion of the host cell and viral membranes [20,21,22].
Given the vital role of glycoproteins in virus entry, this review seeks to provide a comprehensive analysis of the structural features of HIV entry proteins, explicitly focusing on gp120 and gp41 and exploring their functional roles in the viral entry process. By examining the detailed architecture of these proteins, the paper aims to elucidate how their structural properties facilitate HIV’s ability to invade host cells, thereby enhancing our understanding of the mechanisms underlying viral infection.
2. Proteins Engaged in HIV Entry
HIV demonstrates a pronounced binding affinity between the viral glycoprotein gp120 and the initial domain of the CD4 receptor. Subsequently, CXCR4 and CCR5 coreceptors exert pivotal roles in mediating HIV entry by dictating tropism among CD4+ cells. Coreceptors interact with gp120, eliciting conformational alterations in the glycoprotein. These structural shifts lead to the dissociation of gp120 from gp41 and expose previously concealed epitopes on gp41. Subsequent exposure of these cryptic epitopes on gp41 triggers the activation of fusogenic components, initiating the process of receptor-mediated fusion known as virus–cell membrane coalescence [20,21,22]. Concurrently, various studies have documented the entry of HIV into receptor cells independent of CD4, CXCR4, and CCR5 receptors [9,23,24,25,26]. Therefore, HIV-1 gains entry into these cells by utilizing alternative receptors such as C-type lectin or mannose (Man) receptors in astrocytes [23,27]. The subsequent discussion will thoroughly examine the intricate interplay between HIV and host receptors crucial for viral entry.
2.1. Env/gp160
HIV-1 enters the host cell by fusing its lipid envelope with the host cell’s plasma membrane. This fusion process is facilitated by the HIV-1 envelope (Env) glycoprotein, specifically the gp160 complex, which is embedded in the viral lipid envelope. The Env glycoprotein serves as a key antigen, eliciting robust antibody responses, and is a primary focus in developing HIV-1 vaccines and therapeutic agents [28]. The viral env gene encodes the Env protein. It is initially translated as the precursor transmembrane protein gp160, which subsequently undergoes cleavage into two subunits: gp120 and gp41. Structurally, Env forms a trimeric spike-like protein on the virion surface, characterized by its low density per virion, constituting 50% carbohydrate mass. Each trimer consists of three identical molecules, each comprising a cap-like gp120 region and a stem-like gp41 region [29,30,31,32,33].
2.1.1. Synthesis and Trafficking of Env/gp160
The HIV-1 Env glycoprotein is initially synthesized as a 160-kDa precursor protein known as gp160 within the rough endoplasmic reticulum (RER), derived from a single vpu/env bicistronic mRNA [31]. At the N-terminus of the unprocessed gp160, an endoplasmic reticulum (ER) signal sequence is present, guiding the Env protein to the RER membrane. During synthesis and for approximately 15–30 min afterward, a transient signal anchor attaches the N-terminus of HIV-1 gp160 to the ER membrane (Figure 1). Cysteine residues in the signal peptide facilitate disulfide isomerization, promoting conformational flexibility, mainly via the 54–74 disulfide bond. This process aids in completing the inner-domain β sandwich and folding the gp120 molecule, enabling the assembly of gp120 C and N termini (Figure 2) [34].
During translation within the ER, cellular signal peptidases enzymatically remove the signal peptide from gp160, cleaving it at the C28 residue. This cleavage halts further disulfide isomerization and stabilizes the N-terminus of gp120. Intramolecular quality-control processes are influenced by single charge reversals in the gp120 inner domain, which can impair or restore this process [34]. Additionally, a hydrophobic stop-transfer signal within the transmembrane domain (TMD) of gp41 prevents gp160 full release into the ER lumen [31,35]. Consequently, portions of the Env extracellular domain extend into the ER lumen, while the gp41 cytoplasmic tail (CT) first extends into the cytoplasm and later into the virion lumen [35].
During translation, gp160 undergoes glycosylation, where N-linked (and some O-linked) oligosaccharide side chains are added. gp160 monomers typically assemble into trimers within the ER, although dimers and tetramers have also been observed [36]. N-linked oligosaccharide modification begins in the ER, where one out of nine Man residues and all three glucose (Glc) residues are enzymatically removed from each sugar block. Correctly folded and assembled glycoproteins in the ER form oligomers, after which N-linked oligosaccharides undergo extensive and heterogeneous processing in the Golgi complex. Three Man residues are removed, N-acetylglucosamine (GlcNAc) residue is added, and another two Man residues are removed. Subsequently, additional sugars such as fucose (Fuc), galactose (Gal), N- GlcNAc, and sialic acid are removed in the trans-Golgi compartment, further contributing to glycoprotein heterogeneity [37]. The oligomerization process facilitates the transport of gp160 to the Golgi. Modifications in the trans-Golgi network (TGN) primarily affect high-Man oligosaccharide side chains as gp160 traverses the secretory pathway. Through ubiquitin-mediated proteasomal degradation, the HIV-1 accessory protein Vpu downregulates its expression upon binding with CD4 and inhibits premature interaction between oligomerized Env and CD4 within the secretory pathway [31].
N-linked glycosylation involves the attachment of high-Man chains to asparagine (Asn) residues at either Asn-X-Thr or Asn-X-Ser glycosylation sites [38]. Pentasaccharides comprising Man3GlcNAc2, with the Asn residue linked to their side chains’ amide nitrogen, are termed Asn-linked oligosaccharides. The term “trimannosidic core” refers to the three-Man residues at the ends of the core pentasaccharide. N-linked carbohydrates sharing a pentasaccharide core can be categorized as high-Man, hybrid, or complex, all present in the HIV-1 envelope gp120 protein. Structural analysis of oligosaccharide moieties has revealed their composition: approximately 33% high-Man, 4% hybrid, and 63% complex. Among the complex oligosaccharides, around 90% are fucosylated, and 94% are sialylated. Furthermore, these structures include approximately 4% monoantennary, 61% biantennary, 19% triantennary, and 16% tetraantennary arrangements. Complex oligosaccharides exhibit variations in the types and numbers of residues attached to their outer branches compared to high-Man oligosaccharides, which typically have 2–6 Man residues linked to the core [37,39].
The inner N-acetylglucosamine residue can attach to a Fuc residue with bi-, tri-, or tetra-antennary chains, typically forming a sequence known as sialyllactosamine. Hybrid oligosaccharides contain complex carbohydrate and high-Man structures in 1→6 or 1→3 branches, respectively. To produce Man8GlcNAc2 oligosaccharides, carbohydrate moieties are transferred to Asn residues in the RER. The Golgi apparatus can further process these hybrid oligosaccharides into complex carbohydrates, incorporating sugars such as Man, Gal, GlcNAc, N-acetylgalactosamine (GalNAc), L-Fuc, and sialic acids. Complex oligosaccharides can exhibit di-, tri-, tetra-, or penta-antennary structures. Notable differences exist between amino acid side chains and N-linked glycans. N-linked glycans possess an average molecular weight of more than 20 times greater than that of amino acid side chains. They occupy a larger volume, exhibit higher structural complexity, and have greater bulk. Pentasaccharides generally have surface areas comparable to antibody footprints [37,39]. In a study, Thr499 showed no O-linked carbohydrates, as it is exposed on the surface of virions. This result suggests caution when interpreting post-translational analyses utilizing recombinant forms of the envelope protein [40].
In the Golgi complex, furin and furin-like proteases cleave the gp160 glycoprotein at a highly conserved K/R-X-K/R-R motif, yielding mature gp120 and transmembrane gp41 [41,42,43]. In the HIV-1 HXB2 strain, the primary furin cleavage site is located at position 511, which is C-terminal to arginine. Carboxypeptidase removes two amino acids from the gp120 C-terminus, corresponding to HIV-1 HXB2 R511. A secondary dibasic cleavage site at HIV-1 HXB2 R504 results in the cleavage of approximately 10% of the HIV-1 gp160 precursor protein [40,44]. The proteolytic processing of gp160 activates the fusogenic activity of Env, which is essential for viral infectivity. Following cleavage, gp120 and gp41 remain noncovalently associated and assemble into heterotrimeric HIV-1 glycoprotein spikes. Upon arrival at the plasma membrane, cellular clathrin adaptor complex interactions facilitate rapid endocytic recycling of Env [45,46]. Env internalization and shedding of gp120 from the cell surface are crucial for the relatively low levels of Env incorporation into virus particles (∼10 spikes/virion), attributed to the weak and noncovalent interaction between gp120 and gp41 [47]. Maintaining low levels of gp120 and gp41 on both the cell and virion surfaces enables HIV-1 to reduce virus-induced cytopathicity and evade host immune responses.
Virological or infectious synapses refer to cell–cell contacts that efficiently propagate HIV-1 from one cell to another. This process involves the accumulation of Gag, Env, CD4, actin, adhesion molecules, tetraspanins, and coreceptors at virological synapses [31,48,49,50]. The induction of virological synapses occurs through Env-receptor contacts by HIV-1 in T cells and murine leukemia virus (MLV) in fibroblasts [51,52]. Env and Gag are concentrated at virological synapses in T cells and macrophages, although Gag localization to cell–cell contacts appears relatively independent of Env [53,54]. Conversely, in the MLV system, the CT of Env seems to dictate the direction of Gag assembly toward virological synapses [55]. A fundamental question remains about how essential HIV-1 transfer across the virological synapses directed by the Env to the synapse in vivo. An elemental inquiry remains regarding how crucial HIV-1 transfer across virological synapses, guided by Env, is orchestrated in vivo.
Figure 1.
HIV-1 Env trafficking. A detailed mechanism. The HIV-1 envelope glycoprotein (Env) undergoes a complex trafficking pathway. Initially, Env is synthesized and glycosylated within the rough endoplasmic reticulum (RER) as a 160-kDa precursor protein, designated gp160. This precursor protein predominantly oligomerizes into trimers. Concurrently, the precursor Gag protein (Pr55 Gag) is synthesized on cytosolic ribosomes and directed to the plasma membrane (PM), where it multimerizes within lipid rafts (not depicted here) to form nascent virus particles. The oligomerized gp160 is subsequently transported to the Golgi apparatus and the trans-Golgi network (TGN). Here, gp160 undergoes proteolytic processing to generate the mature surface glycoprotein gp120 and the transmembrane glycoprotein gp41. The gp120/gp41 complexes proceed through the secretory pathway, eventually reaching the plasma membrane, where they are incorporated into virus particles as trimeric spikes. At the plasma membrane, Env can undergo endocytosis mediated by clathrin adaptor complexes, leading to its internalization into early endosomes (EE). Within the cell, internalized Env has two potential fates: it can be routed to late endosomes/multivesicular bodies (LE/MVBs) for subsequent degradation in lysosomes, or it can be recycled back to the plasma membrane via recycling endosomes. The domains of Gag and Env are detailed in the inset located at the top left of the diagram. Reprinted (adapted) with permission from [31,56] Copyright Elsevier (2024).
Figure 1.
HIV-1 Env trafficking. A detailed mechanism. The HIV-1 envelope glycoprotein (Env) undergoes a complex trafficking pathway. Initially, Env is synthesized and glycosylated within the rough endoplasmic reticulum (RER) as a 160-kDa precursor protein, designated gp160. This precursor protein predominantly oligomerizes into trimers. Concurrently, the precursor Gag protein (Pr55 Gag) is synthesized on cytosolic ribosomes and directed to the plasma membrane (PM), where it multimerizes within lipid rafts (not depicted here) to form nascent virus particles. The oligomerized gp160 is subsequently transported to the Golgi apparatus and the trans-Golgi network (TGN). Here, gp160 undergoes proteolytic processing to generate the mature surface glycoprotein gp120 and the transmembrane glycoprotein gp41. The gp120/gp41 complexes proceed through the secretory pathway, eventually reaching the plasma membrane, where they are incorporated into virus particles as trimeric spikes. At the plasma membrane, Env can undergo endocytosis mediated by clathrin adaptor complexes, leading to its internalization into early endosomes (EE). Within the cell, internalized Env has two potential fates: it can be routed to late endosomes/multivesicular bodies (LE/MVBs) for subsequent degradation in lysosomes, or it can be recycled back to the plasma membrane via recycling endosomes. The domains of Gag and Env are detailed in the inset located at the top left of the diagram. Reprinted (adapted) with permission from [31,56] Copyright Elsevier (2024).
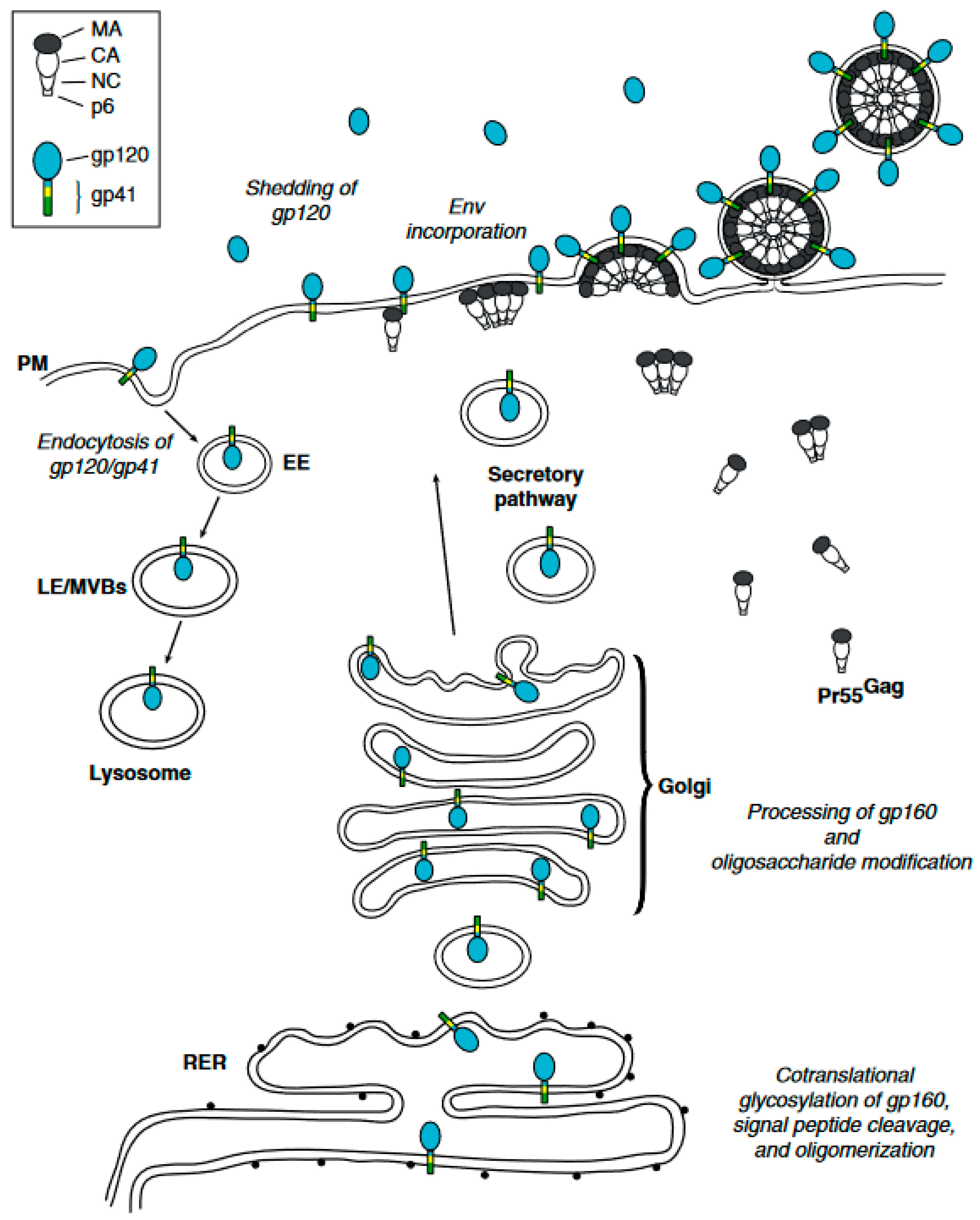
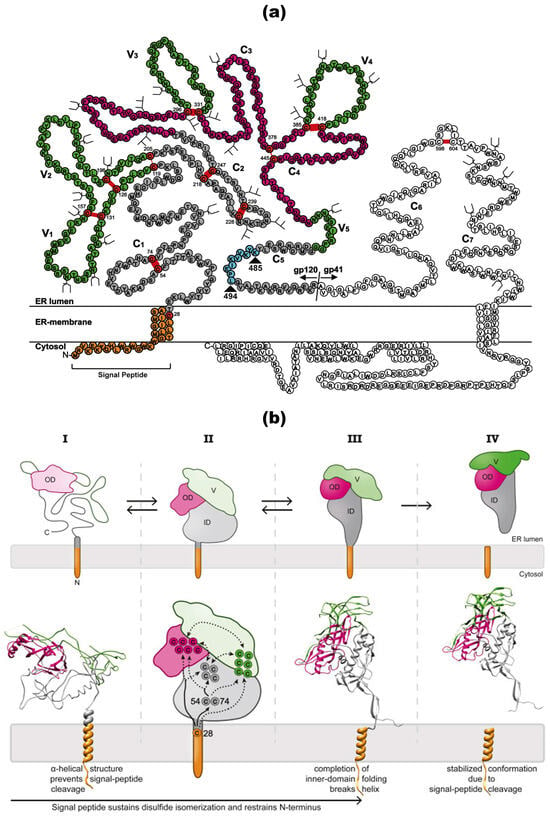
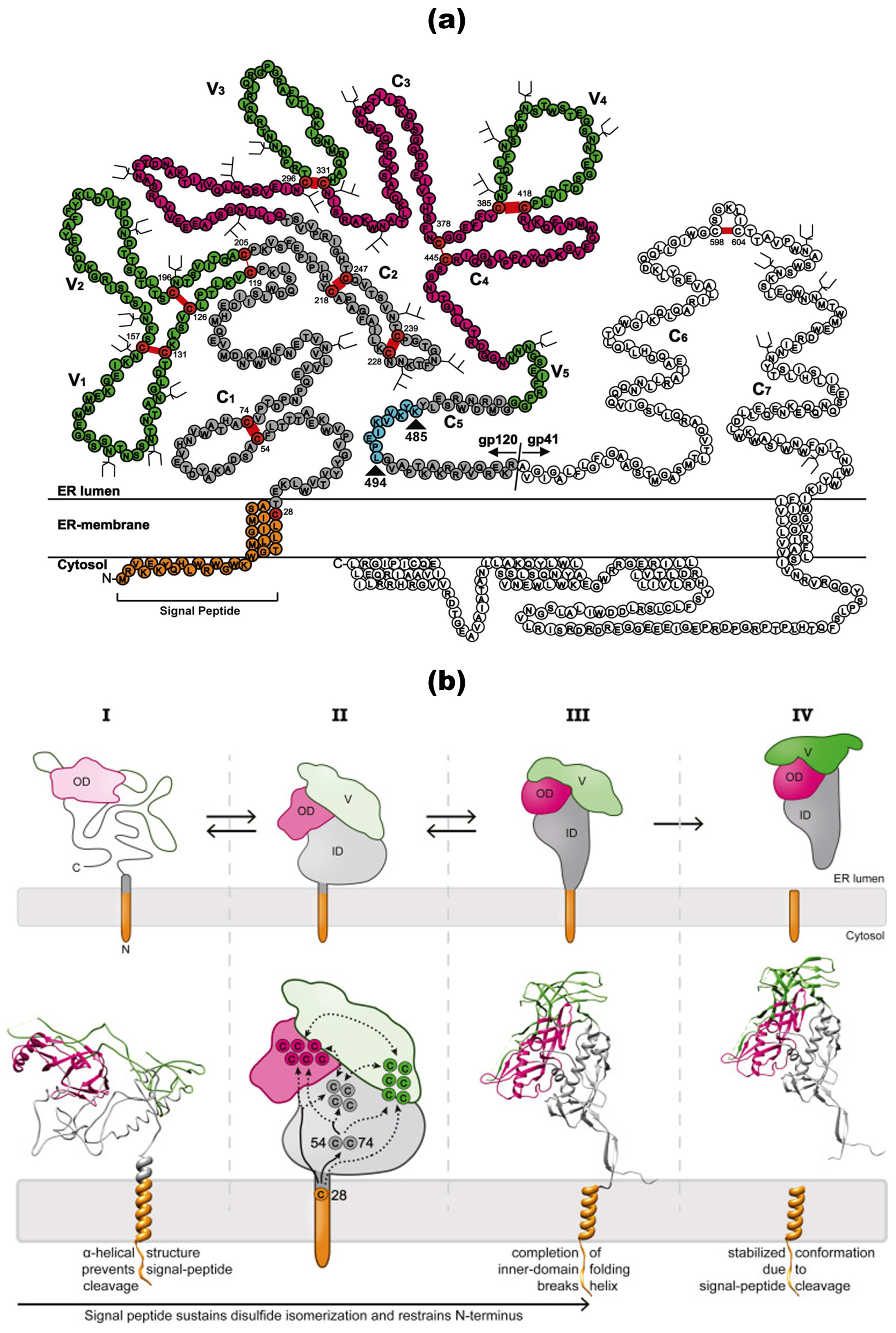
Figure 2.
HIV-1 gp160 protein structure and post-translational modifications. (a) Structure and processing of precursor gp160. The precursor gp160 protein of HIV-1 includes a signal peptide (SP) that is cleaved during translation. Post-translationally, gp160 is processed into the surface subunit (gp120) and the transmembrane subunit (gp41) within the Golgi complex at a specific furin cleavage site. The gp120 subunit comprises five variable domains (V1–V5) and five constant domains (C1–C5). In contrast, the gp41 subunit features extracellular constant domains (C6 and C7) that includes the fusion peptide (FP), heptad repeats (HR1 and HR2), the membrane-proximal external region (MPER), a transmembrane domain (TMD), and a cytoplasmic tail (CT). An enlarged depiction of the gp41 CT highlights several critical motifs: the internalization signal YSPL, the Kennedy sequence, the amphipathic α-helices LLP-1, LLP-2 and LLP-3, and a C-terminal dileucine motif (LL) implicated in the endocytosis and intracellular distribution of Env. The glycosylation sites on various HIV-1 gp120 variants are shown in a structure-based sequence alignment. These sites are called complex or high-mannose glycosylation sites. The domains are color-coded, with the N and C termini indicated. Disulfide bonds are represented as red lines, and the inner-domain β sandwich is boxed. Oligomannose and complex glycans are symbolized by three or two-pronged forked symbols, respectively. Reprinted (adapted) from [34,57,58] under a Creative Commons license. (b) Conformational changes during gp120 folding and signal-peptide cleavage. Stage I: Upon the completion of translation, gp120 remains largely unfolded. An α-helical structure around the signal peptide impedes the cleavage of the signal peptide. Stage II: As long as the signal peptide is attached, the cysteine at position 28 (C28) promotes intramolecular disulfide isomerization by interacting with downstream cysteine residues. The tethering of the N-terminus restricts conformational freedom, potentially aiding the folding of the N-terminal region. Stage III: The folding and integration of the inner-domain β sandwich disrupt the helical structure of the signal peptide, exposing the consensus cleavage site. Stage IV: The cleavage of the signal peptide stabilizes the conformation of gp120 by removing the free sulfhydryl group of C28, thus halting further disulfide isomerization. In the schematic, the inner domain is shaded in gray, the outer domain is pink, the variable loops are green, and the signal peptide is orange. Solid lines represent experimentally determined interactions, while dashed lines denote predicted interactions. Reprinted (adapted) from [34] under a Creative Commons license.
Figure 2.
HIV-1 gp160 protein structure and post-translational modifications. (a) Structure and processing of precursor gp160. The precursor gp160 protein of HIV-1 includes a signal peptide (SP) that is cleaved during translation. Post-translationally, gp160 is processed into the surface subunit (gp120) and the transmembrane subunit (gp41) within the Golgi complex at a specific furin cleavage site. The gp120 subunit comprises five variable domains (V1–V5) and five constant domains (C1–C5). In contrast, the gp41 subunit features extracellular constant domains (C6 and C7) that includes the fusion peptide (FP), heptad repeats (HR1 and HR2), the membrane-proximal external region (MPER), a transmembrane domain (TMD), and a cytoplasmic tail (CT). An enlarged depiction of the gp41 CT highlights several critical motifs: the internalization signal YSPL, the Kennedy sequence, the amphipathic α-helices LLP-1, LLP-2 and LLP-3, and a C-terminal dileucine motif (LL) implicated in the endocytosis and intracellular distribution of Env. The glycosylation sites on various HIV-1 gp120 variants are shown in a structure-based sequence alignment. These sites are called complex or high-mannose glycosylation sites. The domains are color-coded, with the N and C termini indicated. Disulfide bonds are represented as red lines, and the inner-domain β sandwich is boxed. Oligomannose and complex glycans are symbolized by three or two-pronged forked symbols, respectively. Reprinted (adapted) from [34,57,58] under a Creative Commons license. (b) Conformational changes during gp120 folding and signal-peptide cleavage. Stage I: Upon the completion of translation, gp120 remains largely unfolded. An α-helical structure around the signal peptide impedes the cleavage of the signal peptide. Stage II: As long as the signal peptide is attached, the cysteine at position 28 (C28) promotes intramolecular disulfide isomerization by interacting with downstream cysteine residues. The tethering of the N-terminus restricts conformational freedom, potentially aiding the folding of the N-terminal region. Stage III: The folding and integration of the inner-domain β sandwich disrupt the helical structure of the signal peptide, exposing the consensus cleavage site. Stage IV: The cleavage of the signal peptide stabilizes the conformation of gp120 by removing the free sulfhydryl group of C28, thus halting further disulfide isomerization. In the schematic, the inner domain is shaded in gray, the outer domain is pink, the variable loops are green, and the signal peptide is orange. Solid lines represent experimentally determined interactions, while dashed lines denote predicted interactions. Reprinted (adapted) from [34] under a Creative Commons license.
2.1.2. Structural Characteristics of Env/gp160
The full-length Env protein consists of several critical components essential for viral infectivity: a spike-shaped glycosylated ectodomain, a trimerized single-pass TMD, and a highly conserved membrane-proximal external region (MPER). Additionally, it features a membrane-interacting amphipathic CT composed of approximately 150 amino acid residues (Figure 2a). Each of these regions plays a pivotal role in the structural integrity and functionality of the Env protein during the HIV-1 infection process [28,59,60,61]. The membrane protein spikes of HIV-1 Env have been challenging to analyze at high resolution for an extended period. Currently, HIV-1 Env spike proteins have only been visualized at relatively low resolution (~20 Å) using cryo-electron tomography (cryo-ET) [62,63].
The trimeric soluble ectodomain Env, SOSIP.664, has been derived from the BG505 isolate of clade A HIV-1 since 2013. This construct includes several specific modifications: isoleucine to proline substitution at residue 559, a disulfide bond that crosslinks gp120 and gp41, and a truncation at residue 664. These alterations enhance the stability and mimic the native structure of the Env protein, making SOSIP.664 a valuable tool in HIV-1 research and vaccine development [64]. The intriguing finding from a study reveals the loss of viral infectivity after the SOSIP modifications [65]. Figure 3a–c shows the structures of BG505 clade A SOSIP.664 and JR-FL EnvΔCT clade B HIV-1, elucidated through X-ray crystallography (XRC) and cryo-electron microscopy (EM), respectively. This comparison sheds light on the structural alterations induced by these modifications and their implications for viral functionality [22,66,67].
The trimeric molecular structures of Env represent distinct prefusion and postfusion states of gp41. They capture the prefusion conformation of gp41 before membrane fusion and the postfusion six-helix bundle configuration after fusion. This distinction is crucial for understanding the dynamic changes that Env undergoes during the viral entry process [22]. In previous studies, the TMD of the Env protein was not typically considered for its role in passive membrane anchoring. However, research has begun investigating the TMDs of HIV-1 Env proteins and their neighboring regions within the lipid bilayer context. Consequently, many HIV-1 Env proteins expressed on human cell surfaces display conformational uniformity, including those derived from challenging-to-neutralize primary isolates of HIV-1. This finding expanded understanding and underscores the importance of considering the entire Env protein structure, including its membrane-interacting regions, for comprehensive analysis and potential therapeutic targeting [68,69].
Antigenicity plays a crucial role in antibody neutralization, where epitopes exposed on the HIV-1 Env are targeted by broadly neutralizing antibodies, while others remain concealed from non-neutralizing antibodies. The truncation of the CT of these uniform Envs causes the loss of fusogenic activity. However, it also leads to the exposure of previously hidden non-neutralizing epitopes on both gp120 and gp41. This alteration highlights the intricate relationship between Env structure, antigenicity, and antibody recognition, providing insights into potential strategies for vaccine design and therapeutic intervention [68]. The extended CT of approximately 150 residues in the Env membrane represents a notable characteristic facilitating its efficient incorporation into virions [28]. Truncation of CTs in specific HIV-1 isolates has been observed to have a minimal impact on the antigenicity of the Env protein [70,71]. In this context, the CT unexpectedly influences the conformational variability of the Env ectodomain by interacting with the TMD. An NMR analysis effectively reconstituted the TMD within bicelles, providing a model mimicking the lipid bilayer environment. This experimental approach, illustrated in Figure 3, has unveiled the intricate relationship between the CT, TMD, and Env ectodomain, offering insights into how their interplay regulates the structural dynamics of the protein. Such findings are pivotal for understanding viral entry mechanisms and designing targeted interventions against HIV-1 [59].
TMDs are anticipated to assemble into an organized trimeric structure, shielding three conserved arginine residues (Rs), including R696, positioned centrally within the transmembrane helix. This arrangement establishes distinct polar and hydrophobic cores within the membrane. Due to the presence of the polar core, R696 can maintain hydration despite its location within the lipid bilayer [59,72,73]. Mutations that disturb the trimeric structure of the TMD lead to changes in antibody sensitivity within the ectodomain. This observation underscores the TMD’s critical role in maintaining the Env protein’s stability and antigenicity. Consequently, recombinant soluble Env preparations lacking the TMD may display antigenic properties distinct from native proteins [64,69,74]. Additionally, employing the bicelle system, NMR analysis has elucidated the structure of both the TMD and MPER (Figure 3d) [75]. Early structural studies of monomeric MPER peptides revealed that the MPER region is buried within the viral membrane [76,77,78]. Contrarily, findings demonstrate that MPER forms a highly structured and exposed trimeric assembly in conjunction with the lipid bilayer and TMD, closely resembling the native Env prefusion conformation. Moreover, mutations within the MPER region have been shown to impact the antigenic properties of the Env ectodomain. These observations support the hypothesis that MPER acts as a regulatory relay, modulating the antigenic structure of the Env trimer [22,75].
A novel structure was discovered featuring the TMD and CT in conjunction with the lentiviral lytic peptide (LLP)-2 region. In this arrangement, the trimerized CT region encircles the TMD, supporting the baseplate within the membrane. The two-dimensional baseplate consists of amphipathic helices in the lipids’ head-group region. As a result of this architectural configuration, lipids are excluded from the inner leaflet of the membrane, leading to significant effects on membrane reshaping [61]. However, substantial portions of the CT remain relatively unexplored, particularly, residues 789–856 (LLP1 and LLP3). There is a belief that deleting the last 30 residues of the CT could open up the Env trimer, thereby altering the antibody binding profile [68]. Concurrently, genetic studies have suggested a direct interaction between residues 802–806 of the Env CT and the matrix domain of the Gag polyprotein during viral assembly [79]. This remaining fragment could extend the baseplate further, impacting lipid partitioning and inducing deformation of the lipid bilayer [28].
Despite the extensive knowledge regarding the Env structure, a comprehensive comprehension of viral entry mechanisms remains an ongoing pursuit. Prefusion Env structures play a pivotal role in this endeavor. Through single-molecule fluorescence resonance energy transfer (smFRET) studies, researchers have delineated three distinct transition conformations of HIV-1 Env. State 1: Low-FRET, representing the prefusion state; state 2: High-FRET, indicative of a default intermediate conformation; and state 3: Intermediate-FRET, encompassing the three CD4-bound conformations.
These findings provide valuable insights into the dynamic conformational changes underlying viral entry, offering potential targets for therapeutic intervention [22,80,81]. Most broadly neutralizing antibodies target the untriggered prefusion state of the HIV-1 Env glycoprotein and demonstrate a preference for binding to the CD4-triggered Env conformation [82]. A study determined the full-length CT NMR structure utilizing protein fragments containing CT and TMD within a lipid bilayer mimic called bicelles. The resulting structure revealed a substantial trimeric baseplate formed by the CT surrounding the trimeric TMD. This baseplate was situated within the lipid bilayer, with the headgroup region interacting. The exclusion of the baseplate was observed in the cytoleaflet of the lipid bilayer (Figure 3e–h) [28]. To address this challenge and facilitate the selection of an appropriate Env trimer immunogen for clinical studies, obtaining a full-length, unmodified Env structure within a membrane environment may be essential.
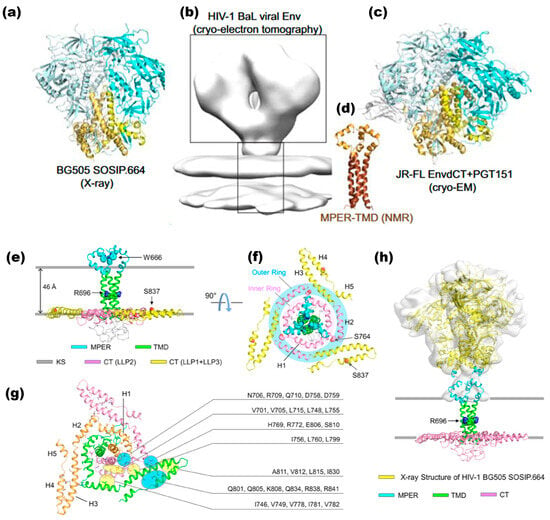
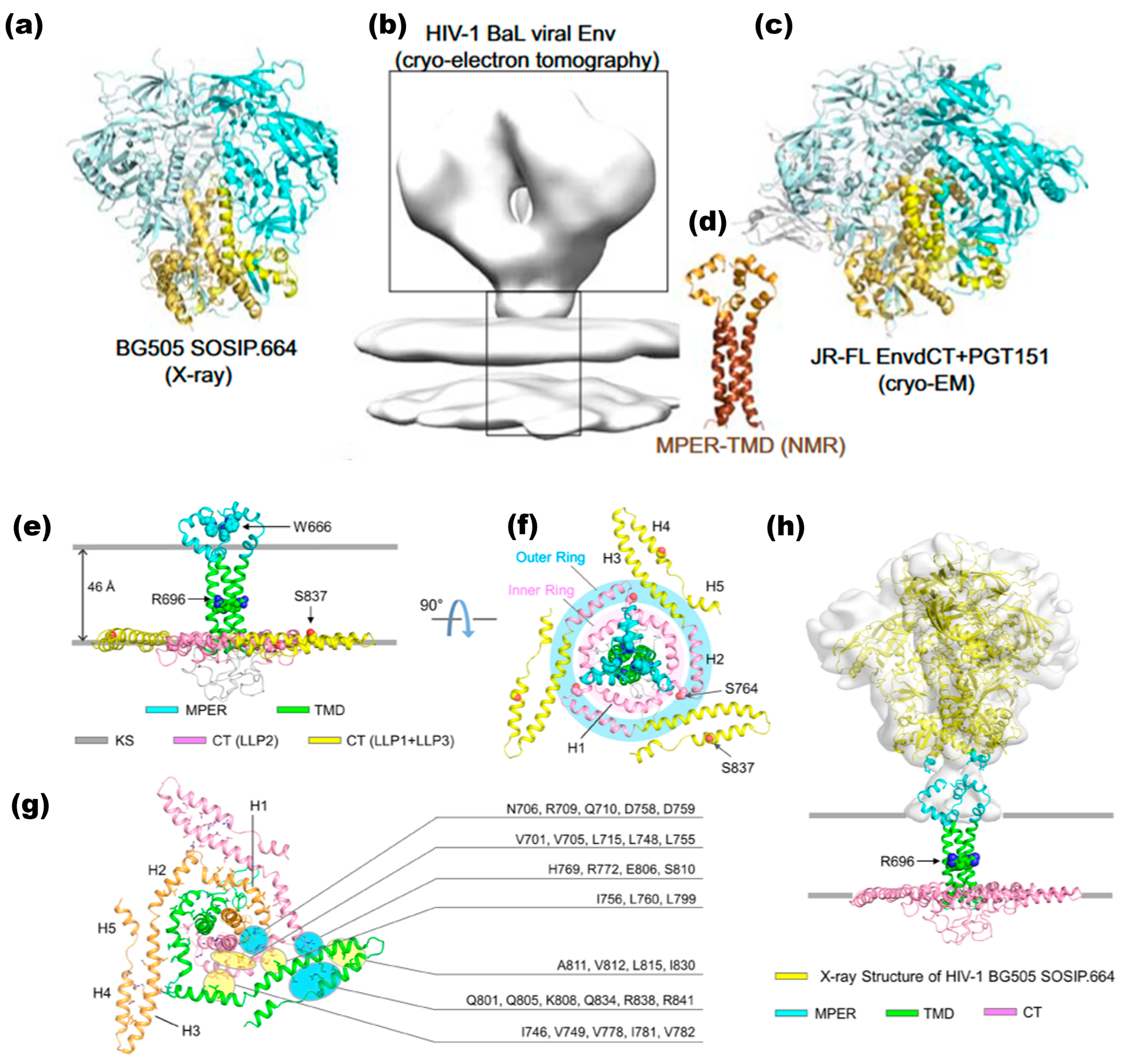
Figure 3.
Structural Analysis of the HIV-1 Entry Protein Env. (a) Crystal structure of unliganded HIV-1 BG505 SOSIP.664 Env trimer: shown in a ribbon diagram, this structure (PDB ID: 4ZMJ [83]) lacks the MPER, TMD, and CT, with gp120 in cyan and gp41 in yellow. (b) EM density of the unliganded HIV-1 BaL Env spike: A 3D reconstruction of the unliganded HIV-1 BaL Env spike on the virion surface by cryo-electron tomography (EMDB ID: EMD-5019 for the Env portion; EMDB ID: EMD-5022 for the membrane portion) is presented in gray. (c) Cryo-EM structure of detergent-solubilized Clade B HIV-1 JR-FL EnvΔCT: This structure, complexed with broadly neutralizing antibody PGT151 (PDB ID: 5FUU [67]), shows gp120 in cyan, gp41 in yellow, and PGT-151 Fab in gray, lacking the CT. (d) NMR structure of MPER–TMD in bicelles: Reconstituted in bicelles mimicking a lipid bilayer, the NMR structure (PDB ID: 6E8W [75]) illustrates the MPER in orange and the TMD in brown. Reprinted (adapted) with permission from [22] Copyright Elsevier (2024). (e) Structural model of the MPER−TMD−CT trimer: This model in q ≈ 0.55 bicelles is derived from integrated NMR data of four fragments: MPER-TMD, TMD, TMD-CT LLP2, and TMD-CT. The position of the structure relative to the bilayer region of the bicelle was determined using the Paramagnetic Probe Technique (PPT). (f) Top View of the Trimeric Complex: The top view from the MPER perspective reveals the inner and outer rings of the baseplate, shaded in pink and blue, respectively. Red spheres indicate the palmitoylation sites at residues 764 and 837. (g) Hydrophobic and polar clusters: Residues forming hydrophobic clusters are shaded in yellow, while polar clusters are shaded in blue. (h) MPER−TMD−CT fitting to EM map density: The fit of the MPER−TMD−CT trimer to the assigned MPER density in the EM map of the HIV-1 Env trimer (EMDB ID: EMD-21412; ~11 Å resolution) is based on a cryo-ET study of the Env on the virion surface. The SOSIP.664 crystal structure (PDB ID: 5T3Z) was used to fit the ectodomain EM density. Reprinted (adapted) with permission from [28] Copyright 2024 American Chemical Society.
Figure 3.
Structural Analysis of the HIV-1 Entry Protein Env. (a) Crystal structure of unliganded HIV-1 BG505 SOSIP.664 Env trimer: shown in a ribbon diagram, this structure (PDB ID: 4ZMJ [83]) lacks the MPER, TMD, and CT, with gp120 in cyan and gp41 in yellow. (b) EM density of the unliganded HIV-1 BaL Env spike: A 3D reconstruction of the unliganded HIV-1 BaL Env spike on the virion surface by cryo-electron tomography (EMDB ID: EMD-5019 for the Env portion; EMDB ID: EMD-5022 for the membrane portion) is presented in gray. (c) Cryo-EM structure of detergent-solubilized Clade B HIV-1 JR-FL EnvΔCT: This structure, complexed with broadly neutralizing antibody PGT151 (PDB ID: 5FUU [67]), shows gp120 in cyan, gp41 in yellow, and PGT-151 Fab in gray, lacking the CT. (d) NMR structure of MPER–TMD in bicelles: Reconstituted in bicelles mimicking a lipid bilayer, the NMR structure (PDB ID: 6E8W [75]) illustrates the MPER in orange and the TMD in brown. Reprinted (adapted) with permission from [22] Copyright Elsevier (2024). (e) Structural model of the MPER−TMD−CT trimer: This model in q ≈ 0.55 bicelles is derived from integrated NMR data of four fragments: MPER-TMD, TMD, TMD-CT LLP2, and TMD-CT. The position of the structure relative to the bilayer region of the bicelle was determined using the Paramagnetic Probe Technique (PPT). (f) Top View of the Trimeric Complex: The top view from the MPER perspective reveals the inner and outer rings of the baseplate, shaded in pink and blue, respectively. Red spheres indicate the palmitoylation sites at residues 764 and 837. (g) Hydrophobic and polar clusters: Residues forming hydrophobic clusters are shaded in yellow, while polar clusters are shaded in blue. (h) MPER−TMD−CT fitting to EM map density: The fit of the MPER−TMD−CT trimer to the assigned MPER density in the EM map of the HIV-1 Env trimer (EMDB ID: EMD-21412; ~11 Å resolution) is based on a cryo-ET study of the Env on the virion surface. The SOSIP.664 crystal structure (PDB ID: 5T3Z) was used to fit the ectodomain EM density. Reprinted (adapted) with permission from [28] Copyright 2024 American Chemical Society.
2.2. gp120
HIV-1 gp120 is a viral membrane spike glycoprotein composed of three molecules anchored in the viral membrane by the linked gp41 protein. gp120 plays a significant role in mediating the entry of HIV into host cells and triggering the T-cell immune response [84]. In various cell types, including lymphocytes, neurons, and cardiomyocytes, soluble gp120 can induce apoptosis. Studies conducted in rat hippocampal slices, cortical cells, and through in vivo intracerebral injections have confirmed that gp120 induces apoptosis. This apoptotic effect is mediated by gp120 binding to neuronal membrane coreceptors such as CCR3, CCR5, and CXCR4. Additionally, soluble gp120 contributes to the release of arachidonate by glial cells, disrupting glutamate reuptake in astrocytes and neurons, prolonging activation of NMDA receptors, and disrupting cellular calcium (Ca2+) homeostasis. Consequently, the generation of superoxide and peroxide species in mitochondria is triggered, leading to the activation of caspases and endonucleases, oxidative stress, and mitochondrial permeabilization, ultimately resulting in neuronal cell death [85].
gp120 Structure
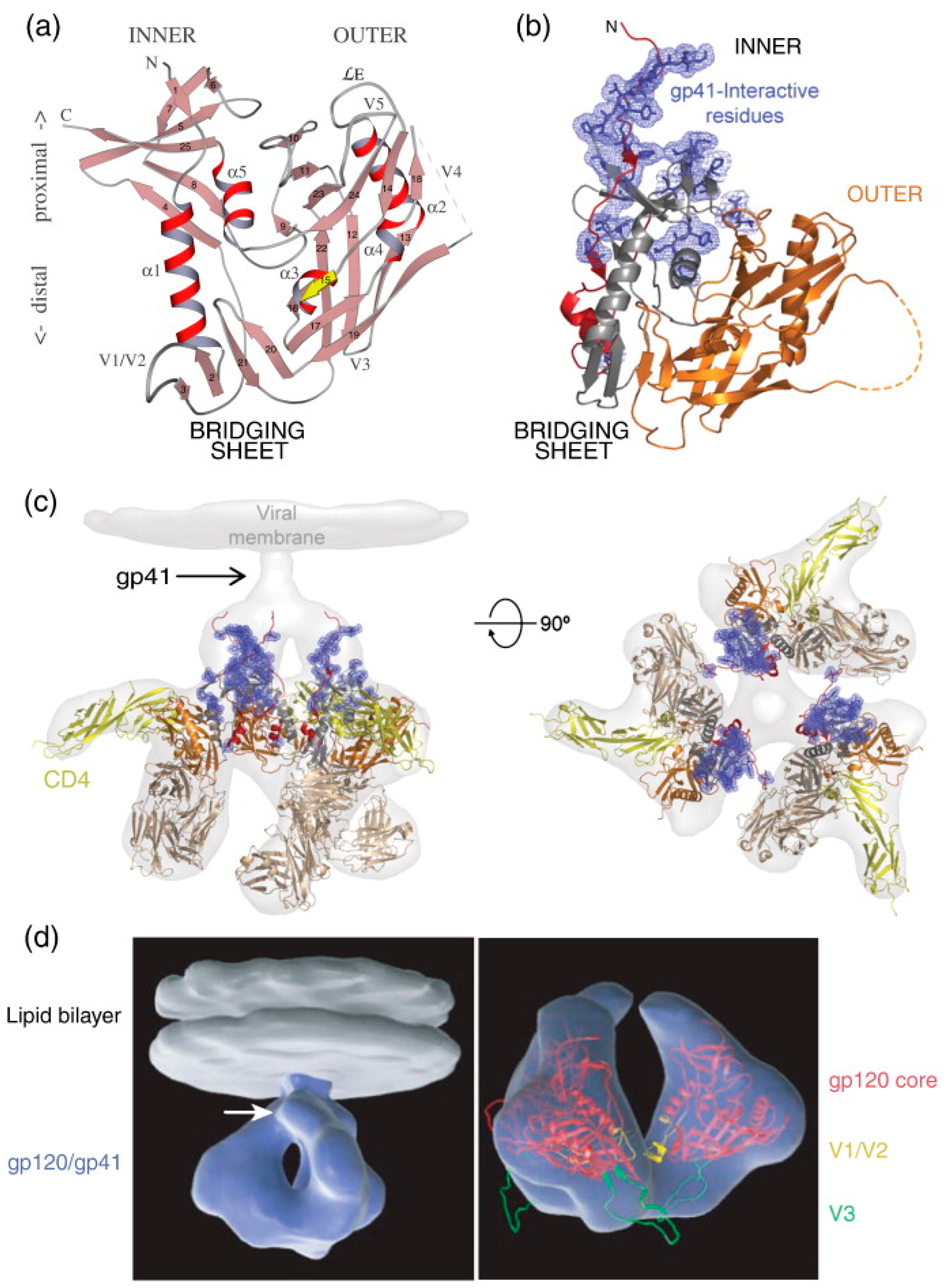
gp120, a globular glycoprotein weighing 120 kDa, is comprised of 480 amino acids with approximately 20–35 N-glycosylation sites, nine disulfide bridges, and five conserved regions (C1–C5) interspersed with five variable regions or loops (V1–V5), (Figure 2a and Figure 4a) [84]. The structure of gp120 folds and generates an outer domain, an inner domain (incorporating the C and N termini associated with gp41), and a bridging sheet that connects these two domains. The CD4 binding pocket is above the bridging sheet and between the outer and inner domains [86]. While the conserved regions constitute the core, the variable regions are positioned near the surface of gp120. Nonetheless, all three domains of gp120 play crucial roles in binding to CD4 and coreceptors. Glycosylation of gp120 predominantly occurs on the protein surface, particularly on the outer domain and the V4 and V5 variable loops, which harbor an immunosilent face [84].
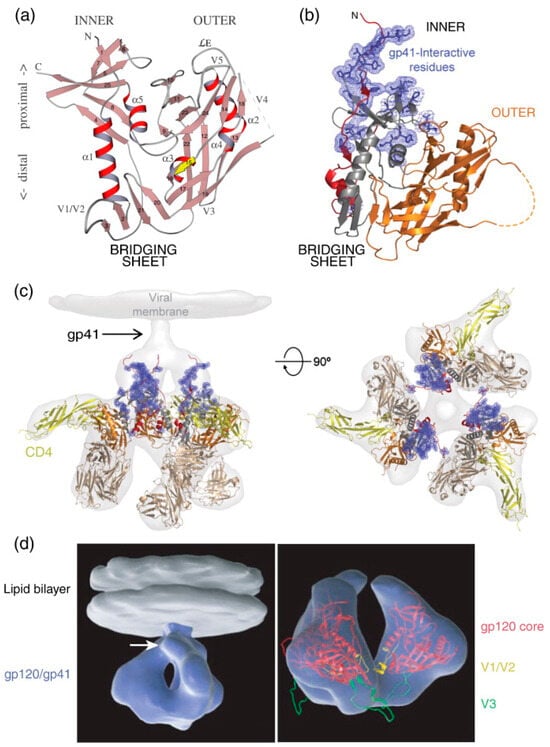
Figure 4.
HIV-1 Env gp120 and gp41 Structures. (a) Ribbon diagram of gp120 core: The ribbon diagram of the gp120 core displays its α-helices (α1–α5) and β-strands (1–25). The relative positions of the variable loops (V1–V5) and the N- and C-termini are indicated. In this orientation, the viral membrane is positioned at the top and the cell membrane at the bottom. Upon binding to CD4, gp120 forms a bridging sheet composed of four β-strands, which separate the inner and outer domains of gp120 with their orientation in the trimeric complex. (b) Ribbon diagram of gp120 core with interaction sites: This diagram shows the gp120 core as in (a), highlighting the N-terminus (red) and the gp41 interaction site (blue). The inner domain is depicted in red and gray, while the outer domain is shown in orange. The bridging sheet, which includes elements from the inner and outer domains, is illustrated in gray and orange. (c) Trimeric gp120 bound to CD4 and antibody Fab: The trimeric gp120 is shown in the same colors as in (b), bound to three molecules of CD4 (yellow) and the Fab fragment from the neutralizing antibody 17b (brown), used to stabilize the gp120 structure. This complex is superimposed onto the electron density observed by cryo-electron tomography (light gray). The right side shows the same structure rotated 90°, positioning the viral membrane in the plane of the page. (d) Three-dimensional representation of HIV-1 Env in CD4-bound conformation: On the left, a trimeric Env spike (blue) is anchored in the lipid bilayer of the viral membrane (gray). The white arrow indicates the predicted location of gp41. On the right, a ribbon diagram of the gp120 core (red) is superimposed on the density map (blue), with the V1/V2 loop in yellow and the V3 loop in green. Reprinted (adapted) with permission from [31] Copyright Elsevier (2024).
Earlier high-resolution structural studies often utilized truncated fragments of gp120 and gp41 to facilitate crystallizing and determining atomic structures. These truncated forms typically consist of the gp120 core, which lacks variable regions V1, V2, and V3, as well as the C- and N-terminal segments. The gp120 core was initially crystallized in two forms: a fully glycosylated unliganded state [87] and a CD4-bound deglycosylated form complexed with an antigen-binding fragment (Fab) capable of recognizing the CD4-induced (CD4i) epitope [88]. Subsequent studies have reported HIV-1 gp120 core crystal structures with C- and N-terminal extensions, an intact V3 loop, and unliganded forms. These modifications aim to provide a more comprehensive understanding of the structural dynamics and functional properties of gp120 [89,90].
gp120 undergoes glycosylation, with approximately half of its molecular mass being glycosylated with N-linked glycans and a smaller fraction bearing O-linked sugars. It harbors 20–35 N-linked glycosylation sites, while gp41 possesses 3–5 N-linked glycosylation sites. These glycosylation sites play crucial roles in host immune recognition, Env folding, and the binding of virions to the host cell surface [31]. Among the 24 N-linked glycosylation sites in gp120, 11 contain hybrid or high-Man structures, while 13 contain complex-type oligosaccharides. Notably, these N-linked glycosylation sites are well conserved in the gp120 amino acid sequence [37]. Additionally, Cysteine (Cys) residues in gp120 and gp41 are highly conserved and play a pivotal role in forming intramolecular disulfide bonds (Figure 2a), which are key components of the Env tertiary structure. gp120 contains nine disulfide bridges formed by covalently bound Cys residues. Among these, two disulfide bonds separate the V1 and V2 loops, while another disulfide bond connects the two loops into a large loop. Additionally, a disulfide bond delineates the V3 and V4 regions. As identified in various studies, the presence and arrangement of Cys residues can significantly impact Env structure and antigenicity by altering their numbers and distribution [31].
Several factors, including deletions, insertions, point mutations, and recombination events, influence the sequence variability of the V domains. Among these, the V1/V2 domain exhibits the highest variability in loop length and number of glycosylation sites [91,92]. The length of V1/V2 loops can range from 50 to 90 amino acids, while the V3 loop typically spans 35 amino acids. Additionally, the lengths of the V4 and V5 domains vary between 19 to 44 and 14 to 36 amino acids, respectively. In contrast, the lengths of the V3, C2, C3, and C4 loops show relatively slight variation [31]. The increase in length and number of glycosylation sites in the V1/V2 domain correlates with disease progression, suggesting a potential role in evading humoral immunity [93]. Furthermore, the secondary structure is influenced by the number of glycosylation sites, particularly in mediating interactions between the V3 loop and neighboring regions.
In the tertiary structure of Env, discontinuous segments containing conserved residues are folded into proximity to form the CD4 receptor binding site. Within gp120, the conserved domains C1, C3, and C4 are responsible for binding to CD4 [94]. The V1, V2, and V3 domains are not directly involved in CD4 binding, as demonstrated by studies where a variant of gp120 with deleted V1, V2, and V3 domains still exhibits high-affinity binding to CD4 [31]. Research has elucidated that the V3 loop plays critical roles in membrane fusion and coreceptor specificity [95] and harbors dominant epitopes recognized by neutralizing antibodies. Mutations within the V3 loop can lead to changes in tropism from R5 to X4, primarily through an increase in net positivity, which facilitates interactions with the negatively charged surface of CXCR4 [31].
In addition to mutations in the V3 loop, modifications in the “bridging sheet” region of gp120 can significantly influence how coreceptors interact [96]. Maraviroc, an FDA-approved drug, specifically targets the interaction between the V3 loop of gp120 and the CCR5 coreceptor. It directly binds to CCR5 and prevents the association between gp120 and CCR5 [97]. However, maraviroc is impractical for viruses that utilize the CXCR4 coreceptor, as expected from a drug targeting the CCR5 receptor. Resistance to maraviroc primarily arises from mutations that allow gp120 to effectively engage with drug-bound CCR5 rather than switching to CXCR4. These mutations enable the virus to circumvent the inhibitory effects of maraviroc by maintaining its dependence on the CCR5 coreceptor [97,98]. Vaccine Research Center (VRC) in NIAID (National Institute of Allergy and Infectious Diseases) has successfully elucidated various structures of HIV-1 gp120 envelope glycoprotein. For instance, one notable structure involves the HIV-1 gp120 “core” lacking the V1/V2 and V3 variable loops and the N- and C-termini. This core structure was observed in complex with a neutralizing antibody and fragments of the CD4 receptor [88]; a gp120 core structure with V3 loop, bound to neutralizing antibodies and the CD4 receptor [96]; unliganded CD4-bound HIV-1 gp120 [89]; CD4-binding-site-specific antibodies poorly neutralizing the gp120 core [99]; CD4-binding-site-specific neutralizing antibodies bound to the gp120 core in a CD4-bound conformation [100]; and gp120’s core bound to gp41’s gp120-interacting region [101].
The research findings provide a detailed understanding of how gp120 interacts with key components involved in HIV-1 entry, including CD4, gp41, coreceptors, entry inhibitors (such as T-20), and neutralizing antibodies. Specifically, gp120’s binding to CD4 initiates conformational changes that enable its interaction with coreceptors (CCR5 or CXCR4) and gp41, facilitating membrane fusion and viral entry. Additionally, these interactions expose epitopes on gp120 that are targeted by neutralizing antibodies, making this process critical for developing entry inhibitors and vaccines (as shown in Figure 4) [88,102,103,104,105]. In contrast to unbound gp120 CD4-bound structures, the binding of CD4 triggers a significant structural rearrangement in gp120. Post-CD4 binding, previously spatially distant residues undergo conformational changes to form the coreceptor binding surface [87]. When the viral complex interacts closely with the target cell, steric hindrance impedes the efficient access of neutralizing antibodies. This action enables the virus to delay exposure of the vulnerable coreceptor binding surface. Models depicting gp120 trimers in unbound and CD4-bound states can be generated using X-ray data and electron tomography maps (Figure 4c,d) [62]. These structural investigations suggest that HIV-1 Env glycoproteins are highly adapted to conceal critical functional surfaces from antibody recognition while maintaining flexibility and tolerance to sequence variations to evade immune responses.
gp120 engages the CD4 receptor via the CD4 binding site located on its surface. This binding site predominantly comprises conserved regions between the β inter-domain sheet and two domains of gp120. Positioned on the opposite side of the HLA type II binding domain within the D1 domain of the Complementarity Determining Region 2 (CDR2) loop, the CD4 binding domain plays a pivotal role. Residues Phe43 and Arg59 are particularly crucial, as they establish significant interactions with the residues Glu370, Trp427, and Asp368 within the CD4 binding site of gp120. The varying affinity of HIV Env for CD4 is contingent upon factors such as tropism and the monomeric or oligomeric quaternary structures. Moreover, the flexibility of gp120 in terms of conformation enables its binding to a conformationally inert site on CD4 [38]
2.3. gp41
The viral lipid envelopes establish binding with host cell membranes primarily through the gp41 TM glycoprotein. The gp41 subunit comprises approximately 345 amino acids. It is organized into three main domains: a TMD spanning 21 amino acid residues, an extracellular domain (or ectodomain) containing 172 amino acid residues, and a C-terminal CT consisting of 142 amino acid residues, as shown in Figure 5 [106]. The ectodomain harbors a pivotal fusion determinant comprised of distinct components: a poly region, a hydrophobic fusion peptide positioned at the N-terminus, the MPER abundant in tryptophan residues, and two hydrophobic heptad-repeat regions referred to as HR1 and HR2 (also called N-helix and C-helix, respectively). These elements collaborate to form α-helical coiled-coil structures, playing a crucial role in mediating the fusion process necessary for viral entry into host cells [107]. The fusion process is primarily driven by the interaction between HR1 and HR2, facilitated by a disulfide bridge within a hydrophilic loop. In typical gp120 and gp41 quaternary complexes, the fusion peptide is concealed. However, upon binding gp120 to CD4 and coreceptor, the fusion peptide is exposed, leading to membrane destabilization. Subsequently, the fusion pore is formed, allowing the fusion peptide to penetrate tissues [108,109].
Each trimer consists of three HR1 motifs aligned in parallel, folding over an antiparallel hydrophobic groove with three HR2 domains, culminating in forming a stable six-helix bundle capable of facilitating fusion [110]. Cellular fusion proteins, such as SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) and other viral fusion proteins, form prompt fusion mechanisms via gp41 [31]. HR1 and HR2 peptides can obstruct the formation of the gp41 six-helix bundle. HIV-1-infected individuals can employ HR2-derived peptides like enfuvirtide (Fuzeon or T-20) for therapeutic intervention; however, due to its elevated cost and limited oral bioavailability, its clinical applicability is restricted. The gp41 extracellular domain encompasses 24 amino acids in the MPER. Although the precise mechanism through which this region fosters membrane fusion remains elusive, it is imperative for fusogenicity and virus propagation. Neutralizing antibodies such as 2F5, 4E10, and Z13 target the highly conserved MPER region [110].
The Env protein is anchored to the lipid bilayer by the gp41 TMD, which comprises approximately 25 conserved amino acids. Mutations within the “core” region of the gp41 TMD can influence Env-mediated fusion due to this domain’s high degree of conservation [111]. Traditionally, the topology of gp41 suggests that the TMD forms a single α-helix spanning the membrane, while the CT resides within the viral particle (Figure 5, left). Alternative topologies, proposing that gp41 spans the membrane three times (initially based on monoclonal antibodies targeting an epitope within the gp41 CT), have also been suggested (Figure 5, right, known as the “Kennedy” sequence (KS)) [112]. Findings by Lu et al. demonstrate that the LLP2 region in the gp41 CT may occasionally become exposed on the cell surface during cell fusion [113]. Due to its critical role in the fusion process, LLP2 is considered a promising target for antiviral therapies, as disrupting its interactions could prevent HIV-1 from successfully merging with host cells and thereby inhibit infection. Located in the cytoplasmic domain alongside other lytic peptide regions like LLP1 and LLP3, LLP2 is characterized by amphipathic helices facilitating lipid bilayer interactions. This membranotropic region influences the fusogenic activity of gp41 by interacting with both the viral core and host membranes, thereby regulating fusion efficiency. The surface-exposed nature of LLP2 allows it to modulate interactions within the gp41 structure, optimizing conditions for viral entry [114,115]. Similarly, SERINC proteins, particularly SERINC5, play a key role in restricting HIV-1 infectivity by integrating into the viral membrane, altering the viral Env conformation, and disrupting the membrane fusion process. SERINC5 is the most potent of the SERINC family (SERINC5 highest; SERINC3 moderate and SERINC2 zero), significantly reducing HIV-1 infectivity, especially in Nef-deficient viruses. Its action primarily interferes with Env clustering during virus entry, potentially exposing the virus to neutralizing antibodies. These features highlight the importance of SERINC5 in host defense and HIV-1 evasion strategies [116,117].
The membrane topology of the gp41 CT remains debated, with two primary models: the single membrane-spanning domain (MSD) model, placing the KE sequence (residues 731–744) intracellularly, and an alternative model suggesting multiple MSDs that expose the KE on the virion surface [60,112,118,119]. For instance, an antibody targeting the KS on cells expressing Env does not bind to Env on intact virions [112]. HIV-1 resistance to the cholesterol-binding compound amphotericin B methyl ester (AME) was found to be further enhanced by viral protease (PR) cleavage sites flanking the KS [31]. Recent NMR studies support the single MSD model, showing an unstructured, non-membrane-associated gp41 CT. However, studies indicating that anti-KE antibodies can neutralize HIV-1 suggest surface exposure of the KE sequence during viral entry. This dynamic exposure, possibly occurring during membrane fusion, requires further investigation to fully understand gp41’s role in HIV-1 entry [120,121,122]. This finding implies that a region of gp41 within virions can be cleaved by PR, supporting the notion that this region is located internally [31].
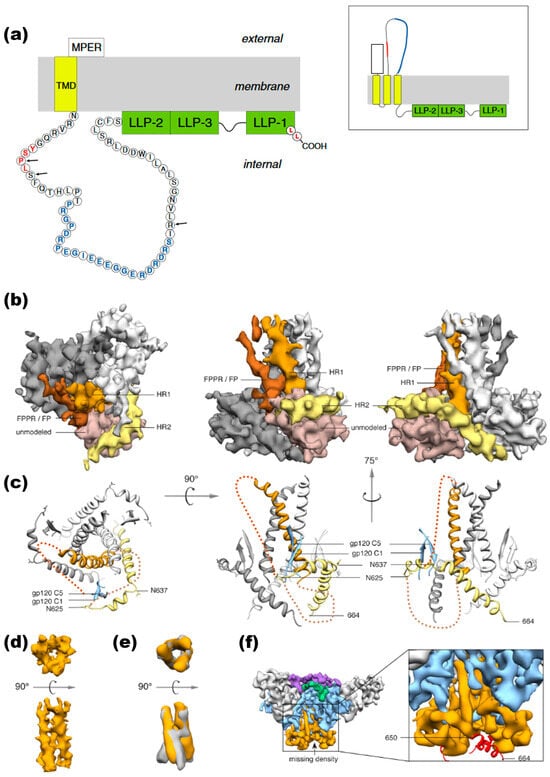
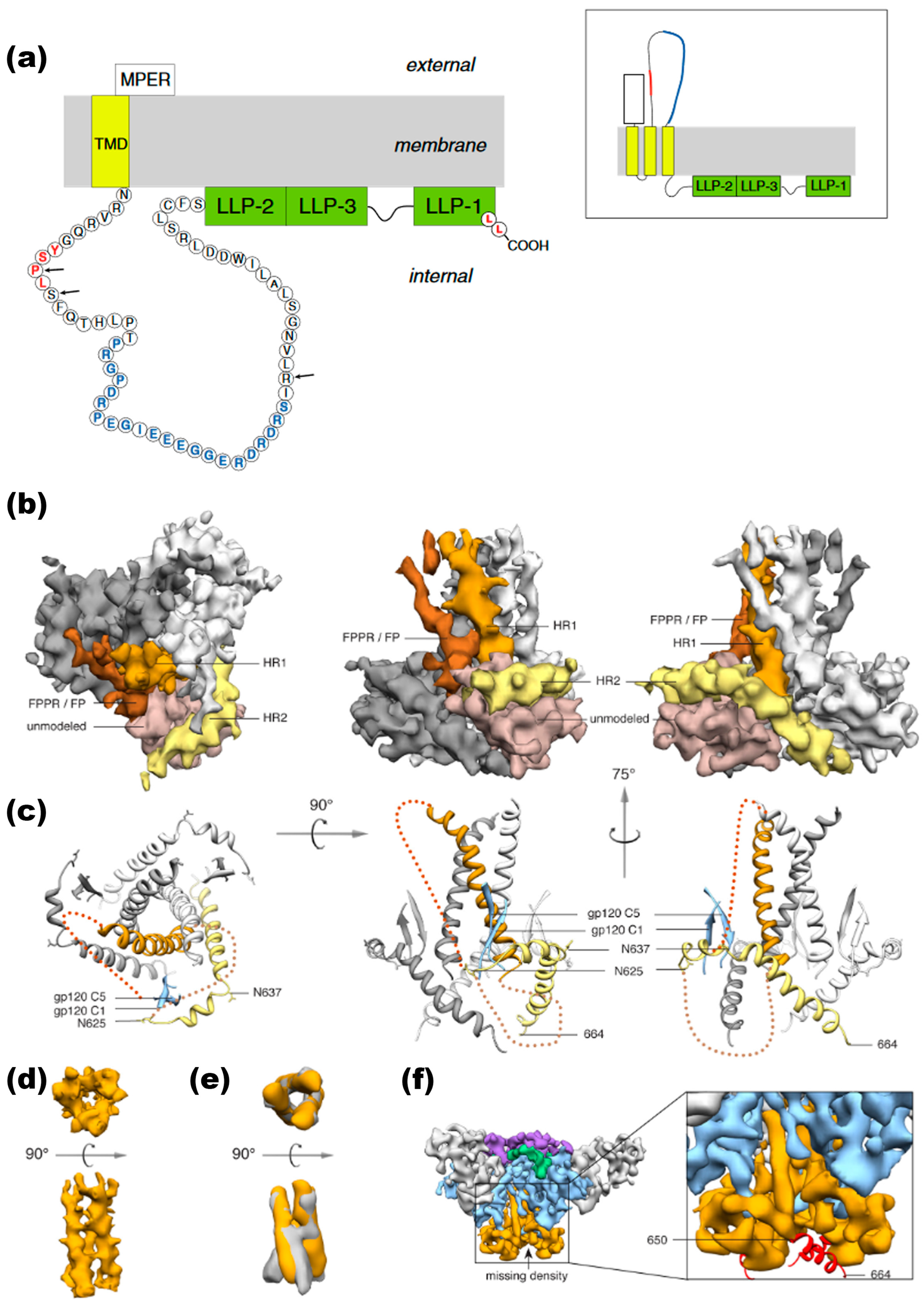
Figure 5.
HIV-1 gp41 CT Topology and Structure. (a) gp41 CT topology. Traditional model (left): The conventional model of the gp41 CT posits a single membrane-spanning domain, likely found in virions. This single-pass model places the entire CT inside the virion (internal). Sites of mutation that confer resistance to amprenavir (AME), which subsequently became new HIV-1 protease (PR) cleavage sites, are marked by black arrows. The epitope known as the “KS,” recognized by neutralizing antibodies, is shown in blue. Alternative model (right): An alternative topology suggests a three-membrane-spanning configuration for the gp41 CT, which exposes portions of the gp41 CT, including the KS, to the extracellular space. This topology may also occur with a detectable frequency in HIV-1 Env-expressing cells alongside the traditional model. Reprinted (adapted) with permission from [31] Copyright Elsevier (2024). (b) Segmented EM density map of gp41 trimer. The segmented EM density map of the gp41 trimer, with gp120 removed, shows the structural arrangement: The C-terminal half of HR1 (rust) forms a central three-helix bundle. The C-terminal half of HR2 (yellow) wraps helically around the trimer base. Unassigned density (beige) likely corresponds to the intervening region between HR1 and HR2, including the disulfide loop and elements from gp120 (C1 and C5). Density parallel to HR1 (brown) likely corresponds to the N-terminal half of HR1, the fusion peptide proximal region (FPPR), and the fusion peptide (FP). (c) Modeled portion corresponding to EM density maps shows the modeled portion of gp41. (d) Three-helix bundle in PGV04-bound trimer. The EM density map illustrates the three-helix bundle formed by HR1 in the structure bound by the PGV04 antibody. (e) Overlay of EM densities compares the three-helix bundle EM density (orange) from the PGV04-bound structure, filtered to 9.5 Å, with the 9 Å reconstruction of a 17b-bound SOSIP gp140 trimer (gray, EMDB-5462). (f) Reconstruction of SOSIP trimer with deletions. An 8.2 Å reconstruction of a SOSIP trimer from which the last 14 amino acids were deleted (SOSIP.650:PGV04) is shown. The difference between the SOSIP.650 and SOSIP.664 maps corresponds to a short helical segment (red) at the end of HR2 that projects toward the adjacent protomer. Reprinted (adapted) with permission from [123] Copyright Science (2024).
Figure 5.
HIV-1 gp41 CT Topology and Structure. (a) gp41 CT topology. Traditional model (left): The conventional model of the gp41 CT posits a single membrane-spanning domain, likely found in virions. This single-pass model places the entire CT inside the virion (internal). Sites of mutation that confer resistance to amprenavir (AME), which subsequently became new HIV-1 protease (PR) cleavage sites, are marked by black arrows. The epitope known as the “KS,” recognized by neutralizing antibodies, is shown in blue. Alternative model (right): An alternative topology suggests a three-membrane-spanning configuration for the gp41 CT, which exposes portions of the gp41 CT, including the KS, to the extracellular space. This topology may also occur with a detectable frequency in HIV-1 Env-expressing cells alongside the traditional model. Reprinted (adapted) with permission from [31] Copyright Elsevier (2024). (b) Segmented EM density map of gp41 trimer. The segmented EM density map of the gp41 trimer, with gp120 removed, shows the structural arrangement: The C-terminal half of HR1 (rust) forms a central three-helix bundle. The C-terminal half of HR2 (yellow) wraps helically around the trimer base. Unassigned density (beige) likely corresponds to the intervening region between HR1 and HR2, including the disulfide loop and elements from gp120 (C1 and C5). Density parallel to HR1 (brown) likely corresponds to the N-terminal half of HR1, the fusion peptide proximal region (FPPR), and the fusion peptide (FP). (c) Modeled portion corresponding to EM density maps shows the modeled portion of gp41. (d) Three-helix bundle in PGV04-bound trimer. The EM density map illustrates the three-helix bundle formed by HR1 in the structure bound by the PGV04 antibody. (e) Overlay of EM densities compares the three-helix bundle EM density (orange) from the PGV04-bound structure, filtered to 9.5 Å, with the 9 Å reconstruction of a 17b-bound SOSIP gp140 trimer (gray, EMDB-5462). (f) Reconstruction of SOSIP trimer with deletions. An 8.2 Å reconstruction of a SOSIP trimer from which the last 14 amino acids were deleted (SOSIP.650:PGV04) is shown. The difference between the SOSIP.650 and SOSIP.664 maps corresponds to a short helical segment (red) at the end of HR2 that projects toward the adjacent protomer. Reprinted (adapted) with permission from [123] Copyright Science (2024).
Unlike other retroviruses, lentiviral envelope (Env) glycoproteins feature an unusually long CT in their transmembrane (TM) subunit [124]. Typically, the CT of gp41 in lentiviruses is approximately 150 amino acids in length, whereas the equine infectious anemia virus (EIAV) has even longer CTs, about 200 amino acids [125]. In contrast, other retroviruses such as Rous sarcoma virus (RSV), Mason-Pfizer monkey virus, and murine leukemia virus (MLV) have TM CTs that are only 20–40 amino acids long. In animal models, truncation of the lentiviral CT has been shown to inhibit virus replication. This observation supports the hypothesis that the long CT in lentiviral TM Env glycoproteins plays a role in suppressing viral transmission [126]. The CT of HIV-1 gp41 influences various viral properties, including Env incorporation, shedding of gp120, viral infectivity, cell surface expression of Env, and Env-induced membrane fusion [31]. Mutations in the CT of HIV-1 and SIV gp41 also affect the conformation of gp120 and the ectodomain, impacting antibody recognition and neutralization. Some HIV-1 isolates that have evolved CD4 independence exhibit truncated gp41 CTs, suggesting that this domain is crucial for regulating gp120 conformation [127,128].
The gp41 CT contains several functional and structural domains that modulate its activity (Figure 2 and Figure 5b–f). The adaptor protein complex 2 (AP-2) mediates clathrin-dependent endocytosis of the Env protein from the plasma membrane via a membrane-proximal tyrosine-based sorting signal, YxxL [129,130]. This motif facilitates the release of viral particles from the basolateral surface of polarized epithelial cells and supports cell-to-cell transmission in T cells [131]. Mutation of the analogous signal in simian immunodeficiency virus (SIV) significantly attenuates viral replication in vivo [132]. Additionally, the clathrin adaptor protein complex 1 (AP-1) interacts with a dileucine motif in the gp41 CT to regulate the intracellular distribution of Env [133]. However, the YxxL motif is not involved in the endocytosis of Env [113,114]. As shown in Figure 5a, The central and C-terminal regions of the gp41 CT contain three conserved amphipathic α-helical segments known as LLP-1, LLP-2, and LLP-3 [31]. The α-helices of LLP-1 and LLP-2 possess arginine residues on one face, conferring a high positive charge. LLP-3 is situated between these segments. These LLP domains are highly conserved among lentiviruses and are linked to various functions, including Env fusogenicity, multimerization, cell-surface expression, protein stability, and incorporation into virions. Additionally, LLP fragments can bind to and perturb membranes, causing cytolysis, hence the designation “lytic peptide” [31].
Substituting arginine (Arg) residues in LLP-1 with glutamic acid (Glu) preserves this segment’s secondary structure and hydrophilicity. Nonetheless, it abolishes its cell lysis activity, indicating that these residues are crucial for LLP function [134]. The CT of gp41 also contains two cysteine (Cys) residues that undergo palmitoylation, a post-translational modification posited to target Env to lipid rafts, although this remains somewhat controversial [135]. Furthermore, the LLP-1 segment appears to contain determinants for lipid raft association, as truncation of LLP-1 diminishes Env localization to these membrane microdomains [136]. These findings highlight the diverse biological functions of the gp41 CT, many of which are not fully understood. Elucidating the role of this region of Env necessitates investigating its biological properties in relevant primary cell types, assessing its role in cell–cell communication, and identifying its cellular partners.
At the base of the trimer, gp41 forms a pedestal structure (Figure 5b,c). Each protomer within the trimer features two prominent helices. HR1, with its C terminus near the viral membrane, forms a three-helix bundle with the HR1 regions of adjacent protomers in the trimer core. Meanwhile, HR2 encircles the trimer base, with its C terminus angling downward (Figure 5b,c). The three-helix bundle formed by the C-terminal half of HR1 resembles the structure of gp41 in its postfusion state [137], as well as the open, intermediate state observed in single-particle EM studies (Figure 5d,e) [138]. An independent 12.7 Å reconstruction of the unliganded trimer was performed to determine whether the three-helix bundle results from PGV04 binding. This structure also exhibited the three-helix bundle, suggesting that the observed gp41 conformation likely represents the closed prefusion state rather than an activated intermediate [138]. Above the three-helix bundle and below the trimer apex, a small opening continuous with the exterior is visible. This opening contrasts previous models, which reported a large hole in the trimer core—likely a consequence of lower resolution reconstructions exceeding 20 Å [62]. The C terminus of gp41 was identified through an ~8 Å resolution reconstruction of a trimer with the last 14 amino acids removed (referred to as SOSIP.650). Difference maps indicated that SOSIP.650 lacked a segment corresponding to approximately 3.5 helical turns, or about 14 amino acids (Figure 5f). Additionally, HR2 residues around position 650 show strong interactions with HR1 at the base of the trimer [123].
The fusion peptide proximal region (FPPR) and fusion peptide (FP) emerge from the apex of the internal coiled-coil structure surrounding the trimer’s threefold axis, curving downward toward the trimer base within the N-terminal portion of HR1, featuring a short helix (Figure 5b). Additional density is observed at the trimer base, which is not definitively attributed but speculated to correspond to residues located between HR1 and HR2 and segments within gp120’s C1 and C5 regions (Figure 5b,c). This interpretation shows interactions between gp120’s C1/C5 and residues 589–610 of gp41 across different subunits [139]. In SOSIP gp140s, this gp41 segment encompasses an internal disulfide-bonded loop and includes engineered cysteine substitutions facilitating covalent bonding between gp120 residue 501 and gp41 residue 605. Within the trimer’s midpoint, the hydrophobic ends of gp120 and nearby gp41 residues are secluded (Figure 5c). This region, inaccessible to solvents, is tentatively designated as the hydrophobic fusion peptide (FP); similarities are noted with fusion glycoproteins from influenza, respiratory syncytial virus, and the Ebola virus. Upon triggering membrane fusion through conformational changes induced by CD4 and coreceptors, the FP is anticipated to be liberated [123].
3. gp120–gp41 Interaction
The HIV surface exhibits trimeric Env spikes formed through noncovalent interactions between gp120 and gp41 [140]. During the entry of HIV into host cells, a dynamic structural rearrangement takes place (Figure 5). Initially, gp120 binds to the CD4 receptor on the host cell, causing a conformational change that disrupts the noncovalent interactions between gp120 and gp41 and reveals the coreceptor-binding sites [141]. This disclosure leads to the reorganization of gp41 helices within the Env spikes into open structural conformations, allowing the rest of the Env complex to undergo subsequent conformational changes [29]. In the prefusion state, gp41’s hydrophobic core binds tightly to the extended N-terminal domain (NTD) and C-terminal domain (CTD) of gp120, forming the gp41-tryptophan clasp [66,83]. The rearrangements in the gp41 spike result in the opening of the gp41-tryptophan clasp, facilitating the enlargement of the fusion pore necessary for viral entry by displacing the gp120 termini [66,142]. Observations indicate that the structural transition from prefusion to postfusion conformations of gp41 is critical for this process. While most HIV strains require two to three Env spikes for entry stoichiometry, constructing the fusion pore may involve one to seven Env spikes [143,144].
Numerous studies have investigated the interaction between gp120 and gp41 to elucidate their interaction domains [29,58,65,66,83,86,101,123,145,146]. There is a consensus that the inner domain, N-terminal domain, and disulfide-bonded domain of gp120 form noncovalent interactions with the heptad repeat 1 region of gp41. Specifically, the inner domain of gp120 can modulate its interaction with gp41 and its binding to CD4 [86], while the terminal regions of gp120 primarily interact with the disulfide-bonded regions of gp41 [147]. gp120–gp41 interactions can be disrupted through amino acid substitutions in the disulfide-bonded region of gp41, such as W596A and W610A [148]. Additionally, broad neutralizing antibodies, such as 3BC315, have been identified to interfere with these interactions. However, the dynamic nature of Env trimers may influence the exposure of epitopes targeted by these antibodies [147,149,150].
4. gp41Env–Matrix
The HIV-1 matrix in Gag precursors (matrixGag) physically interacts with gp41Env [79,147,151,152,153], a relationship crucial in several viral processes. This interaction enhances Env packaging during viral budding and facilitates the rearrangement of Env pre-bundle structures during viral entry [154,155,156]. The gp41Env-matrixGag interaction performs multiple functions, including the interaction of gp41CT with unprocessed Gag in immature HIV-1 particles, suppressing HIV-1 entry [157]. A study used cryo-ET to analyze the Env protein and matrix in HIV-1 particles, achieving a 9.1 Å resolution. It revealed unexpected features, such as a variable central core in gp41, diverse glycosylation, and a flexible stalk that affects Env tilting and epitope exposure. In immature particles, Env positioning differed from its placement in mature particles, highlighting the key role of Gag-Env interactions in virus assembly. However, this study used viruses with a truncated Env CT, which is important for interacting with the matrix [32]. Therefore, the exact accommodation of functional Env into the matrix lattice remains to be determined, although some studies have suggested models for this interaction. These findings and future studies elucidating the Env-matrix interface enhance our understanding of HIV assembly and are crucial for developing vaccines and therapies [30,158].
Upon cleavage of Gag and GagPol precursors by HIV-1 protease, these immature particles transition into mature HIV-1 particles [157]. The proteolytic cleavage of the Gag polyprotein during HIV-1 maturation leads to a reorganization of the matrix lattice, which is crucial for viral entry. This process is dependent on the differential localization of Env glycoprotein trimers, mediated by the interaction between the gp41Env-matrixGag interaction [159,160]. Additionally, this interaction prevents the biotinylation of gp41CT by matrixGag [161]. The interaction domains between the basic and C-terminal regions of HIV-1 matrixGag and gp41CT involve a physical interaction [152,162,163]. The matrix substitution L49D in gp120–gp41 can destabilize this interaction, but the Y710S substitution in gp41CT can reverse this destabilization [164]. The gp41Env-matrixGag interaction relies explicitly on the last 13 to 43 amino acids in the gp41CT region [165]. Furthermore, mutations in gp41CT can contribute to resistance against HIV protease inhibitors (PIs) [166].
The HIV-1 Tat protein significantly interacts with Env, playing a key role in viral entry and pathogenesis. Tat’s binding to gp120 promotes its conformational change and enhances viral entry efficiency by facilitating binding to CD4 and coreceptors [147,167,168,169,170,171]. Tat forms a complex with native trimeric Env glycoproteins on HIV-1 virions, enhancing the virus’s ability to enter dendritic cells via an integrin-mediated endocytic pathway, distinct from the canonical C-type lectin receptor pathway. This interaction, primarily involving the V3 loop of Env, is crucial for viral entry, with modifications to this loop affecting the Tat/Env complex’s stability. Shortening Env’s V1/V2 loop also enhances this stability, commonly seen in founder viruses at mucosal entry points [172,173].
Physical interaction between HIV-1 Tat and gp120 has been observed using techniques, including isothermal titration calorimetry, electron cryomicroscopy, ELISAs, pulldown assays, and surface plasmon resonance analyses [167,171,174,175]. Extracellular Tat also interacts with chemokine receptors such as CCR2 and CCR3, recruiting chemokine receptor-expressing macrophages and monocytes to HIV-infected cells [176]. Additionally, Tat released from infected cells can physically interact with the extracellular membranes of uninfected cells [167]. The gp120-Tat interaction can alter viral coreceptor tropism, allowing X4-tropic virus gp120 to interact efficiently with CXCR4 and CCR5 [170,177]. While this interaction affects viral entry, it does not impact Tat-mediated transactivation [167]. Molecular docking analyses suggest that the CD4-binding site and the V3 loop of gp120 may interact with the cysteine-rich domain of Tat [171,175]. Other studies propose that the V1/V2 loop of gp120 may also serve as a binding site for Tat’s second exon [167,174]. One significant consequence of the extracellular Tat-gp120 interaction is enabling HIV to evade neutralization by anti-Env antibodies, as Tat binding to Env spikes blocks their recognition [169,171,177].
5. Conclusions
The complexities of HIV-1 entry represent a crucial and intriguing challenge in molecular virology. Understanding the precise mechanisms of this process is vital for developing effective vaccines and therapeutic interventions. This review has highlighted the critical roles of HIV’s entry proteins, gp120 and gp41, in facilitating the viral invasion of host cells, emphasizing the need for a thorough understanding of their structural and functional dynamics. However, several limitations persist in our current knowledge. For instance, much of the research on HIV-1 entry has been conducted on isolated protein fragments, which may not fully represent the behavior of the intact Env protein in its native membrane environment. Additionally, despite advancements in imaging and structural biology, the transient and dynamic nature of the interactions between HIV-1 Env, cellular receptors, and the HIV-1 matrix remains only partially understood. Addressing these gaps is crucial for translating structural insights into therapeutic success. Future research should focus on the full-length Env protein within its native membrane context to understand its conformational changes during membrane fusion and interactions with cellular receptors and the HIV-1 matrix. Furthermore, more comprehensive models integrating the contributions of accessory proteins and host factors are needed to capture the full complexity of the viral entry process. Recent advancements in cryo-EM and live-cell imaging techniques offer promising avenues to overcome these challenges. Cryo-EM provides high-resolution visualization of macromolecular structures in a near-native state, offering unprecedented insights into Env’s architecture and conformational states. Live-cell imaging techniques enable real-time observation of molecular interactions and dynamics within the cellular environment. Together, these advanced imaging technologies have the potential to transform theoretical models into real-time molecular movies, offering a dynamic and detailed view of the HIV-1 entry process. By addressing these limitations and leveraging these emerging technologies, future research can significantly enhance our understanding of HIV-1 biology and facilitate the development of more effective therapeutic strategies targeting viral entry mechanisms.
Author Contributions
Conceptualization, A.E.; methodology, A.E. and H.M.; validation, A.E., H.M. and A.Y.R.; formal analysis, A.E.; data curation, H.M. and A.Y.R.; writing—original draft preparation, A.E.; writing—review and editing, H.M. and A.Y.R.; visualization, A.E., H.M. and A.Y.R.; supervision, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV Infection. Nat. Rev. Dis. Prim. 2015, 1, 15305. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Kong, D.; Yang, X.; Zhang, T.; Kuang, Y.Q. Mucosal-Associated Invariant T Cells: A Cryptic Coordinator in HIV-Infected Immune Reconstitution. J. Med. Virol. 2022, 94, 3043–3053. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Quispe, C.; Giordo, R.; Kostić, A.; Katanić Stanković, J.S.; Tsouh Fokou, P.V.; Carbone, K.; Martorell, M.; Kumar, M.; Pintus, G.; et al. Natural Products and Synthetic Analogues against HIV: A Perspective to Develop New Potential Anti-HIV Drugs. Eur. J. Med. Chem. 2022, 233, 114217. [Google Scholar] [CrossRef] [PubMed]
- HIV.gov. The Global HIV and AIDS Epidemic. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 30 May 2024).
- Meissner, M.E.; Talledge, N.; Mansky, L.M. Molecular Biology and Diversification of Human Retroviruses. Front. Virol. 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.; Rousso, I. The HIV-1 Capsid and Reverse Transcription. Retrovirology 2021, 18, 29. [Google Scholar] [CrossRef]
- Joseph, S.B.; Arrildt, K.T.; Sturdevant, C.B.; Swanstrom, R. HIV-1 Target Cells in the CNS. J. Neurovirol. 2015, 21, 276–289. [Google Scholar] [CrossRef]
- Rojas-Celis, V.; Valiente-Echeverría, F.; Toro-Ascuy, D.; Soto-Rifo, R. New Challenges of HIV-1 Infection: How HIV-1 Attacks and Resides in the Central Nervous System. Cells 2019, 8, 1245. [Google Scholar] [CrossRef]
- Coffin, J.M.; Hughes, S.H.; Varmus, H.E. Cellular Targets of Infection. Retroviruses. 2011; pp. 1991–1993. Available online: https://www.ncbi.nlm.nih.gov/books/NBK19434/ (accessed on 15 June 2024).
- Vijayan, K.V.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Schiff, A.E.; Linder, A.H.; Luhembo, S.N.; Banning, S.; Deymier, M.J.; Diefenbach, T.J.; Dickey, A.K.; Tsibris, A.M.; Balazs, A.B.; Cho, J.L.; et al. T Cell-Tropic HIV Efficiently Infects Alveolar Macrophages through Contact with Infected CD4+ T Cells. Sci. Rep. 2021, 11, 3890. [Google Scholar] [CrossRef]
- Maina, E.K.; Adan, A.A.; Mureithi, H.; Muriuki, J.; Lwembe, R.M. A Review of Current Strategies Towards the Elimination of Latent HIV-1 and Subsequent HIV-1 Cure. Curr. HIV Res. 2020, 19, 14–26. [Google Scholar] [CrossRef]
- Woodham, A.W.; Skeate, J.G.; Sanna, A.M.; Taylor, J.R.; Da Silva, D.M.; Cannon, P.M.; Martin Kast, W. Human Immunodeficiency Virus Immune Cell Receptors, Coreceptors, and Cofactors: Implications for Prevention and Treatment. AIDS Patient Care STDS 2016, 30, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Mørch, A.M.; Bálint, Š.; Santos, A.M.; Davis, S.J.; Dustin, M.L. Coreceptors and TCR Signaling—The Strong and the Weak of It. Front. Cell Dev. Biol. 2020, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Artyomov, M.N.; Lis, M.; Devadas, S.; Davis, M.M.; Chakraborty, A.K. CD4 and CD8 Binding to MHC Molecules Primarily Acts to Enhance Lck Delivery. Proc. Natl. Acad. Sci. USA 2010, 107, 16916–16921. [Google Scholar] [CrossRef]
- Leddon, S.A.; Sant, A.J. Generation of MHC Class II-Peptide Ligands for CD4 T-Cell Allorecognition of MHC Class II Molecules. Curr. Opin. Organ Transplant. 2010, 15, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Glatzová, D.; Cebecauer, M. Dual Role of CD4 in Peripheral T Lymphocytes. Front. Immunol. 2019, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.S.; Amrein, K.E.; Hammond, C.; Stern, D.F.; Sefton, B.M.; Rose, J.K. The Ick Tyrosine Protein Kinase Interacts with the Cytoplasmic Tail of the CD4 Glycoprotein through Its Unique Amino-Terminal Domain. Cell 1989, 59, 627–636. [Google Scholar] [CrossRef]
- Claeys, E.; Vermeire, K. The CD4 Receptor: An Indispensable Protein in T Cell Activation and A Promising Target for Immunosuppression. Arch. Microbiol. Immunol. 2019, 3, 133–150. [Google Scholar] [CrossRef]
- Klasse, P.J. The Molecular Basis of HIV Entry. Cell. Microbiol. 2012, 14, 1183–1192. [Google Scholar] [CrossRef]
- Sattentau, Q.J.; Moore, J.P. The Role of CD4 in HIV Binding and Entry. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1993, 342, 59–66. [Google Scholar]
- Chen, B. Molecular Mechanism of HIV-1 Entry. Trends Microbiol. 2019, 27, 878–891. [Google Scholar] [CrossRef]
- Chauhan, A.; Mehla, R.; Vijayakumar, T.S.; Handy, I. Endocytosis-Mediated HIV-1 Entry and Its Significance in the Elusive Behavior of the Virus in Astrocytes. Virology 2014, 456, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Schweighardt, B.; Atwood, W.J. HIV Type 1 Infection of Human Astrocytes Is Restricted by Inefficient Viral Entry. AIDS Res. Hum. Retroviruses 2001, 17, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Canki, M.; Thai, J.N.F.; Chao, W.; Ghorpade, A.; Potash, M.J.; Volsky, D.J. Highly Productive Infection with Pseudotyped Human Immunodeficiency Virus Type 1 (HIV-1) Indicates No Intracellular Restrictions to HIV-1 Replication in Primary Human Astrocytes. J. Virol. 2001, 75, 7925–7933. [Google Scholar] [CrossRef] [PubMed]
- Boutet, A.; Salim, H.; Taoufik, Y.; Lledo, P.M.; Vincent, J.D.; Delfraissy, J.F.; Tardieu, M. Isolated Human Astrocytes Are Not Susceptible to Infection by M- and T- Tropic HIV-1 Strains despite Functional Expression of the Chemokine Receptors CCR5 and CXR4. Glia 2001, 34, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Deiva, K.; Khiati, A.; Hery, C.; Salim, H.; Leclerc, P.; Horellou, P.; Tardieu, M. CCR5-, DC-SIGN-Dependent Endocytosis and Delayed Reverse Transcription after Human Immunodeficiency Virus Type 1 Infection in Human Astrocytes. AIDS Res. Hum. Retroviruses 2006, 22, 1152–1161. [Google Scholar] [CrossRef]
- Piai, A.; Fu, Q.; Sharp, A.K.; Bighi, B.; Brown, A.M.; Chou, J.J. NMR Model of the Entire Membrane-Interacting Region of the HIV-1 Fusion Protein and Its Perturbation of Membrane Morphology. J. Am. Chem. Soc. 2021, 143, 6609–6615. [Google Scholar] [CrossRef]
- Bartesaghi, A.; Merk, A.; Borgnia, M.J.; Milne, J.L.S.; Subramaniam, S. Prefusion Structure of Trimeric HIV-1 Envelope Glycoprotein Determined by Cryo-Electron Microscopy. Nat. Struct. Mol. Biol. 2013, 20, 1352–1357. [Google Scholar] [CrossRef]
- Carlon-Andres, I.; Malinauskas, T.; Padilla-Parra, S. Structure Dynamics of HIV-1 Env Trimers on Native Virions Engaged with Living T Cells. Commun. Biol. 2021, 4, 1228. [Google Scholar] [CrossRef]
- Checkley, M.A.; Luttge, B.G.; Freed, E.O. HIV-1 Envelope Glycoprotein Biosynthesis, Trafficking, and Incorporation. J. Mol. Biol. 2011, 410, 582–608. [Google Scholar] [CrossRef]
- Mangala Prasad, V.; Leaman, D.P.; Lovendahl, K.N.; Croft, J.T.; Benhaim, M.A.; Hodge, E.A.; Zwick, M.B.; Lee, K.K. Cryo-ET of Env on Intact HIV Virions Reveals Structural Variation and Positioning on the Gag Lattice. Cell 2022, 185, 641–653.e17. [Google Scholar] [CrossRef]
- Pan, J.; Peng, H.; Chen, B.; Harrison, S.C. Cryo-EM Structure of Full-Length HIV-1 Env Bound With the Fab of Antibody PG16. J. Mol. Biol. 2020, 432, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- McCaul, N.; Quandte, M.; Bontjer, I.; van Zadelhoff, G.; Land, A.; Crooks, E.T.; Binley, J.M.; Sanders, R.W.; Braakman, I. Intramolecular Quality Control: HIV-1 Envelope gp160 Signal-Peptide Cleavage as a Functional Folding Checkpoint. Cell Rep. 2021, 36, 109646. [Google Scholar] [CrossRef]
- Fenouillet, E.; Jones, I.M. The Glycosylation of Human Immunodeficiency Virus Type 1 Transmembrane Glycoprotein (gp41) Is Important for the Efficient Intracellular Transport of the Envelope Precursor gp160. J. Gen. Virol. 1995, 76, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Lerner, G.; Weaver, N.; Anokhin, B.; Spearman, P. Advances in HIV-1 Assembly. Viruses 2022, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Botos, I.; Wlodawer, A. Proteins That Bind High-Mannose Sugars of the HIV Envelope. Prog. Biophys. Mol. Biol. 2005, 88, 233–282. [Google Scholar] [CrossRef]
- Benjelloun, F.; Genin, C.; Paul, S. HIV-1 Glycoprotein Immunogenicity. In Recent Translational Research in HIV/AIDS; InTech: London, UK, 2011. [Google Scholar]
- Cao, L.; Diedrich, J.K.; Kulp, D.W.; Pauthner, M.; He, L.; Park, S.K.R.; Sok, D.; Su, C.Y.; Delahunty, C.M.; Menis, S.; et al. Global Site-Specific N-Glycosylation Analysis of HIV Envelope Glycoprotein. Nat. Commun. 2017, 8, 14954. [Google Scholar] [CrossRef]
- Stansell, E.; Panico, M.; Canis, K.; Pang, P.C.; Bouché, L.; Binet, D.; O’Connor, M.J.; Chertova, E.; Bess, J.; Lifson, J.D.; et al. gp120 on HIV-1 Virions Lacks O-Linked Carbohydrate. PLoS ONE 2015, 10, e0124784. [Google Scholar] [CrossRef]
- Freed, E.O.; Myers, D.J.; Risser, R. Mutational Analysis of the Cleavage Sequence of the Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Precursor gp160. J. Virol. 1989, 63, 4670–4675. [Google Scholar] [CrossRef]
- McCune, J.M.; Rabin, L.B.; Feinberg, M.B.; Lieberman, M.; Kosek, J.C.; Reyes, G.R.; Weissman, I.L. Endoproteolytic Cleavage of gp160 Is Required for the Activation of Human Immunodeficiency Virus. Cell 1988, 53, 55–67. [Google Scholar] [CrossRef]
- Hallenberger, S.; Bosch, V.; Angliker, H.; Shaw, E.; Klenk, H.D.; Garten, W. Inhibition of Furin-Mediated Cleavage Activation of HIV-1 Glycoprotein Gpl60. Nature 1992, 360, 358–361. [Google Scholar] [CrossRef]
- Shaw, T.I.; Zhang, M. HIV N-Linked Glycosylation Site Analyzer and Its Further Usage in Anchored Alignment. Nucleic Acids Res. 2013, 41, W454. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.A.; Carruth, L.M.; Rowell, J.F.; Yu, X.; Siliciano, R.F. Human Immunodeficiency Virus Type 1 Envelope Protein Endocytosis Mediated by a Highly Conserved Intrinsic Internalization Signal in the Cytoplasmic Domain of gp41 Is Suppressed in the Presence of the Pr55gag Precursor Protein. J. Virol. 1996, 70, 6547–6556. [Google Scholar] [CrossRef] [PubMed]
- Rowell, J.F.; Ruff, A.L.; Guarnieri, F.G.; Staveley-O'Carroll, K.; Lin, X.; Tang, J.; August, J.T.; Siliciano, R.F. Lysosome-Associated Membrane Protein-1-Mediated Targeting of the HIV-1 Envelope Protein to an Endosomal/Lysosomal Compartment Enhances Its Presentation to MHC Class II-Restricted T Cells. J. Immunol. 1995, 155, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chertova, E.; Bess, J.; Lifson, J.D.; Arthur, L.O.; Liu, J.; Taylor, K.A.; Roux, K.H. Electron Tomography Analysis of Envelope Glycoprotein Trimers on HIV and Simian Immunodeficiency Virus Virions. Proc. Natl. Acad. Sci. USA 2003, 100, 15812–15817. [Google Scholar] [CrossRef] [PubMed]
- Mothes, W.; Sherer, N.M.; Jin, J.; Zhong, P. Virus Cell-to-Cell Transmission. J. Virol. 2010, 84, 8360–8368. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C. T Cell Polarization at the Virological Synapse. Viruses 2010, 2, 1261–1278. [Google Scholar] [CrossRef]
- Sattentau, Q.J. Cell-to-Cell Spread of Retroviruses. Viruses 2010, 2, 1306–1321. [Google Scholar] [CrossRef]
- Sherer, N.M.; Jin, J.; Mothes, W. Directional Spread of Surface-Associated Retroviruses Regulated by Differential Virus-Cell Interactions. J. Virol. 2010, 84, 3248–3258. [Google Scholar] [CrossRef]
- Sherer, N.M.; Lehmann, M.J.; Jimenez-Soto, L.F.; Horensavitz, C.; Pypaert, M.; Mothes, W. Retroviruses Can Establish Filopodial Bridges for Efficient Cell-to-Cell Transmission. Nat. Cell Biol. 2007, 9, 310–315. [Google Scholar] [CrossRef]
- Gousset, K.; Ablan, S.D.; Coren, L.V.; Ono, A.; Soheilian, F.; Nagashima, K.; Ott, D.E.; Freed, E.O. Real-Time Visualization of HIV-1 GAG Trafficking in Infected Macrophages. PLoS Pathog. 2008, 4, e1000015. [Google Scholar] [CrossRef]
- Waki, K.; Freed, E.O. Macrophages and Cell-Cell Spread of HIV-1. Viruses 2010, 2, 1603–1620. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sherer, N.M.; Heidecker, G.; Derse, D.; Mothes, W. Assembly of the Murine Leukemia Virus Is Directed towards Sites of Cell-Cell Contact. PLoS Biol. 2009, 7, e1000163. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Freed, E.O. Role of Lipid Rafts in Virus Replication. Adv. Virus Res. 2005, 64, 311–358. [Google Scholar] [PubMed]
- Leonard, C.K.; Spellman, M.W.; Riddle, L.; Harris, R.J.; Thomas, J.N.; Gregory, T.J. Assignment of Intrachain Bisulfide Bonds and Characterization of Potential Glycosylation Sites of the Type 1 Recombinant Human Immunodeficiency Virus Envelope Glycoprotein (gp120) Expressed in Chinese Hamster Ovary Cells. J. Biol. Chem. 1990, 265, 10373–10382. [Google Scholar] [CrossRef]
- Pancera, M.; Majeed, S.; Ban, Y.E.A.; Chen, L.; Huang, C.C.; Kong, L.; Kwon, Y.D.; Stuckey, J.; Zhou, T.; Robinson, J.E.; et al. Structure of HIV-1 gp120 with gp41-Interactive Region Reveals Layered Envelope Architecture and Basis of Conformational Mobility. Proc. Natl. Acad. Sci. USA 2010, 107, 1166–1171. [Google Scholar] [CrossRef]
- Dev, J.; Park, D.; Fu, Q.; Chen, J.; Ha, H.J.; Ghantous, F.; Herrmann, T.; Chang, W.; Liu, Z.; Frey, G.; et al. Structural Basis for Membrane Anchoring of HIV-1 Envelope Spike. Science 2016, 353, 172–175. [Google Scholar] [CrossRef]
- Murphy, R.E.; Samal, A.B.; Vlach, J.; Saad, J.S. Solution Structure and Membrane Interaction of the Cytoplasmic Tail of HIV-1 gp41 Protein. Structure 2017, 25, 1708–1718.e5. [Google Scholar] [CrossRef]
- Piai, A.; Fu, Q.; Cai, Y.; Ghantous, F.; Xiao, T.; Shaik, M.M.; Peng, H.; Rits-Volloch, S.; Chen, W.; Seaman, M.S.; et al. Structural Basis of Transmembrane Coupling of the HIV-1 Envelope Glycoprotein. Nat. Commun. 2020, 11, 2317. [Google Scholar] [CrossRef]
- Liu, J.; Bartesaghi, A.; Borgnia, M.J.; Sapiro, G.; Subramaniam, S. Molecular Architecture of Native HIV-1 gp120 Trimers. Nature 2008, 455, 109–113. [Google Scholar] [CrossRef]
- Zanetti, G.; Briggs, J.A.G.; Grünewald, K.; Sattentau, Q.J.; Fuller, S.D. Cryo-Electron Tomographic Structure of an Immunodeficiency Virus Envelope Complex in Situ. PLoS Pathog. 2006, 2, e83. [Google Scholar] [CrossRef]
- Sanders, R.W.; Derking, R.; Cupo, A.; Julien, J.P.; Yasmeen, A.; de Val, N.; Kim, H.J.; Blattner, C.; de la Peña, A.T.; Korzun, J.; et al. A Next-Generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 Gp140, Expresses Multiple Epitopes for Broadly Neutralizing but Not Non-Neutralizing Antibodies. PLoS Pathog. 2013, 9, e1003618. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, N.; Debbeche, O.; Sodroski, J.; Finzi, A. Effects of the I559P gp41 Change on the Conformation and Function of the Human Immunodeficiency Virus (HIV-1) Membrane Envelope Glycoprotein Trimer. PLoS ONE 2015, 10, e0122111. [Google Scholar] [CrossRef] [PubMed]
- Pancera, M.; Zhou, T.; Druz, A.; Georgiev, I.S.; Soto, C.; Gorman, J.; Huang, J.; Acharya, P.; Chuang, G.Y.; Ofek, G.; et al. Structure and Immune Recognition of Trimeric Pre-Fusion HIV-1 Env. Nature 2014, 514, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ozorowski, G.; Ward, A.B. Cryo-EM Structure of a Native, Fully Glycosylated, Cleaved HIV-1 Envelope Trimer. Science 2016, 351, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kovacs, J.M.; Peng, H.; Rits-Volloch, S.; Lu, J.; Park, D.; Zablowsky, E.; Seaman, M.S.; Chen, B. Effect of the Cytoplasmic Domain on Antigenic Characteristics of HIV-1 Envelope Glycoprotein. Science 2015, 349, 191–195. [Google Scholar] [CrossRef]
- Cai, Y.; Karaca-Griffin, S.; Chen, J.; Tian, S.; Fredette, N.; Linton, C.E.; Rits-Volloch, S.; Lu, J.; Wagh, K.; Theiler, J.; et al. Antigenicity-Defined Conformations of an Extremely Neutralization-Resistant HIV-1 Envelope Spike. Proc. Natl. Acad. Sci. USA 2017, 114, 4477–4482. [Google Scholar] [CrossRef]
- Stano, A.; Leaman, D.P.; Kim, A.S.; Zhang, L.; Autin, L.; Ingale, J.; Gift, S.K.; Truong, J.; Wyatt, R.T.; Olson, A.J.; et al. Dense Array of Spikes on HIV-1 Virion Particles. J. Virol. 2017, 91, 10–1128. [Google Scholar] [CrossRef]
- Castillo-Menendez, L.R.; Witt, K.; Espy, N.; Princiotto, A.; Madani, N.; Pacheco, B.; Finzi, A.; Sodroski, J. Comparison of Uncleaved and Mature Human Immunodeficiency Virus Membrane Envelope Glycoprotein Trimers. J. Virol. 2018, 92, e00277-18. [Google Scholar] [CrossRef]
- Hollingsworth, L.R.; Lemkul, J.A.; Bevan, D.R.; Brown, A.M. HIV-1 Env gp41 Transmembrane Domain Dynamics Are Modulated by Lipid, Water, and Ion Interactions. Biophys. J. 2018, 115, 84–94. [Google Scholar] [CrossRef]
- Piai, A.; Dev, J.; Fu, Q.; Chou, J.J. Stability and Water Accessibility of the Trimeric Membrane Anchors of the HIV-1 Envelope Spikes. J. Am. Chem. Soc. 2017, 139, 18432–18435. [Google Scholar] [CrossRef]
- Kovacs, J.M.; Nkolola, J.P.; Peng, H.; Cheung, A.; Perry, J.; Miller, C.A.; Seaman, M.S.; Barouch, D.H.; Chen, B. HIV-1 Envelope Trimer Elicits More Potent Neutralizing Antibody Responses than Monomeric gp120. Proc. Natl. Acad. Sci. USA 2012, 109, 12111–12116. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Shaik, M.M.; Cai, Y.; Ghantous, F.; Piai, A.; Peng, H.; Rits-Volloch, S.; Liu, Z.; Harrison, S.C.; Seaman, M.S.; et al. Structure of the Membrane Proximal External Region of HIV-1 Envelope Glycoprotein. Proc. Natl. Acad. Sci. USA 2018, 115, E8892–E8899. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Sun, Z.Y.J.; Coleman, K.E.; Zwick, M.B.; Gach, J.S.; Wang, J.H.; Reinherz, E.L.; Wagner, G.; Kim, M. Broadly Neutralizing Anti-HIV-1 Antibodies Disrupt a Hinge-Related Function of gp41 at the Membrane Interface. Proc. Natl. Acad. Sci. USA 2009, 106, 9057–9062. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sun, Z.Y.J.; Rand, K.D.; Shi, X.; Song, L.; Cheng, Y.; Fahmy, A.F.; Majumdar, S.; Ofek, G.; Yang, Y.; et al. Antibody Mechanics on a Membrane-Bound HIV Segment Essential for gp41-Targeted Viral Neutralization. Nat. Struct. Mol. Biol. 2011, 18, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Y.J.; Oh, K.J.; Kim, M.; Yu, J.; Brusic, V.; Song, L.; Qiao, Z.; Wang, J.H.; Wagner, G.; Reinherz, E.L. HIV-1 Broadly Neutralizing Antibody Extracts Its Epitope from a Kinked gp41 Ectodomain Region on the Viral Membrane. Immunity 2008, 28, 52–63. [Google Scholar] [CrossRef]
- Murakami, T.; Freed, E.O. Genetic Evidence for an Interaction between Human Immunodeficiency Virus Type 1 Matrix and α-Helix 2 of the gp41 Cytoplasmic Tail. J. Virol. 2000, 74, 3548–3554. [Google Scholar] [CrossRef]
- Munro, J.B.; Gorman, J.; Ma, X.; Zhou, Z.; Arthos, J.; Burton, D.R.; Koff, W.C.; Courter, J.R.; Smith, A.B.; Kwong, P.D.; et al. Conformational Dynamics of Single HIV-1 Envelope Trimers on the Surface of Native Virions. Science 2014, 346, 759–763. [Google Scholar] [CrossRef]
- Lu, M.; Ma, X.; Castillo-Menendez, L.R.; Gorman, J.; Alsahafi, N.; Ermel, U.; Terry, D.S.; Chambers, M.; Peng, D.; Zhang, B.; et al. Associating HIV-1 Envelope Glycoprotein Structures with States on the Virus Observed by SmFRET. Nature 2019, 568, 415–419. [Google Scholar] [CrossRef]
- Ivan, B.; Sun, Z.; Subbaraman, H.; Friedrich, N.; Trkola, A. CD4 Occupancy Triggers Sequential Pre-Fusion Conformational States of the HIV-1 Envelope Trimer with Relevance for Broadly Neutralizing Antibody Activity. PLoS Biol. 2019, 17, e3000114. [Google Scholar] [CrossRef]
- Do Kwon, Y.; Pancera, M.; Acharya, P.; Georgiev, I.S.; Crooks, E.T.; Gorman, J.; Joyce, M.G.; Guttman, M.; Ma, X.; Narpala, S.; et al. Crystal Structure, Conformational Fixation and Entry-Related Interactions of Mature Ligand-Free HIV-1 Env. Nat. Struct. Mol. Biol. 2015, 22, 522–531. [Google Scholar] [CrossRef]
- Yoon, V.; Fridkis-Hareli, M.; Munisamy, S.; Lee, J.; Anastasiades, D.; Stevceva, L. The gp120 Molecule of HIV-1 and Its Interaction with T Cells. Curr. Med. Chem. 2010, 17, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, J.P.; Strayer, D.S. Gene Delivery of Antioxidant Enzymes in HIV-1-Associated Neurocognitive Disorder. In HIV/AIDS: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–123. ISBN 9780128098547. [Google Scholar]
- Finzi, A.; Xiang, S.H.; Pacheco, B.; Wang, L.; Haight, J.; Kassa, A.; Danek, B.; Pancera, M.; Kwong, P.D.; Sodroski, J. Topological Layers in the HIV-1 gp120 Inner Domain Regulate gp41 Interaction and CD4-Triggered Conformational Transitions. Mol. Cell 2010, 37, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Vogan, E.M.; Gong, H.; Skehel, J.J.; Wiley, D.C.; Harrison, S.C. Structure of an Unliganded Simian Immunodeficiency Virus gp120 Core. Nature 2005, 433, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV Gp 120 Envelope Glycoprotein in Complex with the CD4 Receptor and a Neutralizing Human Antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.D.; Finzi, A.; Wu, X.; Dogo-Isonagie, C.; Lee, L.K.; Moore, L.R.; Schmidt, S.D.; Stuckey, J.; Yang, Y.; Zhou, T.; et al. Unliganded HIV-1 gp120 Core Structures Assume the CD4-Bound Conformation with Regulation by Quaternary Interactions and Variable Loops. Proc. Natl. Acad. Sci. USA 2012, 109, 5663–5668. [Google Scholar] [CrossRef]
- Huang, C.C.; Tang, M.; Zhang, M.Y.; Majeed, S.; Montabana, E.; Stanfield, R.L.; Dimitrov, D.S.; Korber, B.; Sodroski, J.; Wilson, I.A.; et al. Structural Biology: Structure of a V3-Containing HIV-1 gp120 Core. Science 2005, 310, 1025–1028. [Google Scholar] [CrossRef]
- Chohan, B.; Lang, D.; Sagar, M.; Korber, B.; Lavreys, L.; Richardson, B.; Overbaugh, J. Selection for Human Immunodeficiency Virus Type 1 Envelope Glycosylation Variants with Shorter V1-V2 Loop Sequences Occurs during Transmission of Certain Genetic Subtypes and May Impact Viral RNA Levels. J. Virol. 2005, 79, 6528–6531. [Google Scholar] [CrossRef]
- Sagar, M.; Wu, X.; Lee, S.; Overbaugh, J. Human Immunodeficiency Virus Type 1 V1-V2 Envelope Loop Sequences Expand and Add Glycosylation Sites over the Course of Infection, and These Modifications Affect Antibody Neutralization Sensitivity. J. Virol. 2006, 80, 9586–9598. [Google Scholar] [CrossRef]
- Curlin, M.E.; Zioni, R.; Hawes, S.E.; Liu, Y.; Deng, W.; Gottlieb, G.S.; Zhu, T.; Mullins, J.I. Hiv-1 Envelope Subregion Length Variation during Disease Progression. PLoS Pathog. 2010, 6, e1001228. [Google Scholar] [CrossRef]
- Lasky, L.A.; Nakamura, G.; Smith, D.H.; Fennie, C.; Shimasaki, C.; Patzer, E.; Berman, P.; Gregory, T.; Capon, D.J. Delineation of a Region of the Human Immunodeficiency Virus Type 1 gp120 Glycoprotein Critical for Interaction with the CD4 Receptor. Cell 1987, 50, 975–985. [Google Scholar] [CrossRef]
- Blumenthal, R.; Durell, S.; Viard, M. HIV Entry and Envelope Glycoprotein-Mediated Fusion. J. Biol. Chem. 2012, 287, 40841–40849. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.-H.; Finzi, A.; Pacheco, B.; Alexander, K.; Yuan, W.; Rizzuto, C.; Huang, C.-C.; Kwong, P.D.; Sodroski, J. A V3 Loop-Dependent gp120 Element Disrupted by CD4 Binding Stabilizes the Human Immunodeficiency Virus Envelope Glycoprotein Trimer. J. Virol. 2010, 84, 3147–3161. [Google Scholar] [CrossRef] [PubMed]
- Woollard, S.M.; Kanmogne, G.D. Maraviroc: A Review of Its Use in Hivinfection and Beyond. Drug Des. Devel. Ther. 2015, 9, 5447–5468. [Google Scholar] [PubMed]
- Garcia-Perez, J.; Staropoli, I.; Azoulay, S.; Heinrich, J.T.; Cascajero, A.; Colin, P.; Lortat-Jacob, H.; Arenzana-Seisdedos, F.; Alcami, J.; Kellenberger, E.; et al. A Single-Residue Change in the HIV-1 V3 Loop Associated with Maraviroc Resistance Impairs CCR5 Binding Affinity While Increasing Replicative Capacity. Retrovirology 2015, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Migueles, S.A.; Welcher, B.; Svehla, K.; Phogat, A.; Louder, M.K.; Wu, X.; Shaw, G.M.; Connors, M.; Wyatt, R.T.; et al. Broad HIV-1 Neutralization Mediated by CD4-Binding Site Antibodies. Nat. Med. 2007, 13, 1032–1034. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, T.; Yang, Z.; Svehla, K.; O’Dell, S.; Louder, M.K.; Xu, L.; Mascola, J.R.; Burton, D.R.; Hoxie, J.A.; et al. Enhanced Exposure of the CD4-Binding Site to Neutralizing Antibodies by Structural Design of a Membrane-Anchored Human Immunodeficiency Virus Type 1 gp120 Domain. J. Virol. 2009, 83, 5077–5086. [Google Scholar] [CrossRef][Green Version]
- Guttman, M.; Lee, K.K. A Functional Interaction between gp41 and gp120 Is Observed for Monomeric but Not Oligomeric, Uncleaved HIV-1 Env Gp140. J. Virol. 2013, 87, 11462–11475. [Google Scholar] [CrossRef]
- Scharf, L.; West, A.P.; Sievers, S.A.; Chen, C.; Jiang, S.; Gao, H.; Gray, M.D.; McGuire, A.T.; Scheid, J.F.; Nussenzweig, M.C.; et al. Structural Basis for Germline Antibody Recognition of HIV-1 Immunogens. eLife 2016, 5, e13783. [Google Scholar] [CrossRef]
- DeLaitsch, A.T.; Keeffe, J.R.; Gristick, H.B.; Lee, J.A.; Ding, W.; Liu, W.; Skelly, A.N.; Shaw, G.M.; Hahn, B.H.; Björkman, P.J. Neutralizing Antibodies Elicited in Sequentially Immunized Macaques Recognize V3 Residues on Altered Conformations of HIV-1 Env Trimer. bioRxiv 2024. [Google Scholar] [CrossRef]
- Ladinsky, M.S.; Zhu, L.; Ullah, I.; Uchil, P.D.; Kumar, P.; Kay, M.S.; Bjorkman, P.J. Electron Tomography Visualization of HIV-1 Virions Trapped by Fusion Inhibitors to Host Cells in Infected Tissues. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dam, K.M.A.; Fan, C.; Yang, Z.; Bjorkman, P.J. Intermediate Conformations of CD4-Bound HIV-1 Env Heterotrimers. Nature 2023, 623, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Carravilla, P.; Cruz, A.; Martin-Ugarte, I.; Oar-Arteta, I.R.; Torralba, J.; Apellaniz, B.; Pérez-Gil, J.; Requejo-Isidro, J.; Huarte, N.; Nieva, J.L. Effects of HIV-1 gp41-Derived Virucidal Peptides on Virus-like Lipid Membranes. Biophys. J. 2017, 113, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Lozada, C.; Barlow, T.M.A.; Gonzalez, S.; Lubin-Germain, N.; Ballet, S. Identification and Characteristics of Fusion Peptides Derived From Enveloped Viruses. Front. Chem. 2021, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Cai, Y.; Chen, B. Hiv-1 Entry and Membrane Fusion Inhibitors. Viruses 2021, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Murin, C.D.; Wilson, I.A.; Ward, A.B. Antibody Responses to Viral Infections: A Structural Perspective across Three Different Enveloped Viruses. Nat. Microbiol. 2019, 4, 734–747. [Google Scholar] [CrossRef]
- Shi, W.; Bohon, J.; Han, D.P.; Habte, H.; Qin, Y.; Cho, M.W.; Chance, M.R. Structural Characterization of HIV gp41 with the Membrane-Proximal External Region. J. Biol. Chem. 2010, 285, 24290–24298. [Google Scholar] [CrossRef]
- Apellániz, B.; Rujas, E.; Serrano, S.; Morante, K.; Tsumoto, K.; Caaveiro, J.M.M.; Jiménez, M.Á.; Nieva, J.L. The Atomic Structure of the HIV-1 gp41 Transmembrane Domain and Its Connection to the Immunogenic Membrane-Proximal External Region. J. Biol. Chem. 2015, 290, 12999–13015. [Google Scholar] [CrossRef]
- Steckbeck, J.D.; Sun, C.; Sturgeon, T.J.; Montelaro, R.C. Topology of the C-Terminal Tail of HIV-1 gp41: Differential Exposure of the Kennedy Epitope on Cell and Viral Membranes. PLoS ONE 2010, 5, e15261. [Google Scholar] [CrossRef]
- Lu, L.; Zhu, Y.; Huang, J.; Chen, X.; Yang, H.; Jiang, S.; Chen, Y.H. Surface Exposure of the HIV-1 Env Cytoplasmic Tail LLP2 Domain during the Membrane Fusion Process: Interaction with gp41 Fusion Core. J. Biol. Chem. 2008, 283, 16723–16731. [Google Scholar] [CrossRef]
- Aisenbrey, C.; Bechinger, B. Structure, Interactions and Membrane Topology of HIV gp41 Ectodomain Sequences. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183274. [Google Scholar] [CrossRef]
- He, L.; McAndrew, R.; Barbu, R.; Gifford, G.; Halacoglu, C.; Drouin-Allaire, C.; Weber, L.; Kristensen, L.G.; Gupta, S.; Chen, Y.; et al. Structure and Interactions of HIV-1 gp41 CHR-NHR Reverse Hairpin Constructs Reveal Molecular Determinants of Antiviral Activity. J. Mol. Biol. 2024, 436, 168650. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Sood, C.; Marin, M.; Aaron, J.; Gratton, E.; Salaita, K.; Melikyan, G.B. Super-Resolution Fluorescence Imaging Reveals That Serine Incorporator Protein 5 Inhibits Human Immunodeficiency Virus Fusion by Disrupting Envelope Glycoprotein Clusters. ACS Nano 2020, 14, 10929–10943. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, S.A.; Purdy, M.D.; Grover, J.R.; Yang, Z.; Poulos, S.; McIntire, W.E.; Tatham, E.A.; Erramilli, S.K.; Nosol, K.; Lai, K.K.; et al. Antiviral HIV-1 SERINC Restriction Factors Disrupt Virus Membrane Asymmetry. Nat. Commun. 2023, 14, 4368. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kondo, N.; Long, Y.; Xiao, D.; Iwamoto, A.; Matsuda, Z. Membrane Topology Analysis of HIV-1 Envelope Glycoprotein gp41. Retrovirology 2010, 7, 100. [Google Scholar] [CrossRef]
- Kennedy, R.C.; Henkel, R.D.; Pauletti, D.; Allan, J.S.; Lee, T.H.; Essex, M.; Dreesman, G.R. Antiserum to a Synthetic Peptide Recognizes the HTLV-III Envelope Glycoprotein. Science 1986, 231, 1556–1559. [Google Scholar] [CrossRef]
- Hollier, M.J.; Dimmock, N.J. The C-Terminal Tail of the gp41 Transmembrane Envelope Glycoprotein of HIV-1 Clades A, B, C, and D May Exist in Two Conformations: An Analysis of Sequence, Structure, and Function. Virology 2005, 337, 284–296. [Google Scholar] [CrossRef]
- Cleveland, S.M.; McLain, L.; Cheung, L.; Jones, T.D.; Hollier, M.; Dimmock, N.J. A Region of the C-Terminal Tail of the gp41 Envelope Glycoprotein of Human Immunodeficiency Virus Type 1 Contains a Neutralizing Epitope: Evidence for Its Exposure on the Surface of the Virion. J. Gen. Virol. 2003, 84, 591–602. [Google Scholar] [CrossRef]
- Liu, Q.; Acharya, P.; Dolan, M.A.; Zhang, P.; Guzzo, C.; Lu, J.; Kwon, A.; Gururani, D.; Miao, H.; Bylund, T.; et al. Quaternary Contact in the Initial Interaction of CD4 with the HIV-1 Envelope Trimer. Nat. Struct. Mol. Biol. 2017, 24, 370–378. [Google Scholar] [CrossRef]
- Lyumkis, D.; Julien, J.P.; De Val, N.; Cupo, A.; Potter, C.S.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; Carragher, B.; et al. Cryo-EM Structure of a Fully Glycosylated Soluble Cleaved HIV-1 Envelope Trimer. Science 2013, 342, 1484–1490. [Google Scholar] [CrossRef]
- Tedbury, P.R.; Freed, E.O. The Cytoplasmic Tail of Retroviral Envelope Glycoproteins. In Progress in Molecular Biology and Translational Science; NIH Public Access: Montgomery County, MD, USA, 2015; Volume 129, pp. 253–284. [Google Scholar]
- Wang, X.-F.; Wang, Y.-H.; Bai, B.; Zhang, M.; Chen, J.; Zhang, X.; Gao, M.; Wang, X. Truncation of the Cytoplasmic Tail of Equine Infectious Anemia Virus Increases Virion Production by Improving Env Cleavage and Plasma Membrane Localization. J. Virol. 2021, 95, e0108721. [Google Scholar] [CrossRef]
- Shacklett, B.L.; Weber, C.J.; Shaw, K.E.S.; Keddie, E.M.; Gardner, M.B.; Sonigo, P.; Luciw, P.A. The Intracytoplasmic Domain of the Env Transmembrane Protein Is a Locus for Attenuation of Simian Immunodeficiency Virus SIVmac in Rhesus Macaques. J. Virol. 2000, 74, 5836–5844. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.G.; Wyss, S.; Reeves, J.D.; Zolla-Pazner, S.; Hoxie, J.A.; Doms, R.W.; Baribaud, F. Truncation of the Cytoplasmic Domain Induces Exposure of Conserved Regions in the Ectodomain of Human Immunodeficiency Virus Type 1 Envelope Protein. J. Virol. 2002, 76, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.G.; Hoffman, T.L.; Baribaud, F.; Wyss, S.; LaBranche, C.C.; Romano, J.; Adkinson, J.; Sharron, M.; Hoxie, J.A.; Doms, R.W. Relationships between CD4 Independence, Neutralization Sensitivity, and Exposure of a CD4-Induced Epitope in a Human Immunodeficiency Virus Type 1 Envelope Protein. J. Virol. 2001, 75, 5230–5239. [Google Scholar] [CrossRef] [PubMed]
- Rowell, J.F.; Stanhope, P.E.; Siliciano, R.F. Endocytosis of Endogenously Synthesized HIV-1 Envelope Protein. Mechanism and Role in Processing for Association with Class II MHC. J. Immunol. 1995, 155, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.; Abada, P.; Nunez-Iglesias, J.; Cannon, P.M. Recruitment of the Adaptor Protein 2 Complex by the Human Immunodeficiency Virus Type 2 Envelope Protein Is Necessary for High Levels of Virus Release. J. Virol. 2006, 80, 2924–2932. [Google Scholar] [CrossRef]
- Chapuy-Regaud, S.; Allioux, C.; Capelli, N.; Migueres, M.; Lhomme, S.; Izopet, J. Vectorial Release of Human RNA Viruses from Epithelial Cells. Viruses 2022, 14, 231. [Google Scholar] [CrossRef]
- Fultz, P.N.; Vance, P.J.; Endres, M.J.; Tao, B.; Dvorin, J.D.; Davis, I.C.; Lifson, J.D.; Montefiori, D.C.; Marsh, M.; Malim, M.H.; et al. In Vivo Attenuation of Simian Immunodeficiency Virus by Disruption of a Tyrosine-Dependent Sorting Signal in the Envelope Glycoprotein Cytoplasmic Tail. J. Virol. 2001, 75, 278–291. [Google Scholar] [CrossRef][Green Version]
- Byland, R.; Vance, P.J.; Hoxie, J.A.; Marsh, M. A Conserved Dileucine Motif Mediates Clathrin and AP-2-Dependent Endocytosis of the HIV-1 Envelope Protein. Mol. Biol. Cell 2007, 18, 414–425. [Google Scholar] [CrossRef]
- Tencza, S.B.; Miller, M.A.; Islam, K.; Mietzner, T.A.; Montelaro, R.C. Effect of Amino Acid Substitutions on Calmodulin Binding and Cytolytic Properties of the LLP-1 Peptide Segment of Human Immunodeficiency Virus Type 1 Transmembrane Protein. J. Virol. 1995, 69, 5199–5202. [Google Scholar] [CrossRef]
- Rousso, I.; Mixon, M.B.; Chen, B.K.; Kim, P.S. Palmitoylation of the HIV-1 Envelope Glycoprotein Is Critical for Viral Infectivity. Proc. Natl. Acad. Sci. USA 2000, 97, 13523–13525. [Google Scholar] [CrossRef]
- Yang, P.; Ai, L.-S.; Huang, S.-C.; Li, H.-F.; Chan, W.-E.; Chang, C.-W.; Ko, C.-Y.; Chen, S.S.-L. The Cytoplasmic Domain of Human Immunodeficiency Virus Type 1 Transmembrane Protein gp41 Harbors Lipid Raft Association Determinants. J. Virol. 2010, 84, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Weissenhorn, W.; Dessen, A.; Harrison, S.C.; Skehel, J.J.; Wiley, D.C. Atomic Structure of the Ectodomain from HIV-1 gp41. Nature 1997, 387, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.E.H.; Borgnia, M.J.; Kuybeda, O.; Schauder, D.M.; Bartesaghi, A.; Frank, G.A.; Sapiro, G.; Milne, J.L.S.; Subramaniam, S. Structural Mechanism of Trimeric HIV-1 Envelope Glycoprotein Activation. PLoS Pathog. 2012, 8, e1002797. [Google Scholar] [CrossRef] [PubMed]
- Binley, J.M.; Sanders, R.W.; Clas, B.; Schuelke, N.; Master, A.; Guo, Y.; Kajumo, F.; Anselma, D.J.; Maddon, P.J.; Olson, W.C.; et al. A Recombinant Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Complex Stabilized by an Intermolecular Disulfide Bond between the gp120 and gp41 Subunits Is an Antigenic Mimic of the Trimeric Virion-Associated Structure. J. Virol. 2000, 74, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, B.; Alsahafi, N.; Debbeche, O.; Prévost, J.; Ding, S.; Chapleau, J.-P.; Herschhorn, A.; Madani, N.; Princiotto, A.; Melillo, B.; et al. Residues in the gp41 Ectodomain Regulate HIV-1 Envelope Glycoprotein Conformational Transitions Induced by gp120-Directed Inhibitors. J. Virol. 2017, 91, e02219-16. [Google Scholar] [CrossRef]
- Moscoso, C.G.; Sun, Y.; Poon, S.; Xing, L.; Kan, E.; Martin, L.; Green, D.; Lin, F.; Vahlne, A.G.; Barnett, S.; et al. Quaternary Structures of HIV Env Immunogen Exhibit Conformational Vicissitudes and Interface Diminution Elicited by Ligand Binding. Proc. Natl. Acad. Sci. USA. 2011, 108, 6091–6096. [Google Scholar] [CrossRef]
- Melikyan, G.B. Common Principles and Intermediates of Viral Protein-Mediated Fusion: The HIV-1 Paradigm. Retrovirology 2008, 5, 111. [Google Scholar] [CrossRef]
- Brandenberg, O.F.; Magnus, C.; Regoes, R.R.; Trkola, A. The HIV-1 Entry Process: A Stoichiometric View. Trends Microbiol. 2015, 23, 763–774. [Google Scholar] [CrossRef]
- Brandenberg, O.F.; Magnus, C.; Rusert, P.; Regoes, R.R.; Trkola, A. Different Infectivity of HIV-1 Strains Is Linked to Number of Envelope Trimers Required for Entry. PLoS Pathog. 2015, 11, e1004595. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, L.; Gu, C.; Herschhorn, A.; Désormeaux, A.; Finzi, A.; Xiang, S.H.; Sodroski, J.G. Molecular Architecture of the Uncleaved HIV-1 Envelope Glycoprotein Trimer. Proc. Natl. Acad. Sci. USA 2013, 110, 12438–12443. [Google Scholar] [CrossRef]
- Julien, J.P.; Cupo, A.; Sok, D.; Stanfield, R.L.; Lyumkis, D.; Deller, M.C.; Klasse, P.J.; Burton, D.R.; Sanders, R.W.; Moore, J.P.; et al. Crystal Structure of a Soluble Cleaved HIV-1 Envelope Trimer. Science 2013, 342, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; De Clercq, E. HIV Genome-Wide Protein Associations: A Review of 30 Years of Research. Microbiol. Mol. Biol. Rev. 2016, 80, 679–731. [Google Scholar] [CrossRef] [PubMed]
- York, J.; Nunberg, J.H. Role of Hydrophobic Residues in the Central Ectodomain of gp41 in Maintaining the Association between Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Subunits gp120 and gp41. J. Virol. 2004, 78, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R.; Mascola, J.R. Antibody Responses to Envelope Glycoproteins in HIV-1 Infection. Nat. Immunol. 2015, 16, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Leaman, D.P.; Kim, A.S.; Torrents De La Penã, A.; Sliepen, K.; Yasmeen, A.; Derking, R.; Ramos, A.; De Taeye, S.W.; Ozorowski, G.; et al. Antibodies to a Conformational Epitope on gp41 Neutralize HIV-1 by Destabilizing the Env Spike. Nat. Commun. 2015, 6, 8167. [Google Scholar] [CrossRef]
- West, J.T.; Weldon, S.K.; Wyss, S.; Lin, X.; Yu, Q.; Thali, M.; Hunter, E. Mutation of the Dominant Endocytosis Motif in Human Immunodeficiency Virus Type 1 gp41 Can Complement Matrix Mutations without Increasing Env Incorporation. J. Virol. 2002, 76, 3338–3349. [Google Scholar] [CrossRef]
- Tedbury, P.R.; Ablan, S.D.; Freed, E.O. Global Rescue of Defects in HIV-1 Envelope Glycoprotein Incorporation: Implications for Matrix Structure. PLoS Pathog. 2013, 9, e1003739. [Google Scholar] [CrossRef]
- Tedbury, P.R.; Mercredi, P.Y.; Gaines, C.R.; Summers, M.F.; Freed, E.O. Elucidating the Mechanism by Which Compensatory Mutations Rescue an Hiv-1 Matrix Mutant Defective for Gag Membrane Targeting and Envelope Glycoprotein Incorporation. J. Mol. Biol. 2015, 427, 1413–1427. [Google Scholar] [CrossRef]
- Dorfman, T.; Mammano, F.; Haseltine, W.A.; Göttlinger, H.G. Role of the Matrix Protein in the Virion Association of the Human Immunodeficiency Virus Type 1 Envelope Glycoprotein. J. Virol. 1994, 68, 1689–1696. [Google Scholar] [CrossRef]
- Abrahamyan, L.G.; Mkrtchyan, S.R.; Binley, J.; Lu, M.; Melikyan, G.B.; Cohen, F.S. The Cytoplasmic Tail Slows the Folding of Human Immunodeficiency Virus Type 1 Env from a Late Prebundle Configuration into the Six-Helix Bundle. J. Virol. 2005, 79, 106–115. [Google Scholar] [CrossRef]
- Murakami, T.; Freed, E.O. The Long Cytoplasmic Tail of gp41 Is Required in a Cell Type-Dependent Manner for HIV-1 Envelope Glycoprotein Incorporation into Virions. Proc. Natl. Acad. Sci. USA 2000, 97, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Ablan, S.; Freed, E.O.; Tanaka, Y. Regulation of Human Immunodeficiency Virus Type 1 Env-Mediated Membrane Fusion by Viral Protease Activity. J. Virol. 2004, 78, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Ladinsky, M.S.; Gnanapragasam, P.N.P.; Yang, Z.; West, A.P.; Kay, M.S.; Bjorkman, P.J. Electron Tomography Visualization of HIV-1 Fusion with Target Cells Using Fusion Inhibitors to Trap the Pre-Hairpin Intermediate. eLife 2020, 9, e58411. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.; Staudt, T.; Glass, B.; Bingen, P.; Engelhardt, J.; Anders, M.; Schneider, J.; Müller, B.; Hell, S.W.; Kräusslich, H.G. Maturation-Dependent HIV-1 Surface Protein Redistribution Revealed by Fluorescence Nanoscopy. Science 2012, 338, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Ke, Z.; Zila, V.; Anders-Össwein, M.; Glass, B.; Mücksch, F.; Müller, R.; Schultz, C.; Müller, B.; Kräusslich, H.G.; et al. Maturation of the Matrix and Viral Membrane of HIV-1. Science 2021, 373, 700–704. [Google Scholar] [CrossRef]
- Ritchie, C.; Cylinder, I.; Platt, E.J.; Barklis, E. Analysis of HIV-1 Gag Protein Interactions via Biotin Ligase Tagging. J. Virol. 2015, 89, 3988–4001. [Google Scholar] [CrossRef]
- Bhatia, A.K.; Campbell, N.; Panganiban, A.; Ratner, L. Characterization of Replication Defects Induced by Mutations in the Basic Domain and C-Terminus of HIV-1 Matrix. Virology 2007, 369, 47–54. [Google Scholar] [CrossRef][Green Version]
- Brandano, L.; Stevenson, M. A Highly Conserved Residue in the C-Terminal Helix of HIV-1 Matrix Is Required for Envelope Incorporation into Virus Particles. J. Virol. 2012, 86, 2347–2359. [Google Scholar] [CrossRef]
- Davis, M.R.; Jiang, J.; Zhou, J.; Freed, E.O.; Aiken, C. A Mutation in the Human Immunodeficiency Virus Type 1 Gag Protein Destabilizes the Interaction of the Envelope Protein Subunits gp120 and gp41. J. Virol. 2006, 80, 2405–2417. [Google Scholar] [CrossRef]
- Chan, W.-E.; Wang, Y.-L.; Lin, H.-H.; Chen, S.S.-L. Effect of Extension of the Cytoplasmic Domain of Human Immunodeficiency Type 1 Virus Transmembrane Protein gp41 on Virus Replication. J. Virol. 2004, 78, 5157–5169. [Google Scholar] [CrossRef]
- Rabi, S.A.; Laird, G.M.; Durand, C.M.; Laskey, S.; Shan, L.; Bailey, J.R.; Chioma, S.; Moore, R.D.; Siliciano, R.F. Multi-Step Inhibition Explains HIV-1 Protease Inhibitor Pharmacodynamics and Resistance. J. Clin. Invest. 2013, 123, 3848–3860. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, S.; Alfano, M.; Primo, L.; Gramaglia, D.; Butini, L.; Gennero, L.; De Vivo, E.; Arap, W.; Giacca, M.; Pasqualini, R.; et al. Cell Surface-Associated Tat Modulates HIV-1 Infection and Spreading through a Specific Interaction with gp120 Viral Envelope Protein. Blood 2005, 105, 2802–2811. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, A.; Ivanov, A.; Kumari, N.; Lin, X.; Wang, S.; Kovalskyy, D.; Nekhai, S. Targeting Tat–Tar Rna Interaction for Hiv-1 Inhibition. Viruses 2021, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Gotora, P.T.; van der Sluis, R.; Williams, M.E. HIV-1 Tat Amino Acid Residues That Influence Tat-TAR Binding Affinity: A Scoping Review. BMC Infect. Dis. 2023, 23, 164. [Google Scholar] [CrossRef]
- Poon, S.; Moscoso, C.G.; Yenigun, O.M.; Kolatkar, P.R.; Cheng, R.H.; Vahlne, A. HIV-1 Tat Protein Induces Viral Internalization through Env-Mediated Interactions in Dose-Dependent Manner. AIDS 2013, 27, 2355–2364. [Google Scholar] [CrossRef]
- Monini, P.; Cafaro, A.; Srivastava, I.K.; Moretti, S.; Sharma, V.A.; Andreini, C.; Chiozzini, C.; Ferrantelli, F.; Cossut, M.R.P.; Tripiciano, A.; et al. HIV-1 Tat Promotes Integrin-Mediated HIV Transmission to Dendritic Cells by Binding Env Spikes and Competes Neutralization by Anti-HIV Antibodies. PLoS ONE 2012, 7, e48781. [Google Scholar] [CrossRef]
- Cafaro, A.; Schietroma, I.; Sernicola, L.; Belli, R.; Campagna, M.; Mancini, F.; Farcomeni, S.; Pavone-Cossut, M.R.; Borsetti, A.; Monini, P.; et al. Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis. Int. J. Mol. Sci. 2024, 25, 1704. [Google Scholar] [CrossRef]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New Insights into Pathogenesis Point to HIV-1 Tat as a Key Vaccine Target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef]
- Cardaci, S.; Soster, M.; Bussolino, F.; Marchiò, S. The V1/V2 Loop of HIV-1 gp120 Is Necessary for Tat Binding and Consequent Modulation of Virus Entry. FEBS Lett. 2013, 587, 2943–2951. [Google Scholar] [CrossRef]
- Poon, S.; Moscoso, C.G.; Xing, L.; Kan, E.; Sun, Y.; Kolatkar, P.R.; Vahlne, A.G.; Srivastava, I.K.; Barnett, S.W.; Cheng, R.H. Putative Role of Tat-Env Interaction in HIV Infection. AIDS 2013, 27, 2345–2354. [Google Scholar] [CrossRef]
- Albini, A.; Ferrini, S.; Benelli, R.; Sforzini, S.; Giunciuglio, D.; Aluigi, M.G.; Proudfoot, A.E.I.; Alouani, S.; Wells, T.N.C.; Mariani, G.; et al. HIV-1 Tat Protein Mimicry of Chemokines. Proc. Natl. Acad. Sci. USA 1998, 95, 13153–13158. [Google Scholar] [CrossRef] [PubMed]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell. Infect. Microbiol. 2020, 10, 517857. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).